Abstract
Background
Persons with human immunodeficiency virus (PWH) with persistently low CD4 counts despite efficacious antiretroviral therapy could have higher hospitalization risk.
Methods
In 6 US and Canadian clinical cohorts, PWH with virologic suppression for ≥1 year in 2005–2015 were followed until virologic failure, loss to follow-up, death, or study end. Stratified by early (years 2–5) and long-term (years 6–11) suppression and lowest presuppression CD4 count <200 and ≥200 cells/µL, Poisson regression models estimated hospitalization incidence rate ratios (aIRRs) comparing patients by time-updated CD4 count category, adjusted for cohort, age, gender, calendar year, suppression duration, and lowest presuppression CD4 count.
Results
The 6997 included patients (19 980 person-years) were 81% cisgender men and 40% white. Among patients with lowest presuppression CD4 count <200 cells/μL (44%), patients with current CD4 count 200–350 vs >500 cells/μL had aIRRs of 1.44 during early suppression (95% confidence interval [CI], 1.01–2.06), and 1.67 (95% CI, 1.03–2.72) during long-term suppression. Among patients with lowest presuppression CD4 count ≥200 (56%), patients with current CD4 351–500 vs >500 cells/μL had an aIRR of 1.22 (95% CI, .93–1.60) during early suppression and 2.09 (95% CI, 1.18–3.70) during long-term suppression.
Conclusions
Virologically suppressed patients with lower CD4 counts experienced higher hospitalization rates and could potentially benefit from targeted clinical management strategies.
Keywords: HIV, hospitalization, CD4 lymphocyte count, sustained virologic response, cohort studies
Among patients virologically suppressed on antiretroviral therapy for up to 11 years, those with CD4 counts ≤500 vs >500 cells/µL had higher hospitalization rates, including patients without a history of CD4 count <200 cells/µL prior to virologic suppression.
Among persons with human immunodeficiency virus (PWH) in the United States (US) and Canada, hospitalization rates have decreased in recent years but remain elevated compared to the general population [1–5]. Given improved efficacy and safety of antiretroviral therapy (ART) and greater rates of sustained virologic suppression, work is needed to identify clinical risk factors for hospitalization among persons with well-controlled HIV [6]. A patient population that merits attention is PWH with incomplete CD4 count recovery despite long-term virologic suppression. Initiating ART early in HIV infection improves CD4 recovery [7]. In the US and Canada, approximately 25% of PWH are diagnosed with HIV at CD4 counts <200 cells/μL or with an AIDS-defining condition [8, 9]. Additionally, despite early ART initiation, the CD4 counts of many PWH do not increase to levels comparable to those in the general population or to a threshold of 500 cells/µL [7, 10].
PWH with poor CD4 recovery on ART might have higher hospitalization rates for several reasons. First, lower CD4 counts are associated with higher incidence of AIDS- and non-AIDS-defining infections [11, 12]. Patients with delayed ART initiation or lower CD4 recovery also experience greater burdens of chronic inflammation and immune activation, and higher risk of severe non-AIDS outcomes, including myocardial infarction, end-stage renal disease, and cancer [13–16]. Finally, patients with delayed HIV diagnosis and care might have a higher prevalence of smoking, substance use, and comorbidities such as dyslipidemia and hypertension, which could affect clinical outcomes after ART initiation [17–19].
Prior studies have shown associations between lower CD4 counts and higher hospitalization rates [1, 20–22]. However, few studies of hospitalization rates have focused on virologically suppressed PWH or taken into account ART duration [22, 23]. We examined hospitalization rates by past and current CD4 cell count among PWH on ART with sustained virologic suppression for up to 11 years.
METHODS
Data Source
This study was based in the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a consortium of HIV cohorts [24]. One Canadian and 5 US clinical cohorts, based at academic research centers and integrated health systems, were eligible for this study because they collected hospital admission and discharge data for the period 2005–2015, including International Classification of Diseases, Ninth or Tenth Revision (ICD-9 and ICD-10, respectively) codes for discharge diagnoses. Each cohort collects data from the electronic health records of its medical center, as well as mortality data from linkage to vital statistics registries. Data collection was approved by local institutional review boards (IRBs), and secondary analysis by the University of North Carolina IRB.
Patient Inclusion and Follow-up
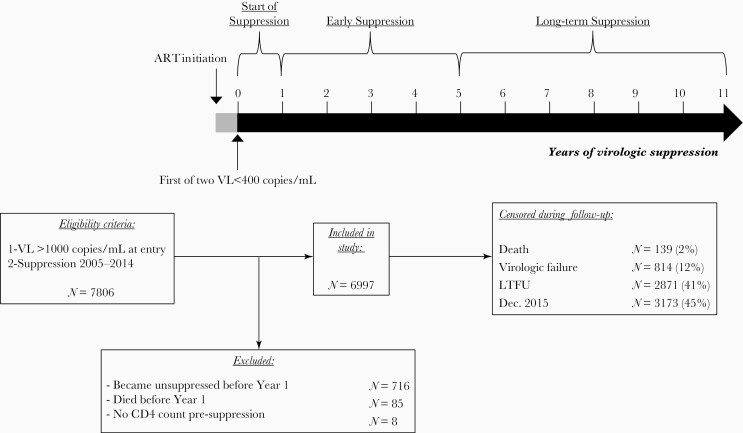
Eligible patients met both of the following criteria: (1) HIV RNA (viral load [VL]) >1000 copies/mL at NA-ACCORD entry; and (2) first virologic suppression between 1 January 2005 and 31 December 2014 (Figure 1, N = 7806). Virologic suppression was defined as 2 consecutive VL measurements <400 copies/mL ≥30 days apart within 12 months. We used 400 copies/mL as that was the highest lower limit of quantification (LLQ) of VL assays used in our study. We excluded patients without a complete year of suppression (n = 716 became unsuppressed; n = 85 died) or available presuppression CD4 count (n = 8). We included patients with evidence of prior ART exposure (13%), defined as any prior VL <1000 copies/mL, as long as they had not previously met our definition of suppression.
