Abstract
Background
Bilastine, a non-sedating H1-antihistamine, is indicated to treat the symptoms of allergic disorders (e.g. rhinoconjunctivitis and urticaria) in adults and adolescents and, more recently, in children. Following its marketing approval, many questions regarding the ideal use of bilastine in various clinical practice situations have been received by the Medical Information Department (MID) of Faes Farma Spain. This article is an update of a previous review, with a focus on recent clinical information on the use of bilastine in paediatric and other populations.
Methods
Results of recent clinical studies in paediatric and other populations as well as questions received and responses provided by the Faes Farma MID.
Results
The information regarding the use of bilastine in paediatric patients is the most relevant aspect of this updated review. The stepwise approval of the paediatric formulations in various countries started with the European Medicines Agency approval in 2017 in accordance with a 2009 Paediatric Investigation Plan, followed by approval in other countries. The queries that are most commonly received by the Faes Farma MID include the potential for drug interactions involving bilastine and other frequently used drugs, and the use of bilastine in special populations or to treat specific symptoms related to allergic conditions. As the concomitant use of many medications is not permitted during clinical trials, the advice provided regarding the concomitant use of other medications with bilastine considers the pharmacological properties of both the drug in question and bilastine, as well as expert opinion. Likewise, advice regarding the use of bilastine in special populations (e.g. patients with renal impairment, obesity, lactose intolerance, and elderly or pregnant individuals) or to treat specific symptoms (e.g. treatment-resistant urticaria, pruritus or BASCULE syndrome) considers the best evidence from a variety of sources, including clinical studies, real-world experience, guideline recommendations and expert opinion.
Conclusion
This updated review provides current data regarding the best use of bilastine in specific situations and patients and identifies areas in which further knowledge is required. Although decisions regarding the use of bilastine may be aided by expert opinion that relies on knowledge of the underlying science, additional research and evidence are required to answer certain queries regarding the use of bilastine.
Keywords: allergic rhinoconjunctivitis, antihistamines, bilastine, drug information, drug interactions, medical information services, paediatrics, safety, urticaria
Introduction
Pharmaceutical companies often receive queries regarding their medicinal products from the public, healthcare professionals, researchers and others.1,2 Such queries are answered by specialists in the Medical Information Department (MID) of the pharmaceutical company, who must be able to provide accurate, appropriate and succinct responses that incorporate balanced information from several sources (e.g. the best evidence from the current literature, practical experience from everyday use, and expert opinion regarding the drug’s pharmacological properties2,3).
In this update to a 2017 review by Leceta et al.,2 real-world queries, responses and supporting data related to the clinical use of bilastine, a non-sedating H1-antihistamine, are presented. These queries were made by healthcare professionals and the medical departments of authorized cross-licensing pharmaceutical companies and were responded to by the MID of Faes Farma Spain, underscoring the practical nature of the services provided by this department. Of note, some of the queries and responses are related to the use of bilastine in indications or at dosages that are not approved in certain countries. Although the Faes Farma MID provided answers to such queries based on the best evidence currently available, their responses should not be taken as an endorsement of the unapproved use of bilastine by Faes Farma Spain.
Review
Use in paediatric patients
The most relevant aspect of this updated review is the information regarding the use of once-daily bilastine 10 mg to treat symptomatic allergic rhinoconjunctivitis (AR) and urticaria in paediatric patients. Since the previous review, the initial 2010 approval of bilastine to treat symptomatic AR or urticaria in adults and adolescents aged ≥12 years has been expanded to include paediatric patients in the European Union4 and elsewhere. The expanded indication was based on the results of the Paediatric Investigation Plan (PIP) agreed upon with the European Medicines Agency (EMA) in 2009. PIPs are developed to ensure that EMA authorizations of medicines for use in paediatric patients are supported by the necessary clinical data from studies in this population.5 The stepwise approval of the paediatric formulations of once-daily bilastine 10 mg in various countries started with the EMA approval in 2017, with subsequent approval in other countries
As with other histamine H1-antihistamines, the efficacy of bilastine in clinical trials in adults and adolescents can be extrapolated to children and there is no need to perform efficacy trials in paediatric populations. It has been demonstrated that the systemic exposure to bilastine provided by bilastine 10 mg in children aged 6–11 years weighing ≥20 kg is equivalent to that provided by bilastine 20 mg in adults.4 The extrapolation from adult and adolescent data is deemed appropriate for bilastine as the pathophysiology of ARs and urticaria is the same for all age groups.
The PIP included trials in children aged 2–11 years, with results supporting the use of bilastine 10 mg/day across this age range. However, the approved age of use of bilastine in children currently varies somewhat between countries, depending on their approach to paediatric use. Paediatric approval of bilastine in the EMA followed the usual stepwise procedure of initial approval in older children (i.e. those aged 6–11 years) and, therefore, bilastine is currently approved to treat children aged 6–11 years who weigh ≥20 kg in the European Union according to the current Summary of Product Characteristics (SmPC).4 Based on the PIP evidence in younger children, bilastine 10 mg is approved to treat children aged 2–11 years in Mexico and countries in Africa. Where approved for use in paediatric patients, bilastine 10 mg is available as a 10 mg oral dispersible tablet (ODT) and/or as 4 mL of the 2.5 mg/mL oral solution.4,6,7 The reader is advised to consult local prescribing information for further details on the current status of the paediatric use of bilastine in various countries.
Bilastine 10 mg/day was considered suitable for use in clinical studies in children aged 2–11 years based on modelled data.8 Analysis of clinical pharmacokinetic data in children aged 2–11 years with AR or urticaria confirmed the validity of the bilastine 10-mg dose in this population due to the achievement of pharmacokinetic, pharmacodynamic and integrative results comparable to those in adults receiving bilastine 20 mg/day.9 These results were supported in a sub-group analysis of the data in children aged 6–11 years.10 The oral bioequivalence of bilastine 10 mg ODT and bilastine 10 mg oral solution was shown in a crossover study in healthy individuals, which also indicated that the bioavailability of the ODT was not affected by taking it with or without water.11
The safety of once-daily bilastine 10 mg in children aged 2–11 years with AR or chronic urticaria was established in a 12-week trial.12 In this double-blind trial, 509 children aged >2 to <12 years (116 aged >2 to <6 years (23%), 200 aged >6 to <9 years (39%) and 193 aged >9 to <12 years (38%))12 received once-daily bilastine 10 mg ODT (n=260) or placebo (n=249). The mean patient age was 7.5 years, 62.5% were male and almost all patients (94%) had AR. During the 12-week trial, bilastine 10 mg and placebo displayed similar safety and tolerability profiles, with almost identical proportions of bilastine and placebo recipients reporting any treatment-emergent adverse event (TEAE; 65.85% versus 67.5%; primary analysis) or treatment-related adverse event (TRAE; 5.8% versus 8.0%); no serious TEAEs in either group was considered to be related to treatment. Across all paediatric data, the most common TRAEs in 291 children receiving bilastine 10 mg were headache, allergic conjunctivitis, rhinitis and abdominal pain, with comparable types and frequencies of TRAEs in 249 children receiving placebo. In the safety trial, there were no significant differences between bilastine and placebo with regard to TEAEs and TRAEs in any of the age sub-groups, with bilastine being equally well tolerated in young children aged >2 to <6 years and the older age groups.12 In addition, there were no clinically relevant differences between study groups in TEAEs by System Organ Class, cardiac safety or somnolence/sedation (measured using the Paediatric Sleep Questionnaire).
In the safety trial, only 30 children (6%) had chronic urticaria in agreement with the much higher prevalence of AR than chronic urticaria in children as well as in adults in the real-world setting. As most of the available paediatric data are in patients with AR, a review of the pharmacological, efficacy, tolerability and safety profile of bilastine in the treatment of chronic urticaria was conducted. It concluded that bilastine 10 mg/day is a suitable treatment in children aged 6–12 years.13 In particular, the lack of potential for bilastine to induce sedation allows it to be used for prolonged periods without interference with learning, performance and cognitive abilities.13
The single fixed 10-mg dose of bilastine for use in children of all ages is convenient and, as dosages do not need to be calculated by physicians or caregivers, minimizes the likelihood of errors in dose calculation, measurement and administration. Such errors are common problems in paediatric drug administration and may result in ‘accidental poisonings’.14,15 The ODT formulation is particularly convenient as no measurement is required and it can be easily administered to children of all ages who are unable/unwilling to swallow traditional oral medications. When necessary, particularly for younger children, the ODT can be dissolved in a spoonful of water before administration. Of note, bilastine ODT was the formulation used in the paediatric safety trial, where it was dissolved in a spoonful of water prior to administration to very young children. Treatment compliance was high in bilastine ODT and placebo recipients (98.5% and 98.6%, respectively),11 indicating that the ODT formulation was well accepted and convenient for children, even the very young, and their parents.
