Abstract
Background
Clostridioides difficile infection (CDI) is a common healthcare-associated infection and is often used as an indicator of hospital safety or quality. However, healthcare exposures occurring prior to hospitalization may increase risk for CDI. We conducted a case-control study comparing hospitalized patients with and without CDI to determine if healthcare exposures prior to hospitalization (ie, clinic visits, antibiotics, family members with CDI) were associated with increased risk for hospital-onset CDI, and how risk varied with time between exposure and hospitalization.
Methods
Records were collected from a large insurance-claims database from 2001 to 2017 for hospitalized adult patients. Prior healthcare exposures were identified using inpatient, outpatient, emergency department, and prescription drug claims; results were compared between various CDI case definitions.
Results
Hospitalized patients with CDI had significantly more frequent healthcare exposures prior to admission. Healthcare visits, antibiotic use, and family exposures were associated with greater likelihood of CDI during hospitalization. The degree of association diminished with time between exposure and hospitalization. Results were consistent across CDI case definitions.
Conclusions
Many different prior healthcare exposures appear to increase risk for CDI presenting during hospitalization. Moreover, patients with CDI typically have multiple exposures prior to admission, confounding the ability to attribute cases to a particular stay.
Keywords: Clostridioides difficile, healthcare-associated infection, epidemiologic surveillance, infection control, hospitalization
This retrospective case-control study evaluated exposures prior to hospital admission associated with risk for Clostridioides difficile infection (CDI). Multiple healthcare exposures, including prior hospitalization, emergency department visits, outpatient care, long-term care, and antibiotics, were associated with increased risk for CDI during a hospital stay.
Clostridioides difficile is a leading cause of healthcare-associated infections and a major cause of morbidity, mortality, and healthcare costs [1–3]. In recent decades, the incidence of C. difficile infection (CDI) has increased [4–6]. CDI prevention is now an important goal of infection control and antibiotic stewardship programs [7], and institutional CDI incidence is considered a reportable measure of hospital quality and safety [8]. Yet measuring healthcare quality using CDI incidence assumes that CDI cases can be accurately attributed to specific healthcare exposures.
To increase the comparability of CDI rates across different institutions, CDI cases are classified as 1 of 3 mutually exclusive types: healthcare-facility onset, community-onset healthcare-facility associated, and community associated [6]. These definitions standardize surveillance and disease reporting, but identifying the source of a CDI case remains a challenge. Even with genotyping, it is difficult to identify epidemiological links between patients diagnosed with CDI [9–11]. Furthermore, patients at risk of acquiring CDI [12, 13] often have multiple prior healthcare exposures (eg, antibiotic use, healthcare facility visits). Thus, a substantial proportion of cases diagnosed during a hospital admission may actually be attributable to prior exposures. There is a need to explore the effects of multiple healthcare and antibiotic exposures prior to hospitalizations to more clearly understand why hospitalized patients develop CDI.
The goal of this study was to estimate the risk for CDI diagnosed during hospitalizations associated with prior healthcare and antibiotic exposures. Specifically, we conducted a retrospective case-control study using a large insurance-claims dataset that captures inpatient care, outpatient care, and outpatient prescriptions. We also investigated how CDI risk changed as a function of time between healthcare exposures.
METHODS
Data
We conducted a retrospective case-control study using the Truven MarketScan Commercial Claims and Encounters and Medicare Supplemental databases from 2001 to 2017. These represent the largest databases of longitudinal commercial insurance claims in the United States, and contain claims from inpatient, outpatient, and emergency department (ED) visits along with outpatient prescription medications. Information is available for diagnoses, procedures, charges, enrollment status, and other visit characteristics. Individual patients are deidentified, but a unique enrollee identification number (ID) allows claims from the same enrollee to be linked across time, and an enrollment family ID links claims among family members enrolled in the same plan.
Study Population
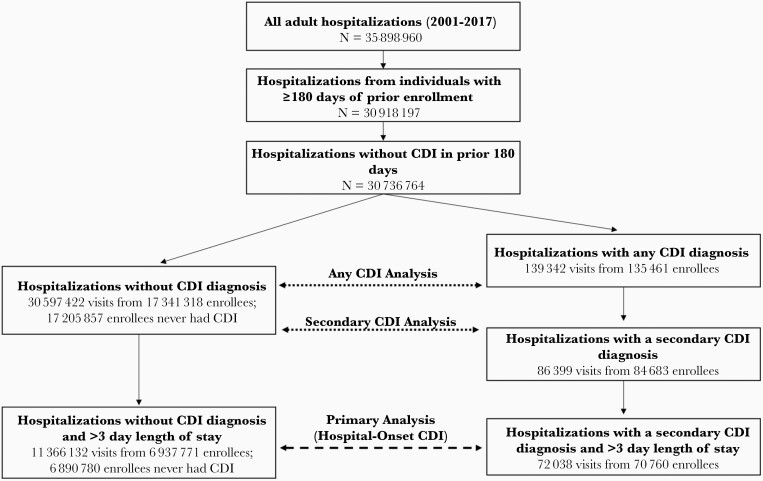
Figure 1 presents the selection criteria for this study. Our initial population included all potential hospitalizations during 2001–2017 for patients 18 years of age or older. We restricted the analysis to visits where the patient was enrolled for at least 180 days prior to admission, and we focused on potential exposures and risk factors for CDI occurring up to 180 days prior to an index hospitalization. To avoid recurrent and unresolved cases of CDI, we excluded hospitalizations from patients with any CDI diagnosis in the previous 180 days. We divided hospitalizations into cases or controls based on having a CDI event during the hospitalization. We identify cases of CDI using diagnosis codes 008.45 (International Classification of Diseases, Ninth Revision, Clinical Modification) or A04.7, A04.71, and A04.72 (International Classification of Diseases, Tenth Revision, Clinical Modification), and record whether the code was listed as the principal (first) or a secondary (second or later) diagnosis.
Figure 1.
Breakdown of study inclusion criteria for case and control patients along with study population sizes. The primary study analysis of hospital-onset Clostridioides difficile infection (CDI) (secondary CDI with >3 day length of stay) is highlighted by the dashed arrow, and the 2 secondary analyses (any secondary CDI cases and all CDI cases) are highlighted by the dotted arrows. The number of distinct enrollees in each group is also described; our primary analysis is restricted to 1 hospitalization per enrollee.