Figure 1.
Flowchart of patient inclusion and follow-up. Abbreviations: ART, antiretroviral therapy; LTFU, loss to follow-up; VL, viral load.
Person-time began at the first VL <400 copies/mL and was censored at death, loss to follow-up (LTFU), virologic failure, or 31 December 2015, whichever occurred first. Virologic failure was defined as the first of 2 consecutive VL ≥400 copies/mL ≥2 weeks apart within 90 days, or 1 VL ≥400 copies/mL with no confirmatory VL within 15–90 days. LTFU was defined as 12 months with no outpatient CD4 count or VL measurement. In this study’s 6 cohorts, LTFU was 8%–11% annually over 2005–2015, consistent with other HIV clinical cohort studies in North America [24, 3].
Patients could not reenter the analysis after censoring. Person-time was divided into 6-month intervals starting from the first VL <400 copies/mL. Days spent in the hospital were not counted as person-time at risk.
Study Measures
The exposure of interest was time-updated CD4 count, categorized as <200, 200–350, 351–500, and >500 cells/μL. For each 6-month interval, for each patient, we calculated a weighted moving average using the 3 most recent CD4 counts in the prior 24 months. Moving averages have been used to study CD4 count trajectories [25]. Ninety-two percent of calculations had 3 CD4 measurements available, 7% had 2 measurements available, and 2% had 1 measurement available. Weights were calculated as 1 divided by the number of days from CD4 measurement to interval start date. We excluded CD4 counts measured during a hospitalization to avoid capturing immune changes related to acute illness. We only used CD4 counts measured ≥30 days apart to avoid overweighting measurements taken close together. We excluded 48 person-years (PY) (<1%) without any CD4 count in the prior 24 months.
The outcome of interest was all-cause hospitalization, allowing patients to contribute >1 hospitalization. We did not count hospitalizations with same-day discharge, which were rare events and undistinguishable from outpatient procedures (eg, endoscopies). To examine reasons for hospitalization, we categorized primary discharge diagnosis ICD-9 codes using Clinical Classifications Software, modified to create an “AIDS-defining illness” category and include other infections in a “non-AIDS-defining infection” category [1, 26]. Using validated methodology, if the primary discharge diagnosis was a code for HIV or chronic hepatitis C infection, we used the next code [27]. ICD-10 codes, when used, were first converted to ICD-9 using General Equivalence Mappings from the Centers for Medicare and Medicaid Services [28].
The covariates in the analysis were NA-ACCORD cohort, age, gender, calendar year, duration of virologic suppression, and lowest known CD4 count measured prior to first VL <400 copies/mL. Continuous covariates were modeled as linear variables. We selected only major covariates associated with both CD4 recovery and hospitalizations, to avoid overadjusting for factors potentially explaining the mechanism between CD4 count and hospitalization rates.
Statistical Analysis
We estimated hospitalization rates per 100 PY by CD4 count category, stratified by lowest presuppression CD4 count <200 or ≥200 cells/μL. To avoid immortal person-time, we did not examine the first year of virologic suppression. We stratified analyses into an early suppression period (2–5 years) and long-term suppression period (6–11 years) (Figure 1). Year 6 was selected as the cutoff based on studies showing minimal additional CD4 recovery beyond this point [10, 29]. This approach allowed us to compare patients according to CD4 count trajectories over time, for example, among patients with lowest presuppression CD4 <200 cells/μL, comparing patients with a current CD4 count 200–350 vs >500 cells/μL during long-term suppression.
We used Poisson regression models to estimate hospitalization incidence rate ratios (IRRs) comparing patients by current CD4 categories, using generalized estimating equations with an independent correlation matrix to account for patients contributing >1 outcome. Unadjusted models only included the NA-ACCORD cohort as covariate. Models estimating adjusted IRRs included all covariates. For rate and IRR estimation, we excluded patients in strata with <100 PY to avoid unstable estimates. Because there were only 35 transgender patients in our study, models including gender as a covariate (categorized as cisgender men, cisgender women, or transgender persons) did not converge to produce statistical estimates. Therefore, we excluded transgender patients in adjusted analyses. Analyses were conducted using SAS version 9.4 software (SAS Institute, Cary, North Carolina). P values were 2-sided with a prespecified α = .05.
Sensitivity Analyses
We conducted 6 sensitivity analyses. First, we censored the person-time of patients with a CD4 decline after the first VL <400 copies/mL while remaining suppressed, as they might have non-HIV-related immunodeficiency (Supplementary Figure 1). A CD4 decline was defined as 2 consecutive decreases ≥15% in unweighted 24-month moving averages using 3 CD4 measurements, updated at each measurement [25]. In this analysis, we also censored person-time after a hospitalization for chemotherapy (ICD-9 code V58.11), which can cause decreases in CD4 count [30]. Second, we counted only the first hospitalization in each 6-month interval, to evaluate the possible impact of a small number of patients contributing many hospitalizations. Third, because defining virologic suppression as VL <400 copies/mL might misclassify patients with low-level viremia as suppressed, we defined virologic suppression as VL <75 copies/mL. As assays with lower LLQs were only widely used in more recent years, this analysis was restricted to 2008–2015. Fourth, we excluded ART-experienced patients, defined above, as they might have higher CD4 counts due to previous periods of suppression. Fifth, since age is an important hospitalization risk factor, we examined whether using restricted quadratic splines with 4 equidistant knots led to better adjustment. And finally, we conducted subgroup analyses of patients with lowest presuppression CD4 count 200–350 and >350 cells/µL.
