Abstract
Background
Ebola virus disease (EVD) supportive care strategies are largely guided by retrospective observational research. This study investigated the effect of EVD supportive care algorithms on duration of survival in a controlled nonhuman primate (NHP) model.
Methods
Fourteen rhesus macaques were challenged intramuscularly with a target dose of Ebola virus (1000 plaque-forming units; Kikwit). NHPs were allocated to intensive care unit (ICU)–like algorithms (n = 7), intravenous fluids plus levofloxacin (n = 2), or a control group (n = 5). The primary outcome measure was duration of survival, and secondary outcomes included changes in clinical laboratory values.
Results
Duration of survival was not significantly different between the pooled ICU-like algorithm and control groups (8.2 vs 6.9 days of survival; hazard ratio; 0.50; P = .25). Norepinephrine was effective in transiently maintaining baseline blood pressure. NHPs treated with ICU-like algorithms had delayed onset of liver and kidney injury.
Conclusions
While an obvious survival difference was not observed with ICU-like care, clinical observations from this model may aid in EVD supportive care NHP model refinement.
Keywords: Ebola virus disease, hemorrhagic fevers, viral, models, animal, Filoviridae, Mononegavirales, intensive care
We investigated supportive care algorithms for Ebola virus disease (EVD) in nonhuman primates (NHPs). Although no obvious survival difference was observed with intensive care unit–like care, clinical observations may aid in refining an NHP model for EVD supportive care.
Observational data suggests that improved outcomes can be achieved with resource-intensive supportive care measures for treating Ebola virus (EBOV) disease (EVD). The 2013–2016 epidemic of EVD in West Africa demonstrated differences in mortality rates between sites, with case fatality rates from 40% to 70%, and improvement occurring over the duration of the outbreak may have been related to wider use of supportive care [1]. Individuals receiving care in intensive care units (ICU) in the United States and Europe had a case fatality rate of 18.5%, which was relatively low compared with the rate among those receiving care in Africa [2]. After the 2013–2016 epidemic, there was an increased emphasis on providing evidence-based supportive care, and new guidelines were developed [3–6]. The breakthrough for treatment for EVD came with the identification of effective monoclonal (mAb114) or pooled (REGN-EB3) monoclonal antibody–based therapeutics in the PALM trial [7]. Though outcomes were substantially improved, mortality rates remain unacceptably high (34%–35%) among those who received the most effective therapeutics and up to 64% (with REG-EB3) to 70% (with monoclonal antibody 114) among those with high viral loads [7].
These findings highlight the need to optimize supportive care to decrease the risk of death due to EVD. Optimal supportive care has not been rigorously studied in patients with EVD for several reasons: resource limitations, infectious risk, iatrogenic complications, and ethical concerns. Animal models provide an opportunity for controlled EVD research to advance supportive care treatments [5]. EVD in nonhuman primates (NHPs) resembles human EVD, with fever, rash, immunopathology, coagulopathy, and end organ failure. However, supportive care research in NHP models in the biosafety level 4 environment presents significant biosafety and logistical challenges [8]. Prior research in an NHP model examined the impact of bolus intravenous fluid replacement on survival [9]. That study was limited to a single intervention of fluid resuscitation, and there were challenges in assessing the animals continuously, as would be done in an ICU setting for humans. The study team saw no impact on survival, but treatment did lower serum blood urea nitrogen and creatinine levels in infected animals [9].
Other groups later attempted to replicate an ICU-like treatment bundle for NHPs, which demonstrated the feasibility of ICU-like care for EBOV infection [10]. ICU-like care had no significant impact on the clinical course, but conclusions were limited by the absence of a control group [8]. In the current article, we describe a model incorporating measures to simulate an algorithmic approach for managing EVD with ICU-like supportive care treatment, including continuous monitoring, intravenous fluids, antibiotics, corticosteroids, vasopressors, and electrolyte repletion.
MATERIALS AND METHODS
Ethics Statement
Research was conducted under an Institutional Animal Care and Use Committee (IACUC)–approved protocol in compliance with the Animal Welfare Act, Public Health Service policy, and other federal statutes and regulations relating to animals and experiments involving animals. The experiments were performed in a biosafety level 4 facility at the US Army Medical Research Institute of Infectious Diseases. This facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Animal Use and Viral Inoculation
The rhesus macaque (Macaca mulatta) 1000–plaque-forming unit (PFU) intramuscular challenge model with EBOV/H.sapiens-tc/COD/1995/Kikwit-9510621 was chosen as it is commonly used to evaluate EBOV therapeutics [11–13]. NHPs were initially divided into a levofloxacin plus intravenous fluids group (n = 2), an ICU-like care group (n = 7), and a control group (n = 5). We were unable to randomize NHPs to control and intervention groups owing to unanticipated difficulties with telemetry devices. Groups were evenly distributed by sex and age. The ICU-like care group was subsequently subdivided into an initial algorithm (n = 2) and revised algorithm (n = 5) groups (Supplementary Figure 1). The ICU-like revised algorithm group was subsequently subdivided further for descriptive purposes and designated based on whether or not norepinephrine was administered (revised ICU-like with norepinephrine [n = 2]; revised ICU-like without norepinephrine [n = 3]).
