Abstract
Purpose:
Prior reports suggest that renin-angiotensin system inhibition may decrease nonmuscle invasive bladder cancer recurrence. We evaluated whether angiotensin converting enzyme inhibitor or angiotensin receptor blocker treatment at initial surgery was associated with decreased recurrence or progression in patients with nonmuscle invasive bladder cancer.
Materials and Methods:
Using an institutional bladder cancer database we identified 340 patients with data available on initial transurethral resection of bladder tumor. Progression was defined as an increase to stage T2. Cox proportional hazards models were used to evaluate associations with recurrence-free and progression-free survival.
Results:
Median patient age was 69.6 years. During a median followup of 3 years (IQR 1.3–6.1) 200 patients (59%) had recurrence and 14 (4.1%) had stage progression. Of those patients 143 were receiving angiotensin converting enzyme inhibitor/angiotensin receptor blockers at the time of the first transurethral resection. On univariate analysis factors associated with improved recurrence-free survival included carcinoma in situ (p = 0.040), bacillus Calmette-Guérin therapy (p = 0.003) and angiotensin converting enzyme inhibitor/angiotensin receptor blocker therapy (p = 0.009). Multivariate analysis demonstrated that patients treated with bacillus Calmette-Guérin therapy (HR 0.68, 95% CI 0.47–0.87, p = 0.002) or angiotensin converting enzyme inhibitor/angiotensin receptor blocker therapy (HR 0.61, 95% CI 0.45–0.84, p = 0.005) were less likely to experience tumor recurrence. The 5-year recurrence-free survival rate was 45.6% for patients treated with angiotensin converting enzyme inhibitor/angiotensin receptor blockers and 28.1% in those not treated with angiotensin converting enzyme inhibitor/angiotensin receptor blockers (p = 0.009).Subgroup analysis was performed to evaluate nonmuscle invasive bladder cancer pathology (Ta, T1 and carcinoma in situ) in 85 patients on bacillus Calmette-Guérin therapy alone and in 52 in whom it was combined with angiotensin converting enzyme inhibitor/angiotensin receptor blocker. Multivariate analysis revealed that patients treated with bacillus Calmette-Guérin alone (HR 2.19, 95% CI 1.01–4.77, p = 0.04) showed worse recurrence-free survival compared to patients treated with bacillus Calmette-Guérin and angiotensin converting enzyme inhibitor/angiotensin receptor blocker (stage Ta HR 0.45, 95% CI 0.21–0.98, p = 0.04).
Conclusions:
Pharmacological inhibition of the renin-angiotensin system is associated with improved outcomes in patients with bladder cancer. Renin-angiotensin system inhibitor administration in nonmuscle invasive bladder cancer cases should be studied in a prospective randomized trial.
Keywords: urinary bladder neoplasms; neoplasm recurrence, local; renin-angiotensin system; angiotensin converting enzyme inhibitors; angiotensin receptor antagonists
LIKE most genitourinary cancers, transitional cell cancer relies on proangiogenic factors for growth and metastasis.1 Yet despite this knowledge many currently available intravesical therapies for NMIBC disregard this potential avenue of treatment. Most diagnosed bladder cancers are NMIBC and the most commonly reported pathological finding is stage Ta following TUR.2 Standard treatment of NMIBC after TUR includes intravesical instillation of chemotherapy or BCG immunotherapy.3 Despite its proven success in decreasing bladder cancer recurrence BCG failure remains a distinct possibility following an initial response according to long-term data.4 Cookson et al randomized 86 patients to TUR for NMIBC with or without BCG.4 The 15-year followup data revealed no significant difference in PFS rates (53%). BCG and intravesical therapies are pivotal for long-term treatment of NMIBC. However, the results of that study and others5 highlight the need to generate new treatment options for NMIBC that can be used alone or in conjunction with BCG.
While AT1 receptor expression has been demonstrated in bladder cancer, a recent report outlined the effect of RAS blockade on RFS.6 RAS blockade with ACE-Is or ARBs delayed time to recurrence in patients with NMIBC (Ta, T1 and CIS). This effect was seen when the medications were administered alone or in combination with BCG therapy. Therefore, we sought to validate in our data set whether inhibiting RAS with ACE-Is and ARBs would provide a clinical benefit on recurrence and progression of NMIBC.