RESULTS
Study Sample
The 6997 included patients were 81% cisgender men, 40% white, and 33% black. At the first VL <400 copies/mL, the median age was 42 years (interquartile range [IQR], 33–49 years), and the median lowest presuppression CD4 count was 228 cells/µL (IQR, 93–342 cells/μL; range, 0–1227 cells/μL; 44% <200 cells/µL). Demographic characteristics varied by lowest presuppression CD4 <200 vs ≥200 cells/µL (Table 1, all P < .01). Overall, during the first year of suppression, the hospitalization rate was 17.3 per 100 PY (95% confidence interval [CI], 15.7–19.0 per 100 PY).
Table 1.
Demographic and Clinical Characteristics of 6997 Patients Who First Achieved and Maintained Virologic Suppression for at Least 1 Year in 6 North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Cohorts, 2005–2014, Stratified by Lowest Known CD4 Cell Count Prior to the Start of Virologic Suppression
| Characteristic at First HIV VL <400 Copies/mL | Lowest Known Prior CD4 Count <200 Cells/µLa (n = 3069) |
Lowest Known Prior CD4 Count ≥200 Cells/µLa (n = 3928) |
P Valueb |
|---|---|---|---|
| Gender | <.01 | ||
| Cisgender men | 2407 (78) | 3257 (83) | |
| Cisgender women | 643 (21) | 655 (17) | |
| Transgenderc | 19 (1) | 16 (<1) | |
| Race/ethnicity | <.01 | ||
| Black, non-Hispanic | 1128 (37) | 1167 (30) | |
| White, non-Hispanic | 1127 (37) | 1672 (43) | |
| Hispanic | 547 (18) | 544 (16) | |
| Other or missing | 267 (9) | 445 (11) | |
| HIV acquisition risk factor | <.01 | ||
| MSM | 1450 (47) | 2423 (62) | |
| Heterosexual or other | 1242 (40) | 1155 (29) | |
| IDU | 377 (12) | 350 (9) | |
| Calendar year, median (IQR) | 2009 (2007–2011) | 2010 (2008–2012) | <.01 |
| Current age, y | 44 (37–50) | 40 (31–48) | <.01 |
| Lowest known prior CD4 count, cells/µL, median (IQR) | 76 (24–140) | 325 (264–426) | <.01 |
| ART experiencedd | 469 (15) | 426 (11) | <.01 |
| Current CD4 count, cells/µLe, median (IQR) | 191 (110–285) | 467 (361–607) | <.01 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IDU, injection drug use; IQR, interquartile range; MSM, men who have sex with men; VL, viral load.
aLowest observed CD4 count prior to the first VL <400 copies/mL, a proxy for nadir CD4 count.
bP values from χ 2 (categorical variables) and Wilcoxon rank-sum (continuous variables) tests.
cTransgender adults were identified from local data, or from having female sex and MSM as risk factor.
dDefined as having a history of VL <1000 copies/mL without meeting study criteria for suppression (2 VLs <400 copies/mL within 12 months).
eBased on weighted average of up to 3 measurements in the prior 24 months. Weights were calculated as 1 divided by the number of days to the first VL <400 copies/mL. Patients could have a current CD4 count value greater than their lowest prior CD4 count because of previous ART use or because of rapid increases between ART initiation and the first VL <400 copies/mL.
For these analyses, patients contributed 19 980 virologically suppressed person-years, including 15 612 during early suppression (years 2–5) and 4368 during long-term suppression (years 6–11). In years 2–11, patients contributed a median of 2.1 suppressed PY (IQR, 0.8–4.4), with 41% censored at LTFU, 12% at virologic failure, and 2% at death (Figure 1). Of 2871 patients LTFU, 90 (3%) were known to have subsequently died. During years 2–11, 1231 (18%) patients were hospitalized at least once. Among these, 73% were hospitalized once, 18% twice, 5% 3 times, 3% 4 times, and 1% 5 or more times, for a total of 2035 hospitalizations. The 3 most frequent hospitalization diagnostic categories were non-AIDS-defining infection (24% of hospitalizations; most frequent diagnosis: sepsis/bacteremia), cardiovascular (13%; acute cardiac ischemic event), and liver/gastrointestinal (10%; acute or chronic pancreatitis) (Supplementary Table 1).
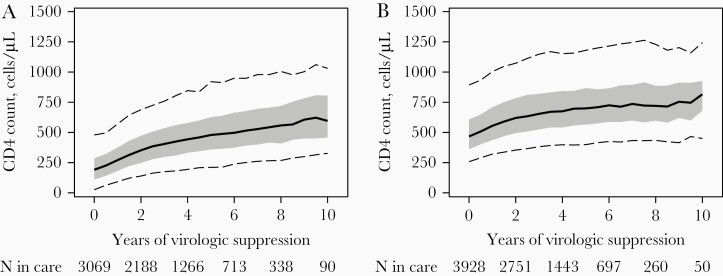
From the first VL <400 copies/mL to year 10 of sustained suppression, median CD4 counts increased from 191 cells/μL (IQR, 110–285 cells/μL) to 596 cells/μL (IQR, 457–805 cells/μL) among patients with lowest presuppression CD4 <200 cells/µL, and from 467 (IQR, 361–607 cells/μL) to 816 cells/μL (IQR, 680–927 cells/μL) among patients with lowest presuppression CD4 ≥200 cells/µL (Figure 2A and 2B). By the fifth year of virologic suppression, 46% had a CD4 count >500 cells/μL among patients with lowest presuppression CD4 <200 cells/µL, and 86% among those with lowest presuppression CD4 ≥200 cells/µL.
Figure 2.
CD4 cell count distribution from the first human immunodeficiency virus RNA load <400 copies/mL to year 10 of sustained virologic suppression, among patients with a lowest presuppression CD4 count <200 cells/µL (A) or ≥200 cells/µL (B). Shown are the median (solid line), interquartile range (band), and 5th and 95th percentiles (dashed lines). CD4 counts are 24-month weighted moving averages of up to 3 measurements, updated every 6 months of virologic suppression. Values for year 11 are not displayed due to small sample sizes.