All NHPs had access to oral hydration, food, and environmental enrichment per standard procedures. NHPs were euthanized if they met standardized euthanasia criteria, as described elsewhere [14]. All NHPs (control and intervention) with a responsiveness score of ≥2 received intravenous buprenorphine (0.01–0.02 mg/kg twice daily) for analgesia until the animal reached euthanasia criteria. Before viral inoculation, all NHPs had telemetry devices implanted (TSE Stellar PPBTA-XXL; or DSI L21), according to manufacturer specifications. Telemetry implants were configured to measure mean arterial pressure (MAP), body temperature, heart rate, electrocardiogram, and intrathoracic pressure or left ventricular pressure. Baseline data were generated for 7 days before viral inoculation.
Telemetry data were collected using antennas located in proximity to the cages, connected to radiofrequency receivers in the animal holding room and wired to data acquisition computers for storage and analysis. Data were captured and archived as digital data in proprietary file format (Notocord Structured Storage) and processed using the Notocord-hem Evolution software platform (version 4.3.0.75). All NHPs were fitted into jackets (Lomir) and tethers a minimum of 3 days before central venous catheter (CVC) surgery to acclimate animals to their presence, facilitate patency of CVCs, and ensure animal safety after CVC implantation. Each animal had a single-lumen Hickman-Broviac catheter placed in an internal jugular vein, and either a catheter-in-catheter system (AVA Biomedical) or a single-lumen Hickman-Broviac catheter was placed into a femoral vein. Blood collection procedures from CVCs are described in the Supplementary Methods.
Supportive Care Algorithm Design and Treatment Triggers
Animals in the control group received no specific supportive care interventions. The ICU-like supportive care algorithms for this study were designed based on human Surviving Sepsis guidelines, World Health Organization guidelines, and retrospective observations of data from rhesus macaques exposed to EBOV (Supplementary Figure 1 and Supplementary Table 1) [15–17]. Medications were evaluated in vitro to ensure that no direct antiviral effects of treatments were incorporated into the supportive care bundles (Supplementary Methods and Supplementary Table 3) [13]. A simple, nonadaptive supportive care algorithm consisted of maintenance fluids (lactated Ringer’s solution) and levofloxacin. Maintenance fluid rates were initially based on the human, weight-based pediatric Holliday-Segar method [18].
Treatments were initiated at the onset of fever or hypotension to simulate management of human disease. NHPs were allocated to groups based on telemetry functionality while balancing age, sex, and weight before challenge. However, 1 NHP assigned to ICU-like care (D5) had a malfunctioning blood pressure probe 4 days after inoculation. Hydrocortisone was subsequently initiated empirically that day, and the NHP did not receive norepinephrine during the study. To replace this NHP, another NHP (D3) was reassigned to the intensive treatment group from a control group after IACUC permission. Fever was defined as a body temperature 1.5°C above an animal’s baseline value or values 3 standard deviations (SDs) above the baseline value and sustained for 1 hour [9]. Fever was chosen as the first threshold to initiate interventions for all treatment groups, because it is the most common sign in human patients with EVD [19]. Hypotension was defined as a drop in MAP of either 20 mm Hg or <3 SDs below baseline [9].
For the ICU-like intervention groups, once hypotension occurred, NHPs were eligible for an intravenous fluid bolus, followed by norepinephrine titration toward baseline MAP. Hydrocortisone was initiated for hypotension refractory to intravenous fluid therapy and vasopressor treatment. Remote-controlled infusion pumps (IRADIMED; MRidium 3860+) were used to immediately adjust fluid rates, deliver fluid boluses, and adjust vasopressor infusions. The well-being of NHPs was continuously monitored through real-time video at the telemetry station. Other interventions limited to the ICU-like group are summarized in Supplementary Data I and II. Owing to the complexity of this study, it was conducted over 3 separate iterations. We attempted to improve care of the NHPs between iterations with veterinarian consultation and IACUC approval. Lessons learned from iteration 1 were applied to generate a “revised ICU-like” supportive care algorithm for iterations 2 and 3. Revisions included a decrease in the intravenous fluid initiation rate after fever onset, an increase in the vasopressor maximum rate, an increase in acetaminophen frequency, adjustments in electrolyte threshold and response, and the potential to make changes with veterinary consultation (Supplements I and II).