Our focus was hospital-onset CDI; however, our claims data do not allow us to directly identify the date of symptom onset. Thus, we restricted our primary analysis to plausible hospital-onset cases—where the CDI diagnosis was (1) listed as a secondary diagnosis code (ie, non–principal diagnosis) and (2) the patient had a length of stay >3 days. We henceforth refer to these cases as hospital-onset CDI cases. Control visits were defined as hospitalizations with no CDI diagnosis that also had a stay >3 days. Because secondary diagnostic codes are an imperfect marker for symptom onset after admission, and we cannot directly identify date of symptom onset, we conducted 2 secondary analyses. First, we repeated our analysis using all secondary CDI diagnoses and all controls regardless of length of stay. Second, we repeated our analysis using all CDI cases (identified by principal or secondary diagnosis) and all controls regardless of length of stay.
The unit of analysis is individual hospitalizations. Individual enrollees may have multiple (likely correlated) hospitalizations during the study period, thereby violating the independence assumptions of our statistical models (described below). Because of the large size of our dataset, we account for this independence violation by restricting our primary analysis to a single randomly drawn index hospitalization per enrollee. We subsequently conducted a sensitivity analysis where we included all hospitalizations from patients with multiple hospitalizations.
Identification of Exposures Prior to Hospitalization
We identified “prior-exposure” events occurring within 180 days before the hospital admission. We considered 3 types of prior exposures: (1) healthcare visits, (2) antibiotic use, and (3) CDI in a family member. Prior healthcare visits were subdivided into 5 types of facilities: inpatient hospital, ED, nursing home or long-term care facility, ambulatory surgery center, or other outpatient facility. We also included indicators for exposure to either “low-CDI-risk” antibiotics (penicillins, macrolides, sulfonamides, trimethoprim, tetracyclines, and first-generation cephalosporins) or “high-CDI-risk” antibiotics (clindamycin, fluoroquinolones, cephalosporins, carbapenems, ampicillin-sulbactam, piperacillin-tazobactam, later-generation cephalosporins) identified by outpatient prescription medication claims [14–17]. Finally, we created an indicator for familial CDI (ie, family member diagnosed with CDI in the previous 180 days) in either inpatient or outpatient settings, because such individuals may be at increased risk for CDI [18–20]. We use the enrollment family ID to identify enrollees in the same insurance plan (note that we could only link members enrolled in the same healthcare plan).
Statistical Analysis
We used logistic regression to estimate the likelihood of CDI during a given hospitalization as a function of prior exposures and other patient characteristics. Our hypothesis was that prior exposures (ie, healthcare visits, antibiotics) would (1) increase the risk for CDI during a given hospital stay and (2) this risk would decay with time from the most recent prior exposure (ie, patients become decolonized over time). For each hospitalization, we found the most recent previous exposure in each category (ie, 5 healthcare types, 2 antibiotic types, familial CDI). Exposures were assorted into disjoint, contiguous, 30-day bins based on time prior to hospitalization. For example, a patient who last visited an ED 40 days prior to hospitalization would receive “1” for the 31- to 60-day ED indicator and “0” for all other ED indicators. If that same patient received a high-risk antibiotic 20 days prior to hospitalization, they would receive “1” for the 0- to 30-day high-risk-antibiotic indicator as well. We compared trends across estimates for these intervals as time before the index hospitalization increased.
In addition to prior exposures, we controlled for patient age, sex, length of stay, and comorbidities. We binned age into the groups 18–26, 27–44, 45–64, and ≥65. We used the Elixhauser comorbidity indicators to identify individual comorbidities (eg, diabetes, congestive heart failure) and included each indicator in the model [21]. Finally, because CDI incidence and testing have changed over time [22–24] and often exhibit seasonal patterns [25, 26], we included separate indicators for the year and month in which the hospitalization occurred.
RESULTS
Selection of our study participants is described in Figure 1. There were 30 736 764 hospitalizations among adult patients enrolled for 180 days prior without CDI in the prior period. Of these, 139 342 had a CDI diagnosis, 86 399 of which were secondary diagnoses, and 72 038 secondary diagnoses had a length of stay >3 days. A total of 11 366 132 hospitalizations without CDI also had a length of stay >3 days. After restricting analysis to a single hospitalization per enrollee, we were left with 70 760 CDI visits and 6 890 780 non-CDI visits with a length of stay >3 days as our primary case and control populations. Similarly, secondary study populations included 84 683 secondary CDI cases, 135 461 any CDI cases, and 17 205 857 non-CDI controls.
Table 1 provides a summary of the number of individuals with exposures under study and baseline characteristics. In general, hospitalizations with CDI occurred among patients who were older, had more comorbidities, and longer lengths of stay. Patients with CDI were also more likely to have experienced each of the exposures under study within 180 days prior to the index hospitalization. The most common prior exposures were other outpatient visits, hospitalizations, or ED visits, while the least common were ambulatory surgery, nursing home/long-term care, or family CDI.
Table 1.
