Abstract
Objectives:
The purpose of the study was to test the hypothesis that anticholinergic drug exposure is associated with cognitive decline in the Wisconsin Registry for Alzheimer’s Prevention (WRAP) study. Secondary aims were to assess if the effects of anticholinergic drugs on different domains of cognitive functioning varied for the entire sample and by apolipoprotein ε4 status.
Methods:
The WRAP study includes a sample of 1,573 subjects who self-reported medication use and were administered several cognitive tests four times over a decade. Partial correlations assessed relationships between reported days of definite anticholinergic drug exposure with changes in cognitive performance. Linear mixed models were conducted tested main effects for anticholinergic drug use and interaction effects between anticholinergic drug use, apolipoprotein ε4 status, and time on neuropsychological assessment performance.
Results:
Partial correlations indicated that days of anticholinergic drug exposure was associated with a decline in mental status for the entire sample (r=−.043, p=.011), and immediate verbal memory (r=−.066, p=.043), delayed verbal memory (r=−.077, p=.018), psychomotor speed (r=−.066, p=.043), and cognitive flexibility (r=−.067, p=.040) of apolipoprotein ε4 carriers only. The linear mixed model results suggested that anticholinergic drug users had a greater decline than non-users in delayed memory, psychomotor speed, and cognitive flexibility. Apolipoprotein ε4 carrier, anticholinergic drug users performed worse in delayed memory than non-users and non-carrier, anticholinergic drug users.
Conclusions:
Anticholinergic drug use may have deleterious effects on the cognitive functioning of subjects in populations at risk for dementia, especially among apolipoprotein ε4 carriers.
Introduction
Anticholinergic drugs are used to treat allergies, depression, psychosis, muscle and gastrointestinal problems, vertigo, Parkinson’s disease, bladder incontinence, arrhythmia, and other cardiopulmonary issues (Pfistermeister et al., 2017). Approximately 34% of older adult populations have used anticholinergic medications (Britt & Day, 2016). Given their widespread use, it is important to understand their effects on cognitive functioning. Studies have linked anticholinergic drug use to increased risk of falls, bone fractures, poorer grip strength, slower walking speed, reduced appetite, and mobility in elderly populations (Attoh-Mensah et al., 2020; Chatterjee et al., 2016; Lim et al., 2019; Marcum et al., 2016; Wouters et al., 2020).
Recent research studies have supported the hypothesis that anticholinergic medications are associated with a higher risk for dementia (Bottiggi et al., 2006; Britt & Day, 2016; Cai et al., 2013; Chuang et al., 2017; Campbell et al., 2018; Dyer et al., 2020; Fortin et al., 2011; Fox et al., 2011; Jessen et al., 2010; Papenberg et al., 2017; Wu et al., 2017; Ziad et al., 2018). A large majority of studies relied on cut-off scores from a mental status exam among other neuropsychological assessments to substantiate a dementia or Mild Cognitive Impairment (MCI) diagnosis (Fox et al., 2011; Lechevallier-Michel et al., 2004). For example, Naharci et al., (2017), Ancelin et al., (2006), and Chuang et al., (2017) using mental status exam cutoff scores to substantiate dementia diagnoses, found that anticholinergic drug exposure was associated with increased dementia risk. A significant limitation of using cutoff scores of neuropsychological assessments to determine dementia is their susceptibility to both false negatives and positives. For example, Mitchell (2009) found that in a memory clinic, the MMSE has a 77% sensitivity and an 91% specificity, which suggests that a significant portion of the sample may be misdiagnosed. Furthermore, there may be considerable variance in cognitive functioning among the groups diagnosed with and not diagnosed with dementia or Mild Cognitive Impairment (MCI). As a result, it may be more beneficial to assess the effects of anticholinergic drug use on a comprehensive battery of neuropsychological test scores directly.
Several studies also used insurance or medical records associating filling a prescription with anticholinergic properties and healthcare provider diagnosis of dementia to draw linkages (Cai et al., 2013; Campbell et al., 2018; Han et al., 2008). Specifically, Cai et al., (2013), using records from a primary care office, found that anticholinergic drug users were 50% more likely to be diagnosed with dementia than non-users. A limitation of using medical records to draw connections between medication and dementia status is that a diagnosis is commonly made by a single provider, typically in the absence of cognitive testing (Han et al., 2008). As a result, studies that use these methodologies may have underestimated the amount of dementia cases in the population. In a more thorough design, Campbell et al., (2018) reviewed registered prescriptions and found that anticholinergic drugs were associated with an increased likelihood of an MCI diagnosis in a predominantly African American population. The previous findings provide a consensus that anticholinergic drug use is associated with greater likelihood of cognitive decline in certain populations.
The apolipoprotein ε4 allele (APOε4) is a genetic risk factor for AD, but few studies have directly assessed differences in the effects of anticholinergic drug use on APOε4 carrier status using a sample with high risk for AD. Montagne et al., (2020) notes that the APOε4 allele has a role in augmenting the effects of AD pathology, which includes neurofibrillary tangles and buildup of amyloid plaques. The APOε4 allele is also associated with other cardiovascular risk factors, which may weaken the blood brain barrier thus enhancing the effects of anticholinergic medications (Montagne et al., 2020). Nebes et al., (2012) found that anticholinergic drug use had a negative effect on the cognitive functioning of APOε4 carriers. Conversely, two recent studies did not find any evidence to support interaction effects between anticholinergic drug use and APOε4 carrier status on cognitive functioning (Dyer et al., 2019; Limback-Stokin et al., 2018). However, these two studies did not control for familial risk factors for AD.
The purpose of the current study was to use data from the Wisconsin Registry for Alzheimer’s Prevention (WRAP) to test the hypothesis that anticholinergic medication use and exposure is associated with cognitive decline in a population with familial risk factors for dementia. A secondary goal of the study was to investigate the effects of anticholinergic drug use and exposure on different domains of cognitive functioning. In turn, it was hypothesized that anticholinergic drug use would have negative effects on working memory, phonemic fluency, auditory verbal learning and delayed memory, psychomotor speed, and cognitive flexibility. Finally, a tertiary aim of the current research study was to explore differences in the effects of anticholinergic drugs on the cognitive functioning of APOε4 carriers and non-carriers. As such, it was predicted that APOε4 carrier, anticholinergic drug users would have worse performance and show greater declines in neuropsychological test scores than non-carriers, and APOε4 carrier, non-users.
Methods
Participants
The current research study analyzes data from the WRAP study, which is one of the largest and most comprehensive longitudinal studies of cognitive functioning over time. The dataset includes approximately 1,573 subjects who were ages 36 to 73 at the start of the study. Nearly three in four of the studies participants had a parent or close relative with a confirmed case of dementia determined via medical or autopsy reports and all non-AD related causes were ruled out (Johnson et al., 2018). It should be noted that none of the subjects had cognitive impairment at the baseline. The attrition rate was estimated at 33% from visits one to four. Johnson et al., (2018) estimates that over 99% of the original WRAP sample has a high school degree and 54% to 66% had a bachelor’s degree.
Procedure
Participants were recruited through their affiliation with certain clinics associated with the study, attendance of educational presentations, and other resources in the community. Each subject was paid $50 per visit in compensation for their participation in the study. The current analysis includes observations from the first four study visits which started the data collection process in 2001, 2006, 2009, and 2012 respectively. During each visit, subjects completed questionnaires, gave blood for testing, and were administered a wide range of neuropsychological assessments by trained examiners (Johnson et al., 2018). Permission to obtain and analyze the dataset was approved by a research ethics committee at a university in the midwestern United States and was subsequently approved by the WRAP study board.
Instruments
Anticholinergic Drug Use.
Participants self-reported their prescription and non-prescription drugs during each visit, which was used to determine the anticholinergic properties of medications. It should be noted that subjects were not required to take any medications as a part of the WRAP study. The Anticholinergic Cognitive Burden Scale (ACBS) is a list of medications classified by their anticholinergic properties. According to the ACBS, drugs listed as a level one is labeled as possessing ‘possible’ anticholinergic properties, whereas drugs classified as a level two or three have definite anticholinergic properties (Aging Brain Care, 2012).
Health Variables.
During the first visit, subjects provided a saliva sample that was used to determine their apolipoprotein E genotype. Participants were asked during each visit if they had ever been diagnosed by a medical provider with heart disease, diabetes, depression, anxiety, head injury, stroke, Parkinson’s disease, multiple sclerosis, lung disease, liver disease, AIDS, and kidney disease. Frequency of physical activity was assessed by asking subjects how often they engage in physical exercise on a scale of 1(rarely or never) to 4(more than once a week). Smoking behavior was determined by asking about their daily cigarette use on a scale of 1 (non-smoker) to 6 (30 or more cigarettes).
