Supplemental Digital Content is available in the text.
Keywords: latent class analysis, mitral valve, postoperative pain, recovery of function
Abstract
Background:
Postoperative pain after cardiac surgery is a significant problem, but studies often report pain value as an average of the study cohort, obscuring clinically meaningful differences in pain trajectories. We sought to characterize heterogeneity in postoperative pain experiences.
Methods:
We enrolled patients undergoing a cardiac surgery at a tertiary care center between January 2019 and February 2020. Participants received an electronically-delivered questionnaire every 3 days for 30 days to assess incision site pain level. We evaluated the variability in pain trajectories over 30 days by the cohort-level mean with confidence band and latent classes identified by group-based trajectory model. Group-based trajectory model estimated the probability of belonging to a specific trajectory of pain.
Results:
Of 92 patients enrolled, 75 provided ≥3 questionnaire responses. The cohort-level mean showed a gradual and consistent decline in the mean pain level, but the confidence bands covered most of the pain score range. The individual-level trajectories varied substantially across patients. Group-based trajectory model identified 4 pain trajectories: persistently low (n=9, 12%), moderate declining (initially mid-level, followed by decline; n=26, 35%), high declining (initially high-level, followed by decline; n=33, 44%), and persistently high pain (n=7, 9%). Persistently high pain and high declining groups did not seem to be clearly distinguishable until approximately postoperative day 10. Patients in persistently low pain trajectory class had a numerically lower median age than the other 3 classes and were below the lower confidence band of the cohort-level approach. Patients in the persistently high pain trajectory class had a longer median length of hospital stay than the other 3 classes and were often higher than the upper confidence band of the cohort-level approach.
Conclusions:
We identified 4 trajectories of postoperative pain that were not evident from a cohort-level mean, which has been a common way of reporting pain level. This study provides key information about the patient experience and indicates the need to understand variation among sites and surgeons and to investigate determinants of different experience and interventions to mitigate persistently high pain.
What Is Known
Evolution of patient experience, such as pain, after cardiac surgery has not been well characterized and an optimal way to summarize and report complex time-series data is unclear.
Digital platforms hold promise in capturing patient experiences, but the feasibility of capturing postoperative experience after cardiac surgery is limited.
What the Study Adds
This study demonstrated that capturing patients’ postoperative experience at a frequent interval (every 3 days) is feasible using a digital platform.
We demonstrated that patients may follow 4 dominant trajectories of pain within 30 days of cardiac surgery.
The most concerning trajectory of persistent pain became distinguishable around postoperative day 10, informing the potential timing for a selective follow-up to optimize the pain experience.
Postoperative pain is an outcome important to patients1 and physicians2 as poorly controlled pain may result in prolonged rehabilitation, reduced activity level, and complications.3,4 Although it is expected that pain level evolves after surgery, there is great variability in how pain has been studied in terms of assessment frequency and duration,5 making it difficult to draw a collective inference. Additionally, such studies commonly report pain level as an average of the entire study cohort,5,6 potentially obscuring individual variations in the longitudinal experience of pain and identification of patient characteristics denoting those with either desirable or concerning progression of pain level. A more granular understanding of pain trajectories can guide judicious pain management strategies at hospital discharge and inform individualized timing of postoperative visits. Electronic platform to measure patients’ pain level may enable frequent measurement even beyond hospital discharge to provide novel insight.1
We aimed to characterize heterogeneity of pain trajectories and explore clinical characteristics of patients with persistently low and high pain over time, using longitudinal data of patient-reported postoperative pain after cardiac surgery captured using electronic platform. We also aimed to assess how measuring responses more frequently than prior studies may relate to precisely capturing patients’ pain experience.
Methods
The de-identified data that support the findings of this study are available from the first author upon reasonable request at makoto.mori@yale.edu.
Patient Selection Criteria and Data Source
We studied a convenience sample of patients who underwent cardiac surgery at Yale New Haven Hospital between January 2019 and March 2020. Postoperative, as opposed to preoperative, enrollment allowed us to enroll nonelective cases. Inclusion criteria were patients undergoing isolated or concomitant coronary artery bypass graft, aortic valve replacement, mitral valve replacement, mitral valve repair, or aortic operation who were discharged from the intensive care unit within 5 days of the operation. This 5-day threshold ensured that time of initiation of pain assessments would be standardized, as patients could not be enrolled while in the intensive care unit. Patients were enrolled with written informed consent upon discharge from the intensive care unit after surgery. Because we did not have access to language translation services for research purposes, patients were not considered a candidate if they did not speak or read English. We excluded those who do not own a smartphone or a tablet because the electronic platform for patient-reported outcome measure data collection relied on patients responding to surveys displayed on a web browser. Although the survey link was sent via email or text, and the patients could, in theory, respond to the question via a computer without using a smartphone, patients who did not own a smartphone rarely brought a computer to the hospital. Therefore, collecting predischarge responses via computer in this patient group was deemed challenging and we excluded them. Despite the need for these exclusion criteria, we chose to use the electronic platform for the automated electronic delivery of surveys that allowed for seamless collection of patient-reported outcome measure data even after the patient’s hospital discharge. We also excluded those who could not complete the enrollment process (Figure 1). Details of the protocol have been published.7 The cardiac surgery service did not have a formalized Enhanced Recovery After Surgery pathway at the time of the study. Pain regimens were individualized to the patients’ needs during the hospitalization and at the time of discharge. The Yale Institutional Review Board approved the study.