Clinical Laboratory Values, Quantitative Real-Time Polymerase Chain Reaction, and EBOV Immunoglobulins
Blood samples were obtained on day 0 (day of inoculation) and then daily beginning on day 3. Serum chemistry was analyzed using the Vitros 350 (Ortho Clinical Diagnostics), and hematology using Advia 120 with multispecies software (Siemens Healthcare Diagnostics). The VetScan i-STAT 1 Handheld Analyzer (Abaxis) was used for blood gas/lactate (CG4+ cartridge), troponin I (troponin cartridge), and electrolyte (CHEM8+ cartridge) analysis. We used quantitative real-time polymerase chain reaction to determine viral RNA copy numbers in plasma samples collected on day 0, and then daily starting day 3 after inoculation. The EBOV quantitative real-time polymerase chain reaction assay used for this study has been described elsewhere [13]. Methods to detect serum EBOV immunoglobulin titers and results are described in the Supplementary Methods and Supplementary Results.
Statistical Analysis
A sample size of 5 control animals and 6 treated animals was selected initially to achieve a 81.2% power to detect a survival difference of 42 hours (SD, 20 hours) with a significance level of .05 using a 2-sided 2-sample t test. Survival time was the time of euthanasia after a predefined end point was met or the time at which the NHP succumbed to disease.
Kaplan-Meier survival curves were created and centered at the time of inoculation, and a log-rank test was performed. After checking proportional hazards assumptions, univariate Cox proportional hazards regression was performed to compare all NHPs in the ICU-like algorithms and intravenous fluid plus antibiotics algorithm with controls. In addition, to compare the slopes of aortic pressure before and after norepinephrine, we performed piecewise multivariable linear regression to evaluate the effect of the start of vasopressors on the change in MAP for each NHP. This was followed by multilevel mixed-effects linear regression to evaluate the effect of vasopressors on aortic pressure, accounting for NHP-level correlation. To decrease the effect of the normal circadian variation and the initiation of other medications, we limited the data to 6 hours before and after the start of norepinephrine. All analyses were performed using Stata software, version 15.0 (StataCorp), and figures were created using and figures were created using Stata software or the R statistical platform, version 4.0.1 (R Foundation).
RESULTS
Clinical Experience With Supportive Care Interventions
NHP clinical parameters were prospectively followed, and supportive treatment modalities administered (Figure 1). Table 1 summarizes NHP level interventions during the study and additional clinical details for all NHPs are in Supplement II. During the initial iteration of ICU-like care, there were clinical observations that guided revisions of the ICU-like treatment algorithm. First, there was evidence of potential iatrogenic worsening of respiratory status from clinical observations of increased respiratory rates and peripheral edema after fluid resuscitation, compared with controls (Figure 2).
Figure 1.
Clinical laboratory values and supportive treatments in the longest living nonhuman primate (NHP) in the intensive care unit (ICU)–like care group (NHP D4) (A) and the longest living NHP assigned as a control (NHP A3) (B). Abbreviations: CRP, C-reactive protein; VL, viral load.
Table 1.
Nonhuman Primate–Level Intravenous Interventions and Duration of Survival
| Variable | NHPs by Treatment Groupa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intravenous Fluid + Levofloxacin (n = 2) | Initial ICU-Like Algorithm (n = 2) | Revised ICU-Like + Algorithm | |||||||
| With NE (n = 2) | Without NE (n = 3) | ||||||||
| B1 | B2 | C1 | C2 | D3 | D4 | D5 | D1 | D2 | |
| Starting time, d after inoculation | |||||||||
| Crystalloids | 4 | 3 | 3 | 4 | 4 | 4 | 4 | 3 | 4 |
| Levofloxacin | 4 | 3 | 3 | 4 | 4 | 4 | 4 | 3 | 4 |
| Norepinephrine | … | … | 5 | 7 | 5 | 4 | … b | … b | … b |
| Hydrocortisone | … | … | 6 | 7 | 5 | 5 | 4 | 5 | 5 |
| Imipenem | … | … | 6 | 7 | 5 | 5 | 8 | 5 | 5 |
| Intravenous fluid variable | |||||||||
| Daily volume, mean, mLc | 493 | 602 | 661 | 696 | 229 | 540 | 407 | 273 | 301 |
| No. of fluid boluses | 0 | 0 | 4 | 4 | 2 | 2 | 2 | 0 | 0 |
| Total volume received, mL | 1971 | 1806 | 3967 | 4179 | 2352 | 5939 | 2033 | 902 | 1363 |
| Weight change from time of challenge to death, kgd | +0.98 | +0.86 | +0.23 | +0.58 | +0.95 | +1.91 | +0.51 | −0.02 | … e |
| Potassium repletionf | |||||||||
| Duration of administration, time after inoculation, d | … | … | 7g | 4, 6–8 | 5–7 | 4–11 | 4–8 | 3–5 | 4–5 |
| Daily dose range, mEq | … | … | … g | 14–25 | 24–48 | 24–48 | 24–48 | 24–48 | 24–48 |
| No. of doses | … | … | … g | 7 | 4 | 11 | 5 | 3 | 2 |
| Duration of survival, dh | 6.5 | 6.5 | 7.3 | 9.0 | 8.2 | 13.0 | 9.2 | 5.2 | 8.2 |
Abbreviations: ICU, intensive care unit; NE, norepinephrine; NHP, nonhuman primate.