Baseline Characteristics for Final Study Populations Used for Primary and Secondary Analysis: 2001–2017 Nationwide Hospitalizations in IBM MarketScan Research Databasea
| Characteristic | Primary Case/Control Definitions (LOS >3 d) |
Secondary Analysis | |||
|---|---|---|---|---|---|
| HO-CDI Cases (Secondary CDI) | No CDI | All Secondary CDI Cases | Any CDI Case | No CDI Controls | |
| No. of patients | 70 760 (100) | 6 890 780 (100) | 84 683 (100) | 135 461 (100) | 17 205 857 (100) |
| Age, y | |||||
| 18–26 | 2241 (3.17) | 478 965 (6.95) | 3013 (3.56) | 5094 (3.76) | 1 668 118 (9.7) |
| 27–44 | 6391 (9.03) | 1 301 627 (18.89) | 8456 (9.99) | 14 796 (10.92) | 5 258 516 (30.56) |
| 45–64 | 29 046 (41.05) | 2 569 394 (37.29) | 34 587 (40.84) | 55 104 (40.68) | 6 137 416 (35.67) |
| ≥65 | 33 082 (46.75) | 2 540 794 (36.87) | 38 627 (45.61) | 60 467 (44.64) | 4 141 807 (24.07) |
| Female sex | 38 469 (54.37) | 3 931 557 (57.06) | 46 828 (55.3) | 80 205 (59.21) | 11 086 895 (64.44) |
| Length of stay, d | |||||
| 1–3 | … | … | 14 066 (16.61) | 34 858 (25.73) | 11 902 198 (69.18) |
| 4–6 | 18 707 (26.44) | 4 329 780 (62.83) | 18 652 (22.03) | 35 882 (26.49) | 3 335 259 (19.38) |
| 7–9 | 13 882 (19.62) | 1 271 083 (18.45) | 13 842 (16.35) | 20 360 (15.03) | 969 607 (5.64) |
| 10–12 | 8907 (12.59) | 498 691 (7.24) | 8889 (10.5) | 11 540 (8.52) | 382 302 (2.22) |
| 13–15 | 6596 (9.32) | 276 068 (4.01) | 6590 (7.78) | 7945 (5.87) | 212 810 (1.24) |
| >15 | 22 668 (32.04) | 515 158 (7.48) | 22 644 (26.74) | 24 876 (18.36) | 403 681 (2.35) |
| Exposures within 180 d of hospitalization | |||||
| Prior hospitalization | 38 317 (54.15) | 1 475 433 (21.41) | 44 177 (52.17) | 64 705 (47.77) | 1 805 205 (10.49) |
| Prior nursing home/LTC visit | 13 200 (18.65) | 285 438 (4.14) | 15 079 (17.81) | 21 199 (15.65) | 354 003 (2.06) |
| Prior ED visit | 23 370 (33.03) | 1 609 488 (23.36) | 28 289 (33.41) | 46 020 (33.97) | 3 326 621 (19.33) |
| Prior ambulatory surgery | 5715 (8.08) | 455 667 (6.61) | 6907 (8.16) | 11 707 (8.64) | 960 698 (5.58) |
| Other prior outpatient visit | 68 137 (96.29) | 6 468 856 (93.88) | 81 653 (96.42) | 131 107 (96.79) | 16 215 109 (94.24) |
| Prior family member with CDI | 121 (0.17) | 3492 (0.05) | 144 (0.17) | 276 (0.2) | 7016 (0.04) |
| Low-risk antibiotic use | 17 082 (24.14) | 1 265 832 (18.37) | 20 876 (24.65) | 34 638 (25.57) | 2 717 009 (15.79) |
| High-risk antibiotic use | 27 504 (38.87) | 1 787 244 (25.94) | 33 672 (39.76) | 75 710 (55.89) | 3 694 760 (21.47) |
| No. of comorbidities | |||||
| 0 | 6638 (9.38) | 1 383 724 (20.08) | 8876 (10.48) | 16 663 (12.3) | 6 279 682 (36.5) |
| 1 | 14 442 (20.41) | 1 809 495 (26.26) | 18 012 (21.27) | 31 848 (23.51) | 4 501 401 (26.16) |
| 2 | 17 420 (24.62) | 1 571 702 (22.81) | 20 627 (24.36) | 32 501 (23.99) | 3 156 292 (18.34) |
| 3 | 15 520 (21.93) | 1 098 163 (15.94) | 18 080 (21.35) | 26 811 (19.79) | 1 835 343 (10.67) |
| 4 | 10 026 (14.17) | 628 427 (9.12) | 11 507 (13.59) | 16 798 (12.4) | 917 555 (5.33) |
| 5 | 4669 (6.6) | 280 488 (4.07) | 5319 (6.28) | 7735 (5.71) | 371 667 (2.16) |
| 6 | 1610 (2.28) | 92 634 (1.34) | 1767 (2.09) | 2454 (1.81) | 114 138 (0.66) |
| 7 | 380 (0.54) | 22 139 (0.32) | 431 (0.51) | 557 (0.41) | 25 364 (0.15) |
| ≥8 | 55 (0.08) | 4008 (0.06) | 64 (0.08) | 94 (0.07) | 4415 (0.03) |
Data are presented as No. (%).
Abbreviations: ED, emergency department; HO-CDI, hospital-onset Clostridioides difficile infection; LTC, long-term care; LOS, length of stay.
aSimilar counts corresponding to all hospitalizations (ie, not restricted to 1 hospitalization per enrollee) can be found in Supplementary Table 2.
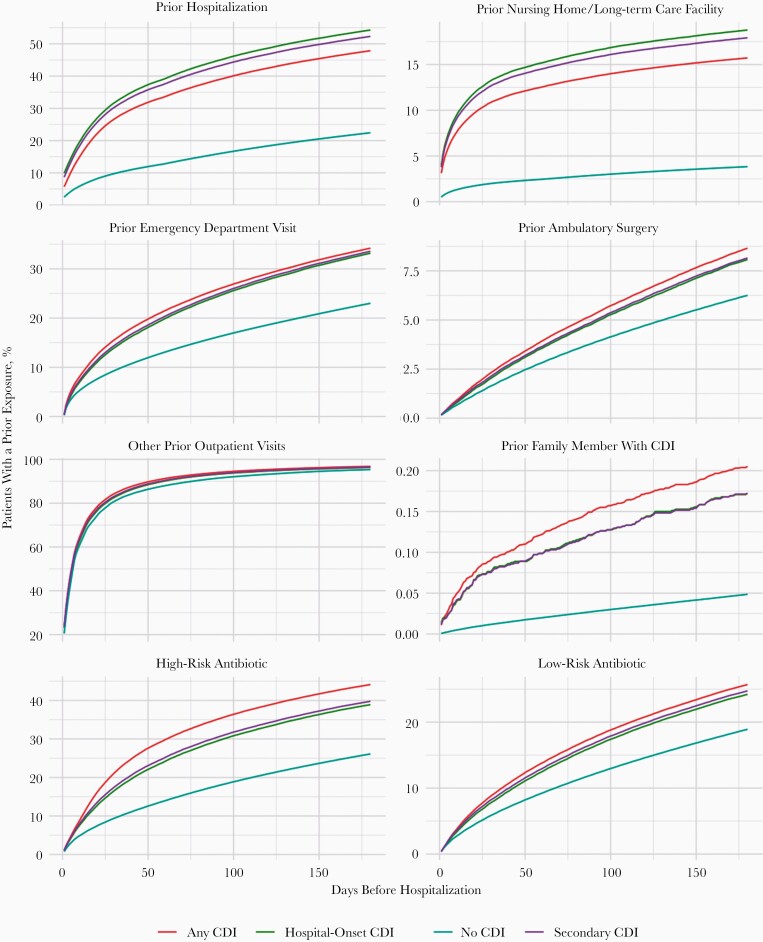
Figure 2 presents the cumulative percentage of patients who had each of the prior health exposures within a number of days prior to hospitalization. Exposure percentages are broken down for hospital-onset CDI cases, secondary CDI cases, all CDI cases, and controls without CDI. With the exception of other outpatient visits, CDI cases look dramatically different than non-CDI controls in terms of the percentage of patients who experienced each prior exposure: a much greater percentage of CDI cases experienced health-related exposures prior to admission. For exposures such as prior hospitalization, nursing home or long-term care, high-risk antibiotic use, or familial CDI, cases were more than twice as likely to have the exposure compared to controls for each period prior to admission. This was consistent for all 3 CDI case definitions.