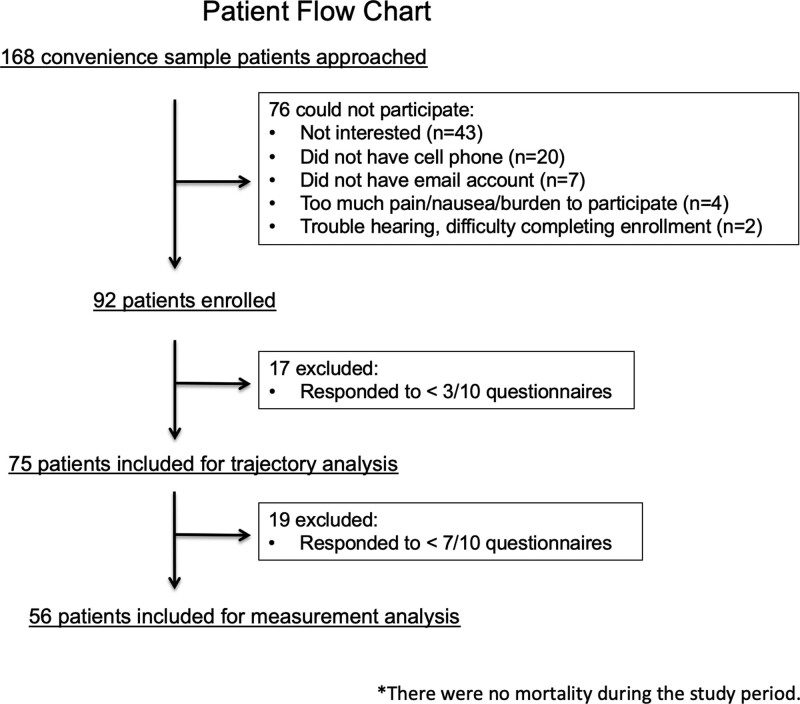
Figure 1.
CONSORT-style patient flow chart. The figure shows the flow of patient exclusion and enrollment. We included 75 patients for trajectory analysis and 56 patients for analysis evaluating the optimal interval and number of measurements. There was no mortality during the study period.
Questionnaire and Data Collected
Quality of Recovery, a 24-item questionnaire assessing postoperative recovery8–10 adapted from the original QoR-40,11 was emailed every 3 days for 30 days. The questionnaire item for pain read “During the last 24 hours, I have been having pain in the surgical wound,” with possible responses ranging from 0 to 10 with 0 corresponding to “none of the time” and 10 corresponding to “all of the time.” Variables describing patient characteristics were prespecified in the protocol article7 and were collected via the institutional Society of Thoracic Surgeon’s Adult Cardiac Surgery Database using the data version 2.91 definitions. Prescription of opioid medication at the time of hospital discharge was collected via chart review and standardized to morphine milligram equivalents.
Statistical Analysis
We evaluated the variability in pain trajectories over 30 days by visualizing the plot by (1) individual, (2) cohort-level mean, and (3) latent class group-based trajectory model. For this step, we excluded patients with fewer than 3 responses to as done previously to minimize the influence of extremely sparse observations.2 We applied a group-based trajectory model, a family of latent class analysis, which estimated the probability of belonging to a specific trajectory of pain.12,13 This is a semiparametric finite mixture model for longitudinal data using a maximum likelihood method fitting the pain score with a censored normal distribution. We fitted the model from 1 to 5 trajectories with polynomial order of up to a cubic term. Attrition from the study was not modeled together, as there was no mortality during the study period.
We determined the optimal number of trajectory classes based on the Bayesian information criterion and average posterior probability of assignment (>0.9 indicated excellent fit and <0.7 indicated poor fit) among the models with 1 to 5 trajectory classes and incrementally increasing the polynomial order.14 Resulting trajectory classes were labeled with simple terms that authors deemed to capture the main trajectory features. We used adjectives such as “high” and “low” to describe the pain trajectory relative to other trajectory classes.
