Abstract
We report a case of bilateral acute angle closure glaucoma developing after prone position ventilation for severe COVID-19 pneumonia. A 53-year-old diabetic and hypertensive male developed blurred vision and ocular discomfort in both eyes after prone position ventilation for severe COVID-19 pneumonia. At initial examination he was noted to have diffuse corneal edema with shallow anterior chambers and mid dilated non reacting pupils. His intraocular pressure was 48 and 54 mm Hg in right and left eye, respectively. Following intravenous mannitol (20%) infusion, oral acetazolamide 250 mg 3 times daily, along with topical therapy with combination Brimonidine and Brinzolamide eye drops and Fluoromethalone eye drops his corneal edema resolved and subsequent to laser iridotomy his intraocular pressures lowered significantly and could be maintained below 16 mm Hg in both eyes with topical therapy alone. With prone position ventilation being a commonly used adjuvant treatment for acute respiratory distress syndrome associated with COVID-19 pneumonia, acute angle closure may be precipitated in these patients if they have pre-existing narrow angles. Awareness of the possibility and its recognition may allow prompt ophthalmic referral, early treatment and minimize visual consequences.
Key Words: prone position ventilation, COVID-19 pneumonia, acute angle closure glaucoma
BACKGROUND
Prone position testing has been used by ophthalmologists as a provocative test for angle closure.1,2 In susceptible eyes the increase in intraocular pressure (IOP) during the test may be caused by a relative pupillary block or the blockage of the anterior chamber angle caused by a forward shift of the lens-iris diaphragm. Bilateral acute angle closure glaucoma has been previously reported after face down spinal surgery and general anesthesia, probably associated with the prolonged prone position during surgery.3,4 Prone position and angle closure glaucoma following vitreoretinal surgery has also been reported.5 Early prone position ventilation for severe COVID-19 pneumonia has been found to reduce the mortality associated with the condition and is a part of the protocol for management of severe pneumonia with impaired oxygenation.6 Prone ventilation for severe pneumonia has been noted as a risk factor for angle closure.7,8 Bilateral periorbital edema, optic disc edema, and retinal hemorrhages with elevated IOPs (orbital compartment syndrome) following prolonged prone position ventilation in COVID-19 patients have been recently reported.9 However, an actual case of acute angle closure following prone ventilation for COVID-19 pneumonia has not been reported to the best of our knowledge.
CASE REPORT
We report a case of bilateral acute angle closure glaucoma following prone position ventilation for severe COVID-19 pneumonia.
A 53-year-old diabetic and hypertensive male patient was admitted with COVID-19 pneumonia. A week after admission he was shifted to the intensive care unit (ICU) when he developed acute respiratory distress syndrome. He was managed with prone position ventilation along with intravenous Dexamethasone, Remdesivir, and anticoagulant Enoxaparin. He also received antibiotics (Sulphmethoxazole with trimethoprim, Levofloxaxin, Meropenem) and antifungals (Voriconazole and Caspofungin) for secondary bacterial and fungal infections. Silodosin was given for his urine retention and Glycopyrrolate granules were used to reduce the viscosity of bronchial secretions. In the initial 2 weeks of his ICU stay he received pressor agents like Vasopressin and Noradrenaline as well as Salbutamol for bronchodilation. He was kept sedated with midazolam during this period. After 22 days his oxygen saturation levels gradually improved and were maintained with oxygen support alone and he was shifted out of intensive care. The same day an ophthalmology referral was sought for a complaint of blurred vision and discomfort in both his eyes. On bedside examination, visual