MATERIALS AND METHODS
We reviewed our institutional NMIBC database at University of Wisconsin and identified 421 patients from 1998 to 2014. All patients were identified as having NMIBC following TUR. Patients were excluded from analysis if they had no medication history, and if they were not on RAS blockade at the time of the first TUR. After excluding 81 patients who did not meet inclusion criteria 340 were available for analysis.
Tumor recurrence was identified on subsequent TUR and confirmed after pathological review. Stage progression was defined as evidence of tumor invasion of the muscularis propria on followup TUR. CIS was defined as histologically confirmed CIS in the presence or absence of Ta/T1 tumors as defined by the EORTC (European Organisation for Research and Treatment of Cancer). Independent variables assessed included patient age; gender; diabetes mellitus type II; smoking status; tumor grade, stage and multiplicity; CIS; BCG; and intravesical therapy. Medication history, ie use of ACE-I/ARBs, was obtained from electronic medical records according to the patient medical records.
Univariate and multivariate Cox proportional hazard regression models with backward selection were used to analyze associations of variables with tumor recurrence and progression. RFS and PFS curves were generated by the Kaplan-Meier method and compared with the log rank test. Variables at p <0.05 were considered significant. Statistical analysis was done using SAS®, version 9.2.
RESULTS
We reviewed our institutional NMIBC database and identified 421 patients, of whom 340 met study inclusion criteria. Patients identified as having received ACE-I/ARBs were only included if they were on the medications at the time of the first TUR. Table 1 lists clinical and pathological characteristics of the 340 patients. Median followup was 3 years (IQR 1.3–6.1) and median patient age was 69.6 years. At last followup 253 patients (74%) were alive, 55 (16%) had died and 32 (9%) were lost to followup. Overall 143 patients were receiving ACE-I/ARBs, including ACE-Is in 102 (71%), ARBs in 36 (25%) and the 2 therapies in 5 (3.4%). Median duration of treatment with ACE-I/ARBs was 4.7 years. In the entire population 248 patients had a smoking history, including 190 (56%) former and 58 (17%) current smokers.
Table 1.
Clinical and pathological characteristics of patients by ACE-I/ARB administration
| No. ACE-I/ARB (%) | |||
|---|---|---|---|
| No | Yes | p Value | |
| Age: | 0.006 | ||
| Less than 65 | 87 (44.2) | 42 (29.4) | |
| Greater than 65 | 110 (55.8) | 101 (70.6) | |
| Gender: | 0.76 | ||
| M | 153 (77.7) | 113 (79) | |
| F | 44 (22.3) | 30 (21) | |
| Smoking: | 0.4 | ||
| Current | 37 (18.9) | 21 (14.7) | |
| Former | 104 (53.1) | 86 (60.1) | |
| Never | 55 (28.1) | 36 (25.2) | |
| Multifocality: | 0.67 | ||
| Yes | 80 (41) | 62 (43.4) | |
| No | 115 (59) | 81 (56.6) | |
| Intravesical chemotherapy: | 0.11 | ||
| Yes | 32 (16.2) | 33 (23.1) | |
| No | 165 (3.8) | 110 (76.9) | |
| BCG therapy: | 0.21 | ||
| Yes | 85 (43.1) | 52 (36.4) | |
| No | 112 (56.9) | 91 (63.3) | |
| Stage: | 0.05 | ||
| Ta | 130 (66) | 99 (69.2) | |
| T1 | 44 (22.3) | 38 (26.6) | |
| CIS | 23 (11.7) | 6 (4.2) | |
| Grade: | 0.51 | ||
| Low | 88 (47.7) | 69 (48.3) | |
| High | 109 (55.3) | 74 (51.7) | |
| Tumor size (cm): | 0.73 | ||
| Less than 3 | 115 (61.2) | 87 (63) | |
| Greater than 3 | 73 (38.8) | 51 (37) | |
| Muscle in specimen: | 0.48 | ||
| No | 134 (68) | 92 (64.3) | |
| Yes | 63 (32) | 51 (35.7) | |
| Second TUR: | 0.08 | ||
| No | 179 (90) | 121 (84.6) | |
| Yes | 18 (9.1) | 22 (15.4) | |
| Mitomycin C: | 0.01 | ||
| No | 180 (91.4) | 117 (81.8) | |
| Yes | 17 (8.6) | 26 (18.2) | |
Multifocal disease (2 or more tumors) was reported in 142 patients (42%). The stage of all bladder tumors was Ta in 229 (67%), T1 in 82 (24%) and Tis in 29 (9%). Tumor grade was classified as low in 182 cases (53%) and high in 158 (46%). BCG therapy was administered after initial TUR in 137 patients (40%) while 65 (19.1%) received intravesical chemotherapy. Tumor recurred during the study period in 180 patients (53%) and 14 (4.1%) progressed.