Potential drug or food/fruit juice interactions with bilastine
The concurrent use of a drug with other drugs or food (including fruit juices) may result in pharmacokinetic or pharmacodynamic drug–drug interactions (DDIs) or food–drug interactions that have the potential to interfere with the drug’s clinical activity, tolerability and/or safety.16 Clinically relevant interactions are often due to alterations in drug metabolism, mostly involving the cytochrome P450 (CYP) isozyme system, leading to changes in drug exposure and, thus, in clinical efficacy and safety. Drugs are classified by their activity as substrates, inducers or inhibitors of CYP and other enzymes as well as of transport protein complexes (e.g. P-glycoprotein (P-gp)), with drugs with minimal activity being less likely to be associated with clinically relevant interactions.17 Of note, elderly patients are at particular risk of DDIs due to their number of concurrent prescriptions and comorbid conditions as well as changes in hepatic function, renal function, nutritional status and/or body weight related to increasing age and between-individual differences in pharmacokinetics/pharmacodynamics.2,18,19 The elderly may also have impaired psychomotor function, leading to an increased risk of DDIs with drugs that affect cognition and other psychomotor function, with such DDIs increasing the risk of falls, incurring the potential for serious consequences such as head injuries, broken hips and other bone fractures, and injury-related death.20,21
Can bilastine be administered with other drugs?
Recommendations for managing potential DDIs with bilastine in response to questions received by the MID at Faes Farma are summarized in Table 1 and are updated from the previous review.2 These recommendations include managing potential DDIs between bilastine and drugs with a narrow therapeutic index, including acenocoumarol, direct (novel) oral anticoagulants, digoxin, antiretrovirals and antituberculosis agents as well as other drugs.
Table 1.
Potential drug interactions between bilastine and other drugs based on queries received by the Medical Information Department of Faes Farma Spain. Table adapted and updated with permission from Leceta et al.2
| Drug | Mechanism of potential DDI | Potential effect of/on BIL | Potential DDI with BIL |
|---|---|---|---|
| Conventional anticoagulants | |||
| Acenocoumarol | Interaction mainly via: CYP system; displacement from 98.7% plasma protein binding; decreased vitamin K bioavailability | BIL does not inhibit or induce CYP enzymes; no available data on its effects on plasma protein binding or vitamin K bioavailability | Acenocoumarol SmPC: only a small risk of clinically significant DDI but caution advised BIL SmPC: does not specify any risk of a DDI |
| Direct-acting (novel) oral anticoagulants and antiplatelet drugs | |||
| Dabigatran | P-gp substrate; exposure can be affected by concomitant P-gp inhibitors/inducers | BIL does not inhibit or induce P-gp; acts as a P-gp substrate (as does digoxin) | BIL not expected to cause any relevant changes in dabigatran exposure (as with digoxin) |
| Rivaroxaban | Metabolized via CYP3A4 or P-gp pathways; plasma concentrations may increase with concomitant CYP3A4 or P-gp inhibitors | BIL is not metabolized; does not inhibit or induce CYP enzymes | BIL not expected to cause any relevant changes in rivaroxaban exposure |
| Apixaban | Metabolized via CYP3A4 or P-gp pathways; plasma concentrations may increase with CYP3A4 or P-gp inhibitors; do not use with strong CYP3A4 or P-gp inhibitors; use with caution with strong CYP3A4 or P-gp inducers | BIL does not affect CYP pathways; does not inhibit or induce P-gp | BIL not expected to cause any relevant changes in apixaban exposure |
| Clopidogrel | Metabolized via CYP2C19 to its active form; avoid use with moderate-to-strong CYP2C19 inhibitors (clinical significance uncertain) | BIL does not affect CYP pathways | BIL not expected to cause any relevant changes in clopidogrel exposure |
| Drugs used to treat asthma or rhinitis | |||
| Chlorphenamine | First-generation sedating antihistamine | Chlorphenamine SmPC: specific warning to not use with other antihistamines | No scientific evidence for use of chlorphenamine + BIL; if an increase in antihistamine effects is needed (e.g. to treat urticaria), an increase in BIL dosage is recommended |
| Corticosteroids | Very rapidly metabolized in the liver | BIL does not inhibit or induce CYP enzymes | No apparent reason to avoid use of BIL + corticosteroids if use considered appropriate by the physician |
| Other drugs | |||
| Cyclosporine (ciclosporin) and other potent P-gp inhibitors | May increase plasma concentrations of drugs that undergo P-gp-mediated elimination | Potential for a decrease in BIL elimination and an increase in BIL plasma levels, especially in patients with renal impairment | BIL SmPC: avoid use of BIL + P-gp inhibitors in patients with moderate/severe renal impairment; concomitant use recommended only in closely monitored patients with normal or mildly impaired renal function |
| Digoxin | Has a narrow therapeutic window; as it is a P-gp substrate, P-gp inhibitors decrease its renal tubular elimination | BIL is a P-gp substrate but not a P-gp inhibitor; BIL should not affect digoxin bioavailability | In the absence of clinical data, exercise caution when using BIL + digoxin; however, probability of a DDI appears to be low |
| Other drug classes | |||
| Anti-TB drugs (e.g. rifampicin, isoniazid, pyrazinamide, ethambutol, quinolones, rifabutin) | Many anti-TB drugs induce P-gp and/or are renally eliminated | Cannot rule out potential for anti-TB drugs to decrease BIL elimination/increase BIL exposure | As PK data for BIL + anti-TB drugs are not currently available, carefully assess the overall risk–benefit when considering such treatment |
| ARTs (large number of drugs, drug classes and drug combinations) | Many ARTs have narrow therapeutic windows; DDIs may be important | ART metabolism via CYP pathways: usually will not be affected by BIL Coadministration of BIL + P-gp inhibitor ARTs: may increase BIL bioavailability but generally not to a clinically significant extent; use caution in renal impairment |
To make an informed risk–benefit assessment, must know the precise ART regimen being taken; should not use BIL in patients with renal impairment receiving P-gp inhibitors |
| Oral contraceptives | Metabolized by CYP pathways, explaining many of their DDIs and potential risk of unwanted pregnancies; also inhibit CYP enzymes, which may affect the metabolism of other drugs | BIL does not inhibit or induce CYP pathways; as BIL is not metabolized, BIL elimination will not be affected by concomitant therapy | No anticipated DDIs with BIL; no DDIs observed in the BIL clinical trial programme, in which women were required to use effective contraception, including oral contraceptives |
| PPIs | Inhibit CYP enzymes, explaining many of their DDIs; also inhibit P-gp (not apparently clinically relevant) | As BIL is not metabolized, it is unlikely to be affected by concomitant therapy | No anticipated DDIs between BIL and PPIs |
ARTs, antiretrovirals; BIL, bilastine; CYP, cytochrome P450; DDI, drug–drug interaction; P-gp, P-glycoprotein efflux transporter; PK, pharmacokinetic; PPIs, proton-pump inhibitors; SmPC, Summary of Product Characteristics; TB, tuberculosis.
Informed decisions regarding the risk–benefit balance of bilastine with many other drugs can be made based on the available data, even when direct experience of their concurrent use is lacking.2 Bilastine has an overall favourable pharmacokinetic profile as it undergoes negligible metabolism and almost exclusive elimination by renal excretion.2 Moreover, as bilastine does not induce or inhibit the in vitro activity of several CYP isozymes,22–24 it can be concurrently administered with acenocoumarol, direct oral anticoagulants (i.e. apixaban, clopidogrel and rivaroxaban), corticosteroids, proton-pump inhibitors, oral contraceptives and other drugs without the risk of clinically relevant DDIs (Table 1).2
As bilastine is a substrate for the P-gp efflux transporter, interactions between it and drugs that are eliminated via the P-gp pathway (e.g. digoxin, apixaban, rivaroxaban and several antituberculosis agents; Table 1) may occur.2 However, the potential for such DDIs is considered minimal as bilastine is not a P-gp inhibitor.22,25 Nevertheless, plasma concentrations of bilastine may increase when administered with potent P-gp inhibitors such as cyclosporine (ciclosporin) and their concomitant use should be avoided in patients with impaired renal function (Table 1).4
In vitro, the active transport of bilastine was considered to be affected to a potentially clinically relevant extent only with regard to interactions between the multidrug resistance protein 1 (MDR1) transporter system, with no effects on breast cancer resistance protein (BCRP), organic anion transporter 1 (OAT1), OAT3 and organic cation transporter 2 (OCT2) systems.25 Furthermore, as bilastine had no clinically relevant inhibitory effects on 12 human transporters and did not seem to be a BRCP, OCT2, OAT1 or OAT3 substrate, it is unlikely that clinically relevant DDIs related to the inhibition of these drug transporters by bilastine would occur.2
Can bilastine be taken with food?