CD4 Cell Count and Hospitalization Rates
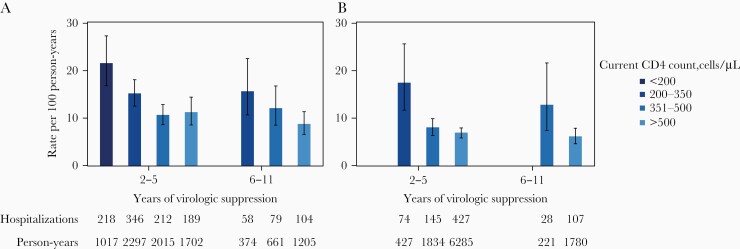
During years 2–11, the overall hospitalization rate was 10.2 hospitalizations per 100 PY (95% CI, 9.3–11.1 per 100 PY). Hospitalization rates were highest during early suppression among patients in the lowest current and presuppression CD4 group, with an estimate of 21.4 per 100 PY (95% CI, 16.8–27.3 per 100 PY), and lowest during long-term suppression among patients in the highest current and presuppression CD4 group, with an estimate of 6.0 per 100 PY (95% CI, 4.6–7.9 per 100 PY) (Figure 3, Supplementary Table 2).
Figure 3.
Unadjusted all-cause hospitalization rates by CD4 cell count category, stratified by early (years 2–5) or long-term (years 6–11) virologic suppression, among patients with a lowest presuppression CD4 count <200 cells/µL (A) or ≥200 cells/µL (B). Error bars are the 95% confidence intervals. Rates were not estimated for categories with <100 person-years. CD4 counts are 24-month weighted moving averages of up to 3 measurements, updated every 6 months of virologic suppression.
Among patients with lowest presuppression CD4 count <200 cells/μL (Table 2), having a lower current CD4 during early suppression was associated with higher hospitalization rates, with an unadjusted IRR of 2.03 (95% CI, 1.42–2.89) for current CD4 count <200 vs >500 cells/µL, and 1.44 (95% CI, 1.05–1.97) for current CD4 count 200–350 vs >500 cells/µL. Patients with a current CD4 count 351–500 cells/µL did not have lower rates than those with a CD4 count >500 cells/µL. During long-term suppression, compared to patients with a current CD4 count >500 cells/µL, the unadjusted IRR was 1.84 (95% CI, 1.17–2.89) for patients with a CD4 count 200–350 cells/µL, and 1.50 (95% CI, 1.01–2.24) for patients with a CD4 count 351–500 cells/µL. In adjusted models, estimates were similar but less precise. The IRR for patients with a current CD4 count 351–500 vs >500 cells/µL during long-term suppression (1.40 [95% CI, .93–2.10]) was no longer statistically significant.
Table 2.
Incidence Rate Ratios for All-Cause Hospitalizations Comparing CD4 Cell Count Categories Among 6997 Patients, Stratified by Lowest Presuppression CD4 Cell Count and by Duration of Virologic Suppression
| Lowest Presuppression CD4 Count <200 Cells/µL | Lowest Presuppression CD4 Count ≥200 Cells/µL | |||
|---|---|---|---|---|
| Current CD4 Count, Cells/µLa | Unadjusted IRR (95% CI)b |
Adjusted IRR (95% CI)c |
Unadjusted IRR (95% CI)b |
Adjusted IRR (95% CI)c |
| Early suppression (years 2–5) | ||||
| <200 | 2.03 (1.42–2.89) | 2.04 (1.28–3.27) | d | d |
| 200–350 | 1.44 (1.05–1.97) | 1.45 (1.01–2.06) | 2.58 (1.69–3.93) | 2.47 (1.52–4.02) |
| 351–500 | 1.00 (.74–1.36) | 1.01 (.73–1.40) | 1.18 (.92–1.53) | 1.22 (.93–1.60) |
| >500 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Long-term suppression (years 6–11) | ||||
| <200 | d | d | d | d |
| 200–350 | 1.84 (1.17–2.89) | 1.67 (1.03–2.72) | d | d |
| 351–500 | 1.50 (1.01–2.24) | 1.40 (.93–2.10) | 2.15 (1.20–3.83) | 2.09 (1.18–3.70) |
| >500 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
aWeighted 24-month moving average updated every 6 months of virologic suppression. Weights are the inverse of the distance to the 6-month interval start date.
bEstimates and 95% CIs from separate Poisson regression models adjusted for North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) cohort, with generalized estimating equations to account for patients contributing >1 time interval to the analysis.
cEstimates and 95% CIs from separate Poisson regression models with generalized estimating equations, adjusted for NA-ACCORD cohort, age, gender, calendar year, years of virologic suppression, and lowest prior CD4 count. We excluded transgender individuals.
dBecause of small sample size, we did not estimate IRR for this category (see Supplementary Table 1 for distribution of hospitalizations and person-time).
Among patients with lowest presuppression CD4 count ≥200 cells/μL (Table 2), we observed similar patterns. During early suppression, compared to having a current CD4 >500 cells/µL, a current CD4 count 200–350 cells/µL was associated with an unadjusted IRR of 2.58 (95% CI, 1.69–3.93), and a CD4 count 351–500 cells/µL was associated with an unadjusted IRR of 1.18 (95% CI, .92–1.53). Adjusted estimates were comparable. For example, during long-term suppression, patients with a CD4 count 351–500 cells/µL had an unadjusted IRR of 2.15 (95% CI, 1.20–3.83) and adjusted IRR of 2.09 (95% CI, 1.18–3.70) compared to those with a CD4 count >500 cells/µL.