Univariate and multivariate Cox proportional regression hazards models were used to determine characteristics associated with disease recurrence and progression (supplementary table, http://jurology.com/). On univariate analysis factors associated with improved RFS included CIS (p = 0.040), BCG therapy (p = 0.003) and ACE-I/ARB therapy (p = 0.009). Multivariate analysis demonstrated that patients treated with BCG (HR 0.68, 95% CI 0.47–0.87, p = 0.002) or ACE-I/ARB (HR 0.61, 95% CI 0.45–0.84, p = 0.005) were less likely to experience tumor recurrence. On multivariate analysis patients with tumor larger than 3 cm were 1.5 time more likely to experience recurrence (HR 1.5, 95% CI 1.1–2, p = 0.01).
There was no significant association of RAS blockade with decreased disease progression. ACE-I/ARB treatment was not associated with improved PFS (HR 0.6, 95% CI 0.19–1.97, p = 0.4). Variables associated with decreased PFS on univariate analysis were multifocal disease (HR 3.5, 95% CI 1.1–11.2, p = 0.03) and patient age (HR 4.5, 95% CI 0.99–2.01, p = 0.05).
As determined by Kaplan-Meier estimates the 5-year RFS rate was 45.6% and 28.1% in patients treated and not treated with ACE-I/ARBs, respectively (p = 0.01, part A of figure). Subgroup analysis was performed to evaluate the effect on NMIBC pathology (Ta, T1 and CIS) in 85 patients on BCG alone and 52 in whom it was combined with ACE-I/ARB. Multivariate analysis revealed that BCG alone (HR 2.19, 95% CI 1.01–4.77, p = 0.04) was associated with worse RFS than BCG plus ACE-I/ARB (stage Ta HR 0.45, 95% CI 0.21–0.98, p = 0.04, table 2 and part B of figure). Further sub-classification of Ta disease into high and low grade in 229 and 166 patients, respectively, demonstrated no significance regarding recurrence between these 2 groups (HR 0.934, 95% CI 0.63–1.384, p = 0.73).
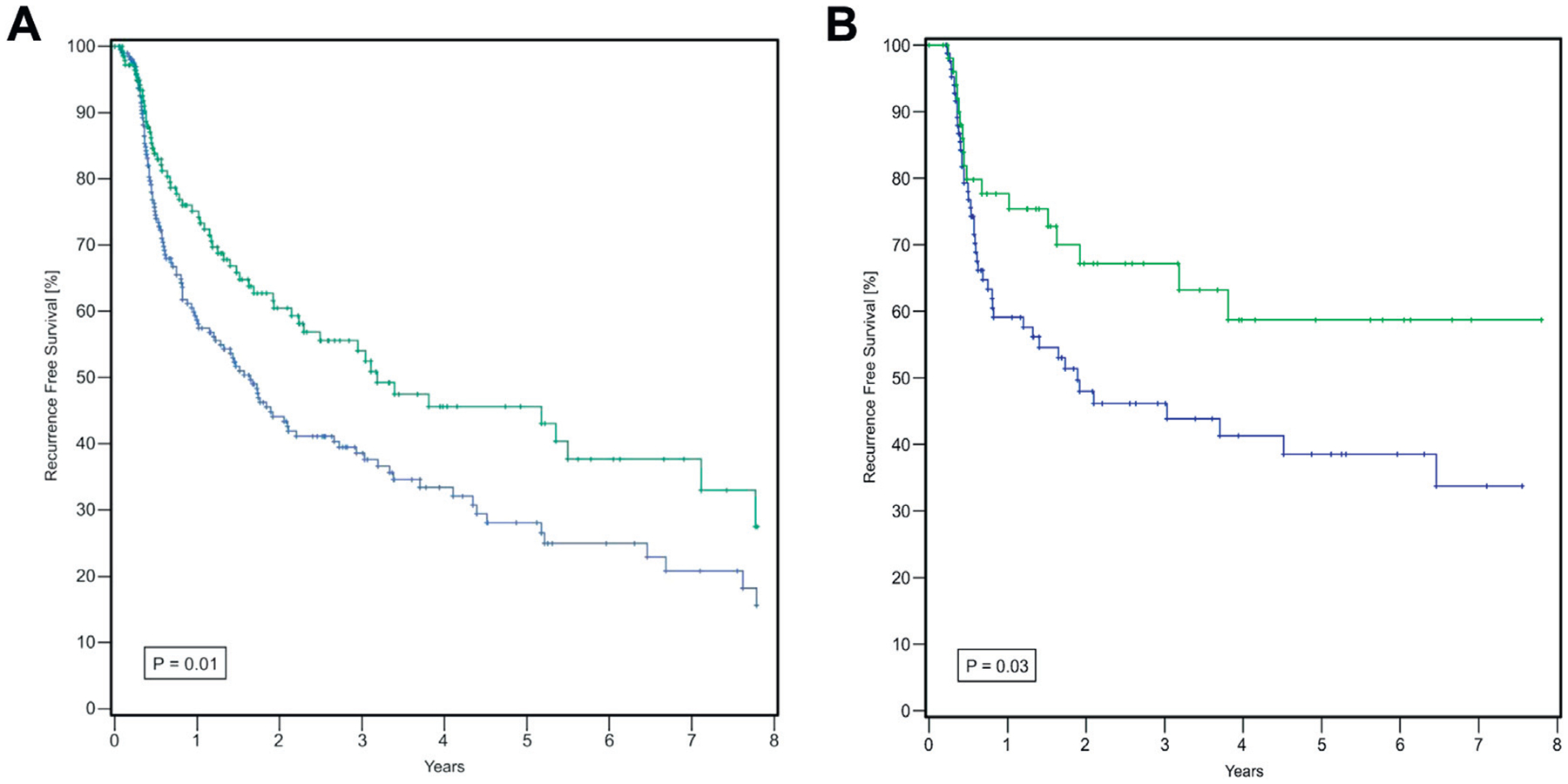
Kaplan-Meier curves of RFS. A, with (green curve) and without (blue curve) ACE-I/ARBs. B, by BCG with and without ACE-I/ARB.
Table 2.
Multivariate Cox regression analysis of RFS in patients treated with BCG with or without ACE-I/ARB