Psychological Assessments
The Center for Epidemiological Studies-Depression (CES-D) was used as a measure of depressive symptomology during each study visit (Radloff et al., 1977). The Verbal Intelligence Quotient (VIQ) of the Wechsler Abbreviated Scale of Intelligence (WASI) was used as an assessment of verbal intelligence during the first study visit (Wechsler et al., 1999). Given that years of education was not included in the dataset, VIQ was used as a proxy for years of education since the two variables are highly correlated (Abad et al., 2016). The Mini-Mental State Exam (MMSE) was used as a measure of mental status and severity of cognitive impairment (Folstein et al., 1983). In turn, the Digit Span subtest of the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) was used as an assessment of working memory capacity (Wechsler, 1997). Phonemic fluency was assessed using the Benton Controlled Oral Word Association (COWA) test (Benton, Hamsher, & Sivan, 1994), whereas immediate (AVLTI) and delayed (AVLTD) verbal learning and memory was assessed using the Auditory Verbal Learning Test (AVLT) (Schmidt et al., 1996). Finally, the Trail Making Test, Part A (TMTA) was used as an assessment of psychomotor speed and Part B (TMTB) was used as an indicator of cognitive flexibility (Reitan, 1958)
Data Analysis
Data Selection and Classification.
Subjects who were using a definite anticholinergic drug (level two or three anticholinergic drug according to the ACBS) were classified as anticholinergic drug users for the purposes of the study. To reduce the effects of possible anticholinergic drugs on the outcome (level one anticholinergic drugs according to the ACBS), the sample excluded observations of non-users who reported using two or more possible anticholinergic drugs. The effects of non-users who reported using one possible anticholinergic drug was accounted for in the statistical models, and it should also be noted that the significance of the statistical tests did not change when all possible anticholinergic drug users were removed from the dataset.
Observations of subjects who reported using definite anticholinergic drugs that were not intended for regular use (i.e. diphenhydramine) were also excluded from the analysis. Furthermore, subjects who reported a history of head injuries, stroke, Parkinson’s disease, multiple sclerosis, kidney disease, liver disease, lung disease, and AIDS were also not included. Specific observations in which participants who reported use of narcotic analgesics, benzodiazepines, acetylcholinesterase inhibitors, and psychostimulant medication use were also not included to limit the influence of non-anticholinergic psychotropic medications that may influence cognitive functioning.
Partial Correlations.
Partial correlations were conducted to test the hypothesis that days of anticholinergic drug exposure was associated with neuropsychological test performance. Partial correlations were chosen over alternative methods because of their ability to compare two continuous variables that adjusted for covariate effects and provide an estimate of effect size. The first set of partial correlations assessed relationships between reported days of anticholinergic drug exposure and raw neuropsychological test scores. Each correlation controlled for heart disease, diabetes, depression, anxiety, prevalence of physical activity, cigarette use, reported days on a possible anticholinergic, CESD ratings of depression, gender, age, and verbal intelligence.
A second set of partial correlations was conducted to test the hypothesis that reported days of anticholinergic drug exposure is associated with a decline in neuropsychological test performance. Specifically, these correlations tested the relationships between change in neuropsychological test scores and reported days of anticholinergic drug use during the most recent visit. The change in neuropsychological test scores variable was computed by subtracting the raw score during the earlier visit from the score during the most recent visit for all tests except TMTA and TMTB. Since TMTA and TMTB are timed tests, the scores during the recent visit were subtracted from the scores during the earlier visit so positive values would suggest an improvement in performance.
A limitation with assessing differences in repeated administrations of neuropsychological tests is that subjects who score high on a prior visit may have less opportunity to increase scores in comparison to those who scored lower. To control for this effect, neuropsychological assessment scores during the earlier visit were included as a covariate in correlations with change in performance as a dependent variable. It should be noted that partial correlation values were reported for the entire sample, APOε4 carriers, and non-carriers separately to assess the hypothesis that anticholinergic drug exposure has a more detrimental effect on the cognitive functioning of APOε4 carriers than non-carriers.
Linear Mixed Models.
Linear mixed models were conducted using the same covariates as the partial correlations to test the second set of hypotheses that definite anticholinergic drug users would perform worse and show greater decline in neuropsychological functioning than non-users. Specifically, each model assessed main effects for time, APOε4 carrier status, anticholinergic drug use, and an interaction effect between anticholinergic drug use and time. To assess the hypothesis that APOε4 carrier status has an impact on the influence of anticholinergic drugs on cognitive functioning, the models also tested interaction effects between anticholinergic drug use and APOε4 carrier status, along with anticholinergic drug use, APOε4 carrier status, and time on neuropsychological test performance.
A second set of linear mixed models was conducted testing the same effects and covariates with change in neuropsychological assessment scores as the dependent variable. Consistent with the partial correlations, the linear mixed models with change scores as the dependent variable included scores during the earlier visit as a covariate to control for extreme scores regressing toward the mean. Each model was tested at an alpha level of .05 and false discovery rate (FDR) corrections were applied to adjust for multiple testing (Benjamini & Yekutieli, 2005). The data was analyzed using IBM SPSS Statistics, Version 26.0 (IBM Corp, Armonk, NY, 2019).
Missing Values.
Little’s MCAR test was significant for all models, which suggests that missing values were not at random. As a result, a chained equations imputation was conducted using SPSS to fill the missing values. The chained equations imputation algorithm uses logistical and linear regression to fill in the missing values in the dataset (Azur et al., 2011). Five imputations were used, and the statistical significance of the results across the five imputations were compared to that of the original dataset. Overall, all the imputations yielded consistent results with the original sample and as such the original dataset was used in the analysis.
Results
Analysis of the Sample
During visits one through four, the sample included 67, 61, 60, and 35 subjects using one or more definite anticholinergic medications respectively. Anticholinergic drug users made up 6.8% of the sample during visit one (non-users, n=1056), 1% during visit two (non-users, n=924) and three (non-users, n=872), and 4% during visit four (non-users, n=569). Chi-squares tests, t-tests, and non-parametric Mann-Whitney U tests were conducted to determine if any differences in the covariates existed between anticholinergic drug users and non-users using the baseline observations. Non-parametric tests were used due to the small percentage of anticholinergic drug users compared to non-users.
Table 1 highlights the count and percentage of anticholinergic drug users by categorical covariate along with chi-square test results during the first study visit. During the baseline observation, there was no significant differences between anticholinergic drug users and non-users in prevalence of APOε4 carriers, monthly physical exercise, cigarette use, reported history of heart disease, and diabetes. Conversely, anticholinergic drug users had a significantly greater proportion of females and were more likely to report a history of depression and anxiety disorders than non-users.
Table 1.
Description of the Sample of Anticholinergic Drug Users (AC+) and Non-users (AC−) by Demographic and Health Related Variables During Baseline Visit
| Variable1 | AC Use2 | N | %3 | χ2 | p |
|---|---|---|---|---|---|
|
| |||||
| Heart Disease | AC− | 83 | 7.9 | .02 | .884 |
| AC+ | 8 | 11.9 | |||
|
| |||||
| Diabetes | AC− | 44 | 4.2 | .04 | .581 |
| AC+ | 3 | 4.5 | |||
|
| |||||
| Depression | AC− | 181 | 17.2 | 56.95 | <.001** |
| AC+ | 35 | 52.2 | |||
|
| |||||
| Anxiety | AC− | 60 | 5.7 | 20.66 | <.001** |
| AC+ | 15 | 22.4 | |||
|
| |||||
| Phys Act | AC− | 788 | 74.8 | 3.93 | .269 |
| AC+ | 43 | 64.2 | |||
|
| |||||
| Cigarette Use | AC− | 76 | 7.2 | 1.73 | .786 |
| AC+ | 3 | 4.5 | |||
|
| |||||
| APOε4+ | AC− | 381 | 36.1 | .95 | .330 |
| AC+ | 29 | 43.3 | |||
|
| |||||
| APOε4− | AC− | 637 | 60.4 | ||
| AC+ | 38 | 56.7 | |||
|
| |||||
| Male | AC− | 322 | 30.6 | 10.90 | .001** |
| AC+ | 10 | 14.9 | |||
|
| |||||
| Female | AC− | 732 | 69.4 | ||
| AC+ | 57 | 85.1 | |||
|
| |||||
| Total | AC− | 1054 | 100 | ||
| AC+ | 67 | 100 | |||
Heart Disease=Reported history of heart disease, Diabetes=Reported history of diabetes, Depression=Subject reported history of depression, Anxiety=Subject reported a history of anxiety disorders, Phys Act=Subject reported that they engaged in physical activity four times a month or greater, Cigarette Use=Subjects reported that they had smoked cigarettes in the previous month, APOε4+= APOε4 carrier, APOε4−=non-carrier
AC−=non-user, AC+=anticholinergic drug user
%=the percentage of subjects who belonged to that group among anticholinergic drug users or non-users. For example, 7.9% in the AC− row of heart disease in the visit one column suggests that 7.9% of non-users reported a history of heart disease.
Table 2 includes the mean, standard deviation, minimum, maximum of raw neuropsychological test scores and continuous covariates along with independent samples t-test and Mann-Whitney U results by anticholinergic use during the baseline visit (and visit two for MMSE since it was not administered during visit one). According to the t-test and Mann-Whitney U results, there was no significant differences between anticholinergic drug users and non-users in Digit Span, AVLTI, AVLTD, TMTA, TMTB, and MMSE scores, age, and verbal intelligence during the baseline observation and visit two for the MMSE. However, definite anticholinergic drug users had significantly better COWA performance, higher CESD scores, and reported more days on a level one anticholinergic drug than non-users during visit one.