Representativeness and Number of Measurements
We tested whether increasing the number of measurements improves the representativeness of pain level. Specifically, we compared the average pain level for each patient based on k number of measurements, against the reference of individual’s average pain level over 10 measurements. This analysis was performed on 56 patients who responded to ≥7 surveys. In this subgroup, pain level was missing in 16% (93/560) of the surveys. The difference between patient-level average of pain score over 10 measurements was compared with the patient-level average of pain score over k measurements and the difference was plotted along the k measurement to visualize the relationship between the number of measurement and representativeness of the measurement.
Missing Data
For the analysis of representativeness of pain measurement, we used data from those who responded to ≥7 surveys. We used a higher threshold for survey response in this analysis to minimize bias introduced by imputing missing values. We used linear interpolation to estimate the missing value for missing responses, because in this dataset, missing data were sparse across the time-series.15 Such imputation was not used for the trajectory model that used observations with ≥3 responses, as group-based trajectory model’s full information maximum likelihood estimation allowed for integration of all available information based on missing-at-random assumption.16 Missing data for the Society of Thoracic Surgeon’s data occurred in <2% of participants and missing values were conditionally estimated as described by Shahian et al17 in the Society of Thoracic Surgeon’s risk model development, classifying missing values to those in the lowest risk category for categorical variables and using age and sex-specific means for continuous variables.
We did not compare groups in terms of statistically significant differences because of the limited sample size. We used Traj package for a group-based trajectory model and calculated k means via Proc Univariate procedure in SAS 9.4 (SAS Institute, Inc Cary, NC). We used Python 3.8 for data preprocessing including linear interpolation.
Results
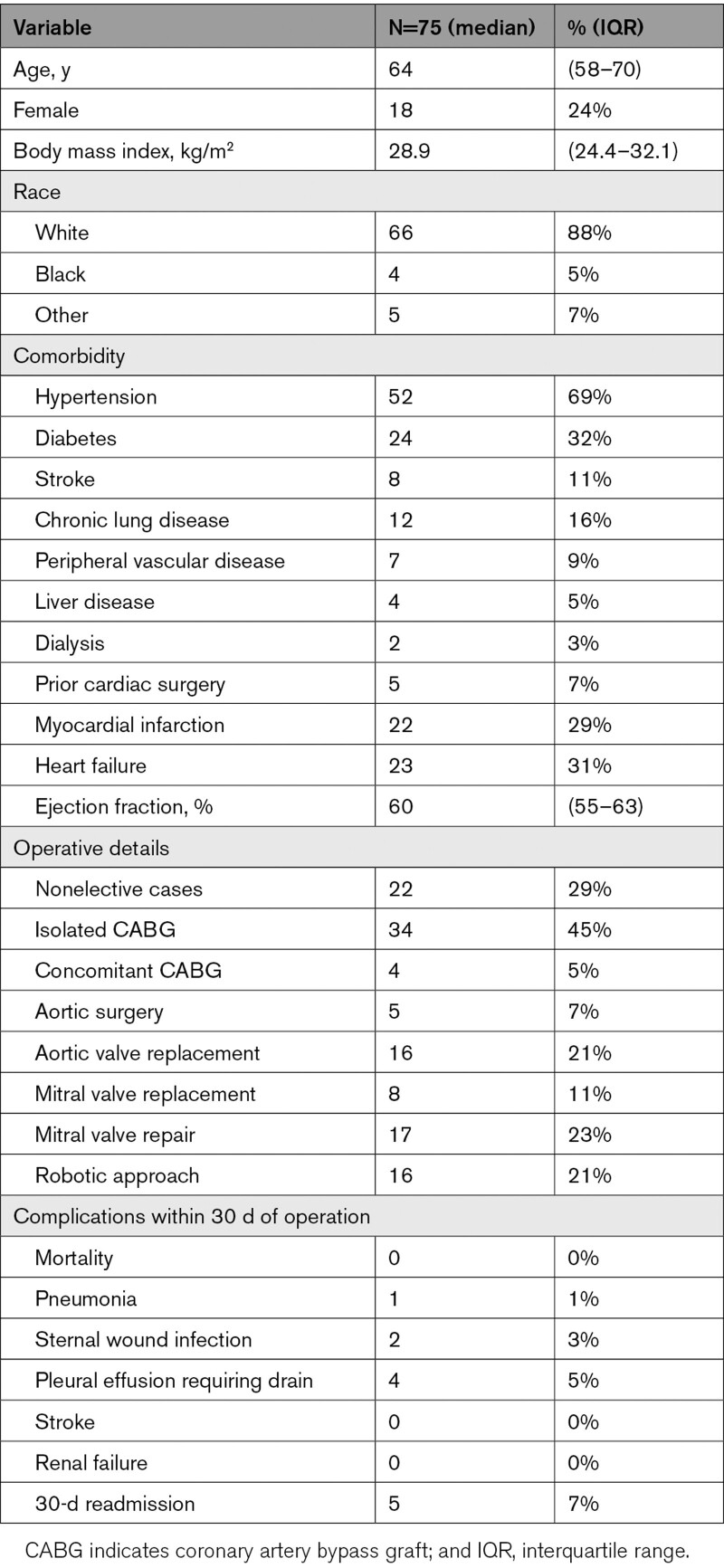
Of 92 patients enrolled, there were 75 (82%) with ≥3 responses and 56 (61%) with ≥7 responses. Characteristics of the 75 patients are summarized in Table 1. The characteristics of patients did not differ significantly between those included and excluded based on completing ≥3 responses, except the frequencies of coronary artery bypass graft operations, which was more frequent in the included patients (Table I in the Data Supplement). The median age of the patients was 64 (interquartile range: 58–70) years with 57 (76%) men, and 66 (88%) White participants. Thirty-four patients underwent isolated coronary artery bypass graft, which was the most common case type.