| Stage (treatment) | HR (95% CI) | p Value |
|---|---|---|
| Ta: | 0.04 | |
| BCG | Referent | |
| BCG + ACE-I/ARB | 0.45 (0.21–0.98) | |
| T1: | 0.19 | |
| BCG | Referent | |
| BCG + ACE-I/ARB | 0.53 (0.21–1.39) | |
| CIS: | 0.82 | |
| BCG | Referent | |
| BCG + ACE-I/ARB | 1.19 (0.25–5.78) |
DISCUSSION
RAS endocrine function primarily targets the regulation of blood pressure and fluid homeostasis. It produces a potent vasoactive peptide, angiotensin II, which acts to promote vasoconstriction and adrenal production of aldosterone to reabsorb sodium and water from the distal renal tubules and collecting duct. AT1, which is responsible for the signal transduction accounting for the vasoconstrictive properties of angiotensin II, can be found in blood vessels and kidneys. Similarly there is increasing evidence that AT1 expression can be found in numerous tumors.7,8 Specifically genitourinary tumors such as bladder, prostate and kidney cancers have been found to express AT1.9 Malignant disease necessitates neovascularization and angiogenesis for local proliferation and distant metastatic potential.10,11 Aside from its endocrine capabilities RAS and AT1 stimulates angiogenesis, resulting in chronic proliferative inflammation.12,13 Moreover, tumors that are highly dependent on angiogenic proliferation may demonstrate growth retardation after receptor antagonism.14
RAS blockade with ACE-I/ARB therapy demonstrates improved NMIBC RFS rates. Our study supports the findings of Yuge et al, who first noted improved RFS in a NMIBC cohort that underwent TUR, and intravesical and ACE-I/ARB therapy.6 We found that patients treated with RAS blockade at the time of the first TUR had a 5-year RFS rate of 45.6% compared to only 28% in those not treated with ACE-I/ARB. Furthermore, on multivariate subgroup analysis patients with stage Ta NMIBC (67%) who were receiving ACE-I/ARB therapy were less likely to experience tumor recurrence (HR 0.45, 95% CI 0.21–0.98) than those on BCG therapy alone (HR 2.19, 95% CI 1.01–4.77) following TUR. Multivariate analysis did not demonstrate that improved PFS was associated with ACE-I/ARB therapy.