Table 2.
Descriptive Statistics of Raw Neuropsychological Test Scores and Continuous Covariates for Anticholinergic Drug Users (AC+) and Non-users (AC−) During Baseline Observation
| Variable4 | AC Use | SD | Min | Max | t5 | p | U6 | p | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| MMSE7 | AC− | 29.38 | .95 | 23 | 30 | .12 | .906 | 32301 | .672 |
| AC+ | 29.36 | .87 | 27 | 30 | |||||
|
| |||||||||
| DS | AC− | 17.29 | 3.91 | 8 | 30 | −1.51 | .133 | 44888 | .220 |
| AC+ | 17.99 | 4.11 | 9 | 29 | |||||
|
| |||||||||
| COWA | AC− | 44.29 | 10.79 | 11 | 85 | −2.12 | .035* | 47386 | .018* |
| AC+ | 47.00 | 9.96 | 24 | 68 | |||||
|
| |||||||||
| AVLTI | AC− | 50.49 | 8.29 | 23 | 75 | .98 | .328 | 38370 | .255 |
| AC+ | 49.53 | 8.42 | 30 | 69 | |||||
|
| |||||||||
| AVLTD | AC− | 10.32 | 2.95 | 0 | 15 | 1.81 | .071 | 36213 | .057 |
| AC+ | 9.68 | 2.89 | 3 | 15 | |||||
|
| |||||||||
| TMTA | AC− | 26.83 | 8.89 | 10 | 88 | −.42 | 675 | 41724 | .067 |
| AC+ | 27.28 | 9.47 | 16 | 66 | |||||
|
| |||||||||
| TMTB | AC− | 64.14 | 28.99 | 25 | 352 | .034 | .973 | 41819 | .910 |
| AC+ | 64.03 | 25.96 | 33 | 195 | |||||
|
| |||||||||
| Age | AC− | 53.62 | 6.73 | 36 | 73 | 1.80 | .072 | 46675 | .076 |
| AC+ | 55.04 | 6.03 | 36 | 67 | |||||
|
| |||||||||
| CESD | AC− | 6.13 | 6.62 | 0 | 44 | −.38 | <.001** | 49589 | .004** |
| AC+ | 9.18 | 9.84 | 0 | 51 | |||||
|
| |||||||||
| VIQ | AC− | 109.36 | 10.87 | 62 | 137 | .82 | .412 | 43330 | .291 |
| AC+ | 110.43 | 10.91 | 78 | 129 | |||||
|
| |||||||||
| L1 Dur | AC− | 201.08 | 868.90 | 0 | 9790 | −3.04 | .002** | 47823 | <.001** |
| AC+ | 532.24 | 1455.82 | 0 | 9547 | |||||
|
| |||||||||
| AC Dur | AC+ | 2118 | 2261 | 2 | 16308 | ||||
MMSE=Mini-Mental State Examination Total Score, DS=Wechsler Adult Intelligence Scale Third Edition, Digit Span subtest, COWA=Benton Controlled Oral Word Association test (COWA), AVLTI=Combined score of the first five trials on the Auditory Verbal Learning Test, AVLTD=Delayed Recall Portion of the Auditory Verbal Learning Test, TMTA=Trail Making Test, Part A; TMTB=Trail Making Test, Part B; CESD=Center for Epidemiological Studies – Depression, Total Score; Age=Reported Age During Visit, L1 Dur=Number of days subject reported using a level one anticholinergic drug on the Anticholinergic Cognitive Burden Scale; AC Dur=Number of reported days using a level two or three anticholinergic drug on the Anticholinergic Cognitive Burden Scale
Independent Samples t-test statistic
Non-parametric Mann-Whitney U test statistic
The MMSE was not administered during visit one, so the means and analyses represent MMSE scores during the second study visit.
Partial Correlations
Partial Correlations Between Duration of Anticholinergic Drug Use and Neuropsychological Test Scores.
Table 3 shows the partial correlations between reported days of exposure to a definite anticholinergic and neuropsychological assessment performance by APOε4 carrier status. As indicated in Table 3, anticholinergic drug exposure was significantly associated with poorer performance on the MMSE, AVLTI, AVLTD, TMTA, and TMTB for the entire sample. For APOε4 carriers, anticholinergic drug exposure was significantly correlated with worse performance on the AVLTI, AVLTD, TMTA, and TMTB, whereas reported exposure was only significantly associated with poorer performance on the AVLTI and AVLTD among non-carriers. As indicated in Table 3, the negative effect of anticholinergic exposure on TMTA and TMTB performance appears strongest for APOε4 carriers.
Table 3.
Partial Correlations Between Reported Days of Definite Anticholinergic Exposure and Total and Change in Neuropsychological Test Performance by Apolipoprotein ε4 Carrier Status
| Model | Test | Stat | Total | ε4− | ε4+ |
|---|---|---|---|---|---|
|
| |||||
| Days of Exposure to Definite Anticholinergic and Neuropsychological Assessment Performance | MMSE | r | −.043* | −.045 | .044 |
| p | .011 | .070 | .181 | ||
|
| |||||
| DS | r | .007 | .018 | −.006 | |
| p | .668 | .389 | .826 | ||
|
| |||||
| COWA | r | .023 | .015 | .046 | |
| p | .175 | .473 | .092 | ||
|
| |||||
| AVLTI | r | −.075** | −.075** | −.092** | |
| p | <.001 | <.001 | <.001 | ||
|
| |||||
| AVLTD | r | −.081** | −.082** | −.094** | |
| p | <.001 | <.001 | <.001 | ||
|
| |||||
| TMTA | r | .051** | .021 | .095** | |
| p | .002 | .315 | <.001 | ||
|
| |||||
| TMTB | r | .039* | .013 | .076** | |
| p | .021 | .534 | .005 | ||
|
| |||||
| Days of Exposure to Definite Anticholinergics and Change in Neuropsychological Assessment Performance | MMSE | r | −.080** | −.093** | −.059 |
|
| |||||
| p | .001 | .003 | .156 | ||
|
| |||||
| DS | r | .034 | −.026 | .040 | |
| p | .078 | .291 | .220 | ||
|
| |||||
| COWA | r | .003 | .001 | .019 | |
| p | .876 | .968 | .560 | ||
|
| |||||
| AVLTI | r | −.034 | .015 | −.066* | |
| p | .077 | .543 | .043 | ||
|
| |||||
| AVLTD | r | −.034 | −.001 | −.077* | |
| p | .077 | .968 | .018 | ||
|
| |||||
| TMTA | r | −.040* | −.016 | −.066* | |
| p | .038 | .516 | .043 | ||
|
| |||||
| TMTB | r | −.035 | −.003 | −.067* | |
| p | .069 | .903 | .040 | ||
p<.05
p<.01
Partial Correlations Between Duration of Use and Change in Neuropsychological Test Scores.
Table 3 also highlighted the correlations between duration of anticholinergic drug exposure and change in performance throughout the duration of the study. As indicated from Table 3, reported days exposure to anticholinergic drugs was associated with a significant decline in MMSE among all subjects and non-carriers, but did not reach statistical significance for APOε4 carriers. In contrast, reported days of anticholinergic drug exposure was associated with significant declines in AVLTI, AVLTD, TMTA, and TMTB scores among APOε4 carriers only. Supplementary Figures 1,2,3,4, and 5 include scatter plots of change scores for MMSE, AVLTI, AVLTD, TMTA, TMTB respectively by number of reported days on an anticholinergic medication.
Linear Mixed Models
Neuropsychological Test Scores as a Dependent Variable.
Main Effects for Anticholinergic Drug Use.
The results for the first set of linear mixed models that used raw neuropsychological test scores as the dependent variable are highlighted in Table 4. As indicated in Table 4, the findings suggest that anticholinergic drug use was not associated with performance on the Digit Span and COWA. However, the findings from Table 3 show significant differences between anticholinergic drug users and non-users on the AVLTI, AVLTD, TMTA, and TMTB. Supplemental Table 1 highlights the model predicted means for anticholinergic users, and non-users on each neuropsychological assessment by study visit. The model predicted means suggest that anticholinergic users had significantly worse overall performance (regardless of study visit) than non-users on AVLTI, AVLTD, TMTA, and TMTB.
Table 4.