Taking bilastine with food does not significantly reduce the drug’s antihistamine efficacy.26 The SmPC recommendation that bilastine should be taken without food (i.e. 2 hours before or 1 hour after food)4 was based on evidence from in vitro and in vivo pharmacokinetic studies.23,27 However, a recent 4-day crossover wheal and flare inhibition study in 23 healthy volunteers found that the 32–34% reduction in bilastine bioavailability in the fed versus the fasting state was not clinically relevant because no significant pharmacodynamic interaction was observed.26 On day 1, taking bilastine with food delayed the onset of action (as shown by the wheal inhibition response) by only 30 minutes relative to the fasting state, with no subsequent effects.26 Furthermore, after steady-state was achieved on day 4, food was found to have no significant effect on wheal inhibition at any time.26
Can bilastine be taken with fruit juice?
Due to the well-known importance of drug interactions with grapefruit juice, pharmacokinetic investigations of the effects of grapefruit juice on drug bioavailability are required.2 Grapefruit juice and other fruit juices affect the bioavailability of various drugs by inhibiting intestinal CYP3A4 and/or organic anion-transporting polypeptide 1A2 (OATP1A2).27 Due to the inhibition of OATP1A2-mediated uptake transport, concomitant grapefruit juice reduced the oral bioavailability of concomitant bilastine 20 mg by ~30% in adults,28 with other fruit juices also having such effects.4 Therefore, bilastine should be taken 2 hours before or 1 hour after fruit juice, to avoid reductions in the bioavailability of the drug.4
Sedation and psychomotor performance
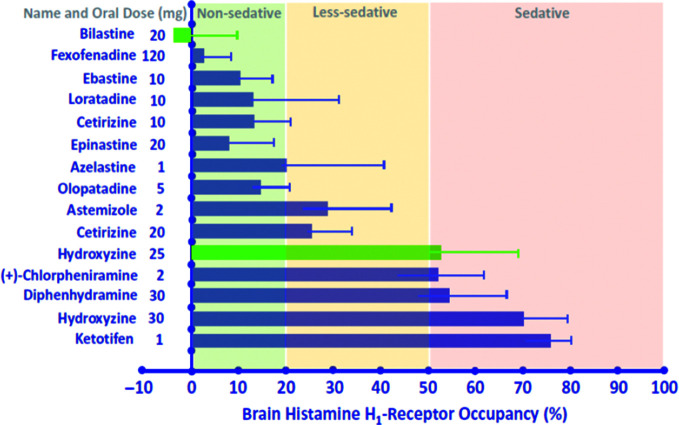
Sedation and other central nervous system (CNS)-related adverse effects associated with H1-antihistamines vary between agents based on their transportation across the blood–brain barrier (BBB) and subsequent H1-receptor occupancy in the brain.29,30 To objectively distinguish between the sedative effects of different antihistamines, brain H1-receptor occupancy data have been used to classify antihistamines as either sedating (defined as brain H1-receptor occupancy of ≥50%), less sedating (brain H1-receptor occupancy of 20–50%) and non-sedating (brain H1-receptor occupancy of <20%).29,30
The sedation classification of bilastine and other H1-antihistamines (Figure 1) was based on the results of positron emission tomography (PET) studies of brain H1-receptor occupancy, which correlates with sedation and other CNS impairments. Bilastine 20 mg and placebo did not differ with regard to mean brain H1-receptor occupancy; however, bilastine 20 mg had significantly lower mean brain H1-receptor occupancy in all five cerebral cortex regions than hydroxyzine 25 mg.31 Moreover, bilastine was also associated with a mean cerebral H1-receptor occupancy that was lower than historical values32 from PET studies of many other second-generation H1-antihistamines (Figure 1).30 For example, the first-generation H1-antihistamines diphenhydramine, chlorphenamine and hydroxyzine are considered sedating; astemizole and cetirizine are considered less sedating; and bilastine, fexofenadine and some of the so-called second-generation antihistamines are considered non-sedating.30 Of note, within the non-sedating category, bilastine and fexofenadine are considered ‘non-brain-penetrating’ antihistamines as they have an H1-receptor occupancy of nearly zero.33 The very low level of binding to cerebral H1 receptors by bilastine and some other non-sedating H1-antihistamines is due to its very limited penetration across the BBB, which, in turn, is due to low lipid solubility and P-gp-mediated efflux from the CNS.25,34–38
Figure 1.
Brain histamine H1-receptor occupancy (mean percentage ± standard deviation) of first-generation and second-generation antihistamines following oral administration.30 Data shown in green were obtained from a positron emission tomography (PET) study of bilastine 20 mg versus hydroxyzine 25 mg; data shown in blue were obtained from a review of PET studies.32 Figure reproduced with permission from Jauregui et al.30
Non-sedating antihistamines should be preferred when treating allergic conditions due to their low CNS penetration and, thereby, their low risk of serious adverse effects.29 When the use of a sedating antihistamine is considered necessary, the impact of its sedative and other potential CNS-adverse effects should be thoroughly considered and patients must be fully educated regarding their pharmacological risks prior to their use.29
Can bilastine be used by pilots and during other complex tasks?
Drugs, whether available over the counter or by prescription, must be safe enough to use whilst piloting an aircraft or engaging in any other activity that involves complex task performance.2 It is up to the individual to be aware of the effects of any medication they are considering taking,39,40 particularly given the intensification of effects that increased altitude may have on a particular drug. Being aware of the potential detrimental effects of drugs is crucial to aviation safety, with the inappropriate use of drugs by pilots being a factor in reports of fatal civil aviation accidents.41,42
Guidance for pilots and air traffic control specialists regarding which antihistamines are acceptable to use whilst performing their duties is available.40,43,44 The Japanese Ministry of Land, Infrastructure, Transport and Tourism has approved bilastine as a drug allowed for use by pilots.40 In the US guidelines, fexofenadine,43,44 loratadine43,44 and desloratadine44 are considered acceptable but cetirizine43,44 and levocetirizine43 are not. Although not specifically mentioned in the current US guidelines, results in simulated altitude condition studies with bilastine were similar to those of the three acceptable antihistamines.
The effect of bilastine on sleepiness and performing tasks associated with flying under conditions of simulated altitude (4000 feet [1250 m]) and cabin pressure have been assessed in placebo-controlled, crossover hypobaric chamber studies in healthy volunteers.45,46 Unlike hydroxyzine, bilastine was not associated with detrimental cognitive effects.45 Bilastine 20 mg and placebo had similar effects on sleepiness, vigilance, tracking and performance of complex tasks throughout the 6-hour study period. Conversely, with hydroxyzine, the ability to perform each of the evaluative tasks was significantly impaired and sleepiness scores increased at all hourly timepoints.45 These results are consistent with those in another study, in which diphenhydramine 50 mg but not desloratadine 5 mg had detrimental effects on both sleepiness and the performance of flying-associated tasks.47
Likewise, relative to placebo, bilastine 20 mg was not associated with impairments in any of the tested abilities (vigilance, ultrashort memory, combined distributive attention and monotony tolerance) at ground level or during simulated conditions of hypobaric hypoxia during flight in a more recent study.46 In contrast, cetirizine 10 mg was associated with an increased number of errors at ground level, with additional impairments in the distributive attention test at simulated altitude. Based on these and similar results, bilastine is preferred over cetirizine as antihistamine treatment in individuals who need to perform complex tasks and maintain constant attention.46
In the real-world setting, psychomotor skills that can be affected commonly include driving a vehicle. The safe use of double-dose (40 mg) or multiple-dose bilastine on driving performance has been shown in on-road driving tests in healthy volunteers48 and high-speed Formula-1 simulator driving tests in patients with AR or urticaria.49 The primary endpoint in both studies was the standard deviation of lateral position (SDLP), which measures the ability to drive in a steady-lane position.