Table 1.
Patient Characteristics
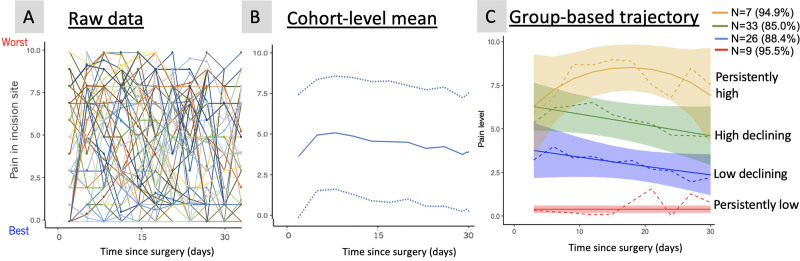
In 75 patients with ≥3 responses, we observed that individual pain scores varied substantially across patients with no dominant mean or pattern (Figure 2A). Cohort-level mean and 95% CI (Figure 2B) showed a gradual and consistent decline in the mean pain level over time, but the confidence bands covered most of the pain score range. Based on the best BIC value (Table I in the Data Supplement), the group-based trajectory model identified 4 trajectories (Figure 2C), all of which had a posterior probability of assignment of 0.85 or higher. We labeled the trajectories according to the observed pattern, with terms high and low referring to the pain level relative to other trajectory classes: persistently low (n=9, 12%), moderate declining (n=26, 35%), high declining (n=33, 44%), and persistently high pain (n=7, 9%). Persistently high pain and high declining groups did not seem to be clearly distinguishable until the third measurement of ≈10 days.
Figure 2.
Various representations of the same longitudinal pain data (n=75). Trajectories of pain 30 days after surgery, at individual level (A), cohort-level (B) mean (solid line) and 95% CI (dotted lines), and by latent class (C). In (C), percentage values in the parenthesis indicate the mean probability of the classified patients belonging to the particular class, dotted lines are the observed mean pain level in each class, solid lines represent fitted lines, and colored band represents 95% CI. The figure shows that cohort-level mean oversimplifies variable trajectories, and the latent class model may offer an interpretable representation of various trajectories.
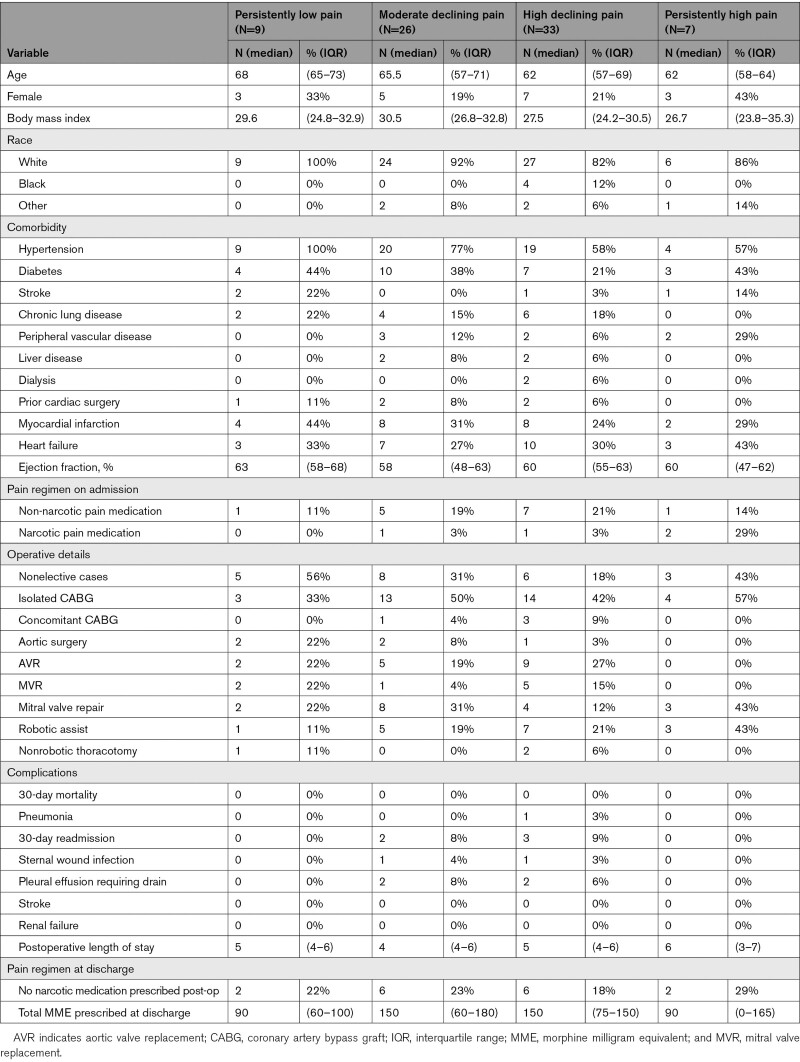
Comparing patient, operative, and postoperative characteristics in the 4 assigned trajectories, patients in the persistently low pain trajectory class were older (numerically higher median age) than the other 3 classes. The proportions of patients who underwent robotic-assisted surgery were 1 (11%) in persistently low, 5 (19%) in moderate declining, 7 (21%) in