Linear Mixed Model Results by Neuropsychological Assessment
| Test8 | Model | Scores at Visit | Change in Scores | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Effect | AC | ε4 * AC | T * AC | T * ε4 * AC | AC | AC * ε4 | AC * ε4*T | |
|
| ||||||||
| MMSE | df | 1, 2383 | 1, 2378 | 2,1530 | 1, 2378 | 1, 1498 | 1, 1494 | 3, 1400 |
| F | 3.30 | .01 | 4.80 | .01 | 7.68 | .01 | .53 | |
| P | .069 | .942 | .008** | .942 | .006** | .934 | .587 | |
| FDR9 | .138 | .942 | .032* | .587 | .018* | .934 | .881 | |
|
| ||||||||
| DS | df | 1, 2004 | 1,1970 | 1,1105 | 1,1122 | 1,2079 | 1, 2076 | 4, 1386 |
| F | .29 | .89 | .90 | .95 | 1.22 | .22 | 2.79 | |
| P | .593 | .345 | .443 | .459 | .270 | .636 | .025* | |
| FDR | .712 | .565 | .636 | .636 | .405 | .636 | .075 | |
|
| ||||||||
| COWA | df | 1,1685 | 1,1652 | 1,1079 | 1,1092 | 1,1978 | 1,1975 | 4, 1277 |
| F | .55 | .68 | .25 | 1.24 | .19 | .92 | .35 | |
| P | .459 | .410 | .861 | .282 | .660 | .337 | .847 | |
| FDR | .751 | .738 | .962 | .564 | .847 | .847 | .847 | |
|
| ||||||||
| AVLTI | df | 1, 1926 | 1,1892 | 1,1118 | 1,1134 | 1,2049 | 1,2048 | 4, 1369 |
| F | 8.78 | 1.9 | 2.32 | .77 | 2.34 | 1.10 | 1.32 | |
| P | .003** | .168 | .074 | .592 | .126 | .294 | .259 | |
| FDR | .009** | .233 | .121 | .592 | .294 | .294 | .294 | |
|
| ||||||||
| AVLTD | df | 1, 2010 | 1,1976 | 1,1118 | 1,1135 | 1,2075 | 1, 2076 | 4, 1397 |
| F | 19.34 | 4.94 | 2.19 | .85 | 11.6 | 8.75 | 3.62 | |
| P | <.001** | .026* | .087 | .530 | .001** | .003** | .006** | |
| FDR | <.001** | .059 | .131 | .681 | .003** | .005** | .006** | |
|
| ||||||||
| TMTA | df | 1, 2073 | 1, 2041 | 1,1107 | 1,1132 | 1,2054 | 1, 2053 | 4, 1409 |
| F | 9.39 | .72 | 1.71 | .78 | 7.04 | .25 | 2.16 | |
| P | .002** | .396 | .163 | .588 | .008** | .616 | .071 | |
| FDR | .007** | .509 | .293 | .706 | .024* | .616 | .107 | |
|
| ||||||||
| TMTB | df | 1,1937 | 1,1903 | 1,1091 | 1,1111 | 1,2052 | 1,2050 | 4, 1453 |
| F | 12.79 | 1.77 | 3.80 | 1.53 | 13.39 | 2.23 | 1.62 | |
| P | <.001** | .184 | .010* | .163 | <.001** | .135 | .168 | |
| FDR | <.001** | .221 | .026* | .219 | <.001** | .168 | .168 | |
MMSE=Mini-Mental State Examination, DS=Digit Span subtest from the Wechsler Adult Intelligence Scale-Third Edition, COWA=Benton Controlled Oral Word Association test, AVLTI=Total number of words recalled from the first five trials or Rey’s Auditory Verbal Learning Test, AVLTD=the total number of words recalled after the delayed period on Rey’s Auditory Verbal Learning Test, TMTA=Trail Making Test, Part A, TMTB=Trail Making Test, Part B.
P-value adjusted for False Discovery Rates (FDR)
Anticholinergic Use and Time.
For the first set of models, anticholinergic drug use interacted significantly with time on MMSE scores. Figure 1 highlights the model predicted mean scores, 95% upper bound confidence intervals for anticholinergic drug users, and the 95% lower confidence interval scores for non-users. As displayed in Figure 1, anticholinergic drug users’ MMSE scores declined from visits three to four, whereas the scores remained consistent for non-users. As indicated in Table 4, anticholinergic drug use interacted significantly with time on TMTB. Figure 2 highlights the model predicted mean TMTB scores along with the 95% confidence interval lower bound scores for non-users and upper bound scores for anticholinergic drug users by visit. As evident by Figure 2, non-users’ model predicted mean TMTB finish times improved at a greater pace during visit one and continued to improve from visits two to four. In contrast, anticholinergic drug users’ performance improved less than non-users from visits one to two, and model predicted mean TMTB finish times increased from visits two to four suggesting worse performance with repeated trials. In contrast to the findings for MMSE and TMTB, anticholinergic use did not interact significantly with time on Digit Span, COWA, AVLTI, AVLTD, and COWA.
Figure 1.
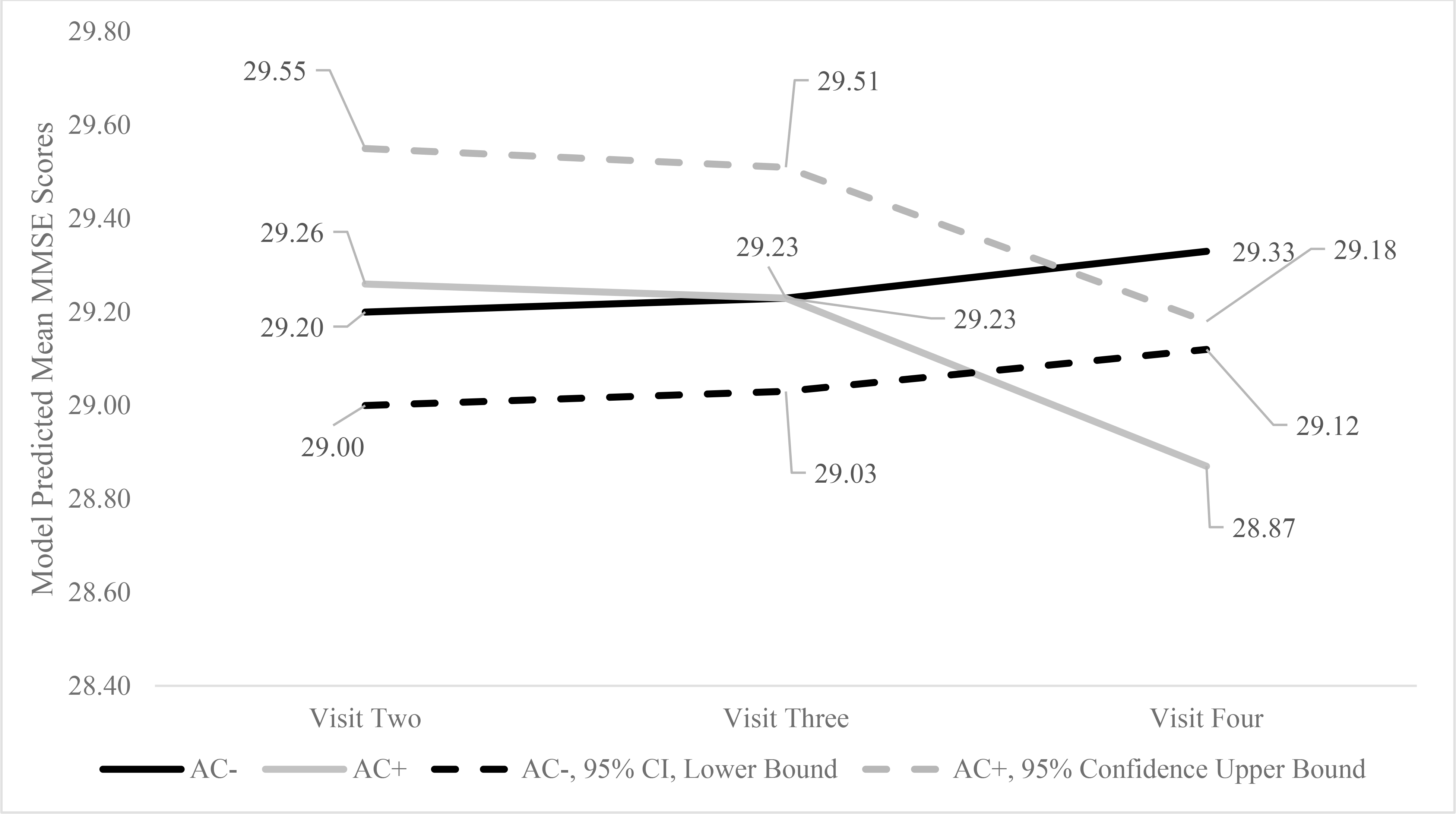
Model Predicted Mean and 95% Confidence Interval Mini Mental State Examination Scores by Anticholinergic Drug Use and Visit
Figure 2.