Sensitivity Analyses
Censoring person-time after a persistent CD4 decline or hospitalization for chemotherapy (1% and <1% of patients, respectively; Supplementary Figure 1), results were similar to the main findings (Supplementary Table 3). Counting only 1 hospitalization per 6-month interval (n = 513 hospitalizations excluded, n = 1522 remaining), effect estimates were attenuated and less precise but showed comparable trends of increasing hospitalization rates with decreasing CD4 count (Supplementary Tables 4 and 5). For example, among patients with lowest presuppression CD4 <200 cells/μL, the adjusted IRR was 1.32 (95% CI, .99–1.74) for a CD4 count 200–350 vs >500 cells/µL during early suppression, and 1.25 (95% CI, .85–1.83) for a CD4 count 351–500 vs >500 cells/µL during long-term suppression. Defining virologic suppression as VL <75 copies/mL, restricted to 2008–2015, sample sizes were reduced by 38% (n = 1908) and 25% (n = 2957) for patients with lowest presuppression CD4 count <200 and ≥200 copies/mL, respectively (Supplementary Table 6). Given reduced sample size and limited long-term follow-up, we were unable to estimate a number of associations, and others were imprecise. Where we had statistical support to draw inferences, findings using <75 copies/mL were comparable to primary analyses (Supplementary Table 7). Results for remaining sensitivity analyses were similar to the main findings (Supplementary Tables 8–11).
DISCUSSION
Among patients with sustained virologic suppression on ART up to 11 years, lower CD4 counts were consistently associated with higher hospitalization rates. During 2–5 years of suppression, patients with current CD4 <200 or 200–350 cells/μL had higher unadjusted and adjusted hospitalization rates than those with CD4 >500 cells/μL. During 6–11 years of suppression, patients with current CD4 351–500 cells/μL had higher rates than those with CD4 >500 cells/μL. These trends were overall similar for patients with and without a history of CD4 <200 cells/μL prior to suppression. However, we observed the greatest hospitalization rate disparity between patients with CD4 count 200–350 vs >500 cells/µL during early suppression, among those with lowest presuppression CD4 ≥200 cells/µL. Patients whose lowest presuppression CD4 count was <200 cells/µL continued to have the highest absolute hospitalization rates during long-term virologic suppression.
In populations of patients with and without suppression, studies have previously reported higher hospitalization rates in descending CD4 strata below 500 cells/μL [1, 20, 21, 31]. In 1 study following ART initiators for a year, patients with sustained suppression and CD4 increases >100 cells/μL experienced lower hospitalization rates [32]. In a military cohort, among suppressed patients, having a CD4 count ≤200 vs >750 cells/μL was associated with higher hospitalization rates, but not intermediate CD4 count categories [22]. However, patients in this study were 12 years younger than our study population and included 20% elite controllers, who may have a different immunologic profile than other PWH.
In our study of patients with sustained suppression for up to 11 years, patients with even modestly lower CD4 recovery had higher rates of hospitalization, including patients without presuppression CD4 counts <200 cells/μL. One possible explanation is confounding by age. Older patients at ART initiation have poorer CD4 recovery, and older patients are more likely to have multiple comorbidities [10, 33]. Yet estimates adjusted for age were similar to unadjusted results, including in analyses using different parameterizations of age. It is also possible that patients with CD4 counts <500 cells/μL after 5 years of ART would continue to experience CD4 recovery and eventually lower hospitalization rates. While CD4 recovery has been shown to occur up to 6–7 years after ART initiation, substantial increases in CD4 count are largely observed in the first 3 years of suppression [10, 29]. Additionally, if lower CD4 count groups included subsets of severely sick and repeatedly hospitalized patients, these individuals could have contributed to the disparities we observed. However, counting only 1 hospitalization per patient in each time interval, estimates, while attenuated, were consistent with main analyses.
Patients with attenuated CD4 recovery could have higher hospitalization rates through several mechanisms. First, lower CD4 counts put patients at risk of AIDS-defining opportunistic infections and other non-AIDS infections such as bacterial pneumonia, soft tissue infections, and bacteremia, although this risk is lower with sustained virologic suppression [11, 12, 34]. Lower CD4 recovery could be a marker for underlying immune dysfunction and more severe comorbid burden. ART initiation at lower CD4 counts leads to poorer CD4 recovery, persistent gut CD4 cell depletion, chronic inflammation and immune activation, and comorbidities [7, 13–16, 35]. It is possible some patients experienced viremia between consecutive suppressed VL measurements, for example, due to nonadherence, that contributed to lower CD4 counts and comorbidity incidence. In our main analyses, we defined virologic suppression as VL <400 copies/mL, which might have misclassified patients with low-level viremia as suppressed. In a subgroup analysis defining suppression as VL <75 copies/mL, we had limited statistical support for drawing most inferences. For results with sufficient sample size, findings were consistent with analyses using 400 copies/mL. However, low-level viremia has been associated with mortality, non-AIDS morbidity, and lower CD4 recovery, and it will be important with longer follow-up to assess hospitalization rates among patients with low-level viremia [36, 37].
Another potential mechanism is that the use of different antiretroviral agents might have affected CD4 recovery and disease progression, though recent studies comparing contemporary first-line ART regimens reported only small differences in CD4 recovery and similar AIDS and non-AIDS morbidity [38, 39]. Assessing effects of different ART regimens, especially newer agents, should be a priority as long-term data accrue among patients using these regimens. Finally, socioeconomic factors delaying HIV diagnosis, thus leading to lower CD4 counts on ART, might contribute to comorbidity incidence [17, 40]. For example, patients living in poverty have higher rates of smoking and poor nutrition [18, 19]. Additionally, poor insurance coverage can result in financial barriers and care delays, compromise comorbidity management, and increase hospitalization risk [41, 42].