acuity recorded with his regular prescription glasses (+4.75 diopter sphere and +5.5 diopter sphere for right and left, respectively) was noted to be finger counting at 3 m in both the eyes. Torch light examination showed mild conjunctival injection and diffuse corneal edema with shallow anterior chamber and mid dilated non reacting pupil in both the eyes. Using Perkins’ applanation tonometry his IOP was 48 and 52 mm Hg in the right and left eye, respectively. He received 300 mL of intravenous 20%Mannitol over 45 minutes. Oral acetazolamide 250 mg thrice daily was added to his systemic medication. His pulmonary condition limited the type of topical medication that could be safely used, hence he was started on a topical combination eye drop of Brinzolamide with Brimonidine thrice daily in both his eyes and Fluorometholone eye drops 3 times daily were added to reduce inflammation of the iris and facilitate laser iridotomy. He was examined at the ophthalmology outpatient clinic the next day. His visual acuity was recorded to be 20/60 and 20/40 in the right and left eye respectively with +4.0 diopter sphere and +1.0 diopter cylinder at 180 degrees in his right eye and +4.75 diopter sphere with +0.75 diopter cylinder in his left eye. On slit lamp biomicroscopy the corneal epithelial edema had completely resolved with very minimal residual stromal haze. The anterior chamber was peripherally shallow with mid dilated sluggishly reacting pupils and few glaucomflecken were noted. Applanation IOP was 42 and 38 mm Hg, respectively, in the right and left eye and 4 mirror gonioscopy of both eyes showed 360 degrees of appositional angle closure. Fundus examination by slit lamp biomicroscopy with a 90 diopter lens showed both eyes to have normal sized discs with 0.9 vertical cup disc ratio and bipolar rim thinning with pallor. There was generalized arteriolosclerosis but no evidence of diabetic retinopathy. No choroidal detachment was clinically evident. Anterior segment optical coherence tomography confirmed the appositional angle closure (Fig. 1). The lens vault was measured to be 0.58 and 0.62 mm, respectively, in the right and left eye. Since we did not have ultrasound biomicroscopy facility at our center, an ultrasound B scan was performed which did not reveal any anterior or posterior choroidal detachment. Laser iridotomy was performed in both his eyes. At review 1 week later, he was off oxygen support and his applanation IOP was 12 mm Hg in both his eyes on topical antiglaucoma medication and oral acetazolamide. Repeat 4 mirror gonioscopy revealed peripheral anterior synechiae over 180 degrees in the right eye and 120 degrees in the left eye with widening of the angle recess in the remaining area. Anterior segment optical coherence tomography confirmed these findings (Fig. 1). Automated visual field analysis showed advanced glaucomatous damage with residual small central islands of vision in both eyes. At last follow up his best corrected visual acuity was 20/40 in the right eye and 20/30 in the left eye. His IOP was maintained below 14 mm Hg his right eye and 16 mm Hg in his left on topical therapy alone.
FIGURE 1.
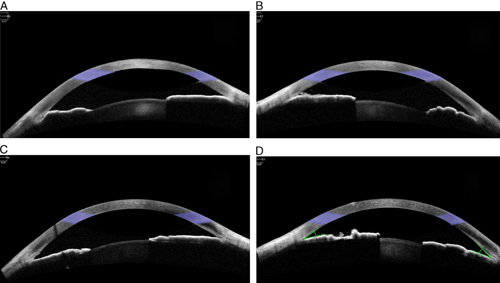
Anterior segment optical coherence tomography of the right and left eye before (A and B) and after (C and D) iridotomy.
DISCUSSION
Prone ventilation has become a part of the treatment protocol for management of acute respiratory distress syndrome associated with COVID-19 pneumonia and patients may be prone ventilated for as long as 12 hours a day. In the prone position, IOP increases and the extent of this increase is related to the amount of time the patient is in the prone position. Acute rises in IOP may affect the ocular perfusion pressure and carries the risk of irreversible vision loss because of ischemic events. A recent report of raised IOP after prone position ventilation for COVID-19 pneumonia mentions orbital compartment syndrome as the probable mechanism for IOP rise.