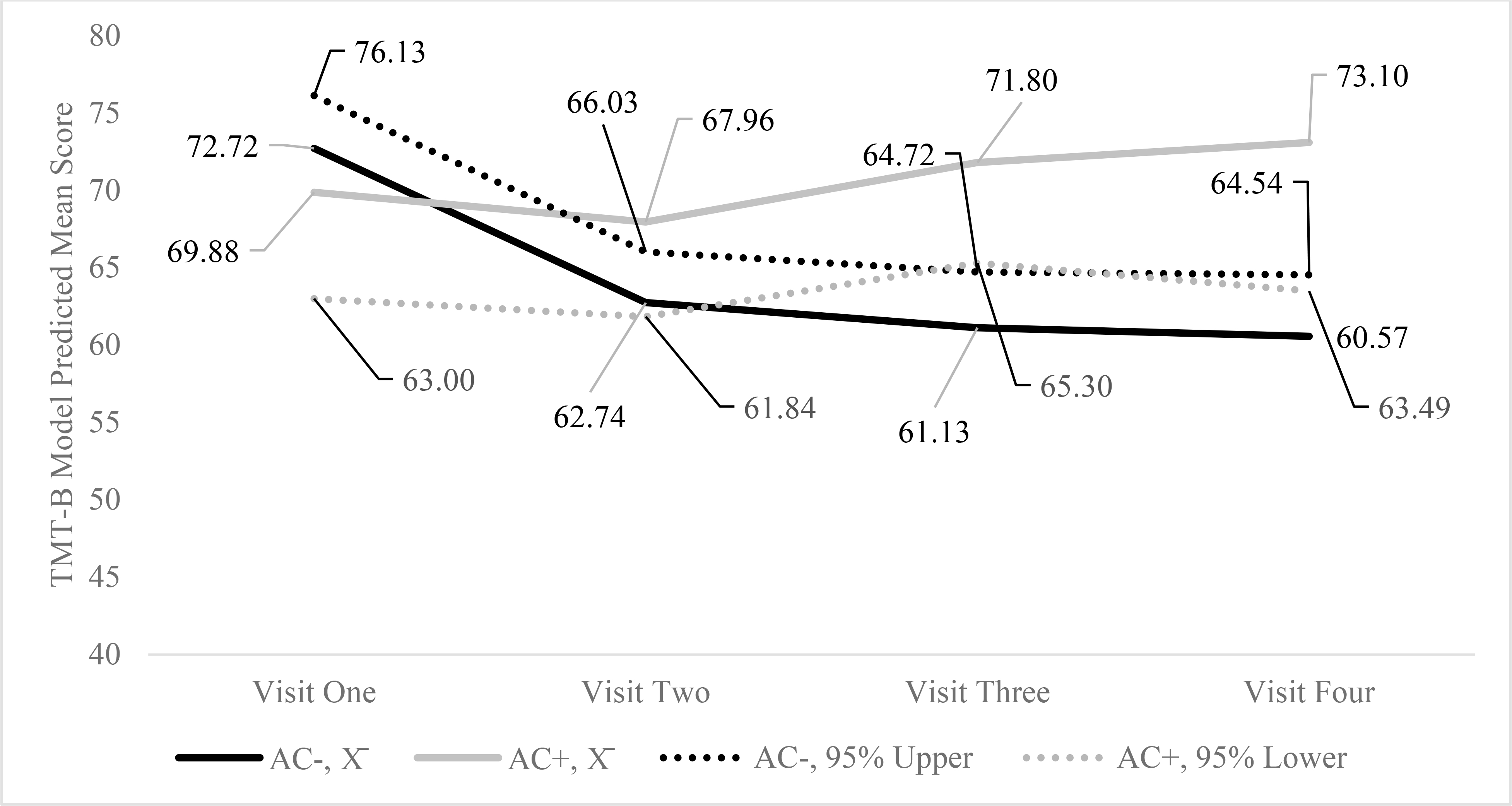
Model Predicted Mean, 95% Upper Bound for Non-users (AC−), and Lower Bound for Anticholinergic Drug Users (AC+) on the Trail Making Test, Part B16
16 Trail Making Test, Part B (TMT-B) is a timed test, so lower scores suggest better performance.
Anticholinergic Drug Use and APOε4 Carrier Status.
As evident from Table 4, anticholinergic drug use did not interact significantly with APOε4 carrier status on MMSE, Digit Span, COWA, AVLTI, TMTA, and TMTB. However, there was a significant association between AVLTD scores and APOε4 carrier status, but not after adjusting for FDR.
Anticholinergic Drug Use, APOε4 Carrier Status, and Time.
As indicated in Table 4, anticholinergic drug use did not significantly interact with APOε4 carrier status and time on performance on any of the neuropsychological tests.
Models with Change in Performance as Dependent Variable.
The second model assesses change in neuropsychological assessment scores while controlling for performance during the previous visit. Table 5 highlights the FDR corrected p-values, the model predicted mean change scores, and the 95% confidence intervals by anticholinergic drug use and APOε4 carrier status.
Table 5.
Model Two Predicted Mean Change Scores and 95% Confidence Interval Scores by Anticholinergic Drug Use10 and Apolipoprotein ε4 Carrier Status11
| Test12 | Group | Total | FDR13 | APOε4− | APOε4+ | FDR14 | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Stat15 | AC− | AC+ | AC− | AC+ | AC− | AC+ | |||
|
| |||||||||
| MMSE | −.07 | −.31 | .018* | −.01 | −.26 | −.13 | −.36 | .934 | |
|
|
|
||||||||
| L | −.34 | −.61 | −.29 | −.59 | −.40 | −.71 | |||
| H | .20 | −.01 | .26 | .08 | .14 | −.01 | |||
|
| |||||||||
| DS | .17 | −.07 | .405 | .27 | −.08 | .08 | −.06 | .636 | |
|
|
|
||||||||
| L | −.31 | −.69 | −.22 | −.77 | −.42 | −.86 | |||
| H | .65 | .55 | .76 | .62 | .57 | .73 | |||
|
| |||||||||
| COWA | .74 | 1.04 | .847 | .79 | .45 | .70 | 1.63 | .847 | |
|
|
|
||||||||
| L | −.73 | −.87 | −.72 | −1.68 | −.81 | −.80 | |||
| H | 2.21 | 2.95 | 2.30 | 2.58 | 2.21 | 4.05 | |||
|
| |||||||||
| AVLTI | −.90 | −1.71 | .294 | −1.08 | −1.34 | −.72 | −2.08 | .294 | |
|
|
|
||||||||
| L | −2.07 | −3.23 | −2.28 | −3.03 | −1.92 | −4.00 | |||
| H | .26 | −.19 | .11 | .34 | .48 | −.15 | |||
|
| |||||||||
| AVLTD | −.49 | −1.12 | .003** | −.52 | −.61 | −.46 | −1.64 | .005** | |
|
|
|
||||||||
| L | −.90 | −1.65 | −.94 | −1.20 | −.88 | −2.31 | |||
| H | −.08 | −.59 | −.10 | −.02 | −.04 | −.96 | |||
|
| |||||||||
| TMTA | −.10 | −1.72 | .024* | .83 | −1.84 | .28 | −1.60 | .616 | |
|
|
|
||||||||
| L | −1.44 | −3.46 | −1.30 | −3.78 | −1.66 | −3.82 | |||
| H | 1.24 | .03 | 1.46 | .11 | 1.10 | .62 | |||
|
| |||||||||
| TMTB | −4.94 | −10.81 | <.001** | −4.23 | −7.72 | −5.65 | −13.89 | .168 | |
|
|
|
||||||||
| L | −8.46 | −15.40 | −7.84 | −12.83 | −9.27 | −19.73 | |||
| H | −1.41 | −6.22 | −.61 | −2.62 | −2.03 | −8.05 | |||
p<.05
p<.01
AC−=Non-user, AC+=Definite Anticholinergic Drug Users
Apolipoprotein ε4 non-carrier=APOε4−, Apolipoprotein ε4 carrier=APOε4+
MMSE=Mini-Mental State Examination, DS=Digit Span subtest from the Wechsler Adult Intelligence Scale-Third Edition, COWA=Benton Controlled Oral Word Association test, AVLTI=Total number of words recalled from the first five trials or Rey’s Auditory Verbal Learning Test, AVLTD=the total number of words recalled after the delayed period on Rey’s Auditory Verbal Learning Test, TMTA=Trail Making Test, Part A, TMTB=Trail Making Test, Part B.
Adjusted p-value for False Discovery Rates (FDR) for linear mixed models assessing main effects for anticholinergic drug use on change in neuropsychological performance
Adjusted p-value for False Discovery Rates (FDR) for linear mixed models assessing interaction effects of anticholinergic drug use and apolipoprotein ε4 carrier status on change in neuropsychological assessment scores
=Linear Mixed Model Predicted Mean, L=Lower Bound 95% Confidence Interval, H=Upper Bound 95% Confidence Interval
Anticholinergic Drug Use.
As indicated in Table 4, anticholinergic use was associated with a greater decline in performance on the MMSE, AVLTD, TMTA, and TMTB than non-users. In contrast to these significant findings, there was no associations between anticholinergic drug use and change in performance on Digit Span, COWA, and AVLTI.
Anticholinergic Use and APOε4 Carrier Status.
Anticholinergic drug use interacted significantly with APOε4 carrier status on the AVLTD. Figure 3 highlights the model predicted mean and 95% confidence interval change in AVLTD scores by APOε4 carrier status and anticholinergic drug use. As indicated in Figure 3, APOε4 carrier anticholinergic drug users showed greater decline in performance over the course of the study than non-carrier, non-users; APOε4 carrier, non-users; and non-carrier, anticholinergic drug users.