aControl NHPs (n = 5) did not receive supportive interventions.
bNorepinephrine administration was attempted but was not successful owing to central line malfunction or malfuntioning blood pressure probe.
cAverage per day after intravenous fluids were started.
dThe weight changes from time of challenge to death among control NHPs were −0.2 kg in A1, +0.3 kg in A2, −0.4 kg in A3, and −0.1 kg in A4; in A5, weight was not obtained before necropsy.
eWeight was not obtained before necropsy in D2.
fMagnesium, phosphate, and calcium repletion detailed in Supplement II.
gThis NHP (C1) did not receive the full dose.
hThe durations of survival for control NHPs were 5.83 (A1), 7.13 (A2), 9.08 (A3), 6.92 (A4), and 6.08 (A5) days; the median duration of survival among controls was 6.9 days
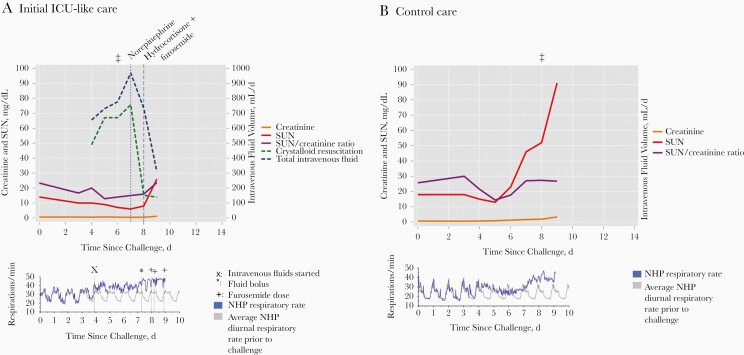
Figure 2.
Renal laboratory values, daily intravenous fluid, respiratory rate, and clinical edema in the longest living nonhuman primate (NHP) that received furosemide (NHP C2) (A) and the longest living NHP in the control group (NHP A3) (B). Facial or extremity edema was observed clinically on day 6 in C2 and on day 8 in A3 (denoted by ‡ above each graph). Abbreviations: ICU, intensive care unit; SUN, serum urea nitrogen.
Second, it was noted that the initial electrolyte repletion thresholds were set too low and were challenging to adequately replete, particularly with potassium and magnesium (Figure 1). Electrolyte repletion and intravenous fluid boluses led to volume overload and elevated respiratory rates (with presumed pulmonary edema) without the ability to intubate and mechanically ventilate. Furosemide dosing was attempted to treat presumed pulmonary edema but potentially exacerbated hypokalemia further and complicated hypotension reversal with norepinephrine.
Third, electrocardiographic (ECG) changes were noted, including ST-segment changes and arrhythmias, potentially due to electrolyte abnormalities (Table 2). Two NHPs had clinically significant hypokalemia despite electrolyte repletion, which likely contributed to the development of arrhythmias (Supplement II). One NHP had a syncopal event associated with transient tachyarrhythmias and the second had ECG findings associated with hypokalemia culminating in ventricular fibrillation. Therefore, a revised algorithm (Supplementary Table 2) decreased maintenance fluid rates after fever onset, made the fluid bolus at hypotension onset at clinician discretion, adjusted electrolyte thresholds, and modified medication formulation plans to concentrate intravenous medications maximally.
Table 2.