Our findings of hospitalization disparities by CD4 recovery on long-term ART raise important questions. It should be further elucidated if immunocompromise marked by lower CD4 counts on ART is a direct cause of morbidity leading to hospitalization, or only a marker of greater comorbidity burden. In particular, additional work is indicated to evaluate whether hospitalization disparities by CD4 count are present when assessing patients with similar comorbidity profiles. Estimating the effect on hospitalization risk of differential clinical management based on longitudinal CD4 count among patients with long-term suppression is also indicated. In our study, the most frequent hospitalization diagnostic categories were non-AIDS-defining infection, cardiovascular conditions, and liver/gastrointestinal conditions. Patients with slower CD4 count recovery following ART initiation, or with CD4 counts below a certain threshold after several years of ART, might benefit from earlier screening and treatment or more frequent follow-up for certain conditions, to prevent disease progression and hospitalization. Implementation of targeted interventions to reduce smoking and other modifiable risk factors might further prevent excess hospitalizations. Infection prevention, including vaccination and harm reduction for people who inject drugs, could also mitigate acute events requiring hospitalization. Other interventions currently being evaluated in PWH, such as prophylactic statin use, if effective, could potentially be particularly beneficial to these patients [43].
This study’s strengths include sufficient sample size and follow-up to examine patients with different CD4 trajectories after >5 years of virologic suppression, although a substantial proportion of patients were censored at 12 months without an HIV RNA measurement. We included PWH from 1 Canadian and 5 US cohorts, representing heterogeneous patient populations and care settings, though cisgender women made up a small proportion of the patient sample. Our findings may not be generalizable to all virologically suppressed PWH in this region or other settings. Hospitalization rates could be slightly underestimated if patients had hospitalizations at external sites that were not captured. However, we have no reason to believe there was differential hospitalization capture by CD4 count category. One possible limitation is that we measured immune recovery using only CD4 counts, which are subject to variability, though using moving averages of 3 measurements might have mitigated this. Other measures such as CD4 percentage and CD4/CD8 ratio were not available in this study. Additionally, some patients might have taken ART prior to entering NA-ACCORD and have a nadir CD4 count lower than what we observed. Finally, we did not have data on comorbidities, insurance, socioeconomic status, or substance use, important risk factors for morbidity and hospitalization.
In conclusion, patients with CD4 counts ≤500 vs >500 cells/μL after several years of virologic suppression experienced higher hospitalization rates, even without a history of CD4 <200 cells/µL. Future studies should elucidate reasons for these disparities and whether specific clinical management approaches among patients with attenuated CD4 count recovery on long-term suppressive ART can prevent acute events requiring inpatient care.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
APPENDIX
NA-ACCORD Collaborating Cohorts and Representatives: AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson and Ronald J. Bosch.
AIDS Link to the IntraVenous Experience: Gregory D. Kirk.
Fenway Health HIV Cohort: Kenneth H. Mayer and Chris Grasso.
HAART Observational Medical Evaluation and Research: Robert S. Hogg, Julio S. G. Montaner, Kate Salters, Viviane D. Lima, Paul Sereda, and Jason Trigg.
HIV Outpatient Study: Kate Buchacz and Jun Li.
HIV Research Network: Kelly A. Gebo and Richard D. Moore.
Johns Hopkins HIV Clinical Cohort: Richard D. Moore.
John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez.
Kaiser Permanente Mid-Atlantic States: Michael A. Horberg.
Kaiser Permanente Northern California: Michael J. Silverberg.
Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne.
MACS/WIHS Combined Cohort Study: Todd Brown, Phyllis Tien, and Gypsyamber D’Souza.
Multicenter Hemophilia Cohort Study–II: Charles Rabkin.
Montreal Chest Institute Immunodeficiency Service Cohort: Marina B. Klein.
Ontario HIV Treatment Network Cohort Study: Abigail Kroch, Ann Burchell, Adrian Betts, and Joanne Lindsay.
Retrovirus Research Center, Bayamon, Puerto Rico: Robert F. Hunter-Mellado and Angel M. Mayor.
Southern Alberta Clinic Cohort: M. John Gill.
Study of the Consequences of the Protease Inhibitor Era: Jeffrey N. Martin.
Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Jun Li and John T. Brooks.
University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig.
University of California at San Diego: William C. Mathews.
University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron, and Sonia Napravnik.
University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane.
Vanderbilt Comprehensive Care Clinic HIV Cohort: Timothy R. Sterling, David Haas, Peter Rebeiro, and Megan Turner.
Veterans Aging Cohort Study: Janet Tate, Robert Dubrow, and David Fiellin.
NA-ACCORD Study Administration:
Executive Committee: Richard D. Moore, Keri N. Althoff, Stephen J. Gange, Mari M. Kitahata, Michael S. Saag, Michael A. Horberg, Marina B. Klein, Rosemary G. McKaig, and Aimee M. Freeman.
Administrative Core: Richard D. Moore, Keri N. Althoff, and Aimee M. Freeman.
Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Liz Morton, Justin McReynolds, and William B. Lober.
Epidemiology and Biostatistics Core: Stephen J. Gange, Keri N. Althoff, Jennifer S. Lee, Bin You, Brenna Hogan, Jinbing Zhang, Jerry Jing, Elizabeth Humes, Lucas Gerace, and Sally Coburn.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the Centers for Disease Control and Prevention (CDC).