In the randomized, double-blind on-the-road trial, patients received once-daily bilastine 20 mg (recommended dose) or 40 mg (double dose), hydroxyzine 50 mg or placebo for 8 days in a crossover manner, with the road tracking test being performed on days 1 and 8 of each treatment period. Relative to placebo, single and repeated doses of bilastine at the usual and double dose (20 and 40 mg) did not affect driving performance, suggesting its safe use in traffic.30 Although there were no significant differences between either bilastine doses and placebo with regard to mean SDLP on either day 1 or day 8, there were significant differences between hydroxyzine 50 mg and placebo in mean SDLP on both days, indicating that tolerance to the sedative effects of hydroxyzine did not develop over time.48
In the open-label Formula-1 simulator driving test, patients performed a baseline 30-minute Formula-1 simulator driving test, received bilastine 20 mg for 7 days, then had a final driving test.49 Bilastine did not have any negative effects on driving performance even at high speed as measured by mean changes from baseline in SDLP, as well as the secondary endpoints of maintenance of constant speed and time to reaction.49
Taken as a whole, the relevant pharmacological and clinical data are sufficient to consider bilastine as a reliable non-sedating antihistamine. As discussed earlier, data from a PET study indicate that bilastine has a very low potential to penetrate the BBB and cause sedation and detrimental psychomotor effects, with the results of clinical studies showing a lack of subjective sedation or objective impairment of psychomotor performance during assessments of flight-related and driving-related performance. This is of particular relevance in patients with difficult-to-treat chronic urticaria who may require higher than normal dosages of antihistamines to control their symptoms.50 Based on the overall results of clinical trials of double-dose bilastine, it is an ideal antihistamine for titrating to up to fourfold in such patients51 (see the ‘Can the dosage of bilastine be increased in chronic urticaria?’ sub-section for further details). Nevertheless, as with all drugs, individuals in whom psychomotor performance is vital should be advised to try bilastine before performing such tasks to determine their response to this drug.2 They should also be informed that, albeit rare, drowsiness, which may negatively affect psychomotor skills, may occur with bilastine.2,4
Use in allergy-related conditions
Can bilastine be used to treat acute urticaria?
The approved indications for bilastine include the ‘symptomatic treatment of urticaria’, including acute urticaria.4 According to current international urticaria guidelines, non-sedating second-generation H1-antihistamines, including bilastine, are recommended for the first-line symptomatic treatment of acute and chronic urticaria due to their favourable safety profile.50 In patients who do not respond to standard dosages of second-generation H1-antihistamines, increasing the dosage to two or even four times the usual dosage is indicated as second-line treatment.50,52 This is based on the overall results of clinical studies of second-generation antihistamines in urticaria, indicating that the majority of patients who do not respond to standard doses will benefit from up-titration.50
Can higher dosages of bilastine be used in chronic urticaria?
The approved dosage of bilastine in the SmPC is 20 mg once daily.4 However, in patients who do not respond to standard dosages of second-generation H1-antihistamines, current urticaria guidelines recommend increasing the dosage to two or even four times the usual dosage based on the overall results of clinical trials that evaluated the effectiveness of higher dosages.50
The key phase III clinical trial evaluating the efficacy of bilastine in the symptomatic treatment of urticaria used the approved dosage of 20 mg/day53; however, the efficacy of higher dosages has also been shown during the clinical development programme. Once-daily bilastine 20, 40 or 80 mg for 7 days effectively reduced the critical temperature threshold of almost all (19 of 20) patients with cold contact urticaria without an increase in sedation at the higher dosages.54 More recently, in an exploratory open-label 8-week study in 115 patients with pruritus associated with chronic spontaneous urticaria (CSU) or other skin diseases, 31 non-responders to 2 weeks of treatment with bilastine 20 mg received up-dosed bilastine 40 mg for the remaining 6 weeks.55 In previous non-responders, up-dosed bilastine significantly improved weekly pruritis severity scores relative to baseline by 45% by week 4 and by 49% by week 8.55
The effectiveness of up-dosed bilastine was also shown in a real-world study in patients with chronic urticaria in whom previous treatment with therapeutic doses of second-generation H1-antihistamines, other than bilastine, had failed.56 Bilastine 40 mg/day relieved chronic urticaria symptoms in the majority of these difficult-to-treat patients, with only some of the more severely affected patients requiring an increase to 80 mg/day.56
Supratherapeutic doses of bilastine have been also studied and found to be well tolerated in phase I studies.23,51,57–60 As previously reviewed,23,51 clinically significant trends in ECG, vital signs or physical examination findings were not observed when single and multiple supratherapeutic bilastine doses (up to ~10-fold higher than normal) were administered. Bilastine 20–80 mg was also well tolerated in CNS safety studies,45,46,48,49,57,59 with no impairment in CNS function with bilastine 20 or 40 mg across a variety of psychomotor tests45,46,48,49,57 and no increase in the depressant CNS effects of either alcohol or lorazepam with concomitant bilastine 20 mg. In the ‘thorough QT/QTc’ study, treatment with bilastine 100 mg and combination treatment with bilastine 20 mg plus ketoconazole were not associated with issues related to cardiac safety.58,60 Moreover, the clinical development programme did not show any differences between the adverse event profiles of bilastine and placebo.4
Overall, the results of these studies confirm the safety of bilastine at therapeutic (even when combined with ketoconazole) and supratherapeutic doses and support current urticaria guidelines for up-titration of its dosage when necessary. If a physician, after considering the risk–benefit ratio, decides to up-titrate the dosage of a second-generation antihistamine based on the current urticaria guidelines,50,52 they should consider bilastine as a possible option due to its excellent tolerability and safety profiles.2
Is bilastine useful in treating ‘pruritus’?
Pruritus is a common dermatological condition, especially in the elderly, and may adversely affect sleep and quality of life.61 Itching is most commonly caused by skin disease but may also be due to a variety of underlying systemic conditions (e.g. renal disease, cholestasis, myeloproliferative disorders, hyperthyroidism) or may have neuropathic or psychogenic causes.2,61 Itch is associated with several chemical mediators, of which histamine is only one and is primarily associated with allergic conditions.62
Management of pruritus should target the underlying cause, including general measures (e.g. emollient creams) and pharmacotherapy (e.g. calcineurin inhibitors, capsaicin inhibitors, serotonin reuptake inhibitors, gabapentin and/or antihistamines).2,63 Although classically used to treat itching, or rather to provide night-time sedation to assist sleep, first-generation antihistamines do not provide uniform effectiveness in managing all causes of pruritus, may cause daytime drowsiness and anticholinergic adverse effects (especially in the elderly), affect ‘morning-after’ driving performance and impair work and school productivity.2,61 In Japan, bilastine is indicated to treat pruritus associated with skin diseases (dermatitis/eczema and cutaneous pruritus);64 this approval was based on the results of clinical trials, in which bilastine improved symptoms and quality of life and had a favourable safety profile in these patient populations.2
Bilastine 20 mg significantly reduced itching sensation relative to desloratadine 5 mg, rupatadine 10 mg and placebo in a single-dose crossover study assessing their effects in suppressing responses to intradermal histamine in healthy volunteers.65 Improvements in itching sensation scores were significantly better with bilastine than with placebo within 1 hour of administration; desloratadine within 2–12 hours or rupatadine within 2–9 hours. In contrast, compared placebo, desloratadine and rupatadine did not reduce itching sensation scores to a significant extent at any timepoint.65
In the previously mentioned 8-week study in 115 patients with pruritis associated with CSU or other skin diseases, bilastine (20 mg or up-dosed to 40 mg) significantly decreased mean weekly pruritus scores by 71% in the overall population and 85% in the sub-group of 34 patients with CSU.55 In all populations, there was a progression and significant reduction in weekly pruritus severity scores from baseline at all earlier timepoints (weeks 1, 2 and 4). In the overall and CSU populations, significant improvements from baseline were shown for other pruritus-related outcomes, including pruritus visual analogue scale scores at weeks 1, 2, 4 and 8 and Dermatology Life Quality Index scores at weeks 4 and 8. Of note, in CSU patients, a single dose of bilastine significantly improved mean daily Urticaria Activity Scores, with continued treatment providing significant and progressive improvements from baseline in weekly scores at weeks 1, 2, 4 and 8.55
Can patients with asthma and allergic rhinitis use bilastine?
According to the recent Interasthma (a global asthma association) manifesto on united airway diseases,66 ~80% of asthmatics have rhinitis, with rhinitis significantly increasing the odds of asthma by 3.5. Antihistamines are the key class of drugs used to treat AR: bilastine, a non-brain-penetrating antihistamine, effectively decreases allergy symptoms and does not cause night-time sleep disturbances or related adverse events.66
Treatment with bilastine improves the symptoms of AR in patients with asthma.67 A randomized trial in 419 patients with seasonal AR and mild-to-moderate asthma partially controlled by beclomethasone dipropionate ≤500 μg/day or equivalent (as monotherapy or in combination with long-term β2-adrenergic bronchodilators) evaluated the efficacy of monotherapy with once-daily bilastine 20 mg or montelukast 10 mg versus combination treatment with both drugs on seasonal AR symptoms.67 Mean nasal/ocular total symptom scores significantly improved from baseline in all treatment groups at week 4 (primary endpoint); mean daytime nasal and non-nasal symptom scores also significantly improved in all groups at week 4. Improvements from baseline in all these seasonal AR symptom scores were significantly better with bilastine 20 mg than with montelukast 10 mg during early treatment (weeks 1 and 2), which is consistent with the more rapid onset of action of bilastine. Results in the combination treatment group did not differ from those in the monotherapy groups, perhaps reflecting the relatively low severity of seasonal AR at baseline.67 Thus, bilastine may provide rapid relief of seasonal AR in patients with asthma who need intermittent control of seasonal AR symptoms.67
Is bilastine effective in the real-world setting?