Summary of Clinical Cardiac Findings and Electrolytes During the Study
| NHP ID by Group | ECG Findings | Electrolytesa | Peak Troponin I, ng/mL |
|---|---|---|---|
| Controls | |||
| A1 | ST-segment depressions | ↑ K+, Mg2+ DPI 6 | 0.80 |
| A2 | ST-segment depressions, T-wave morphologic change | ↓↓ K+ DPI 4,6; ↑ K+, DPI 7 | 0.10 |
| A3 | Nothing other than sinus tachycardia | ↓ K+ DPI 5–9; ↑ Mg2+, DPI 7–9 | 0.70 |
| A4 | Nothing other than sinus tachycardia | ↓↓ K+ DPI 4–6 | 0.46 |
| A5 | ST-segment depressions, T-wave morphologic changes followed by loss of perfusion; these findings accompanied seizure activity | ↓ K+ DPI 5 | 0.06 |
| Intravenous fluids + levofloxacin | |||
| B1 | T-wave inversions | ↑ K+ Mg2+, DPI 7 | 0.25 |
| B2 | ST-segment elevations and T-wave changes | ↓ K+ DPI 5, 6 | 1.17 |
| ICU-like algorithms | |||
| Initial | |||
| C1b,c | Progression of changes consistent with hypokalemia leading to ventricular tachycardia and ventricular fibrillation | ↓↓ K+ DPI 7; K+, Mg2+ repletion initiated but not completed | 3.72 |
| C2b,c | Syncopal event observed on camera was associated with hypotension (MAP <50 mm Hg) and tachyarrhythmias | ↓↓ K+ DPI 4, 6–8; K+, Mg2+ repletion DPI 4,6-8 | 0.26 |
| Revised | |||
| D1c,d | Hyperacute T waves | ↓↓ K+ DPI 5; K+, Mg2+ repletion DPI 3–5 | 0.31 |
| D2d,e | ST depressions, T-wave morphologic change | K+ repletion DPI 4–5, Mg2+ repletion DPI 3–7 | 1.48 |
| D3b,c | ST depressions, T-wave morphologic change | ↓↓ K+ DPI 3,6, 7; K+ repletion DPI 5–7, Mg2+ repletion DPI 4–7 | 1.65 |
| D4b,c | Prominent ST-segment elevations | ↓↓ K+ DPI 6–9; K+, Mg2+ repletion DPI 4–11 | >50 |
| D5c,d | Decreased QRS amplitude, subtle ST-segment morphologic change | ↓↓ K+ DPI 7; ↑ K+, DPI 9; K+, Mg2+ repletion DPI 4–8 | >50 |
Abbreviations: DPI, days post inoculation; ECG, electrocardiographic; ICU, intensive care unit; ID, identifier; K+, potassium; MAP, mean arterial pressure; Mg2+, magnesium; NHP, nonhuman primate.
aThe focus here is on K+ and Mg2+. Other electrolytes are detailed in Supplement II by NHP. Single arrows (↓ and ↑) denote K+ levels outside 3.2–5.3 mmol/L or Mg2+ levels outside 1.7–2.3 mmol/L; double arrows (↓↓), an instance of severe hypokalemia (defined as K+ <3 mmol/L).
bNorepinephrine administered.
cHydrocortisone administered.
dNo norepinephrine administered.
eNo hydrocortisone administered.
Fourth, while norepinephrine appeared to stabilize blood pressure, the initial dose for the protocol was observed to be too low in practice. Thus, the revised ICU-like algorithm increased the maximum norepinephrine continuous infusion dosing from 4 µg/min to up to 12 µg/min initially, and to a maximum of 30 µg/min with veterinary consultation.
During the second iteration, the most significant challenge in conducting ICU-like care was disconnection of CVC tubing in NHP backpacks, leading to the inability to deliver norepinephrine (Supplement II). Subsequently, iteration 3 was complicated by loss of critical telemetry functionality (methods above; Supplement II). Despite this, we were able to deliver the revised ICU-like algorithm with norepinephrine successfully to 2 NHPs, with a third NHP receiving all of the revised ICU-like algorithm except norepinephrine (Table 1).
With the revised ICU-like algorithm the challenges observed with the first and second iterations were minimized. Electrolyte repletion needed frequent and continuous attention (Supplement II). However, despite this, the treated NHPs in the third iteration (D3, D4, and D5) developed multiorgan failure complicated by significantly elevated creatine kinase (presumed rhabdomyolysis) and myocardial injury. Myocardial injury was identified by elevated troponin I in all 3 of these NHPs (Table 2). ECG evidence of myocardial injury included prominent ST-segment elevations in NHP D4, and ST depressions in NHP D3 (Table 2). There were multiple ECG findings in controls and treated groups, with the most common being T-wave morphologic changes and ST-segment changes (Table 2).