Figure 3.
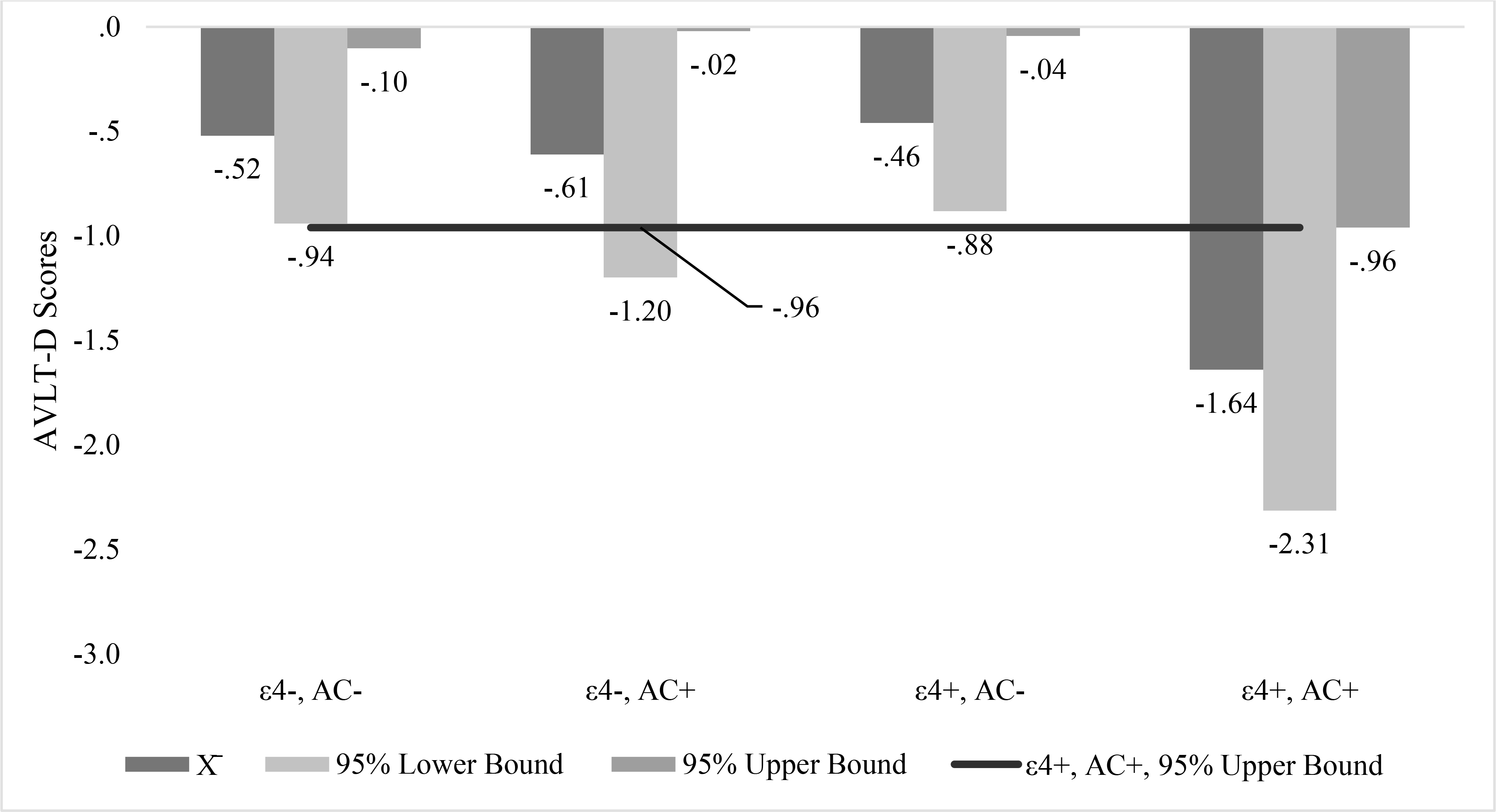
Model Predicted Mean and 95% Confidence Interval Auditory Verbal Learning Test Delayed Recall Scores for Apolipoprotein ε4 Carrier (ε4+) and Non-apolipoprotein ε4 carrier, Anticholinergic Drug Users (AC+) and Non-users (AC−)
Anticholinergic Use, APOε4 Carrier Status, and Time.
Anticholinergic drug use and APOε4 carrier status interacted significantly with time on AVLTD scores. Supplementary Figure 6 highlights the model predicted mean scores by APOε4 carrier status and the 95% upper bound confidence interval AVLTD scores for APOε4 carrier, anticholinergic drug users. As indicated in Supplementary Figure 6, APOε4 carrier, anticholinergic drug users had similar AVLTD change scores from visits one to two and greater decline from visits two to three and three to four than the other three groups.
Discussion
The primary aim of the current study was to use WRAP data to test the hypothesis that anticholinergic exposure is associated with lower and reduced performance on neuropsychological tests. As hypothesized, the findings suggested that anticholinergic drug exposure was associated with declines in cognitive performance in certain domains of cognitive functioning. In turn, a secondary aim of the study was to investigate the effects of anticholinergic drug use and exposure on different domains of cognitive functioning, which included tests of mental status, working memory, phonemic fluency, verbal learning and memory, psychomotor speed, and cognitive flexibility. The study hypothesized that anticholinergic drug use and exposure would be associated with lower and reduced performance in all measured domains of cognitive functioning. However, the results of the study did not support this hypothesis as neither anticholinergic drug use or exposure was associated with lower or reduced performance in working memory or phonemic fluency. Conversely, the results confirmed the hypothesis that anticholinergic drug use and exposure was associated with significantly greater declines in mental status, delayed memory, psychomotor speed, and cognitive flexibility. The results echo the findings of Bottiggi et al., (2006) and Lanctot et al., (2014) who found negative associations between anticholinergic use, mental status, psychomotor speed, and cognitive flexibility. The findings also parallel those of Uusvaara et al., (2013), Wouters et al., (2020), Han et al., (2008), and Papenberg et al., (2017) who found that anticholinergic drug use was associated with lower performance in verbal memory.
The cholinergic system in the basal forebrain is believed to communicate with the parietal regions, the limbic system, and the prefrontal cortex to regulate which sensory stimuli pass through the filter of selective attention into working memory (Bentley et al., 2011). Given that TMTA and TMTB may foster reliance on the cholinergic system (identifying the next appropriate item while ignoring other items), it is plausible that anticholinergic interference with these attention processes may explain anticholinergic drug users’ lower performance on tasks of psychomotor speed and cognitive flexibility. In concert with their influence on attention, cholinergic systems may play an instrumental role in encoding processes, specifically through communications between the basal forebrain, hippocampus, and entorhinal cortex (Bentley et al., 2011). As a result, the association between anticholinergic drug use and declines in delayed verbal memory performance may be due to interference with these encoding processes.
Conversely, the selective attention and encoding skills that are necessary for optimal performance on TMTA, TMTB, and AVLTD are not as essential on Digit Span and COWA, which may explain the insignificant associations between anticholinergic drug use or exposure and working memory or phonemic fluency found in this study. However, it is important to note that the lack of associations between anticholinergic use and working memory does contradict other studies (Motley et al., 2018). Specifically, studies associating acetylcholine with working memory performance focus on nicotinic receptors, while most of the medications used in this study block synaptic transmission at muscarinic receptors (Motley et al., 2018). As a result, it is possible that the type of anticholinergic medications used by the participants in this study did not have a substantial influence on working memory. The lack of negative associations between anticholinergic drug use and phonemic fluency is consistent with other studies (Pompeia et al., 2012). Although cholinergic activity enhances the encoding processes, high levels of acetylcholine have appeared antagonistic to the consolidation and retrieval processes (Bentley et al., 2011). Therefore, its plausible that by lowering levels of acetylcholine, anticholinergic drugs may, as in the case of Pompeia et al., (2012) facilitate or in the current study, at least not attenuate performance on word retrieval tasks like the COWA.
The current study adds to the literature by suggesting that anticholinergic drug use is associated with reduced mental status, executive functioning, and verbal memory performance in a population with familial risk factors for developing AD. Neuroimaging studies suggest the basal forebrain is one of the sites first impacted by neurofibrillary tangles associated with AD (Jessen et al., 2010; Liu et al., 2015). As a result, blocking cholinergic activity in the basal forebrain with long-term anticholinergic drug use may play a role in development of amyloid pathology in individuals susceptible to development of AD. However, studies suggest that anticholinergic drugs do not accelerate the process of AD, but individuals with AD may be more susceptible to the negative cognitive effects of the medications (Jessen et al., 2010).