In addition to its proven efficacy in clinical trials, bilastine has also been effective in treating patients with conditions requiring antihistamine treatment in the real-world setting across multiple disciplines.68
In a series of real-world patient cases in Canada presented by expert panel members, bilastine was effective in relieving symptoms across a range of clinically relevant conditions (perennial and seasonal AR, chronic urticaria, and less common and challenging conditions such as urticarial vasculitis and pruritus associated with inflammatory skin conditions), patient ages (9–76 years) and disciplines (e.g. dermatology, paediatrics and allergy/clinical immunology).68 Moreover, bilastine was well tolerated and safe, even when used over prolonged periods (up to several months) and at higher-than-normal dosages (up to 80 mg/day), across all case reports.68
Could bilastine be effective in treating BASCULE syndrome?
Bilastine may prove effective in the treatment of the symptoms of BASCULE (Bier anaemic spots, cyanosis and urticaria-like eruption) syndrome, a recently described vasomotor dermatosis. In a case report, treatment with a high dosage of bilastine (80 mg/day administered as 40 mg twice daily) completely resolved the symptoms of BASCULE syndrome in a 16-year-old male.69 However, the symptoms reappeared when the dosage was reduced to 40 mg/day. Moreover, previous antihistamine treatment at standard dosages (cetirizine 10 mg/day and bilastine 20 mg/day) were unsuccessful. This is the first documented case of successfully treating BASCULE syndrome with bilastine at higher-than-usual dosages.69
Use in special situations
Can bilastine be used during pregnancy?
To date, there is a lack of data to make an informed decision regarding the safety of bilastine in pregnant women.2,4 The benefits and risks of the use of any antihistamine during pregnancy must be weighed by the physician on an individual basis,70,71 considering all available clinical and non-clinical knowledge of the medicinal product and compounds within the same drug class.72 According to the bilastine SmPC, as a precautionary measure, it is preferable to avoid the use of bilastine during pregnancy as there are no or limited data in pregnant women.4 In animal studies, there were no indications that bilastine had direct or indirect harmful effects on reproductive toxicity, parturition or postnatal development.4
Can elderly patients use bilastine?
Bilastine is safe and effective in elderly patients. As the pharmacokinetic profile of bilastine in healthy individuals aged >65 years is comparable to that in younger individuals, dosage adjustments are not necessary in the elderly.2,4 A 3-month safety study was conducted in 150 poly-medicated patients aged ≥65 years with urticaria and/or AR as well as with a number of other diseases. The incidence of adverse events with bilastine 20 mg/day in this elderly population with other comorbid conditions and pharmacological treatments was similar to that in younger healthier patients, signifying a favourable safety profile with a low incidence of TEAEs.73
Can patients with obesity use bilastine?
Body weight had no impact on the pharmacological profile of bilastine in the clinical development programme. For example, no association between pharmacokinetic parameters and body weight was shown in phase I studies.4,23 There is no current evidence indicating that the efficacy and/or safety of bilastine will differ between patients with or without obesity.
According to the SmPC, increased body weight was uncommon in the bilastine clinical trial programme, being reported in only ~0.5% of adult and adolescent patients receiving bilastine 20 mg or bilastine at any dosage compared with 0.15% of patients receiving placebo.4 The SmPC does not include increased body weight in the list of adverse events at least possibly related to bilastine that were reported in >0.01% of paediatric patients in the clinical trial programme. Of interest, the World Health Organization detected a potential signal that loratadine and desloratadine may increase weight in paediatric patients.74
Can patients with coeliac disease or who are lactose intolerant use bilastine?
According to the EMA,75 the SmPC of drugs must provide a list of excipients considered relevant (e.g. gluten, lactose, glucose, preservatives, flavouring agents, etc.) if they are present in amounts above a predetermined quantity. If the excipient is not listed in the SmPC, it means it is not present in this amount.
The list of excipients for bilastine does not include gluten. Furthermore, recently updated European regulations state that medicinal products with a gluten content of <20 parts per million can be referred to as ‘gluten free’.76 Based on these regulations, bilastine tablets, oral solution and ODTs marketed and/or manufactured by Faes Farma and its authorized license holders (e.g. Menarini, Taiho, Takeda, Pfizer, Aralez) are gluten free as they do not contain wheat starch and are therefore suitable for use by patients with coeliac disease.4
Likewise, as the list of excipients for bilastine formulations marketed and/or manufactured by Faes Farma and its authorized license holders does not include lactose, bilastine may be used by patients with lactose intolerance.
For other intolerances or excipient contents, the reader is advised to please consult their local SmPC or the local marketing authorization holder.
Can patients with a kidney transplant use bilastine?
Overall, in patients who have undergone kidney or other transplantation who are receiving cyclosporine or other immunosuppressant treatment, the use of bilastine is recommended only in closely monitored individuals with normal or mildly impaired renal function.4 Coadministration of bilastine and cyclosporine should be avoided in patients with moderate or severe renal impairment,4 as plasma concentrations of bilastine may increase (Table 1).
Can patients on renal dialysis use bilastine?
As described in the previous review,2 the extent to which an individual drug is affected by dialysis is determined by the properties of the drug as well as by the technical aspects of the dialysis procedure. According to the 2020 Dialysis of Drugs table,77 there is still no data on the effect of any type of haemodialysis on the clearance of bilastine. As the physicochemical characteristics of bilastine are considered similar to those of fexofenadine, its ability to be dialyzed might also be comparable.2 The 2020 recommendations for fexofenadine state that conventional haemodialysis does not have a clinically important effect on plasma clearance and supplemental dosing is not usually required and that peritoneal dialysis is unlikely to result in significant drug removal based on the drug’s physicochemical characteristics; no data exist on the effects of high permeability haemodialysis.77
Of interest, bilastine may be an effective treatment for decreasing the symptoms of uremic pruritus, a common and debilitating problem in patients with end-stage renal disease that has been associated with the release of histamine.78 In a recent open-label study, 10 patients with confirmed uraemic pruritus who were dependent on dialysis received either once-daily bilastine 20 mg or desloratadine 5 mg for 1 month.78 At 1 month, standardized 5-D itch scale questionnaire scores decreased from baseline to a significant extent with bilastine but not with desloratadine. All individual itch parameters (duration, degree, direction, disability and direction of itch) improved in bilastine recipients, and no major adverse events were reported during follow-up.78
Can patients with urinary retention/dysuria use bilastine?
Antihistamines with poor receptor selectivity and/or interactions with muscarinic receptors may cause urinary retention/dysuria.2 However, given bilastine’s well-established high selectivity profile for H1 receptors and the lack of affinity for muscarinic receptors,4,79 it is reasonable to assume that the use of bilastine will not increase the risk of urinary retention/dysuria.2
Can bilastine induce photosensitivity reactions with laser depilation?
Although specific data are lacking, based on its chemical properties, it seems unlikely that bilastine would induce photosensitivity reactions with laser or intense pulsed light, depilation or other types of phototherapy.2 Lasers used in hair removal range from visible light to near infra-red with typical wavelengths of 690–1064 nm depending on the light source; intense pulsed light used in depilation is not a laser and has a wavelength of 640 nm.80 Photosensitivity reactions are acquired, immunologically mediated drug reactions that are caused by the formation of photoproducts when a drug is exposed to ultraviolet (UV) A, UVB or visible light.2 Only drugs that absorb 290–700 nm of the electromagnetic spectrum will be photo-activated and, therefore, only these drugs can be direct photochemical photosensitizers.81 Bilastine has a UV/visible radiation absorption spectrum of 253 nm, which is less than the minimum value for which photo-safety testing is recommended. Therefore, as photosensitivity reactions with bilastine are highly improbable, it has not undergone photo-safety testing.2
Is bilastine available in formulations other than oral?
Allergic conjunctivitis is a common allergic condition that may be treated locally with antihistamine eye drops, especially when it is not associated with relevant allergic rhinitis symptoms. However, the preservatives needed in multidose ophthalmic formulations often act as an irritant, further inflaming the ocular surface.82 To address this problem, preservative-free eye drops are an increasingly popular option. A bilastine preservative-free eye drop is being developed to treat allergic conjunctivitis.