Management of hypotension was challenging during this study. A temporary stabilization of blood pressure with norepinephrine was demonstrated in NHPs treated with ICU-like algorithms (Figure 3 and Supplementary Figure 5A), so this was explored statistically. In these NHPs, MAP decreased with an MAP slope of −2.2 mm Hg/h for 6 hours before norepinephrine initiation (Figure 3). This slope was reversed by an increase of a mean 3.7 mm Hg/h (95% CI, 2.4–5.0 mm Hg/h; P < .001) over the 6 hours after norepinephrine initiation (Supplementary Figure 5B). Administration of hydrocortisone may have led to transient improvements in MAP and a vasopressor sparing effect in some cases (Supplement II; NHPs C2 and D4). It was difficult to demonstrate a benefit of fluid boluses on blood pressure in this study, and the best example was noted in NHP C1 (Supplement II). There may have been a limited benefit because NHPs were already receiving maintenance intravenous fluid before the development of hypotension.
Figure 3.
Comparison of mean arterial pressure between groups by nonhuman primates (NHPs) with functioning telemetry blood pressure (BP) sensors. A, Controls (A3 and A5). B, Intravenous fluids plus levofloxacin treatment (B2) and revised intensive care unit (ICU)–like algorithm without norepinephrine (D1 and D2). C, Initial ICU-like algorithm (C1 and C2). D, Revised ICU-like algorithm with norepinephrine (D3 and D4). Dotted vertical lines indicate when norepinephrine was started in each treated NHP.
Survival Time and Viral Loads
Median survival times (interquartile range) were 6.9 (6.1–7.1) days for controls, 7.3 (7.3–9.0) days for the ICU-like group, and 8.2 (7.1–9.2) days for the ICU-like algorithm (NHPs pooled), and both NHPs in the intravenous fluids plus levofloxacin group survived for 6.5 days (Table 1). There was no difference observed in the survival curves with ICU-like groups (n = 2 or 3; log-rank P = .25) (Figure 4A) or when the ICU-like algorithms were pooled (log-rank P = .11) (Supplementary Figure 6). In addition, no difference was observed when pooled ICU-like care was directly compared with control care, with Cox regression (hazard ratio, 0.49; P = .25). ICU-like care algorithms were observed to have later and lower peaks in viral load (median, 8.0 days after inoculation; 8.3 log10 copies/mL) compared with controls (7.0 days after inoculation; 9.7 log10 copies/mL) and intravenous fluid plus levofloxacin (6 and 6 days after inoculation; 9.7 and 9.9 log10 copies/mL, respectively) (Figure 4B).
Figure 4.
A, Kaplan-Meier curve comparing treatment groups, grouping initial and revised intensive treatment groups. B, Plasma viral load (VL) over time, by treatment group. Abbreviations: ICU, intensive care units; LLOD, lower level of detection; LLOQ, lower level of quantification; NE, norepinephrine.
Effect of the ICU-Like Bundle on Organ Markers
All ICU-like groups appeared to have lower peak organ injury laboratory values than controls. This included median serum aspartate aminotransferase (552 vs 1014 U/L), alanine aminotransferase (123 vs 414 U/L), and creatinine (0.9 vs 4.8 mg/dL) levels (Figure 5). The NHPs that received levofloxacin plus intravenous fluids had peak laboratory markers similar to those in controls (aspartate aminotransferase, 932 and 2439 U/L, respectively; alanine aminotransferase, 285 and 726 U/L; creatinine, 1.6 and 4.3 mg/dL). Peak troponin I values were higher in the groups that received ICU-like care (median, 1.65 ng/mL) than in controls (0.46 ng/mL) or in those that received intravenous fluids plus levofloxacin alone (0.26 and 1.17 ng/mL). The median peak lactic acid was similar between NHPs that received ICU-like care (5.9 mmol/L) and those that received control care (6.3 mmol/L). The NHPs that received intravenous fluids plus levofloxacin had peak lactic acid levels of 3.4 and 4.9 mmol/L. In addition, there were no obvious differences in hemoglobin, white blood count, or platelet trends (Supplementary Figure 7).
Figure 5.
Clinical laboratory kinetics between treatment groups A, Alanine aminotransferase (ALT). B, Creatinine. C, Troponin I. D, Creatine kinase). Red lines represent locally estimated scatterplot smoothing curves of control group values; gray-shaded bands, control group confidence interval; and red dashed horizontal lines, upper limits of normal. No confidence intervals are shown in C because values among controls were low (≤0.8 ng/mL) compared with extreme elevations in 2 nonhuman primate. (Serum urea nitrogen and aspartate aminotransferase plots are included in Supplement I, Supplementary Figure 8.) Abbreviations: ICU, intensive care unit; NE, norepinephrine.