high declining, and 3 (43%) in persistently high pain trajectory classes. Patients in the persistently high pain trajectory class had a numerically higher median length of hospital stay than the other 3 classes. The proportion of patients who were not prescribed any opioid medications at the time of hospital discharge were 2 (22%) in persistently low, 6 (23%) in moderate declining, 6 (18%) in high declining, and 2 (29%) in persistently high pain trajectory classes.
Median morphine milligram equivalent of narcotics prescribed at the time of discharge were 90 (interquartile range: 60–100 mg) in persistently low, 150 (interquartile range: 60–180 mg) in moderate declining, 150 (interquartile range: 75–150 mg) in high declining, and 90 (interquartile range: 0–s165 mg) in persistently high pain trajectory classes (Table 2).
Table 2.
Patient Characteristics by Trajectory Classes
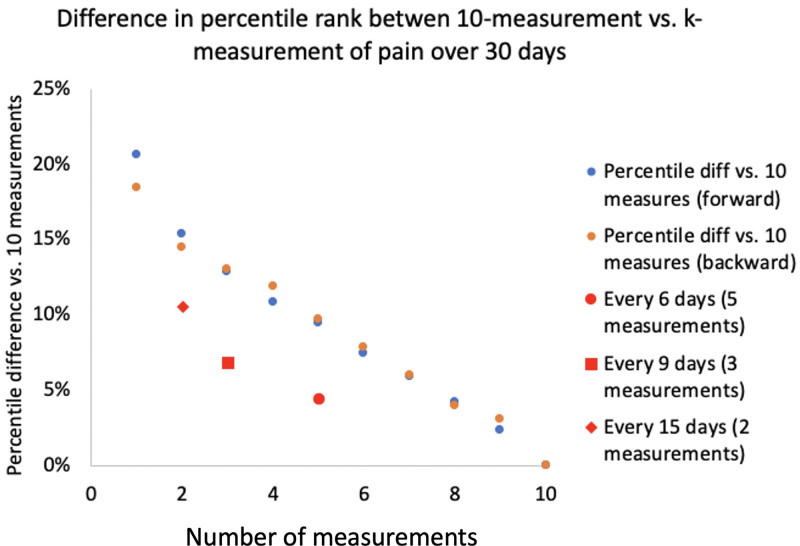
Compared with the patient-level mean of pain score over 10 measurements as a reference, the pain level determined by the first measurement alone differed by 2.1 points on average within the same patient. The pain level determined by the last measurement alone differed by 1.9 points. Increasing the number of measurements decreased the difference incrementally. Obtaining measurements every 6, 9, and 15 days for a total of 5, 3, and 2 measurements was associated with 0.5, 0.7, and 1.0 point differences from the reference, respectively (Figure 3).
Figure 3.
Representativeness of pain experience characterized via variable number of measurements. Difference in patient-level pain scale ranging 0 to 10 (y axis) over 10 measurements vs over k measurements (x axis). Using only one measurement of pain at the beginning or end of the measurement yielded approximately 2-point differences when compared with average pain level of 10 measurements over 30 d. Blue circles are counting number of measurements from the first postoperative survey and orange circles are counting from the last survey. This difference diminished incrementally with every 15, 9, and 6 d of measurements.