A pharmacokinetic study in healthy volunteers found that bilastine is rapidly absorbed following the administration of multiple doses of once-daily bilastine 0.6% preservative-free eye drops, with maximum blood levels being reached within 3 hours of administration at steady state.83 The eye drops were also well tolerated, suggesting that the conjunctival pathway may offer another administration route for bilastine.83 A study evaluating the efficacy of bilastine 0.6% eyedrops relative to that of vehicle and ketotifen 0.025% eye drops for the treatment of the signs and symptoms of allergic conjunctivitis has been conducted as part of the approval process for this formulation.84
Other formulations are also being developed (new oral forms, injectable, intranasal) to provide healthcare professionals and patients with different options to be chosen depending on the kind and severity of symptoms, guideline recommendations, and individual preferences or requirements.
Conclusions
Based on the extensive research of its pharmacological and clinical profiles, bilastine is an effective, well-tolerated and safe non-sedating H1-antihistamine for use in the treatment of allergic disorders, including rhinoconjunctivitis and urticaria, in adults, adolescents and children.23,24,51 Of interest, recent research suggests that, in addition to preventing histamine from binding to the H1 receptor, bilastine is also an inverse H1 agonist, which is more potent than a pure antagonist because it suppresses constitutive H1-receptor activity.85
Large numbers of patients with common allergic diseases were treated with bilastine in studies in the clinical trial programme, which included well-defined participants who received treatment in well-controlled conditions.2 However, in routine clinical practice, many patients have comorbid conditions and/or may be taking concomitant medications and therefore do not meet the strict inclusion criteria used in the clinical trials. Importantly, bilastine has been shown to effectively treat a variety of conditions requiring antihistamine treatment in the real-world setting.68
The queries that are most commonly received by the Faes Farma MID include the potential for drug interactions involving bilastine and other frequently used drugs and the use of bilastine in special populations or to treat specific symptoms related to allergic conditions. As the concomitant use of many medications is not permitted during clinical trials, advice regarding their concomitant use with bilastine needs to consider the pharmacological properties of both the drug in question and bilastine as well as expert opinion. Similar considerations apply to specific patient groups, such as individuals with renal impairment, obesity or lactose intolerance, and those who are elderly or pregnant, and in the treatment of specific symptoms such as treatment-resistant urticaria, pruritus or BASCULE syndrome. As noted in the previous review,2 it appears that the SmPC for bilastine is being poorly used as an information resource in everyday practice as the answer to a number of queries related to the indication, duration of treatment and prophylactic use of bilastine received by the Faes Farma MID were already provided in the SmPC.
Overall, this updated review provides current data regarding the best use of bilastine in specific situations and patients and identifies areas in which further knowledge is required. Although decisions regarding the use of bilastine may be aided by expert opinion that relies on knowledge of the underlying science, additional research and evidence are required to answer certain queries regarding the use of bilastine.
Acknowledgements
The authors thank Katherine Lyseng-Williamson, Content Ed Net, for editorial assistance in the preparation of this manuscript; funding for editorial assistance was provided by Faes Farma SA.
Footnotes
Contributions: All authors contributed equally to the preparation of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors are employees of Faes Farma SA, 48940-Leioa, Bizkaia, Spain. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2021/07/dic.2021-5-1-COI.pdf
Funding declaration: This research was supported by Faes Farma SA.
Correct attribution: Copyright © 2021 Leceta A, García A, Sologuren A, Campo C. https://doi.org/10.7573/dic.2021-5-1. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Shah I, Janajreh I, Fung SM. Medical information practices across the pharma industry: what can we learn from benchmarking surveys? Therapeut Innovat Regul Sci. 2020;54(6):1259–1262. doi: 10.1007/s43441-020-00226-z. [DOI] [PubMed] [Google Scholar]
- 2.Leceta A, Sologuren A, Valiente R, Campo C, Labeaga L. Bilastine in allergic rhinoconjunctivitis and urticaria: a practical approach to treatment decisions based on queries received by the medical information department. Drugs Context. 2017;6:212500. doi: 10.7573/dic.212500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graves DA, Baker RP. The core curriculum for medical communications professionals practicing in the pharmaceutical industry. Drug Inform J. 2000;34(4):995–1008. doi: 10.1177/009286150003400402. [DOI] [Google Scholar]
- 4.Bilaxten (bilastine) 20 mg tablets, 10 mg orodispersible tablets and 2.5 mg/mL oral solution: EU Summary of Product Characteristics. Faes Farma; 2020. [Google Scholar]
- 5.European Medicines Agency (EMA) Paediatric investigation plans. [Accessed June 29, 2021]. https://www.ema.europa.eu/en/human-regulatory/research-development/paediatric-medicines/paediatric-investigation-plans.
- 6.Ilaxten 20 mg tablet, 10 mg orodispersible tablets, 2.5 mg/ml oral solution. Luxembourg: Menarini International Operations Luxembourg SA; 2020. [Google Scholar]
- 7.Blaxitec (bilastine) 10 mg orodispersable tablets: Mexican summary of prescribing information. Mexico City: Faes Farma Mexico SA; 2018. [Google Scholar]
- 8.Vozmediano V, Sologuren A, Lukas JC, Leal N, Rodriguez M. Model informed pediatric development applied to bilastine: ontogenic PK model development, dose selection for first time in children and PK study design. Pharm Res. 2017;34(12):2720–2734. doi: 10.1007/s11095-017-2248-6. [DOI] [PubMed] [Google Scholar]
- 9.Vozmediano V, Lukas JC, Encinas E, et al. Model-informed pediatric development applied to bilastine: analysis of the clinical PK data and confirmation of the dose selected for the target population. Eur J Pharm Sci. 2019;128:180–192. doi: 10.1016/j.ejps.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez M, Vozmediano V, García-Bea A, et al. Pharmacokinetics and safety of bilastine in children aged 6 to 11 years with allergic rhinoconjunctivitis or chronic urticaria. Eur J Pediatr. 2020;179(5):801–805. doi: 10.1007/s00431-019-03559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sádaba B, Azanza JR, García-Bea A, Labeaga L, Campo C, Valiente R. Bioequivalence evaluation of three pediatric oral formulations of bilastine in healthy subjects: results from a randomized, open label, crossover study. Eur J Drug Metab Pharmacokinet. 2020;45(2):265–272. doi: 10.1007/s13318-019-00596-2. [DOI] [PubMed] [Google Scholar]
- 12.Novák Z, Yáñez A, Kiss I, Kuna P, Tortajada-Girbés M, Valiente R Bilastine Paediatric Safety Study Group. Safety and tolerability of bilastine 10 mg administered for 12 weeks in children with allergic diseases. Pediatr Allergy Immunol. 2016;27(5):493–498. doi: 10.1111/pai.12555. [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulos NG, Zuberbier T. The safety and tolerability profile of bilastine for chronic urticaria in children. Clin Transl Allergy. 2019;9:55. doi: 10.1186/s13601-019-0294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green JL, Wang GS, Reynolds KM, et al. Safety profile of cough and cold medication use in pediatrics. Pediatrics. 2017;139(6):e20163070. doi: 10.1542/peds.2016-3070. [DOI] [PubMed] [Google Scholar]
- 15.Wang GS, Reynolds KM, Banner W, et al. Medication errors from over-the-counter cough and cold medications in children. Acad Pediatr. 2020;20(3):327–332. doi: 10.1016/j.acap.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Stockley IH. Stockley’s Drug Interactions. 10th ed. London: Pharmaceutical Press; 2013. [Google Scholar]
- 17.Carpenter M, Berry H, Pelletier AL. Clinically relevant drug-drug interactions in primary care. Am Fam Physician. 2019;99(9):558–564. [PubMed] [Google Scholar]
- 18.Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet. 2007;370:185–191. doi: 10.1016/S0140-6736(07)61092-7. [DOI] [PubMed] [Google Scholar]
- 19.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289(9):1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 20.Enderlin C, Rooker J, Ball S, et al. Summary of factors contributing to falls in older adults and nursing implications. Geriatr Nurs. 2015;36(5):397–406. doi: 10.1016/j.gerinurse.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75(1):51–61. doi: 10.1016/j.maturitas.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Lucero ML, Gonzalo A, Mumford R, Betanzos M, Alejandro A. An overview of bilastine metabolism during preclinical investigations. Drug Chem Toxicol. 2012;35:18–24. doi: 10.3109/01480545.2012.682651. [DOI] [PubMed] [Google Scholar]
- 23.Church MK. Safety and efficacy of bilastine: a new H1-antihistamine for the treatment of allergic rhinoconjunctivitis and urticaria. Expert Opin Drug Saf. 2011;10:779–793. doi: 10.1517/14740338.2011.604029. [DOI] [PubMed] [Google Scholar]