Angiotensin receptor type 1 tumor involvement has been found in prior studies.15–17 Likewise in genitourinary tumors AT1 has been shown to have a role in tumor proliferation.18,19 Medications designed to antagonize AT1 act as antitumor agents through RAS inhibition by regulating angiogenesis, cellular proliferation, immune modulation and fibrosis.19,20 As many organ systems express RAS receptors, its endocrine influence on solid tumor production and proliferation can be widespread. A particular carcinogenic property of genitourinary tumors includes the process of angiogenesis, which is enabled by uninhibited VEGF activity. To combat this distinctive characteristic the importance of antiangiogenic agents was underscored in a bladder cancer xenograft model.18 Through RAS inhibition via the ARB candesartan the investigators found that in vitro human bladder cancer cells had decreased microvessel density and VEGF expression compared to those in the control group. Inhibiting RAS through ACE-I/ARB therapy may provide a possible targeted therapeutic role and account for improved RFS in patients with bladder cancer. Beyond the antiVEGF properties the additive inhibitory effect on inflammation and cellular proliferation offers comprehensive antitumor properties to RAS blockade. In concert with the ability of BCG to generate a T-helper cell response to bladder cancer cells this may provide an explanation for the improved RFS rates in patients with stage Ta disease who are on ACE-I/ARB therapy.
A possible explanation for the decreased recurrence rates seen in Ta NMIBC with RAS inhibition compared to higher stages is that AT1 expression may be lost when investigating invasive tumors.21 Using immunocytochemistry and in situ hybridization techniques on breast biopsies De Paepe et al found that AT1 expression on cell membranes disappeared in invasive breast cancer cells while remaining present in hyperplastic lesions and CIS.21 This study offers insight into the effectiveness of RAS inhibition for Ta bladder cancer, suggesting a regulatory pathway that is lost during disease progression. To our knowledge this has not been found in bladder cancer cell lines. However, further studies of this nature must be performed to confirm the enhanced performance of RAS inhibition in Ta bladder cancer.
The current series validates previously reported findings with improved RFS rates in patients who received ACE-I/ARB therapy. This benefit was also seen in those undergoing BCG instillations. BCG failure occurs in up to 50% of patients and it is associated with poor prognosis. Strategic goals for NMIBC management focus on complete tumor resection at TUR followed by intravesical BCG therapy.22 However, ultimately residual tumor that is left behind following TUR negatively affects the response to BCG and decreases RFS and PFS.23 Therefore, newer approaches to local control of NMIBC to reduce the tumor burden and time to cystectomy may provide an added benefit to the contemporary standard of care. RAS blockade may be considered a nascent form of targeted bladder cancer therapy that adds local control by extending RFS rates.
Previous reports have demonstrated that RAS blockade prolongs RFS and metastasis-free survival rates in patients with localized urothelial carcinoma.6,24 Following nephroureterectomy for upper tract urothelial carcinoma Tanaka et al observed that the absence of RAS inhibition in their cohort of 279 patients was an independent predictor of a decrease in metastasis-free survival.24 The 5-year metastasis-free survival rate in those receiving and not receiving RAS inhibition therapy was 93% and 72.8%, respectively (p = 0.008). This study suggests the possible role of RAS blockade systemically decreasing metastatic disease rates in patients who undergo surgery for local disease. Antitumor impact in this scope most likely reflects the multiple activities that RAS inhibition has regarding immune suppression, inhibition of cellular proliferation and immune regulation.