Discussion
Using a longitudinal patient-reported pain measure collected up to 30 days after cardiac surgery, we identified distinct trajectories of reported pain. These results reveal that people experience very different recoveries after cardiac surgery. Revealing this heterogeneity provides an impetus to understand the determinants of different outcomes and targets of intervention to ensure that more people have less pain. It also provides the possibility of better informing patients about what the recovery experience might entail. To date, little attention has focused on quantifying the variations in the way that pain tracks through the early recovery period.
The heterogeneity of the identified pain trajectories is important because pain and other postoperative recovery domains have mostly been reported as cohort-level average even in studies that measured pain at multiple postoperative time points.18–20 Therefore, the dominant patterns of trajectories and a heterogeneous patient experience after cardiac surgery may be obscured by focusing on average effects.5 This study highlights the opportunity to identify factors that may be related to distinct pain trajectories. These may include factors at the patient, surgeon, and site levels. It also highlights the potential for studying heterogeneity in trajectories of other postoperative outcomes, including mobility, energy level and return to baseline activities.
This study adds to the literature on trajectories of pain following surgery in several notable ways. First, prior studies have included thoracotomy, orthopedic, and general surgery.21–23 Therefore, this study uniquely highlights pain trajectories after cardiac surgery that may differ from noncardiac surgery due to sternotomy and less-invasive approaches. Second, prior studies focused on a much longer timescale with the follow-up at 1 year after the operation.21–23 Our granular measurement within the shorter, 30-day postoperative window may offer different opportunities for timely interventions. Given the current opioid epidemic and increased attention to judicious postoperative narcotics prescription,24 recognizing the pain trajectory variations in the immediate postoperative period may promote more judicious and individualized narcotics prescription. Individualized opioid regimen is especially important in the postoperative period where the risk of opioid misuse is high.3 Given the growing population of patients undergoing valve surgery due to endocarditis in the setting of injection drug use, information to guide pain management after surgery is especially relevant.25,26
Our study highlights the utility of characterizing postoperative recovery and proposes an underutilized approach to measuring and reporting recovery. Because remote patient monitoring can be reimbursed with recent expansion of the rules,4 our study offers timely insights into the frequency of measurements needed to adequately capture patient experience. We demonstrated that relying on a single point of measurement may insufficiently represent a patient’s recovery experience and increasing the number of measurements incrementally improved the representation. In summarizing such data, the cohort-level average of pain level over time alone limits information meaningful to patients and surgeons, obscuring important variations among patients. For example, it would be less informative to patients to know that on average, pain level after cardiac surgery will mostly be between 2 and 8 points around 10 days after the operation than to know that given the first few measurements of pain, the patient is likely to have persistently high or low pain. It is important to recognize that, albeit a small study, we identified this substantial variation among this relatively homogenous patient group of younger, mostly White patients, and male patients.
Although the small sample size limited evaluation of associations between patient characteristics and each trajectory class of pain, it is notable that patients in the persistently high pain trajectory did not differ substantially according to opioid prescription at discharge, readmission, or sternal wound infection within 30 days. The total morphine milligram equivalent of narcotics prescribed was numerically lower compared with low or high-declining groups, but the median value of 90 was equivalent to that of persistently low pain group. Understanding associations of such potentially relevant factors and the pain trajectories require further studies.
Limitations
The single-center design of our study may limit the generalizability of our findings, although the variation in the phenotype of pain trajectories may be a finding applicable to practices in care settings different from ours. Despite the relative homogeneity of our patient group in terms of demographics, low comorbidity profile, and low complication rates, we observed considerable variation in the trajectories of pain. Generalizability of such variation in the trajectories to different patient groups must be evaluated in future studies. The number of approached patients were lower than the number of cases usually performed at our hospital because of the coronavirus disease 2019 (COVID-19) pandemic, gradual acceleration of the enrollment rate at the beginning of the study, and the part-time availability of the research assistant to complete the enrollment. Nevertheless, the bias was toward a more homogeneous sample and yet our results reveal marked heterogeneity in patient experience even among this selected cohort. The sample size limited our ability to make more robust inference for characteristics associated with specific recovery trajectory, including multivariable analysis and evaluation of the longitudinal change in pain regimen including interactions with pain medications and comorbidities. As expected, many patients did not complete all 10 delivered surveys. We delivered a high number of surveys to capture at least 3 responses for the trajectory to be modeled in the latent class analysis. The reported pain levels were the perceived pain level without adjusting for variation in adherence or the prescribed narcotic dose. Therefore, this study did not evaluate whether the observed variation in pain level is associated with variation in individual pain management approaches. Nevertheless, the heterogeneity identified irrespective of the treatment approach inform potential ways to improve postoperative pain experience, including individualized timing of postoperative follow-up.