Discussion
EVD has high case fatality rates that warrant refinement of supportive care to decrease the risk of death. When pooling ICU-like care algorithms, we did not observe an increase in duration of survival compared with controls. NHPs receiving ICU-like supportive care algorithms demonstrated a delayed onset of liver or renal injury compared with controls or NHPs receiving maintenance intravenous fluids and antibiotics. This 1000-PFU intramuscular EBOV Kikwit EVD model successfully simulated aspects of ICU-like EVD care, but it may be too rapid to adequately evaluate the impact on outcomes.
Our initial observations of potential iatrogenic respiratory worsening owing to intravenous fluids and insufficient electrolyte repletion highlight the delicate treatment balance required and the need to refine treatment strategies for EVD. While case fatality rates due to EVD during the West Africa epidemic are thought to have decreased with improved supportive care, including increased fluid resuscitation [1], the case fatality rate remains unacceptably high, even with new therapeutics [20]. Evidence-based fluid resuscitation and electrolyte monitoring strategies are needed for settings where ventilatory support and dialysis are not readily available. In such locations, there may be a benefit to a more judicious strategy for intravenous fluids resuscitation goals [21–23]. Our study emphasizes the importance of close and near-real-time monitoring of vital signs (including ECG monitoring, when possible, in the sickest patients) and laboratory values, given the metabolic and acid-base derangement and volume depletion characteristic of severe disease, balanced with the need to avoid iatrogenic harm.
In our study design, we incorporated technologies that enabled ongoing assessment and care of the animals while minimizing infectious risk to the laboratory staff. A remote infusion device facilitated titration of intravenous fluids and vasopressors with “tele-infusion,” decreasing infectious risk to laboratory personnel while stabilizing blood pressure in real time. Similar measures could be implemented in the treatment of patients with EVD or coronavirus disease 2019, reducing healthcare worker exposure and adding to recent advances in personal protective equipment use [5].
Of note, the current study was the first to our knowledge to measure cardiac troponin I and simultaneously describe ECG changes in an NHP model of EVD. We observed markedly elevated troponin I levels in 3 NHPs, which received the highest cumulative doses of hydrocortisone. Two of these 3 NHPs received the revised ICU-like bundle with norepinephrine, but 1 did not receive norepinephrine. Potential causes of the troponin elevation and ECG changes observed include myocarditis due to direct viral damage, stress-related ischemia in the context of norepinephrine use, or ischemia due to coronary thrombosis. In addition, in 1 NHP ventricular fibrillation developed in the context of hypokalemia. While the small sample size limits our ability to be definitive, these observations support the utility of ECG and electrolyte monitoring during EVD.
Alternative strategies to optimizing supportive care for EVD are needed. An obvious mortality benefit was also absent in prior uncontrolled studies assessing supportive care for EVD in NHPs [8, 10]. However, the 1000-PFU intramuscular Kikwit model, which is nearly 100% fatal in NHPs over a course of a week, may not be ideal to demonstrate supportive care therapies. Alternative exposure methods that lead to a more prolonged disease course, such as intranasal exposure or low-dose intramuscular challenge, may be better suited for evaluating supportive care [9, 24, 25]. In addition, optimization of supportive care should be evaluated together with antibody-based therapeutics or novel small-molecule antivirals initiated after the onset of clinical disease, which would more closely approximate the human clinical experience.
The current study had multiple limitations owing to the complexity of administering advanced supportive treatment in a high-containment laboratory. These algorithms were complex and designed to optimize treatment outcomes and to simulate real world management. This led to heterogeneity of treatment dosing, duration, and onset after inoculation. It is possible that unmeasured confounders were introduced in the study during NHP reassignments. Because of technical difficulties in the ICU-like groups, sample size decreased, limiting conclusions from comparisons. Owing to technical and biosafety considerations, research staff members were not blinded to assignment. However, experiences during the study led to further refinements and improvements in delivery of supportive care in our NHP model.
Further research with supportive care in EVD could build on our findings to optimize components of study design. Expert guidelines for optimal ICU-like care management for viral hemorrhagic fevers have evolved over the past decade, as more clinical information has been obtained and standards of care have improved [3, 6]. Well-designed prospective observational studies, which collect clinical data in greater granularity, are needed to evaluate optimal management approaches and to ensure the NHP model is clinically translatable. Moreover, these results suggest a need to further refine the supportive care animal model for filovirus diseases and argue for the importance of antiviral treatments in addition to optimized supportive care. Recent progress with monoclonal antibody products for EVD has been encouraging, but more research is needed for enhanced supportive care.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: ASTMH 67th Annual Meeting, New Orleans, Louisiana, USA, 28 October–1 November 2018; ASTMH 68th Annual Meeting, National Harbor, Maryland, USA, 20–24 November 2019.