- 24.Bousquet J, Ansótegui I, Canonica GW, et al. Establishing the place in therapy of bilastine in the treatment of allergic rhinitis according to ARIA: evidence review. Curr Med Res Opin. 2012;28(1):131–139. doi: 10.1185/03007995.2011.648263. [DOI] [PubMed] [Google Scholar]
- 25.Lucero ML, Gonzalo A, Ganza A, et al. Interactions of bilastine, a new oral H1 antihistamine, with human transporter systems. Drug Chem Toxicol. 2012;35:8–17. doi: 10.3109/01480545.2012.682653. [DOI] [PubMed] [Google Scholar]
- 26.Coimbra J, Campo C, Labeaga L, et al. Lack of clinical relevance of bilastine-food pharmacokinetic interaction assessed by inhibition of histamine-induced wheal and flare response in healthy volunteers. Skin Allergy Meeting; April 4–6, 2019; Munich, Germany. [Google Scholar]
- 27.Bailey DG. Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol. 2010;70:645–655. doi: 10.1111/j.1365-2125.2010.03722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crean C, Valiente R, Sologuren A, McLaverty D. Effect of grapefruit juice on the pharmacokinetics of bilastine. Abstract 1171. J Clin Pharmacol. 2007;47(9):1198. [Google Scholar]
- 29.Yanai K, Yoshikawa T, Yanai A, et al. The clinical pharmacology of non-sedating antihistamines. Pharmacol Ther. 2017;178:148–156. doi: 10.1016/j.pharmthera.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Jáuregui I, Ramaekers JG, Yanai K, et al. Bilastine: a new antihistamine with an optimal benefit-to-risk ratio for safety during driving. Expert Opin Drug Saf. 2016;15:89–98. doi: 10.1517/14740338.2016.1112786. [DOI] [PubMed] [Google Scholar]
- 31.Farré M, Pérez-Mañá C, Papaseit E, et al. Bilastine vs. hydroxyzine: occupation of brain histamine H1 receptors evaluated by positron emission tomography in healthy volunteers. Br J Clin Pharmacol. 2014;78:970–980. doi: 10.1111/bcp.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanai K, Rogala B, Chugh K, Paraskakis E, Pampura AN, Boev R. Safety considerations in the management of allergic diseases: focus on antihistamines. Curr Med Res Opin. 2012;28(4):623–642. doi: 10.1185/03007995.2012.672405. [DOI] [PubMed] [Google Scholar]
- 33.Kawauchi H, Yanai K, Wang DY, Itahashi K, Okubo K. Antihistamines for allergic rhinitis treatment from the viewpoint of nonsedative properties. Int J Mol Sci. 2019;20(1):21. doi: 10.3390/ijms20010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Y, Sieck DE, Hsu WH. Why are second-generation H1-antihistamines minimally sedating? Eur J Pharmacol. 2015;765:100–106. doi: 10.1016/j.ejphar.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Conen S, Theunissen EL, Vermeeren A, et al. The role of P-glycoprotein in CNS antihistamine effects. Psychopharmacology. 2013;229(1):9–19. doi: 10.1007/s00213-013-3075-z. [DOI] [PubMed] [Google Scholar]
- 36.Chen C, Hanson E, Watson JW, Lee JS. P-glycoprotein limits the brain penetration of nonsedating but not sedating H1-antagonists. Drug Metab Dispos. 2003;31(3):312–328. doi: 10.1124/dmd.31.3.312. [DOI] [PubMed] [Google Scholar]
- 37.Obradovic T, Dobson GG, Shingaki T, Kungu T, Hidalgo IJ. Assessment of the first and second generation antihistamines brain penetration and role of P-glycoprotein. Pharm Res. 2007;24(2):318–327. doi: 10.1007/s11095-006-9149-4. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman P. Histamine, antihistamines, and the central nervous system. Allergy Asthma Proc. 2009;3(5):482–486. doi: 10.2500/aap.2009.30.3264. [DOI] [PubMed] [Google Scholar]
- 39.Introduction to aviation physiology. [Accessed March 8, 2021]. www.faa.gov/pilots/training/airman_education/media/IntroAviationPhys.pdf.
- 40.Japanese Ministry of Land, Infrastructure, Transport and Tourism, Civil Aviation Bureau. [Accessed March 26, 2021]. https://www.mlit.go.jp/koku/content/001312959.pdf.
- 41.Akparibo IY, Stolfi A. Pilot certification, age of pilot, and drug use in fatal civil aviation accidents. Aerosp Med Hum Perform. 2017;88(10):931–936. doi: 10.3357/AMHP.4813.2017. [DOI] [PubMed] [Google Scholar]
- 42.Sen A, Akin A, Craft KJ, Canfield DV, Chaturvedi AK. First-generation H1 antihistamines found in pilot fatalities of civil aviation accidents, 1990–2005. Washington, DC: Federal Aviation Administration, Office of Aerospace Medicine; 2007. [Accessed March 8, 2021]. DOT/FAA/AM-07/12. https://www.faa.gov/data_research/research/med_humanfacs/oamtechreports/2000s/media/200712.pdf. [PubMed] [Google Scholar]
- 43.Federal Aviation Administration. What over-the counter (OTC) medications can I take and still be safe to fly? [Accessed March 4, 2021]. https://www.faa.gov/licenses_certificates/medical_certification/media/OTCMedicationsforPilots.pdf.
- 44.Pilot Medical Solutions, Inc. [Accessed March 5, 2021];Air Traffic Control Specialists (ATCS) https://www.leftseat.com/atc/atcsmeds.htm. [Google Scholar]
- 45.Valk PJ, Simons R, Jetten AM, Valiente R, Labeaga L. Cognitive performance effects of bilastine 20 mg during 6 hours at 8000 ft cabin altitude. Aerosp Med Hum Perform. 2016;87(7):622–627. doi: 10.3357/AMHP.4522.2016. [DOI] [PubMed] [Google Scholar]
- 46.Reményi Á, Grósz A, Szabó SA, Tótka Z, Molnár D, Helfferich F. Comparative study of the effect of bilastine and cetirizine on cognitive functions at ground level and at an altitude of 4,000 m simulated in hypobaric chamber: a randomized, double-blind, placebo-controlled, cross-over study. Expert Opin Drug Saf. 2018;17(9):859–868. doi: 10.1080/14740338.2018.1502268. [DOI] [PubMed] [Google Scholar]
- 47.Valk PJ, Van Roon DB, Simons RM, Rikken G. Desloratadine shows no effect on performance during 6 h at 8,000 ft simulated cabin altitude. Aviat Space Environ Med. 2004;75(5):433–438. [PubMed] [Google Scholar]
- 48.Conen S, Theunissen EL, Van Oers AC, Valiente R, Ramaekers JG. Acute and subchronic effects of bilastine (20 and 40 mg) and hydroxyzine (50 mg) on actual driving performance in healthy volunteers. J Psychopharmacol. 2011;25(11):1517–1523. doi: 10.1177/0269881110382467. [DOI] [PubMed] [Google Scholar]
- 49.Demonte A, Guanti MB, Liberati S, et al. Bilastine safety in drivers why need antihistamines: new evidence from high-speed simulator driving test on allergic patients. Eur Rev Med Pharmacol Sci. 2018;22(3):820–828. doi: 10.26355/eurrev_201802_14318. [DOI] [PubMed] [Google Scholar]
- 50.Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA2 LEN/EDF/WAO guideline for the definition, classification, diagnosis, and management of urticaria: the 2017 revision and update. Allergy. 2018;73(7):1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 51.Church MK, Tiongco-Recto M, Ridolo E, Novák Z. Bilastine: a lifetime companion for the treatment of allergies. Curr Med Res Opin. 2020;36(3):445–454. doi: 10.1080/03007995.2019.1681134. [DOI] [PubMed] [Google Scholar]
- 52.Schaefer P. Acute and chronic urticaria: evaluation and treatment. Am Fam Physician. 2017;95(11):717–724. [PubMed] [Google Scholar]
- 53.Zuberbier T, Oanta A, Bogacka E, et al. Bilastine International Working Group. Comparison of the efficacy and safety of bilastine 20 mg vs levocetirizine 5 mg for the treatment of chronic idiopathic urticaria: a multi-centre, double-blind, randomized, placebo-controlled study. Allergy. 65(4):516–528. doi: 10.1111/j.1398-9995.2009.02217.x. [DOI] [PubMed] [Google Scholar]
- 54.Krause K, Spohr A, Zuberbier T, Church MK, Maurer M. Up-dosing with bilastine results in improved effectiveness in cold contact urticaria. Allergy. 2013;68(7):921–928. doi: 10.1111/all.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serra E, Campo C, Novák Z, et al. Efficacy and safety of bilastine in reducing pruritus in patients with chronic spontaneous urticaria and other skin diseases: an exploratory study. J Dermatolog Treat. 2020;31(3):270–278. doi: 10.1080/09546634.2019.1590522. [DOI] [PubMed] [Google Scholar]
- 56.Weller K, Church MK, Hawro T, et al. Updosing of bilastine is effective in moderate to severe chronic spontaneous urticaria: a real-life study. Allergy. 2018;73(10):2073–2075. doi: 10.1111/all.13494. [DOI] [PubMed] [Google Scholar]
- 57.García-Gea C, Martínez J, Ballester MR, Gich I, Valiente R, Antonijoan RM. Psychomotor and subjective effects of bilastine, hydroxyzine, and cetirizine, in combination with alcohol: a randomized, double-blind, crossover, and positive-controlled and placebo-controlled Phase I clinical trials. Hum Psychopharmacol. 2014;29(2):120–132. doi: 10.1002/hup.2378. [DOI] [PubMed] [Google Scholar]
- 58.Tyl B, Kabbaj M, Azzam S, et al. Lack of significant effect of bilastine administered at therapeutic and supratherapeutic doses and concomitantly with ketoconazole on ventricular repolarization: results of a thorough QT study (TQTS) with QT-concentration analysis. J Clin Pharmacol. 2012;52:893–903. doi: 10.1177/0091270011407191. [DOI] [PubMed] [Google Scholar]
- 59.García-Gea C, Martínez-Colomer J, Antonijoan RM, Valiente R, Barbanoj MJ. Comparison of peripheral and central effects of single and repeated oral dose administrations of bilastine, a new H1 antihistamine: a dose-range study in healthy volunteers with hydroxyzine and placebo as control treatments. J Clin Psychopharmacol. 2008;28(6):675–685. doi: 10.1097/JCP.0b013e31818b2091. [DOI] [PubMed] [Google Scholar]
- 60.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) ICH Harmonized Tripartite Guideline E14. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. 2005. [Accessed March 8, 2021]. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/Step4/E14_Guideline.pdf.