In our patient all the clinical findings were suggestive of pupillary block associated angle closure as the probable cause of raised IOP. Since the patient was sedated and seriously ill in the ICU and was unable to verbalize his symptoms, it was only after he was better and able to communicate that the ophthalmic referral was sought. Thus the exact time of onset of acute closure is not known. If there were recurrent episodes of angle closure associated with very high IOP’s during the 3-week period when he was in the ICU, it is possible that most of the glaucomatous damage occurred during this period. A number of other drugs were administered during this period. Of these Noradrenaline, Glycopyrrolate, and Salbutamol could have precipitated or compounded the acute angle closure and this cannot be ruled out as a contributory factor. Other possible mechanisms of raised IOP besides pupillary block include a steroid induced IOP spike (patient received intravenous dexamethasone while in ICU and was shifted to oral prednisolone thereafter). However, with closed angles on gonioscopy, the steroid component would have at best compounded the IOP spike. An idiosyncratic reaction following the use of Sulphmethoxazole resulting in supra ciliary and choroidal effusion and secondary angle closure is a possibility, but the absence of a significant myopic shift in refraction and the absence of choroidal effusion on clinical examination and ultrasound B scan make this unlikely.
While the angle closure in our patient was not related to the COVID-19 infection directly, it was most likely precipitated or aggravated by prolonged prone position adopted in the treatment of COVID-19 pneumonia. Although prone position related bilateral acute angle closure in various situations has been previously reported, most of these cases are single events of prone position. Also some of these reports have possible confounding factors like role of general anesthetic drugs used or overfill of gas in vitreoretinal surgery. Besides, the patients in these cases were able to report the symptoms soon after their occurrence. In our case, the patient was placed in prone position for 8 hours daily for 2 weeks. The detection and treatment of the angle closure was possibly delayed as the patient was unable to verbalize his symptoms while in the ICU under sedation. Though ophthalmic referral was sought as soon as the patient complained of blurred vision and discomfort, allowing treatment to be initiated and residual visual function to be salvaged, a significant amount of visual field damage had already occurred. In an ICU setting, periorbital edema and chemosis with severe congestion are easier to recognize compared with the more subtle findings of corneal haze and mid dilated pupils which need to be specifically looked for. Sensitizing the ICU staff to this possible complication and incorporating examination of corneal clarity as well as pupil size and reaction into the ICU protocol for prone ventilated patients may result in earlier ophthalmic referral and may help prevent or reduce the risk of potential serious visual consequences.
Footnotes
Disclosure: The authors declare no conflict of interest.
Contributor Information
Roopali R. Nerlikar, Email: nerlikar.roopali@gmail.com.
Aratee C. Palsule, Email: palsulea@gmail.com.
Shantanu Vadke, Email: 0127shantanuvadke@gmail.com.
REFERENCES
- 1.Ichioka I.Short-term prone-position test in angle-closure glaucoma. Jpn J Clin Ophthalmol. 2006;60:1619–1623.
- 2.Kim TW, Park KH, Hong C. Dark-room prone-position test for intermittent angle closure. Korean J Ophthalmol. 2007;21:151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer MS, Salim S. Bilateral acute angle-closure glaucoma as a complication of facedown spine surgery. Spine J. 2010;109:7–9. [DOI] [PubMed] [Google Scholar]
- 4.Gayat E, Gabison E, Devys JM. Bilateral angle closure glaucoma after general anesthesia. Anesth Analg. 2011;112:126–128. [DOI] [PubMed] [Google Scholar]
- 5.Sutter FKP, Smorgon A, McClellan K. Acute angle closure in the fellow eye as a complication of prone positioning after vitreoretinal surgery. Arch Ophthalmol. 2003;121:1057. [DOI] [PubMed] [Google Scholar]
- 6.Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8:1–3. [PMC free article] [PubMed] [Google Scholar]
- 7.Soare C, Nowak VA, Osborne S. Eye care in the intensive care unit during the COVID-19 pandemic and beyond. Anaesthesia. 2021;75:1118–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hearne BJ, Hearne EG, Montgomery H, et al. Eye care in the intensive care unit. J Intensive Care Soc. 2018;19:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L, Hymowitz M, Pomeranz HD. Eye protection for patients with COVID-19 undergoing prolonged prone-position ventilation. JAMA Ophthalmol. 2021;139:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]