Figure 2.
Cumulative percent of hospitalization that had each of the health-related exposures prior to hospitalization. Percentages are broken down for all Clostridioides difficile infection (CDI) cases, secondary CDI cases, secondary CDI cases with a length of stay >3 days, and for hospitalizations without CDI. For each of the exposures and each time period prior to hospitalization, CDI cases were much more likely to have a given exposure regardless of CDI case definition. Counts correspond to all possible hospitalizations (ie, we do not restrict counts to 1 hospitalization per patient).
Table 2 presents counts and percentages of patients who had multiple distinct healthcare-setting exposures prior to hospitalization. We counted how many of the 4 distinct healthcare settings (hospitalization, ED, ambulatory surgery, or nursing home/long-term care) each patient encountered prior to hospitalization (counts of “other outpatient care” were excluded, because nearly all patients had some outpatient care prior to hospitalization). Compared to patients without CDI, those with hospital-onset CDI were more likely to have had at least 1 of these 4 prior exposures or to have had multiple healthcare exposures. Within 30 days prior to hospitalization, 48% of hospital-onset CDI cases had exposure to at least 1 of the 4 settings and 12% had been exposed to 2 or more settings. In comparison, only 22% of the control patients had been exposed to 1 of these settings and <3% had been exposed to multiple settings within 30 days prior to hospitalization. Within 180 days prior to hospitalization, 34% of case patients but only 11% of non-CDI control patients had been exposed to multiple settings.
Table 2.
Patients With Multiple Prior Healthcare Exposures, Excluding “Other Outpatient Care”: 2001–2017 Nationwide Hospitalizations in IBM MarketScan Research Databasea
| Setting | 30 Days | 60 Days | 90 Days | 120 Days | 150 Days | 180 Days |
|---|---|---|---|---|---|---|
| Hospital-onset CDI | ||||||
| No exposure | 37 006 (52.3) | 30 893 (43.66) | 26 773 (37.84) | 23 998 (33.91) | 21 920 (30.98) | 20 226 (28.58) |
| ≥1 setting | 33 754 (47.7) | 39 867 (56.34) | 43 987 (62.16) | 46 762 (66.09) | 48 840 (69.02) | 50 534 (71.42) |
| ≥2 settings | 8430 (11.91) | 13 238 (18.71) | 16 802 (23.75) | 19 637 (27.75) | 22 064 (31.18) | 24 045 (33.98) |
| ≥3 settings | 848 (1.2) | 2004 (2.83) | 3068 (4.34) | 4176 (5.9) | 5175 (7.31) | 6144 (8.68) |
| No CDI (and LOS ≥3 d) | ||||||
| No exposure | 5 386 358 (78.17) | 4 967 053 (72.08) | 4 631 107 (67.21) | 4 363 673 (63.33) | 4 139 714 (60.08) | 3 945 419 (57.26) |
| ≥1 setting | 1 504 422 (21.83) | 1 923 727 (27.92) | 2 259 673 (32.79) | 2 527 107 (36.67) | 2 751 066 (39.92) | 2 945 361 (42.74) |
| ≥2 settings | 170 571 (2.48) | 303 443 (4.4) | 431 516 (6.26) | 553 980 (8.04) | 668 766 (9.71) | 775 793 (11.26) |
| ≥3 settings | 8816 (0.13) | 23 606 (0.34) | 41 189 (0.6) | 61 818 (0.9) | 84 259 (1.22) | 108 363 (1.57) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CDI, Clostridioides difficile infection; LOS, length of stay.
aFor each patient, we counted the number of different types of different healthcare settings (ie, hospitalization, emergency department [ED], ambulatory surgery, or nursing home/long-term care facility) they encountered in a given window prior to admission. For example, a patient who had a hospitalization, ED visit, and ambulatory surgery visit within 30 days of admission would receive a count of 2, whereas a patient who only encountered an ED in the prior 30 days would receive a count of 1.
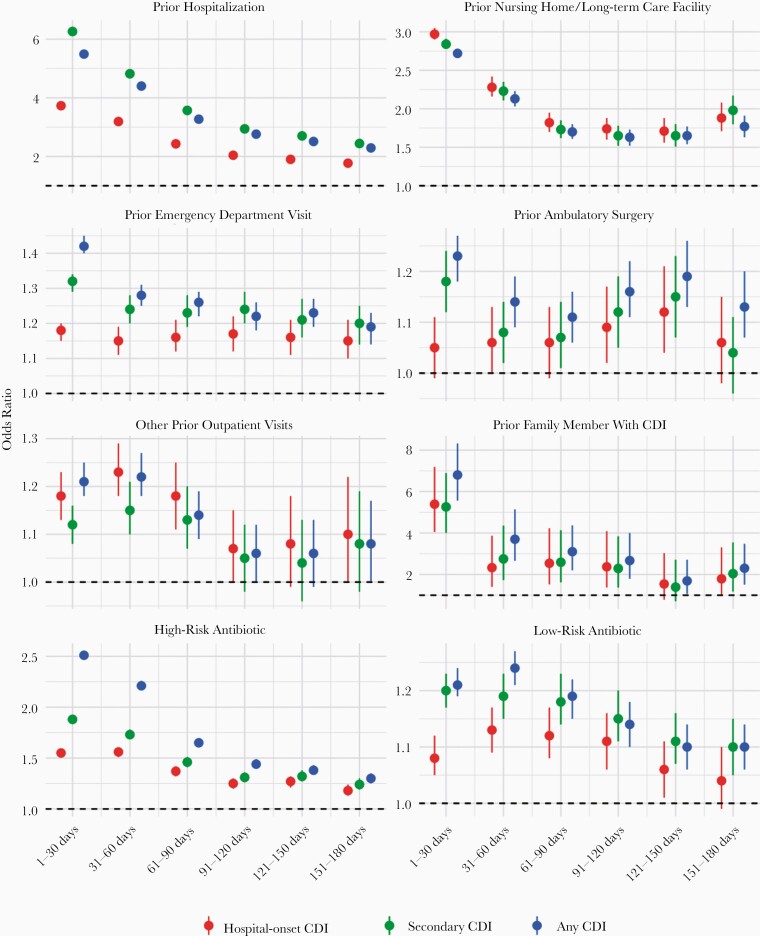
Figure 3 summarizes estimates from the regression models evaluating risk associated with prior exposures; odds ratios (ORs) and corresponding 95% confidence intervals (CIs) are given for each exposure window. There are 2 distinguishable trends in these estimates. First, nearly all of the prior exposure variables considered were associated with increased CDI risk (the exception being “other outpatient” visits for all secondary CDI cases). Second, in general for each prior exposure, the risks associated with exposure diminished with time prior to hospitalization. In general, for most exposures and case definitions, the risk can be seen to decay with time. One exception is ambulatory surgery, which showed a slight increase in risk during the 90- to 150-day interval.