A tertiary aim of the current study was to test the hypothesis that anticholinergic drug use and exposure would have more deleterious effects on the cognitive functioning of APOε4 carriers than non-carriers. APOε4 carrier status is a genetic risk factor for Alzheimer’s disease and has been linked, although not consistently across studies, to lower performance on tasks assessing delayed memory, psychomotor speed, and cognitive flexibility in older adults (O’Donoghue et al., 2018). Coincidentally, the findings from the current study suggested that days of drug exposure was associated with a more deleterious effect on verbal memory, psychomotor speed, and cognitive flexibility of APOε4 carriers than non-carriers. However, when anticholinergic users were compared to non-users directly, anticholinergic drug use was only associated with a decline in delayed verbal memory. Nebes et al., (2012), who found similar results, argues that APOε4 carriers’ blood brain barriers are more sensitive to the effects of anticholinergic drugs. In addition, it was stated that APOε4 carriers have less acetyltransferase enzymes than non-carriers, which may result in lower acetylcholine levels. For example, a study by Allen et al., (1997) who examined brains postmortem found that APOε4 carriers without evidence of AD had reduced acetylcholine activity than non-carriers. As a result, APOε4 carriers may be more sensitive to anticholinergic medications than non-carriers, which provides support for the hypothesis that individuals with a genetic predisposition to AD may be more likely to experience negative cognitive effects from taking anticholinergic medications.
The results of this study support the well-established finding that acetylcholine activity is important to the processes of learning and memory (Hasselmo, 2006). In addition, it should be noted that the clinical presentation of early stage AD often involves memory deficits prior to other domains of cognitive functioning (Lezak et al., 2012). In contrast to the significant findings for delayed memory, there was insufficient evidence to suggest that anticholinergic drugs affected APOε4 carriers and non-carriers differently in working memory and phonemic fluency.
The present study utilized a sample drawn from a population of individuals with a probable family history of AD. Examining the potential effect of anticholinergic medication of an at-risk population is a unique aspect of this study. Additional strengths include the study’s longitudinal design, which includes four observations over a 12 to 15-year period. Furthermore, the study addressed and controlled for non-prescription anticholinergic medication use (i.e. diphenhydramine). The study also excluded observations when subjects reported using definite anticholinergic drugs that were not used on a regular and consistent basis, other medications were also controlled for that affect cognitive functioning like acetylcholinesterase inhibitors, psychostimulants for ADHD, benzodiazepines, anti-parkinsonians, and narcotic analgesics. The study also addressed self-reported history of depression, rating of depression during each visit, accounted for multiple comparisons bias, and controlled for baseline neuropsychological assessment results. Although the current study has some key strengths, it is important to note this is not a randomized controlled study, so it is still difficult to identify causation despite the longitudinal design. In addition, there was a small number of APOε4 carriers and definite anticholinergic drug users. However, the percentage of anticholinergic drug users in the current study compared to non-users was consistent with estimates of potent anticholinergic drug use reported in other studies (Grossi et al., 2020). Furthermore, medications were self-reported so it is possible that participants forgot to report a medication during a study visit and, as a result, the sample may underrepresent anticholinergic use. The dose strength of anticholinergic medications was also not considered in the analysis. In turn, it should be noted that repeated administrations of the neuropsychological assessments can result in improvements due to practice effects. Although practice effects could not be fully addressed in the statistical models, the linear mixed models assessing change in performance controlled for scores during the earlier visit which addressed extreme scores regressing back to the mean. In turn, the study did not include measurements of amyloid or tau pathology, which should be considered in future studies assessing the effects of anticholinergic drugs on the cognitive functioning of APOε4 carriers and non-carriers. The analysis did not include educational level, however VIQ was used as a proxy for years of education since they are highly correlated (Abad et al., 2016). As highlighted in the results, there was no significant difference between anticholinergic drug users and non-users in VIQ. Furthermore, more than 99% of the sample of WRAP subjects had at least a high school diploma, which reduces the variation in years of education among the study sample. It is also important to note that even though years of education has been associated with higher premorbid scores on cognitive tests, there is insufficient evidence to suggest years of education explains or is predictive of changes in cognitive performance independent of its association with premorbid scores, which was controlled for by the current study design (Wilson et al., 2009; Nishita et al., 2013). Given all these considerations, it is unlikely that years of education explained the differences in changes in cognitive functioning between anticholinergic drug users and non-users found in this study.
Future research is needed to address the limitations of this study beginning with a larger and more diverse sample of subjects using anticholinergic medications. Given that much of the medication data was self-reported, future researchers may want to consider using medication databases that are confirmed and supplemented by self-report data. As mentioned, the dose was not considered with the current design, future research should consider measuring serum anticholinergic activity to determine anticholinergic burden rather than the ACBS (Mulsant et al., 2003). Finally, future studies should also consider giving alternative forms of neuropsychological assessment measures to help adjust for practice effects from repeated testing administrations.
Supplementary Material
Key Points.
Question: Does anticholinergic drug use influence the cognitive functioning of subjects with familial and genetic risk factors for Alzheimer’s disease?
Findings: Anticholinergic drug exposure was associated with declines in mental status, verbal learning and memory, psychomotor speed, and cognitive flexibility, especially among apolipoprotein ε4 carriers.
Importance: Medical providers may want to consider anticholinergic drug burden when prescribing definite anticholinergic drugs to patients with familial and genetic risk factors for Alzheimer’s disease.
Next Steps: Future research is needed to test a link between anticholinergic drug use and measured tau or amyloid activity in populations with increased risk for Alzheimer’s disease.
Acknowledgements
The project described was supported by the Clinical Translational Science Award (CTSA) program, though the National Center for Advancing Translational Sciences (NCATS) grant UL1TR00427. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The authors would like to thank the Wisconsin Registry for Alzheimer’s Prevention study team who were responsible for obtaining the dataset.
References
- Abad FJ, Sorrel MA, Román FJ, & Colom R (2016). The relationships between WAIS-IV factor index scores and educational level: A bifactor model approach. Psychological Assessment, 28(8), 987–1000. 10.1037/pas0000228 [DOI] [PubMed] [Google Scholar]
- Aging Brain Care. Anticholinergic Cognitive Burden scale: 2012 update. http://www.agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf.Accessed January 1, 2020.
- Allen SJ, MacGowan SH, Tyler S, Wilcock GK, Robertson AGS, Holden PH, ... & Dawbarn D (1997). Reduced cholinergic function in normal and Alzheimer’s disease brain is associated with apolipoprotein E4 genotype. Neuroscience letters, 239(1), 33–36. [DOI] [PubMed] [Google Scholar]
- Ancelin M, Artero S, Portet F, Dupuy A, Touchon J, & Ritchie K (2006). Non-Degenerative Mild Cognitive Impairment In Elderly People And Use Of Anticholinergic Drugs: Longitudinal Cohort Study. BMJ: British Medical Journal, 332(7539), 455–458. Retrieved April 28, 2020, from www.jstor.org/stable/25456217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attoh-Mensah E, Loggia G, Schumann-Bard P, Morello R, Descatoire P, Marcelli C, & Chavoix C (2020). Adverse Effects of Anticholinergic Drugs on Cognition and Mobility: Cutoff for Impairment in a Cross-Sectional Study in Young–Old and Old–Old Adults. Drugs & Aging, 37(4), 301–310. 10.1007/s40266-019-00743-z [DOI] [PubMed] [Google Scholar]
- Azur MJ, Stuart EA, Frangakis C, & Leaf PJ (2011). Multiple imputation by chained equations: what is it and how does it work?. International journal of methods in psychiatric research, 20(1), 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Yekutieli D (2005). False discovery rate–adjusted multiple confidence intervals for selected parameters. Journal of the American Statistical Association, 100(469), 71–81. [Google Scholar]
- Bentley P, Driver J, & Dolan RJ (2011). Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Progress in neurobiology, 94(4), 360–388. 10.1016/j.pneurobio.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. de S., & Sivan AB (1994). Multilingual Aphasia Examination. Iowa City: AJA Associates. [Google Scholar]
- Bottiggi KA, Salazar JC, Yu L, Caban-Holt AM, Ryan M, Mendiondo MS & Schmitt FA (2006). Long-Term Cognitive Impact of Anticholinergic Medications in Older Adults. American Journal of Geriatric Psychiatry, 14(11), 980–984. doi: 10.1097/01.JGP.0000224619.87681.71. [DOI] [PubMed] [Google Scholar]
- Britt DM, & Day GS (2016). Over-prescribed medications, under-appreciated risks: a review of the cognitive effects of anticholinergic medications in older adults. Missouri medicine, 113(3), 207. [PMC free article] [PubMed] [Google Scholar]