Acknowledgment. A. P. C. thanks Laura Gomba, Sagrario Espinal, and Brian Sauerbry for logistical support necessary to complete this study, and Travis K. Warren, PhD, for his mentorship during protocol development.
Disclaimer. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Financial support. This work was supported by the Joint Project Manager Medical Countermeasure Systems BioDefense Therapeutics (JPM-MCS-BDTX) and the National Institutes of Health (grant T32 HL116275 to P. W. B.).
Potential conflicts of interest. M. G. K. is chair of the Scientific Advisory Board of Integrum Scientifics. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Disclaimer: Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the US Armed Forces or the University of Nebraska Medical Center.
References
- 1.Garske T, Cori A, Ariyarajah A, et al. Heterogeneities in the case fatality ratio in the West African Ebola outbreak 2013–2016. Philos Trans R Soc Lond B Biol Sci 2017; 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uyeki TM, Mehta AK, Davey RT Jr, et al. Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med 2016; 374:636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamontagne F, Fowler RA, Adhikari NK, et al. Evidence-based guidelines for supportive care of patients with Ebola virus disease. Lancet 2018; 391:700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamontagne F, Clément C, Kojan R, Godin M, Kabuni P, Fowler RA. The evolution of supportive care for Ebola virus disease. Lancet 2019; 393:620–1. [DOI] [PubMed] [Google Scholar]
- 5.Fischer WA 2nd, Crozier I, Bausch DG, et al. Shifting the paradigm—applying universal standards of care to Ebola virus disease. N Engl J Med 2019; 380:1389–91. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Optimized supportive care for Ebola virus disease: clinical management standard operating procedures. Geneva, Switzerland: World Health Organization, 2019. [Google Scholar]
- 7.Mulangu S, Dodd LE, Davey RT Jr, et al. ; PALM Writing Group; PALM Consortium Study Team . A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 2019; 381:2293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poliquin G, Funk D, Jones S, et al. Impact of intensive care unit supportive care on the physiology of Ebola virus disease in a universally lethal non-human primate model. Intensive Care Med Exp 2019; 7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kortepeter MG, Lawler JV, Honko A, et al. Real-time monitoring of cardiovascular function in rhesus macaques infected with Zaire ebolavirus. J Infect Dis 2011; 204(suppl 3):S1000–10. [DOI] [PubMed] [Google Scholar]
- 10.Poliquin PG, Biondi M, Ranadheera C, et al. Delivering prolonged intensive care to a non-human primate: a high fidelity animal model of critical illness. Sci Rep 2017; 7:1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reisler RB, Zeng X, Schellhase CW, et al. Ebola virus causes intestinal tract architectural disruption and bacterial invasion in non-human primates. Viruses 2018; 10:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reisler RB, Yu C, Donofrio MJ, et al. Clinical laboratory values as early indicators of Ebola virus infection in nonhuman primates. Emerg Infect Dis 2017; 23:1316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016; 531:381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren TK, Trefry JC, Marko ST, et al. Euthanasia assessment in Ebola virus infected nonhuman primates. Viruses 2014; 6:4666–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. New edition. Geneva, Switzerland: World Health Organization, 2009. [PubMed] [Google Scholar]
- 16.World Health Organization. Clinical management of patients with viral haemorrhagic fever: a pocket guide for front-line health workers—interim emergency guidance for country adaptation. Geneva, Switzerland: World Health Organization, 2016. [Google Scholar]
- 17.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43:304–77 [DOI] [PubMed] [Google Scholar]
- 18.Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957; 19: 823–32. [PubMed] [Google Scholar]
- 19.Uyeki TM, Mehta AK, Davey RT Jr, et al. Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med 2016; 374:636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Ebola health update—DRC, 2019/situation reports. https://www.who.int/emergencies/diseases/ebola/drc-2019/situation-reports. Accessed 1 June 2020.
- 21.Aluisio AR, Yam D, Peters JL, et al. Impact of intravenous fluid therapy on survival among patients with Ebola virus disease: an international multisite retrospective cohort study. Clin Infect Dis 2020; 70:1038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews B, Semler MW, Muchemwa L, et al. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMA 2017; 318:1233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maitland K, Kiguli S, Opoka RO, et al. ; FEAST Trial Group . Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011; 364:2483–95. [DOI] [PubMed] [Google Scholar]
- 24.Speranza E, Bixler SL, Altamura LA, et al. A conserved transcriptional response to intranasal Ebola virus exposure in nonhuman primates prior to onset of fever. Sci Transl Med 2018; 10:eaaq1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfson KJ, Avena LE, Worwa G, Carrion R, Griffiths A. Development of a lethal intranasal exposure model of Ebola virus in the cynomolgus macaque. Viruses 2017; 9:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.