Figure 3.
Logistic regression results: relative odds of Clostridioides difficile infection (CDI) for each type of exposure as a function of the time between prior exposure and admission. Odds ratios are presented for the 3 different regression models based on CDI case definition: hospital-onset CDI (defined as secondary cases with length of stay >3 days; red), all secondary cases (green), and all CDI cases (blue). Regardless of CDI case definition, for each exposure there is a fairly consistent pattern where the risk of CDI decays with increasing time prior to admission. Exact values can be found in Supplementary Table 1. The slight downward trend for low-risk antibiotics in 1–31 days likely reflects the fact that the exposure risk window cannot be fully observed in this period (eg, a 14-day course of antibiotics beginning 7 days prior to admission). See Supplementary Figure 2 for similar trends for high- and low-risk antibiotics broken down by treatment duration ≤14 days or >14 days.
Of the healthcare settings evaluated, prior hospitalization and nursing home/long-term care exhibited the strongest and most consistent trend. For hospital-onset CDI, risk associated with a prior hospitalization declined, nearly monotonically, from an OR of 3.73 (95% CI, 3.66–3.80) in 0–30 days to 1.77 (95% CI, 1.68–1.86) for 151–180 days prior to hospitalization. Risk associated with nursing home/long-term care exposure decreased from an OR of 2.97 (95% CI, 2.90–3.05) at 0–30 days and roughly leveled off to an OR between 1.7 and 1.8 for periods >90 days prior to hospitalization. Risk associated with prior ED visits changed very little with time, beginning at 1.18 (95% CI, 1.15–1.20) for visits 0–30 days prior to hospitalization and remaining around 1.15–1.16 for periods >30 days prior to hospitalization, although risk associated with ED exposure did decline with time since exposure for secondary and all CDI cases. Risk associated with prior ambulatory surgery did not appear to decline with time for hospital-onset CDI, but generally declined for secondary and all CDI cases, notwithstanding a slight uptick between 90 and 150 days. These inconsistent patterns likely reflect the smaller number of observations available for ambulatory surgery, as reflected by the wide CIs. Other prior outpatient visits conveyed increased risk for hospital-onset CDI, secondary CDI, and all CDI cases. For hospital-onset CDI, the OR for other outpatient visits varied between 1.18 (95% CI, 1.13–1.23) and 1.23 (95% CI, 1.18–1.29) for visits between 0 and 90 days prior to hospitalization and diminished to no significant risk for 90 days prior. For all secondary CDI cases, there did not appear to be an increased risk due to other prior outpatient visits.
For each period prior to hospitalization, the risk associated with high-CDI-risk antibiotics was greater than low-CDI-risk antibiotics. For low-CDI-risk antibiotics, the increased risk associated with prior exposure significantly diminished by 60 days prior to hospitalization; this was consistent for all CDI case definitions. However, high-risk antibiotics were associated with significantly increased risk even up to 180 days prior to hospitalization. For hospital-onset CDI cases, the OR for high-CDI-risk antibiotics decreased from 1.55 (95% CI, 1.52–1.59) for 0–30 days prior to hospitalization to 1.18 (95% CI, 1.13–1.24) for 151–180 days prior.
The greatest relative risk, in terms of OR magnitude, was familial exposure. In the period 0–30 days prior to hospitalization, the ORs associated with family exposure were 5.39 (95% CI, 4.05–7.19), 5.26 (95% CI, 4.01–6.89), and 6.80 (95% CI, 5.56–8.32) for hospital-onset, secondary, and all CDI cases, respectively. The large association of family exposure diminished quickly, yielding ORs between 2 and 3 in the 30–120 days prior to hospitalization to slightly below 2 in the 120–180 days prior to hospitalization. However, given the low incidence of family exposure (Table 1), the absolute and attributable risk is very low.
Across many of the prior exposures evaluated, there was a consistent trend between exposure risk and CDI-case definition. In general, for each exposure period, the hospital-onset CDI case cohort had the lowest risk, whereas all secondary or all CDI cases had slightly greater levels of risk (Figure 3). In some cases, such as prior hospitalization or nursing home/long-term care exposures, secondary CDI cases were associated with the highest level of risk, while for others, such as ED, ambulatory surgery, and outpatient antibiotic exposures, all CDI cases had more elevated risk. For outpatient visits and family CDI exposures, there was no major difference between case definitions. The largest difference in terms of risk among case definitions was for outpatient antibiotic exposures (both high and low risk).
Table 3 provides a summary of other patient characteristics from the regression model.
Table 3.