Conclusions
After cardiac surgery, the trajectory of pain is variable within 30 days. This individual variation is not adequately captured unless multiple measurements are obtained. Cohort-level mean, a common way of reporting pain level, fails to capture this variation, while a latent class model can illustrate the heterogeneity. Studies on postoperative pain should consider the time-varying nature of pain and recognize the limitation in capturing patient experience when relying on a small number of measurements.
Sources of Funding
Dr Mori is a PhD Student in the Investigative Medicine Program at Yale, which is supported by Clinical and Translational Science Awards (CTSA) Grant Number UL1 TR001863 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Disclosures
Dr Krumholz works under contract with the Centers for Medicare & Medicaid Services to support quality measurement programs; was a recipient of a research grant, through Yale, from Medtronic and the US Food and Drug Administration to develop methods for post-market surveillance of medical devices; was a recipient of a research grant from Johnson & Johnson, through Yale University, to support clinical trial data sharing; was a recipient of a research agreement, through Yale University, from the Shenzhen Center for Health Information for work to advance intelligent disease prevention and health promotion; collaborates with the National Center for Cardiovascular Diseases in Beijing; receives payment from the Arnold & Porter Law Firm for work related to the Sanofi clopidogrel litigation, from the Martin Baughman Law Firm for work related to the Cook Celect IVC filter litigation, and from the Siegfried and Jensen Law Firm for work related to Vioxx litigation; chairs a Cardiac Scientific Advisory Board for UnitedHealth; was a member of the IBM Watson Health Life Sciences Board; is a member of the Advisory Board for Element Science, the Advisory Board for Facebook, and the Physician Advisory Board for Aetna; and is the co-founder of HugoHealth, a personal health information platform, and co-founder of Refactor Health, an enterprise healthcare AI-augmented data management company. Dr Chaudhry serves as a paid consultant for the CVS Caremark State of Connecticut Clinical Pharmacy Program. Dr Geirsson receives consulting fee for being a member of Medtronic Strategic Surgical Advisory Board. Medtronic produces valve products for valve replacement and repairs.
Supplemental Materials
Table I
Supplementary Material
Footnotes
This manuscript was sent to Marwah Abdalla, MD, MPH, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCOUTCOMES.120.007781.
For Sources of Funding and Disclosures, see page 887 & 888.
References
- 1.Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105:205–221. doi: 10.1213/01.ane.0000268145.52345.55 [DOI] [PubMed] [Google Scholar]
- 2.Green CR, Wheeler JR. Physician variability in the management of acute postoperative and cancer pain: a quantitative analysis of the Michigan experience. Pain Med. 2003;4:8–20. doi: 10.1046/j.1526-4637.2003.03006.x [DOI] [PubMed] [Google Scholar]
- 3.Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth. 2001;87:62–72. doi: 10.1093/bja/87.1.62 [DOI] [PubMed] [Google Scholar]
- 4.Sethares KA, Chin E, Costa I. Pain intensity, interference and patient pain management strategies the first 12 weeks after coronary artery bypass graft surgery. Appl Nurs Res. 2013;26:174–179. doi: 10.1016/j.apnr.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 5.Mori M, Angraal S, Chaudhry SI, Suter LG, Geirsson A, Wallach JD, Krumholz HM. Characterizing patient-centered postoperative recovery after adult cardiac surgery: a systematic review. J Am Heart Assoc. 2019;8:e013546. doi: 10.1161/JAHA.119.013546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Gulik L, Janssen LI, Ahlers SJ, Bruins P, Driessen AH, van Boven WJ, van Dongen EP, Knibbe CA. Risk factors for chronic thoracic pain after cardiac surgery via sternotomy. Eur J Cardiothorac Surg. 2011;40:1309–1313. doi: 10.1016/j.ejcts.2011.03.039 [DOI] [PubMed] [Google Scholar]
- 7.Mori M, Brooks C, Spatz E, Mortazavi BJ, Dhruva SS, Linderman GC, Grab LA, Zhang Y, Geirsson A, Chaudhry SI, et al. Protocol for project recovery after cardiac surgery: a single-center cohort study leveraging digital platform to characterise longitudinal patient-reported postoperative recovery patterns. BMJ Open. 2020;10:e036959. doi: 10.1136/bmjopen-2020-036959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaensson M, Dahlberg K, Eriksson M, Nilsson U. Evaluation of postoperative recovery in day surgery patients using a mobile phone application: a multicentre randomized trial. Br J Anaesth. 2017;119:1030–1038. doi: 10.1093/bja/aex331 [DOI] [PubMed] [Google Scholar]