Financial support. T. D.-M. has received support from the National Institute of Allergy and Infectious Diseases (NIAID; grant number T32AI007001) and the National Institute on Drug Abuse (NIDA; grant number T32DA007250). This work was supported by the National Institutes of Health (NIH) (grant numbers U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01DA011602, R01DA012568, R01 AG053100, R24AI067039, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA03629, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24AA020794,U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, Z01CP010214, and Z01CP010176); the Centers for Disease Control and Prevention (CDC) (contract numbers CDC-200-2006-18797 and CDC-200-2015-63931); the Agency for Healthcare Research and Quality (contract number 90047713); the Health Resources and Services Administration (contract number 90051652); the Canadian Institutes of Health Research (grant numbers CBR-86906, CBR-94036, HCP-97105, and TGF-96118); the Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Heart, Lung, and Blood Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Human Genome Research Institute, the National Institute of Mental Health, NIDA, the National Institute on Aging, the National Institute of Dental and Craniofacial Research, the National Institute of Neurological Disorders and Stroke, the National Institute of Nursing Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and Other Communication Disorders, and the National Institute of Diabetes and Digestive and Kidney Diseases.
Potential conflicts of interest. K. N. A. serves as a consultant to the All of Us study (NIH) and on the scientific advisory board for TrioHealth, outside the scope of this work. J. A. C. has received honoraria from Integritas Communications (Gilead educational grant) and Vindico Medical Education (ViiV education grant). J. J. E. has received grants and personal fees from ViiV, Janssen, and Gilead, and personal fees from Merck. M. J. G. has received honoraria for membership in ad hoc national HIV advisory committee meetings for Merck, Gilead, and ViiV. M. B. K. has received research support from ViiV, AbbVie, Merck, and Gilead and consulting fees from ViiV, AbbVie, and Gilead. M. J. S. has received grants from Gilead. D. v. D. has served on the advisory boards of Allergan, Achaogen, Qpex, Shionogi, Sanofi-Pasteur, T2 Biosystems, NeuMedicine, Roche, MedImmune, Astellas, and Merck. D. A. W. has served on the advisory boards of Gilead, Merck, ViiV, and Janssen, and has received grants from Gilead, ViiV, and Merck. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of the International Epidemiology Databases to Evaluate AIDS (IeDEA):
Constance A Benson, Ronald J Bosch, Gregory D Kirk, Kenneth H Mayer, Chris Grasso, Robert S Hogg, Julio S G Montaner, Kate Salters, Viviane D Lima, Paul Sereda, Jason Trigg, Kate Buchacz, Jun Li, Kelly A Gebo, Richard D Moore, Richard D Moore, Benigno Rodriguez, Michael A Horberg, Michael J Silverberg, Jennifer E Thorne, Todd Brown, Phyllis Tien, Gypsyamber D’Souza, Charles Rabkin, Marina B Klein, Abigail Kroch, Ann Burchell, Adrian Betts, oanne Lindsay, Robert F Hunter-Mellado, Angel M Mayor, M John Gill, Jeffrey N Martin, Jun Li, John T Brooks, Michael S Saag, Michael J Mugavero, James Willig, William C Mathews, Joseph J Eron, Sonia Napravnik, Mari M Kitahata, Heidi M Crane, Timothy R Sterling, David Haas, Peter Rebeiro, Megan Turner, Janet Tate, Robert Dubrow, David Fiellin, Richard D Moore, Keri N Althoff, Stephen J Gange, Mari M Kitahata, Michael S Saag, Michael A Horberg, Marina B Klein, Rosemary G McKaig, Aimee M Freeman, Richard D Moore, Keri N Althoff, Aimee M Freeman, Mari M Kitahata, Stephen E Van Rompaey, Heidi M Crane, Liz Morton, Justin McReynolds, William B Lober, Stephen J Gange, Keri N Althoff, Jennifer S Lee, Bin You, Brenna Hogan, Jinbing Zhang, Jerry Jing, Elizabeth Humes, Lucas Gerace, and Sally Coburn
References
- 1.Berry SA, Fleishman JA, Moore RD, Gebo KA. Trends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001–2008. J Acquir Immune Defic Syndr 2012; 59:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleming J, Berry SA, Moore RD, et al. U.S. hospitalization rates and reasons stratified by age among persons with HIV 2014–15. AIDS Care 2019; 32:1353–62. [DOI] [PubMed] [Google Scholar]
- 3.Davy-Mendez T, Napravnik S, Hogan BC, et al. Hospitalization rates and causes among persons with HIV in the US and Canada, 2005–2015 [manuscript published online ahead of print 21 October 2020]. J Infect Dis 2020. doi: 10.1093/infdis/jiaa661. [DOI]
- 4.Healthcare Cost and Utilization Project. HCUP fast stats.https://www.hcup-us.ahrq.gov/faststats/NationalTrendsServlet. Accessed 12 August 2019.
- 5.Canadian Institute for Health Information. Inpatient hospitalizations: volumes, length of stay and standardized rates.https://apps.cihi.ca/mstrapp/asp/Main.aspx. Accessed 8 April 2020.