Regression Results for Regression Coefficients Not Associated With Prior Exposuresa
| Coefficient | Hospital-Onset CDI (Secondary Diagnosis and >3 Day LOS) | CDI as a Secondary Diagnosis | CDI as Any Diagnosis | |||
|---|---|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Age, y | ||||||
| 18–26 | (reference) | (reference) | (reference) | |||
| 27–44 | 0.99 | (.94–1.04) | 0.97 | (.93–1.01) | 0.98 | (.95–1.01) |
| 45–64 | 1.64 | (1.57–1.72) | 2.01 | (1.94–2.09) | 1.99 | (1.93–2.05) |
| ≥65 | 1.8 | (1.72–1.88) | 2.68 | (2.57–2.79) | 2.68 | (2.6–2.77) |
| Female sex | 1.02 | (1–1.04) | 1 | (.98–1.01) | 1.1 | (1.09–1.12) |
| Length of stay | 1.02 | (1.02–1.02) | 1.03 | (1.03–1.03) | 1.03 | (1.02–1.03) |
| Year | ||||||
| 2001 | (reference) | (reference) | (reference) | |||
| 2002 | 1.43 | (1.21–1.68) | 1.4 | (1.21–1.64) | 1.44 | (1.29–1.62) |
| 2003 | 1.7 | (1.46–1.98) | 1.68 | (1.45–1.94) | 1.51 | (1.36–1.68) |
| 2004 | 2.42 | (2.09–2.8) | 2.37 | (2.06–2.72) | 1.99 | (1.8–2.22) |
| 2005 | 2.89 | (2.49–3.34) | 2.84 | (2.48–3.27) | 2.39 | (2.15–2.65) |
| 2006 | 2.08 | (1.79–2.41) | 2.17 | (1.88–2.49) | 2.28 | (2.06–2.54) |
| 2007 | 2.75 | (2.37–3.18) | 2.81 | (2.45–3.23) | 2.71 | (2.45–3.01) |
| 2008 | 3.3 | (2.85–3.81) | 3.15 | (2.74–3.61) | 2.98 | (2.69–3.3) |
| 2009 | 3.22 | (2.79–3.72) | 3.08 | (2.69–3.53) | 2.79 | (2.52–3.09) |
| 2010 | 3.27 | (2.83–3.78) | 3.13 | (2.73–3.59) | 2.9 | (2.62–3.21) |
| 2011 | 3.77 | (3.27–4.35) | 3.6 | (3.14–4.12) | 3.21 | (2.9–3.56) |
| 2012 | 3.97 | (3.44–4.58) | 3.87 | (3.38–4.44) | 3.4 | (3.07–3.77) |
| 2013 | 4.01 | (3.47–4.64) | 4.07 | (3.55–4.66) | 3.51 | (3.17–3.89) |
| 2014 | 4.28 | (3.71–4.95) | 4.29 | (3.75–4.92) | 3.56 | (3.21–3.94) |
| 2015 | 4.15 | (3.59–4.8) | 4.25 | (3.71–4.88) | 3.46 | (3.12–3.84) |
| 2016 | 4.04 | (3.49–4.67) | 4.08 | (3.56–4.68) | 3.23 | (2.91–3.58) |
| 2017 | 3.34 | (2.89–3.87) | 3.37 | (2.93–3.87) | 2.76 | (2.49–3.06) |
| Month | ||||||
| January | (reference) | (reference) | (reference) | |||
| February | 1 | (.97–1.04) | 1 | (.96–1.03) | 0.99 | (.96–1.02) |
| March | 1 | (.97–1.04) | 0.99 | (.96–1.03) | 1.01 | (.99–1.04) |
| April | 0.99 | (.95–1.03) | 0.98 | (.95–1.02) | 1.01 | (.98–1.04) |
| May | 0.98 | (.95–1.02) | 0.97 | (.94–1.01) | 1 | (.97–1.03) |
| June | 0.98 | (.95–1.02) | 0.95 | (.91–.98) | 0.98 | (.95–1.01) |
| July | 0.95 | (.92–.99) | 0.9 | (.87–.93) | 0.92 | (.9–.95) |
| August | 0.97 | (.93–1.01) | 0.92 | (.89–.95) | 0.94 | (.91–.96) |
| September | 0.97 | (.93–1) | 0.93 | (.9–.96) | 0.96 | (.93–.98) |
| October | 0.98 | (.94–1.01) | 0.95 | (.92–.98) | 0.96 | (.93–.98) |
| November | 0.99 | (.95–1.02) | 0.96 | (.93–.99) | 0.96 | (.93–.99) |
| December | 1 | (.97–1.04) | 0.96 | (.93–.99) | 0.96 | (.93–.98) |
| Comorbidities | ||||||
| CHF | 1.05 | (1.03–1.08) | 1.17 | (1.15–1.2) | 0.97 | (.95–.98) |
| Valvular | 0.76 | (.74–.79) | 0.87 | (.84–.89) | 0.75 | (.74–.77) |
| PHTN | 0.91 | (.88–.95) | 1.03 | (.99–1.07) | 0.84 | (.81–.87) |
| PVD | 1.14 | (1.11–1.18) | 1.31 | (1.27–1.35) | 1.21 | (1.18–1.25) |
| HTN | 0.75 | (.73–.76) | 0.73 | (.72–.74) | 0.76 | (.75–.77) |
| Paralysis | 1.25 | (1.2–1.29) | 1.38 | (1.33–1.43) | 1.15 | (1.11–1.19) |
| Neuro (other) | 0.99 | (.96–1.01) | 1.05 | (1.02–1.07) | 0.88 | (.86–.9) |
| Pulmonary | 0.92 | (.9–.94) | 0.96 | (.94–.98) | 0.86 | (.84–.87) |
| DM | 0.93 | (.91–.96) | 0.94 | (.92–.96) | 0.87 | (.85–.89) |
| DMcx | 1.11 | (1.07–1.14) | 1.19 | (1.15–1.23) | 0.99 | (.96–1.02) |
| Hypothyroid | 0.86 | (.84–.9) | 0.85 | (.82–.88) | 0.92 | (.9–.94) |
| Renal | 1.58 | (1.54–1.62) | 1.7 | (1.66–1.74) | 1.59 | (1.55–1.62) |
| Liver | 1.34 | (1.29–1.4) | 1.53 | (1.47–1.58) | 1.56 | (1.52–1.61) |
| PUD | 0.97 | (.84–1.13) | 1.14 | (.99–1.32) | 0.96 | (.85–1.08) |
| HIV | 1.42 | (1.28–1.57) | 1.67 | (1.51–1.84) | 1.37 | (1.26–1.5) |
| Lymphoma | 1.84 | (1.77–1.91) | 2.03 | (1.95–2.11) | 1.62 | (1.56–1.67) |
| Mets | 0.84 | (.81–.87) | 0.99 | (.96–1.02) | 0.77 | (.75–.79) |
| Tumor | 1 | (.97–1.03) | 1.09 | (1.06–1.12) | 0.9 | (.88–.92) |
| Rheumatic | 1.09 | (1.03–1.15) | 1.2 | (1.14–1.26) | 1.22 | (1.17–1.27) |
| Coagulopathy | 1.34 | (1.3–1.38) | 1.49 | (1.44–1.53) | 1.2 | (1.17–1.23) |
| Obesity | 0.8 | (.77–.83) | 0.78 | (.76–.81) | 0.68 | (.67–.7) |
| Weight loss | 2.22 | (2.17–2.27) | 2.42 | (2.36–2.47) | 2.14 | (2.1–2.19) |
| FluidsLytes | 2.17 | (2.14–2.21) | 2.78 | (2.74–2.82) | 3.81 | (3.76–3.85) |
| Blood loss | 0.87 | (.81–.92) | 0.89 | (.84–.94) | 0.76 | (.72–.8) |
| Anemia | 1.06 | (1.04–1.09) | 1.15 | (1.13–1.18) | 1.14 | (1.12–1.16) |
| Alcohol | 0.84 | (.8–.88) | 0.97 | (.93–1.02) | 0.77 | (.74–.8) |
| Drugs | 0.47 | (.44–.51) | 0.64 | (.6–.69) | 0.63 | (.59–.67) |
| Psychoses | 0.58 | (.56–.61) | 0.73 | (.7–.76) | 0.65 | (.63–.68) |
| Depression | 0.79 | (.76–.82) | 0.85 | (.82–.88) | 0.99 | (.96–1.01) |
Abbreviations: CDI, Clostridioides difficile infection; CHF, congestive heart failure; CI, confidence interval; DM, diabetes mellitus; DMcx, diabetes mellitus with complications; FluidsLytes, Fluid and Electrolyte disorders; HIV, human immunodeficiency virus; HTN, Hypertension; OR, odds ratio; PHTN, Pulmonary Hypertension / Pulmonary Circulation Disorders; PUD, Peptic Ulcer Disease; PVD, Peripheral Vascular Disorders.