Tumor suppression through RAS inhibition is equally seen in prostate cancer.25,26 Similar to the effect of candesartan on in vitro human bladder cancer cells, in vitro prostate cancer cells underwent significant VEGF suppression following treatment.19 In a castrate resistant prostate cancer cell line Kosaka et al noted that androgen independent cells had greater expression of VEGF and AT1.19 After treatment with candesartan VEGF, prostate specific antigen and tumor growth were significantly suppressed. The study further lends support to target angiogenic properties of tumors with significant inhibition of cancer growth while confirming a possible role for RAS blockade in genitourinary cancer management.
Our study has several limitations. Primarily the study was performed in a retrospective manner and so carries the inherent risk of selection bias. We were not able to compare the effects of RAS blockade between ACE-Is and ARBs. The population size was small and increasing our cohort patient numbers would have added to the power and underlined the impact that these medications may have on bladder cancer recurrence. Similarly we did not see the same impact on RFS among patients with stage T1 and CIS, reflecting low patient numbers, which were unable to reveal a variance. We did not find associations with tumor recurrence regarding other common variables such as grade, stage, muscle in the TUR specimen and impact of intravesical therapy, again likely reflecting our small patient population.
However, since we performed our study to validate the findings of Yuge et al,6 we believe that there is a potential for clinical use of ACE-I/ARB therapy in NMIBC management. Our findings continue to support the antitumor effect of these medications. Their use is plausible, considering the improved RFS rates and the relatively mild side effects.
CONCLUSIONS
ACE-I/ARB therapy improved RFS rates in patients treated for NMIBC. The most marked effect was seen in those who received ACE-I/ARB therapy plus intravesical BCG. RAS has proven properties of cellular proliferation and angiogenesis by activating AT1. ACE-I/ARB therapy may decrease recurrence rates in patients with stage Ta NMIBC. Our findings warrant further basic science research to determine the exact mechanism of action that these medications may have on improving RFS rates among patients with NMIBC as well as prospective research in a possible randomized trial to elucidate clinical application.
Supplementary Material
Acknowledgments
Supported by T32 Training Grant 5T32CA009614-24 (MLB).
Abbreviations and Acronyms
- ACE-I
angiotensin converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- AT1
angiotensin receptor type 1
- BCG
bacillus Calmette-Guérin
- CIS
carcinoma in situ
- NMIBC
nonmuscle invasive bladder cancer
- PFS
progression-free survival
- RAS
renin-angiotensin system
- RFS
recurrence-free survival
- TUR
transurethral resection
- VEGF
vascular endothelial growth factor
Footnotes
Study received institutional review board approval.
REFERENCES
- 1.Kopparapu PK, Boorjian SA, Robinson BD et al. : Expression of VEGF and its receptors VEGFR1/VEGFR2 is associated with invasiveness of bladder cancer. Anticancer Res 2013; 33: 2381. [PubMed] [Google Scholar]
- 2.Hansel DE, Miller JS, Cookson MS et al. : Challenges in the pathology of non-muscle-invasive bladder cancer: a dialogue between the urologic surgeon and the pathologist. Urology 2013; 81: 1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall MC, Chang SS, Dalbagni G et al. : Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007; 178: 2314. [DOI] [PubMed] [Google Scholar]
- 4.Cookson MS, Herr HW, Zhang ZF et al. : The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol 1997; 158: 62. [DOI] [PubMed] [Google Scholar]
- 5.Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J et al. : Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol 2000; 164: 680. [DOI] [PubMed] [Google Scholar]
- 6.Yuge K, Miyajima A, Tanaka N et al. : Prognostic value of renin-angiotensin system blockade in non-muscle-invasive bladder cancer. Ann Surg Oncol 2012; 19: 3987. [DOI] [PubMed] [Google Scholar]