- 6.Nance RM, Delaney JAC, Simoni JM, et al. HIV viral suppression trends over time among HIV-infected patients receiving care in the United States, 1997 to 2015: a cohort study. Ann Intern Med 2018; 169:376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368:218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall HI, Tang T, Espinoza L. Late diagnosis of HIV infection in metropolitan areas of the United States and Puerto Rico. AIDS Behav 2016; 20:967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilton J, Light L, Gardner S, et al. Late diagnosis, delayed presentation and late presentation among persons enrolled in a clinical HIV cohort in Ontario, Canada (1999–2013). HIV Med 2019; 20:110–20. [DOI] [PubMed] [Google Scholar]
- 10.Roul H, Mary-Krause M, Ghosn J, et al. ; FHDH-ANRS CO4 . CD4+ cell count recovery after combined antiretroviral therapy in the modern combined antiretroviral therapy era. AIDS 2018; 32:2605–14. [DOI] [PubMed] [Google Scholar]
- 11.Buchacz K, Lau B, Jing Y, et al. Incidence of AIDS-defining opportunistic infections in a multicohort analysis of HIV-infected persons in the United States and Canada, 2000–2010. J Infect Dis 2016; 214:862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia Garrido HM, Mak AMR, Wit FWNM, et al. Incidence and risk factors for invasive pneumococcal disease and community-acquired pneumonia in human immunodeficiency virus-infected individuals in a high-income setting. Clin Infect Dis 2020; 71:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis 2016; 214:S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev 2011; 20:2551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverberg MJ, Leyden WA, Xu L, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr 2014; 65:160–6. [DOI] [PubMed] [Google Scholar]
- 16.Abraham AG, Althoff KN, Jing Y, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) . End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis 2015; 60:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ransome Y, Kawachi I, Braunstein S, Nash D. Structural inequalities drive late HIV diagnosis: the role of black racial concentration, income inequality, socioeconomic deprivation, and HIV testing. Health Place 2016; 42:148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162:335–44. [DOI] [PubMed] [Google Scholar]
- 19.Rehm CD, Penalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA 2016; 315:2542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchacz K, Baker RK, Moorman AC, et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS 2008; 22:1345–54. [DOI] [PubMed] [Google Scholar]
- 21.Yehia BR, Fleishman JA, Hicks PL, Ridore M, Moore RD, Gebo KA. Inpatient health services utilization among HIV-infected adult patients in care 2002–2007. J Acquir Immune Defic Syndr 2010; 53:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowell TA, Ganesan A, Berry SA, Deiss RG, Agan BK, Okulicz JF; Infectious Disease Clinical Research Program (IDCRP) HIV Working Group . Hospitalizations among HIV controllers and persons with medically controlled HIV in the U.S. Military HIV Natural History Study. J Int AIDS Soc 2016; 19:20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowell TA, Gebo KA, Blankson JN, et al. ; HIV Research Network . Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis 2015; 211:1692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol 2007; 36:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helleberg M, Kronborg G, Larsen CS, et al. CD4 decline is associated with increased risk of cardiovascular disease, cancer, and death in virally suppressed patients with HIV. Clin Infect Dis 2013; 57:314–21. [DOI] [PubMed] [Google Scholar]
- 26.Elixhauser A, Steiner C, Palmer L. Clinical Classifications Software (CCS).https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed 31 March 2020.
- 27.Gebo KA, Diener-West M, Moore RD. Hospitalization rates differ by hepatitis C status in an urban HIV cohort. J Acquir Immune Defic Syndr 2003; 34:165–73. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Medicare and Medicaid Services. General equivalence mappings.http://data.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html. Accessed 31 March 2020.
- 29.Palella FJ Jr, Armon C, Chmiel JS, et al. ; HOPS Investigators . CD4 cell count at initiation of ART, long-term likelihood of achieving CD4 >750 cells/mm3 and mortality risk. J Antimicrob Chemother 2016; 71:2654–62. [DOI] [PubMed] [Google Scholar]
- 30.Calkins KL, Chander G, Joshu CE, et al. Immune status and associated mortality after cancer treatment among individuals with HIV in the antiretroviral therapy era. JAMA Oncol 2020; 6:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davy-Mendez T, Napravnik S, Wohl DA, et al. Hospitalization rates and outcomes among persons with human immunodeficiency virus in the southeastern United States, 1996–2016. Clin Infect Dis 2020; 71:1616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berry SA, Manabe YC, Moore RD, Gebo KA. Hospitalization risk following initiation of highly active antiretroviral therapy. HIV Med 2010; 11:289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong C, Gange SJ, Moore RD, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) . Multimorbidity among persons living with human immunodeficiency virus in the United States. Clin Infect Dis 2018; 66:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemmige V, Arias CA, Pasalar S, Giordano TP. Skin and soft tissue infection in people living with human immunodeficiency virus in a large, urban, public healthcare system in Houston, Texas, 2009–2014. Clin Infect Dis 2020; 70:1985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 2003; 77:11708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elvstam O, Marrone G, Medstrand P, et al. All-cause mortality and serious non-AIDS events in adults with low-level HIV viremia during combination antiretroviral therapy: results from a Swedish nationwide observational study [manuscript published online ahead of print 9 April 2020]. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostrowski SR, Katzenstein TL, Thim PT, Pedersen BK, Gerstoft J, Ullum H. Low-level viremia and proviral DNA impede immune reconstitution in HIV-1-infected patients receiving highly active antiretroviral therapy. J Infect Dis 2005; 191:348–57. [DOI] [PubMed] [Google Scholar]
- 38.Edwards JK, Cole SR, Hall HI, et al. ; CNICS Investigators . Virologic suppression and CD4+ cell count recovery after initiation of raltegravir or efavirenz-containing HIV treatment regimens. AIDS 2018; 32:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu H, Cole SR, Westreich D, et al. Clinical effectiveness of integrase strand transfer inhibitor-based antiretroviral regimens among adults with human immunodeficiency virus: a collaboration of cohort studies in the United States and Canada [manuscript published online ahead of print 11 April 2020]. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adedinsewo DA, Wei SC, Robertson M, et al. Timing of antiretroviral therapy initiation in a nationally representative sample of HIV-infected adults receiving medical care in the United States. AIDS Patient Care STDS 2014; 28:613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludema C, Cole SR, Eron JJ Jr, et al. Health insurance type and control of hypertension among US women living with and without HIV infection in the women’s interagency HIV study. Am J Hypertens 2017; 30:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bindman AB, Chattopadhyay A, Auerback GM. Interruptions in Medicaid coverage and risk for hospitalization for ambulatory care-sensitive conditions. Ann Intern Med 2008; 149:854–60. [DOI] [PubMed] [Google Scholar]
- 43.Fichtenbaum CJ, Ribaudo HJ, Leon-Cruz J, et al. ; REPRIEVE Investigators . Patterns of antiretroviral therapy use and immunologic profiles at enrollment in the REPRIEVE trial. J Infect Dis 2020; 222:S8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.