aThese models correspond to results presented in red, green, and blue, respectively, in Figure 3. Similar results corresponding to all hospitalizations (ie, not restricted to 1 hospitalization per enrollee) can be found in Supplementary Table 3 and Supplementary Figure 1.
In general, CDI risk increased with patient age for adults. Each additional day a patient stayed in the hospital was associated with slightly increased risk for CDI, with an OR of 1.02 (95% CI, 1.02–1.02) for hospital-onset cases. For all CDI cases, female patients were at increased risk for CDI; however, this was not statistically significant for hospital-onset and secondary cases. Year was positively associated with CDI risk, increasing from 2001 to 2015 and then decreasing for 2016–2017.
As a sensitivity analysis, we repeated all of our statistical analyses using all patients without restricting enrollees to a single hospitalization. This study population’s baseline characteristics are summarized in Supplementary Table 2, with regression results reported in Supplementary Table 3 and Supplementary Figure 1. In general, all of our primary estimated associations remained consistent when using the complete set of hospitalizations: there was increased risk associated with prior healthcare exposures, and this risk decreased with the amount of time between hospitalization and prior exposure. However, individual estimates decreased slightly when we used multiple observations from each enrollee.
DISCUSSION
Our results demonstrate that exposure to different healthcare environments and antibiotics prior to a hospitalization are associated with an increased likelihood of a CDI diagnosis. Because of our large study population, we could evaluate the CDI risk attributable to different types of exposures (eg, ED visits, antibiotics). In the 30 days before an index hospitalization, prior hospitalizations and long-term care stays have the strongest associations with CDI diagnoses (ORs of 3.73 and 2.97, respectively). In contrast, outpatient visits are associated with lower risk (OR, 1.18). In addition, the risk associated with exposures decays with time, especially for exposures >30 days in the past. However, for some exposures, such as prior hospitalizations or long-term care stays, the increased likelihood of CDI decays much more slowly, and persists up to 180 days.
One of our most striking results is that CDI patients had far greater exposure to multiple healthcare settings prior to the hospitalization where they were diagnosed with CDI, compared with patients who did not develop CDI during a hospital stay. In fact, a majority of patients diagnosed with CDI in hospitals (62%) had at least 1 prior hospitalization, ED visit, ambulatory surgery, or nursing home/long-term care stay within 90 days prior to the CDI-associated hospitalization. By contrast, only 32% of patients without CDI had such an exposure within 90 days prior to hospitalization. For any given time period prior to hospitalization, patients with CDI were 3–4 times more likely to be exposed to 2 or more of these settings. Relatively few people with CDI had no prior exposures to healthcare or antibiotics. Previous research has shown that people at risk for CDI have more preexisting comorbidities and thus have more healthcare exposure; [27, 28]; thus, we cannot rule out the possibility of, or directly quantify the degree to which, the prior-healthcare exposure risk we identify is attributable to overall patient health. However, given that we controlled for a number of other patient comorbidities and identified an exposure risk that decayed with time, it seems unlikely that overall patient morbidity can fully explain our findings. Regardless of whether the risk is associated with underlying patient illness or the healthcare exposure itself, these visits are an important marker for both predicting and adjusting for CDI risk.
Our findings have several important implications. First, our results highlight the importance of considering longitudinal healthcare exposures to understand the natural history of CDI infections. Prior exposure to healthcare settings as far back as 180 days may be linked to increased CDI risk during a current hospital stay, and different types of exposures may confer different levels of risk. Second, our results can inform efforts to model or predict CDI, evaluate potential infection control interventions, or identify individuals at high risk for developing CDI. Our findings suggest that these efforts may need to measure and account for multiple exposures prior to hospitalization. Third, our estimates have direct implications for antimicrobial stewardship efforts. Our approach confirms the findings of others: that some antimicrobials confer higher levels of risk for CDI than others. Our work also shows that risk associated with low-CDI-risk antimicrobials decreases quickly with time prior to hospitalization, whereas risk associated with other antimicrobials is sustained over much longer time intervals, highlighting the importance of prescribing lower-CDI-risk antimicrobials when clinically appropriate.
Our results offer some insight into the role of the environment on the spread of CDI. The environment has long been implicated in the spread of CDI [29–31]. However, studies sequencing CDI strains in geographic regions or hospitals have demonstrated that substantial proportions of isolates from symptomatic CDI patients are not genetically related and epidemiologic links cannot be established for many patients, suggesting a role of asymptomatic spread [9, 10]. However, our results demonstrate that a substantial proportion of patients with hospital-onset CDI have many different exposures (eg, antibiotic use, familial CDI, ED visits) prior to admission where such links may occur. Given the persistent levels of risk we report, future efforts to identify genetic relationships between CDI cases may need to widen the catchment area of potential exposures far beyond the hospital where symptoms present.
Aside from informing future CDI-related research, our results also have direct policy implications. In an effort to motivate prevention efforts, hospital reimbursement is often tied to CDI rates, penalizing institutions with higher CDI incidence. While there is currently evidence that CDI rates may need to be adjusted for patient and healthcare setting characteristics [32, 33], our findings suggest that comparisons of CDI rates also need to account for the mix of exposures that patients may experience prior to hospitalization.
Our observational study design is based exclusively on routinely collected administrative claims data and thus has 3 distinct limitations. First, these data are primarily collected for the purposes of billing, which our research team cannot directly validate. Second, we do not have access to laboratory results to confirm CDI diagnoses, nor can we directly measure CDI colonization. Third, we cannot identify the exact day when symptoms emerge during a hospital stay. Thus, we cannot specifically identify cases of CDI that meet exact surveillance definitions for healthcare facility–onset CDI or community-onset, healthcare facility–associated CDI. However, our analyses considered 3 different case definitions of CDI, based on length of stay and order of diagnosis, and results were consistent across all 3.