- 61.Yosipovitch G, Bernhard JD. Clinical practice: chronic pruritus. N Engl J Med. 2013;368(17):1625–1634. doi: 10.1056/NEJMcp1208814. [DOI] [PubMed] [Google Scholar]
- 62.Green D, Dong X. The cell biology of acute itch. J Cell Biol. 2016;213(2):155–161. doi: 10.1083/jcb.201603042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ward JR, Bernhard JD. Willan’s itch and other causes of pruritus in the elderly. Int J Dermatol. 2005;44:267–273. doi: 10.1111/j.1365-4632.2004.02553.x. [DOI] [PubMed] [Google Scholar]
- 64.Bilanoa tablet 20 mg (bilastine tablets): Japanese product information. Tokyo: Taiho Pharmaceutical Co., Ltd; 2016. [Google Scholar]
- 65.Antonijoan R, Coimbra J, García-Gea C, et al. Comparative efficacy of bilastine, desloratadine and rupatadine in the suppression of wheal and flare response induced by intradermal histamine in healthy volunteers. Curr Med Res Opin. 2017;33(1):129–136. doi: 10.1080/03007995.2016.1240665. [DOI] [PubMed] [Google Scholar]
- 66.Tiotiu A, Novakova P, Baiardini I, et al. Manifesto on united airways diseases (UAD): an Interasma (global asthma association – GAA) document. J Asthma. 2021:1–16. doi: 10.1080/02770903.2021.1879130. [DOI] [PubMed] [Google Scholar]
- 67.Lavorini F, Matucci A, Rossi O, Pistolesi M. SKY study investigators. Concomitant bilastine and montelukast as additive therapy for seasonal allergic rhinoconjunctivitis and mild-to-moderate asthma. The SKY study. Allergy. 2020;75(3):675–677. doi: 10.1111/all.14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lynde CW, Sussman G, Dion PL, et al. Multidisciplinary real-world experience with bilastine, a second generation antihistamine. J Drugs Dermatol. 2020;19(2):145–154. doi: 10.36849/jdd.2020.4835. [DOI] [PubMed] [Google Scholar]
- 69.Cunningham L, Dvorakova V, Browne F, Irvine AD. High-dose bilastine for the treatment of BASCULE syndrome. Clin Exp Dermatol. 2021;46(2):357–358. doi: 10.1111/ced.14377. [DOI] [PubMed] [Google Scholar]
- 70.Keleş N. Treatment of allergic rhinitis during pregnancy. Am J Rhinol. 2004;18:23–28. [PubMed] [Google Scholar]
- 71.Vlastarakos PV, Manolopoulos L, Ferekidis E, Antsaklis A, Nikolopoulos TP. Treating common problems of the nose and throat in pregnancy: what is safe? Eur Arch Otorhinolaryngol. 2008;265(5):499–508. doi: 10.1007/s00405-008-0601-4. [DOI] [PubMed] [Google Scholar]
- 72.Guideline on risk assessment of medicinal products on human reproduction and lactation from data to labelling. London: European Medicines Agency (EMA): Committee for Medicinal Products for Human Use (CHMP); 2008. [Accessed March 9, 2021]. EMEA/CHMP/203927/2005 https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-risk-assessment-medicinal-products-human-reproduction-lactation-data-labelling_en.pdf. [Google Scholar]
- 73.Sologuren A, Viñas R, Cordón E, Elisabeth S, Forés M, Senán M. [Postmarketing study to assess the safety profile of bilastine 20 mg in elderly patients with rhinoconjunctivitis and/or urticaria]. J Investig Allergol Clin Immunol. 2015;25:65. Abstract. [Google Scholar]
- 74.Viola E, Conforti A. Desloratadine, loratadine and weight increase in children. WHO Pharm Newsl. 2017;4:15–19. [Google Scholar]
- 75.Guideline on excipients in the dossier for application for marketing authorisation of a medicinal product. London: European Medicines Agency (EMA) Committee For Medicinal Products For Human Use (CHMP); 2007. [Accessed March 29, 2021]. EMEA/CHMP/QWP/396951/2006 https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-excipients-dossier-application-marketing-authorisation-medicinal-product-revision-2_en.pdf. [Google Scholar]
- 76.Questions and answers of wheat starch (containing gluten) used as an excipient in medicinal products for human use. London: European Medicines Agency Committe for Human Medicinal Products (CHMP); 2017. [Accessed March 29, 2021]. EMA/CHMP/704219/2013 corr. 1. https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-wheat-starch-containing-gluten-used-excipient-medicinal-products-human-use_en.pdf. [Google Scholar]
- 77.Bailie GR, Mason NA. Bailie and Mason’s 2020 Dialysis of Drugs. Renal Pharmacy Consultants LLC; 2021. [Google Scholar]
- 78.Chourdakis V, Papasotiriou M, Ntrinias T, et al. Efficacy of desloratadine versus bilastine on uremic pruritus in patients with end stage renal disease. Nephrol Dial Transplant. 2019;34(Suppl 1):410–411. Abstract SP415. [Google Scholar]
- 79.Corcóstegui R, Labeaga L, Innerárity A, Berisa A, Orjales A. In vivo pharmacological characterisation of bilastine, a potent and selective histamine H1 receptor antagonist. Drugs R D. 2006;7(4):219–231. doi: 10.2165/00126839-200607040-00002. [DOI] [PubMed] [Google Scholar]
- 80.Bashour M, James A. Laser hair removal. [Accessed March 6, 2021]. https://emedicine.medscape.com/article/843831.
- 81.Photosafety evaulation of pharmaceuticals: Guidance for Industry. Silver Spring (MD): US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2015. [Accessed March 26, 2021]. https://www.fda.gov/media/85076/download. [Google Scholar]
- 82.Coroi MC, Bungau S, Tit M. Preservatives from the eye drops and the ocular surface. Rom J Ophthalmol. 59(1):2–5. [PMC free article] [PubMed] [Google Scholar]
- 83.Nieves F, Hernandez G, Arranz P, et al. Bilastine 0.6% once-daily, preservative-free eye drops pharmacokinetics after single and multiple dose administration. Investig Ophthalmol Vis Sci. 2020;61(7):389. [Google Scholar]
- 84.ClinicalTrials.gov. A study evaluating bilastine ophthalmic solution 0.6% in the conjunctival allergen challenge (Ora-CAC®) model. [Accessed March 6, 2021]. https://clinicaltrials.gov/ct2/show/NCT03479307.
- 85.Mizuguchi H, Wakugawa T, Sadakata H, et al. Elucidation of inverse agonist activity of bilastine. Pharmaceutics. 2020;12(6):525. doi: 10.3390/pharmaceutics12060525. [DOI] [PMC free article] [PubMed] [Google Scholar]