- Cai X, Campbell N, Khan B, Callahan C, & Boustani M (2013). Long-term anticholinergic use and the aging brain. Alzheimer’s & dementia : the journal of the Alzheimer’s Association, 9(4), 377–385. 10.1016/j.jalz.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NL, Lane KA, Gao S, Boustani MA, & Unverzagt F (2018). Anticholinergics influence transition from normal cognition to mild cognitive impairment in older adults in primary care. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 38(5), 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Bali V, Carnahan RM, Chen H, Johnson ML, & Aparasu RR (2016). Anticholinergic medication use and risk of fracture in elderly adults with depression. Journal of the American Geriatrics Society, 64(7), 1492–1497. [DOI] [PubMed] [Google Scholar]
- Chuang YF, Elango P, Gonzalez CE, & Thambisetty M (2017). Midlife anticholinergic drug use, risk of Alzheimer’s disease, and brain atrophy in community-dwelling older adults. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 3(3), 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer AH, Murphy C, Segurado R, Lawlor B, Kennelly SP, & NILVAD Study Group. (2020). Is Ongoing Anticholinergic Burden Associated With Greater Cognitive Decline and Dementia Severity in Mild to Moderate Alzheimer’s Disease?. The Journals of Gerontology: Series A, 75(5), 987–994. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Robins LN, & Helzer JE (1983). The Mini-Mental State Examination. Archives of General Psychiatry, 40(7), 812. [DOI] [PubMed] [Google Scholar]
- Fortin M-P, Rouch I, Dauphinot V, Gédéon C, Genthon S, Bonnefoy M, & Krolak-Salmon P (2011). Effects of anticholinergic drugs on verbal episodic memory function in the elderly: A retrospective, cross-sectional study. Drugs & Aging, 28(3), 195–204. 10.2165/11586580-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Fox C, Livingston G, Maidment ID, Coulton S, Smithard DG, Boustani M, & Katona C (2011). The impact of anticholinergic burden in Alzheimer’s dementia-the LASER-AD study. Age and ageing, 40(6), 730–735. [DOI] [PubMed] [Google Scholar]
- Grossi C, Richardson K, Savva G, Fox C, Arthur A, Loke Y, ... & Myint P (2020). Increasing prevalence of anticholinergic medication use in older people in England over 20 years: Cognitive Function and Ageing Study I and II. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Agostini JV, & Allore HG (2008). Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. Journal of the American Geriatrics Society, 56(12), 2203–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME (2006). The role of acetylcholine in learning and memory. Current opinion in neurobiology, 16(6), 710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM. (2019). SPSS Statistics Version 26. Armonk, NY: IBM Corp. [Google Scholar]
- Jessen F, Kaduszkiewicz H, Daerr M, Bickel H, Pentzek M, Riedel-Heller S, Wagner M, Weyerer S, Wiese B, van den Bussche H, Broich K, & Maier W (2010). Anticholinergic drug use and risk for dementia: Target for dementia prevention. European Archives of Psychiatry and Clinical Neuroscience, 260(Suppl 2), S111–S115. 10.1007/s00406-010-0156-4 [DOI] [PubMed] [Google Scholar]
- Johnson SC, Koscik RL, Jonaitis EM, Clark LR, Mueller KD, Berman SE, ... & Asthana S (2018). The Wisconsin Registry for Alzheimer’s Prevention: a review of findings and current directions. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 10, 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctôt KL, O’Regan J, Schwartz Y, Swardfager W, Saleem M, Oh PI, & Herrmann N (2014). Assessing cognitive effects of anticholinergic medications in patients with coronary artery disease. Psychosomatics, 55(1), 61–68. [DOI] [PubMed] [Google Scholar]
- Lechevallier-Michel N, Molimard M, Dartigues JF, Fabrigoule C, & Fourrier-Réglat A (2005). Drugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID Study. British journal of clinical pharmacology, 59(2), 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M, Howieson D, Bigler E, & Tranel D (2012). Neuropsychological Assessment - Fifth Edition. New York, NY: Oxford University Press. [Google Scholar]
- Lim R, Ellett LMK, Widagdo IS, Pratt NL, & Roughead EE (2019). Analysis of anticholinergic and sedative medicine effects on physical function, cognitive function, appetite and frailty: a cross-sectional study in Australia. BMJ open, 9(9), e029221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limback-Stokin MM, Krell-Roesch J, Roesler K, Hansen A, Stonnington CM, Temkit MH, ... & Geda YE (2018). Anticholinergic Medications and Cognitive Function in Late Midlife. Alzheimer Disease & Associated Disorders, 32(3), 262–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AK, Chang RC, Pearce RK, & Gentleman SM (2015). Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta neuropathologica, 129(4), 527–540. 10.1007/s00401-015-1392-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum ZA, Wirtz HS, Pettinger M, LaCroix AZ, Carnahan R, Cauley JA, Bea JW, & Gray SL (2016). Anticholinergic medication use and falls in postmenopausal women: findings from the women’s health initiative cohort study. BMC geriatrics, 16, 76. 10.1186/s12877-016-0251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ (2009). A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. Journal of psychiatric research, 43(4), 411–431. [DOI] [PubMed] [Google Scholar]
- Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, ... & Buennagel DP (2020). APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature, 581(7806), 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley SE (2018). Relationship Between Neuromodulation and Working Memory in the Prefrontal Cortex: It’s Complicated. Frontiers in neural circuits, 12, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulsant Benoit H., et al. “Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance.” Archives of General Psychiatry 60.2 (2003): 198–203. [DOI] [PubMed] [Google Scholar]
- Naharci MI, Cintosun U, Ozturk A, Oztin H, Turker T, Bozoglu E, & Doruk H (2017). Effect of anticholinergic burden on the development of dementia in older adults with subjective cognitive decline. Psychiatry and Clinical Psychopharmacology, 27(3), 269–276. 10.1080/24750573.2017.1358130 [DOI] [Google Scholar]
- Nebes RD, Pollock BG, Perera S, Halligan EM, & Saxton JA (2012). The greater sensitivity of elderly APOE ε4 carriers to anticholinergic medications is independent of cerebrovascular disease risk. The American journal of geriatric pharmacotherapy, 10(3), 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita Y, Tange C, Tomida M, Ando F, & Shimokata H (2013). Does high educational level protect against intellectual decline in older adults?: A 10-year longitudinal study. Japanese Psychological Research, 55(4), 378–389. 10.1111/jpr.12028 [DOI] [Google Scholar]
- O’Donoghue MC, Murphy SE, Zamboni G, Nobre AC, & Mackay CE (2018). APOE genotype and cognition in healthy individuals at risk of Alzheimer’s disease: A review. Cortex, 104, 103–123. [DOI] [PubMed] [Google Scholar]
- Pfistermeister B, Tümena T, Gaßmann KG, Maas R, & Fromm MF (2017). Anticholinergic burden and cognitive function in a large German cohort of hospitalized geriatric patients. PLoS One, 12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenberg G, Bäckman L, Fratiglioni L, Laukka EJ, Fastbom J, & Johnell K (2017). Anticholinergic drug use is associated with episodic memory decline in older adults without dementia. Neurobiology of aging, 55, 27–32. [DOI] [PubMed] [Google Scholar]
- Pompeia S, Rusted JM, & Curran HV (2002). Verbal fluency facilitated by the cholinergic blocker, scopolamine. Human Psychopharmacology: Clinical and Experimental, 17(1), 51–59. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement, 1(3), 385–401. [Google Scholar]
- Reitan RM (1958). Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and motor skills, 8(3), 271–276. [Google Scholar]
- Richardson K, Fox C, Maidment I, Steel N, Loke YK, Arthur A, Myint PK, Grossi CM, Mattishent K, Bennett K, Campbell NL, Boustani M, Robinson L, Brayne C, Matthews FE, & Savva GM (2018). Anticholinergic drugs and risk of dementia: Case-control study. BMJ: British Medical Journal, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M (1996). Rey auditory verbal learning test: A handbook (p. 1996). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Uusvaara J, Pitkala KH, Kautiainen H, Tilvis RS, & Strandberg TE (2011). Association of Anticholinergic Drugs with Hospitalization and Mortality among Older Cardiovascular Patients A Prospective Study. Drugs & Aging, 28(2), 131–138. 10.2165/11585060-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997). Wechsler Adult Intelligence Scale – Third Edition. San Antonio, Texas: The Psychological Corporation. [Google Scholar]
- Wechsler D (1999). Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation: Harcourt Brace & Company. New York, NY. [Google Scholar]
- Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, & Evans DA (2009). Educational attainment and cognitive decline in old age. Neurology, 72(5), 460–465. 10.1212/01.wnl.0000341782.71418.6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters H, Hilmer SN, Gnjidic D, Campen JPV, Teichert M, Meer HGVD, Schaap LA, Huisman M, Comijs HC, Denig P, Lamoth CJ, Taxis K, Van Campen JP, & Van Der Meer HG (2020). Long-Term Exposure to Anticholinergic and Sedative Medications and Cognitive and Physical Function in Later Life. Journals of Gerontology Series A: Biological Sciences & Medical Sciences, 75(2), 357–365. 10.1093/gerona/glz019 [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang C, Hung C, Chen L, Lin M, Wang P, & Chen L (2017). Association between using medications with anticholinergic properties and short-term cognitive decline among older men: A retrospective cohort study in Taiwan. Geriatrics & Gerontology International, 17(Suppl 1), 57–64. 10.1111/ggi.13032 [DOI] [PubMed] [Google Scholar]
- Ziad A, Olekhnovitch R, Ruiz F, Berr C, Bégaud B, Goldberg M, Zins M, & Mura T (2018). Anticholinergic drug use and cognitive performances in middle age: findings from the CONSTANCES cohort. Journal of Neurology, Neurosurgery, And Psychiatry, 89(10), 1107–1115. 10.1136/jnnp-2018-318190 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