- 7.Escobar E, Rodríguez-Reyna TS, Arrieta O et al. : Angiotensin II, cell proliferation and angiogenesis regulator: biologic and therapeutic implications in cancer. Curr Vasc Pharmacol 2004; 2: 385. [DOI] [PubMed] [Google Scholar]
- 8.Deshayes F and Nahmias C: Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab 2005; 16: 293. [DOI] [PubMed] [Google Scholar]
- 9.Miyajima A, Kikuchi E, Kosaka T et al. : Angiotensin II type 1 receptor antagonist as an angiogenic inhibitor in urogenital cancer. Rev Recent Clin Trials 2009; 4: 75. [DOI] [PubMed] [Google Scholar]
- 10.Weidner N, Semple JP, Welch WR et al. : Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1. [DOI] [PubMed] [Google Scholar]
- 11.Weidner N, Carroll PR, Flax J et al. : Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol 1993; 143: 401. [PMC free article] [PubMed] [Google Scholar]
- 12.Katada J, Muramatsu M, Hayashi I et al. : Significance of vascular endothelial cell growth factor up-regulation mediated via a chymase-angiotensin-dependent pathway during angiogenesis in hamster sponge granulomas. J Pharmacol Exp Ther 2002; 302: 949. [DOI] [PubMed] [Google Scholar]
- 13.Muramatsu M, Katada J, Hattori M et al. : Chymase mediates mast cell-induced angiogenesis in hamster sponge granulomas. Eur J Pharmacol 2000; 402: 181. [DOI] [PubMed] [Google Scholar]
- 14.Fujita M, Hayashi I, Yamashina S et al. : Blockade of angiotensin AT1a receptor signaling reduces tumor growth, angiogenesis, and metastasis. Biochem Biophys Res Commun 2002; 294: 441. [DOI] [PubMed] [Google Scholar]
- 15.Okazaki M, Fushida S, Harada S et al. : The Angiotensin II type 1 receptor blocker candesartan suppresses proliferation and fibrosis in gastric cancer. Cancer Lett 2014; 355: 46. [DOI] [PubMed] [Google Scholar]
- 16.Chen YH, Huang CH, Lu HI et al. : Prognostic impact of renin-angiotensin system blockade in esophageal squamous cell carcinoma. J Renin Angiotensin Aldosterone Syst 2014; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Suganuma T, Ino K, Shibata K et al. : Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clin Cancer Res 2005; 11: 2686. [DOI] [PubMed] [Google Scholar]
- 18.Kosugi M, Miyajima A, Kikuchi E et al. : Effect of angiotensin II type 1 receptor antagonist on tumor growth and angiogenesis in a xenograft model of human bladder cancer. Hum Cell 2007; 20: 1. [DOI] [PubMed] [Google Scholar]
- 19.Kosaka T, Miyajima A, Takayama E et al. : Angiotensin II type 1 receptor antagonist as an angiogenic inhibitor in prostate cancer. Prostate 2007; 67: 41. [DOI] [PubMed] [Google Scholar]
- 20.Ager EI, Neo J and Christophi C: The renin-angiotensin system and malignancy. Carcino-genesis 2008; 29: 1675. [DOI] [PubMed] [Google Scholar]
- 21.De Paepe B, Verstraeten VL, De Potter CR et al. : Growth stimulatory angiotensin II type-1 receptor is upregulated in breast hyperplasia and in situ carcinoma but not in invasive carcinoma. Histochem Cell Biol 2001; 116: 247. [DOI] [PubMed] [Google Scholar]
- 22.Ku JH and Lerner SP: Strategies to prevent progression of high-risk bladder cancer at initial diagnosis. Curr Opin Urol 2012; 22: 405. [DOI] [PubMed] [Google Scholar]
- 23.Sfakianos JP, Kim PH, Hakimi AA et al. : The effect of restaging transurethral resection on recurrence and progression rates in patients with nonmuscle invasive bladder cancer treated with intravesical bacillus Calmette-Guérin. J Urol 2014; 191: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka N, Miyajima A, Kikuchi E et al. : Prognostic impact of renin-angiotensin system blockade in localised upper-tract urothelial carcinoma. Br J Cancer 2012; 106: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshino K, Ishiguro H, Teranishi J et al. : Regulation of androgen receptor expression through angiotensin II type 1 receptor in prostate cancer cells. Prostate 2011; 71: 964. [DOI] [PubMed] [Google Scholar]
- 26.Uemura H, Nakaigawa N, Ishiguro H et al. : Antiproliferative efficacy of angiotensin II receptor blockers in prostate cancer. Curr Cancer Drug Targets 2005; 5: 307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