Despite these limitations, our results show that patients diagnosed with CDI in hospital settings typically have had previous—and often multiple—prior healthcare-associated exposures. Furthermore, different types of exposures entail different levels of risk, which decay at different rates with respect to time prior to hospitalization. Collectively, our findings highlight how difficult it is to directly attribute cases of CDI to specific exposures and thus have important implications for CDI research, infection control, antimicrobial stewardship, and efforts to link CDI cases to reimbursement.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. A portion of the preliminary results of this analysis were scheduled to be presented at the Decennial 2020: Sixth International Conference on Healthcare Associated Infections (canceled due to COVID-19). Abstracts for this conference will now be electronically presented in the Journal of Infection Control and Hospital Epidemiology.
Financial support. This work was supported by the Centers for Disease Control and Prevention Modeling Infectious Diseases in Healthcare Network (cooperative agreement number U01CK000531) and by the National Institutes of Health Clinical and Translational Science Awards Program (program grant number UL1TR002537).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lessa FC, Winston LG, McDonald LC; Emerging Infections Program C. difficile Surveillance Team. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:2369–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013; 173:2039–46. [DOI] [PubMed] [Google Scholar]
- 3.Kwon JH, Olsen MA, Dubberke ER. The morbidity, mortality, and costs associated with Clostridium difficile infection. Infect Dis Clin North Am 2015; 29:123–34. [DOI] [PubMed] [Google Scholar]
- 4.Magill SS, Edwards JR, Bamberg W, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005; 353:2433–41. [DOI] [PubMed] [Google Scholar]
- 6.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:987–94. [DOI] [PubMed] [Google Scholar]
- 7.Gerding DN, Muto CA, Owens RC Jr. Measures to control and prevent Clostridium difficile infection. Clin Infect Dis 2008; 46(Suppl 1):S43–9. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare and Medicaid Services. Hospital inpatient quality reporting (IQR) program: IQR measures. 2020. https://www.qualitynet.org/inpatient/iqr/measures. Accessed 3 February 2020.
- 9.Eyre DW, Cule ML, Wilson DJ, et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med 2013; 369:1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curry SR, Muto CA, Schlackman JL, et al. Use of multilocus variable number of tandem repeats analysis genotyping to determine the role of asymptomatic carriers in Clostridium difficile transmission. Clin Infect Dis 2013; 57:1094–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker AS, Eyre DW, Wyllie DH, et al. Characterisation of Clostridium difficile hospital ward-based transmission using extensive epidemiological data and molecular typing. PLoS Med 2012; 9:e1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol 2002; 23:653–9. [DOI] [PubMed] [Google Scholar]
- 13.Rodemann JF, Dubberke ER, Reske KA, Seo DH, Stone CD. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol 2007; 5:339–44. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013; 68:1951–61. [DOI] [PubMed] [Google Scholar]
- 15.Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother 2013; 57:2326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartof SY, Rieg GK, Wei R, Tseng HF, Jacobsen SJ, Yu KC. A comprehensive assessment across the healthcare continuum: risk of hospital-associated Clostridium difficile infection due to outpatient and inpatient antibiotic exposure. Infect Control Hosp Epidemiol 2015; 36:1409–16. [DOI] [PubMed] [Google Scholar]
- 17.Teng C, Reveles KR, Obodozie-Ofoegbu OO, Frei CR. Clostridium difficile infection risk with important antibiotic classes: an analysis of the FDA adverse event reporting system. Int J Med Sci 2019; 16:630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller AC, Segre AM, Pemmaraju SV, Sewell DK, Polgreen PM. Association of household exposure to primary Clostridioides difficile infection with secondary infection in family members. JAMA Netw Open 2020; 3:e208925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loo VG, Brassard P, Miller MA. Household transmission of Clostridium difficile to family members and domestic pets. Infect Control Hosp Epidemiol 2016; 37:1342–8. [DOI] [PubMed] [Google Scholar]
- 20.Pépin J, Gonzales M, Valiquette L. Risk of secondary cases of Clostridium difficile infection among household contacts of index cases. J Infect 2012; 64:387–90. [DOI] [PubMed] [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 22.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis 2006; 12:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubberke ER, Reske KA, Olsen MA, et al. Evaluation of Clostridium difficile-associated disease pressure as a risk factor for C difficile-associated disease. Arch Intern Med 2007; 167:1092–7. [DOI] [PubMed] [Google Scholar]
- 24.Reveles KR, Lee GC, Boyd NK, Frei CR. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001–2010. Am J Infect Control 2014; 42:1028–32. [DOI] [PubMed] [Google Scholar]
- 25.Polgreen PM, Yang M, Bohnett LC, Cavanaugh JE. A time-series analysis of Clostridium difficile and its seasonal association with influenza. Infect Control Hosp Epidemiol 2010; 31:382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown KA, Daneman N, Arora P, Moineddin R, Fisman DN. The co-seasonality of pneumonia and influenza with Clostridium difficile infection in the United States, 1993–2008. Am J Epidemiol 2013; 178:118–25. [DOI] [PubMed] [Google Scholar]
- 27.Furuya-Kanamori L, Stone JC, Clark J, et al. Comorbidities, exposure to medications, and the risk of community-acquired Clostridium difficile Infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2015; 36:132–41. [DOI] [PubMed] [Google Scholar]
- 28.Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol 2011; 8:17–26. [DOI] [PubMed] [Google Scholar]
- 29.Durovic A, Widmer AF, Tschudin-Sutter S. New insights into transmission of Clostridium difficile infection-narrative review. Clin Microbiol Infect 2018; 24:483–92. [DOI] [PubMed] [Google Scholar]
- 30.Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol 1995; 16:459–77. [DOI] [PubMed] [Google Scholar]
- 31.Kim KH, Fekety R, Batts DH, et al. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis. J Infect Dis 1981; 143:42–50. [DOI] [PubMed] [Google Scholar]
- 32.Dudeck MA, Weiner LM, Malpiedi P, Edwards J, Peterson K, Sievert D. Risk adjustment for healthcare facility-onset C. difficile and MRSA bacteremia laboratory-identified event reporting in NHSN. Atlanta, GA: Centers for Disease Control and Prevention, 2013:12. [Google Scholar]
- 33.Thompson ND, Edwards JR, Dudeck MA, Fridkin SK, Magill SS. Evaluating the use of the case mix index for risk adjustment of healthcare-associated infection data: an illustration using Clostridium difficile infection data from the National Healthcare Safety Network. Infect Control Hosp Epidemiol 2016; 37:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.