- 9.Hälleberg Nyman M, Nilsson U, Dahlberg K, Jaensson M. Association between functional health literacy and postoperative recovery, health care contacts, and health-related quality of life among patients undergoing day surgery: secondary analysis of a randomized clinical trial. JAMA Surg. 2018;153:738–745. doi: 10.1001/jamasurg.2018.0672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahlberg K, Jaensson M, Eriksson M, Nilsson U. Evaluation of the Swedish Web-Version of Quality of Recovery (SwQoR): secondary step in the development of a mobile phone app to measure postoperative recovery. JMIR Res Protoc. 2016;5:e192. doi: 10.2196/resprot.5881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myles PS, Weitkamp B, Jones K, Melick J, Hensen S. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84:11–15. doi: 10.1093/oxfordjournals.bja.a013366 [DOI] [PubMed] [Google Scholar]
- 12.Mori M, Krumholz HM, Allore HG. Using latent class analysis to identify hidden clinical phenotypes. JAMA. 2020;324:700–701. doi: 10.1001/jama.2020.2278 [DOI] [PubMed] [Google Scholar]
- 13.Nagin DS, Tremblay RE. Developmental trajectory groups: fact or a useful statistical fiction? Criminology. 2005;43:873–904. doi: 10.1111/j.1745-9125.2005.00026.x [Google Scholar]
- 14.Nagin D. Group-Based Modeling of Development. 2005. Harvard University Press [Google Scholar]
- 15.Newbury J. Linear Interpolation. Basic Numeracy Skills and Practice. 1981. Macmillan Education UK; 67–72. [Google Scholar]
- 16.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413 [DOI] [PubMed] [Google Scholar]
- 17.Shahian DM, O’Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 1-coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S2–S22. doi:10.1016/j.athoracsur.2009.05.053 [DOI] [PubMed] [Google Scholar]
- 18.Diab MS, Bilkhu R, Soppa G, Edsell M, Fletcher N, Heiberg J, Royse C, Jahangiri M. The influence of prolonged intensive care stay on quality of life, recovery, and clinical outcomes following cardiac surgery: a prospective cohort study. J Thorac Cardiovasc Surg. 2018;156:1906–1915.e3. doi: 10.1016/j.jtcvs.2018.05.076 [DOI] [PubMed] [Google Scholar]
- 19.Chapman CR, Zaslansky R, Donaldson GW, Shinfeld A. Postoperative pain trajectories in cardiac surgery patients. Pain Res Treat. 2012;2012:608359. doi: 10.1155/2012/608359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milgrom LB, Brooks JA, Qi R, Bunnell K, Wuestfeld S, Beckman D. Pain levels experienced with activities after cardiac surgery. Am J Crit Care. 2004;13:116–125. [PubMed] [Google Scholar]
- 21.Gjeilo KH, Oksholm T, Follestad T, Wahba A, Rustøen T. Trajectories of pain in patients undergoing lung cancer surgery: a Longitudinal Prospective Study. J Pain Symptom Manage. 2020;59:818–828.e1. doi:10.1016/j.jpainsymman.2019.11.004 [DOI] [PubMed] [Google Scholar]
- 22.Althaus A, Arránz Becker O, Neugebauer E. Distinguishing between pain intensity and pain resolution: using acute post-surgical pain trajectories to predict chronic post-surgical pain. Eur J Pain. 2014;18:513–521. doi: 10.1002/j.1532-2149.2013.00385.x [DOI] [PubMed] [Google Scholar]
- 23.Bishop MO, Bayman EO, Hadlandsmyth K, Lund BC, Kang S. Opioid use trajectories after thoracic surgery among veterans in the United States. Eur J Pain. 2020;24:1569–1584. doi: 10.1002/ejp.1610 [DOI] [PubMed] [Google Scholar]
- 24.Brown CR, Chen Z, Khurshan F, Groeneveld PW, Desai ND. Development of persistent opioid use after cardiac surgery. JAMA Cardiol. 2020;5:889–896. doi: 10.1001/jamacardio.2020.1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geirsson A, Schranz A, Jawitz O, Mori M, Feng L, Zwischenberger BA, Iribarne A, Dearani J, Rushing G, Badhwar V, et al. The evolving burden of drug use associated infective endocarditis in the United States. Ann Thorac Surg. 2020;110:1185–1192. doi: 10.1016/j.athoracsur.2020.03.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori M, Bin Mahmood SU, Schranz AJ, Sultan I, Axtell AL, Sarsour N, Hiesinger W, Boskovski MT, Hirji S, Kaneko T, et al. Risk of reoperative valve surgery for endocarditis associated with drug use. J Thorac Cardiovasc Surg. 2020;159:1262–1268.e2. doi: 10.1016/j.jtcvs.2019.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.