Abstract
Background.
Neoadjuvant therapy is increasingly used for patients with pancreatic ductal adenocarcinoma (PDAC). It is unknown whether neoadjuvant chemoradiotherapy is more effective than chemotherapy (NCRT vs. NAC). We aim to compare pathological and survival outcomes of NCRT and NAC in patients with PDAC.
Patients and Methods.
Single-center analysis of PDAC patients treated with NCRT or NAC followed by resection between December 2008 and December 2018 was performed. Average treatment effect (ATE) was estimated after case–control matching using Mahalanobis distance nearest-neighbor matching. Inverse probability weighted estimates (IPWE)-based ATE was estimated for disease-free survival (DFS) and overall survival (OS).
Results.
Among the 418 patients (mean age 66.8 years, 51% female) included in the study, 327 received NAC and 91 received NCRT. NCRT patients had higher rates of locally advanced disease, number of neoadjuvant chemotherapy cycles, more chemotherapy regimen crossover (gemcitabine and 5-FU based), and were more likely to undergo open surgical procedures and/or vascular resection (all p < 0.05). After matched analysis, NCRT was associated with a significant reduction in lymph node positive disease [ATE = (−)0.24, p = 0.007] and lymphovascular invasion [ATE = (−)0.20, p = 0.02]. While NCRT was associated with significantly improved DFS by 9.5 months (p = 0.006), it did not affect OS by IPWE-based ATE after adjusting for adjuvant therapy (ATE = 5.5 months; p = 0.32).
Conclusion.
Compared with NAC alone, NCRT is associated with improved pathologic surrogates and disease-free survival, but not overall survival in patients with PDAC.
Surgical resection is considered the only curative modality for patients with pancreatic ductal adenocarcinoma (PDAC). Despite this, only 20% of tumors are classified as resectable at diagnosis, and PDAC is associated with a 5-year survival of only 9%.1,2 Neoadjuvant therapy (NAT) has been increasingly adopted to downstage borderline-resectable (BR) and locally advanced tumors (LA), treat micrometastatic disease, and spare patients with aggressive disease the morbidity of futile surgery.3,4 Although survival benefit is yet to be demonstrated on an intent to treat basis,5,6 patients with improved biochemical (CA19-9) and pathological response during NAT exhibit improved survival.7,8
Neoadjuvant therapy may be administered as neoadjuvant chemotherapy (NAC) alone or in combination with radiation (neoadjuvant chemoradiotherapy, NCRT). Common chemotherapy regimens are extrapolated from metastatic and adjuvant settings and include gemcitabine with nab-paclitaxel or 5FU/leucovorin/irinotecan/oxaliplatin (FOLFIRINOX). Radiotherapy (RT) can be given as conventionally fractionated RT (CFRT) using intensity-modulated radiotherapy (IMRT) or 3D conformal techniques, or as hypofractionated RT, specifically stereotactic body radiotherapy (SBRT).9,10 Although chemotherapy is associated with a survival benefit in PDAC, the utility of chemoradiation remains controversial.11-17 Despite this, RT remains a popular component of NAT in the USA, with several studies demonstrating improved tumor downstaging and pathologic response.18,19
To date, a clear survival benefit for NCRT over NAC is yet to be demonstrated. Comparisons between both modalities are limited by selection bias in applying NCRT to more advanced (BR and LA) lesions. Moreover, comparative studies of conventionally fractionated versus hypofractionated NCRT are lacking. In this study, we compare the efficacy of NCRT and NAC in PDAC by examining pathologic and survival outcomes. We hypothesize that following adjustment using a methodology of inverse probability weighted estimators to account for selection bias, NCRT would be associated with improved outcomes over NAC.
PATIENTS AND METHODS
Study Design and Patient Population
This study is a retrospective, single-center analysis of consecutive PDAC patients treated with NAC or NCRT followed by resection at University of Pittsburgh Medical Center (UPMC) between December 2008 and December 2018. The study was approved by the Institutional Review Board. Patients included were identified from a prospectively maintained surgical database and met all of the following criteria: (1) histological diagnosis of PDAC, (2) received NAT, and (3) underwent resection with no evidence of metastatic disease or grossly positive R2 margins. Patients who underwent surgery-first approach for treatment of PDAC were not included in the current analysis. Patients were classified into two groups based on the type of NAT received as NAC or NCRT (consisting of induction chemotherapy followed by chemoradiation). Outcomes examined were biochemical and pathological surrogates of treatment response, disease-free survival (DFS), and overall survival (OS).
Data Collection and Definitions
All data were obtained from a prospectively maintained institutional database, which holds details of all patients who have undergone pancreatic resections at UPMC. Patient and disease variables included age at diagnosis, gender, Charlson comorbidity index (CCI), history of diabetes and cardiovascular disease, prior surgery, body mass index (BMI), American Society of Anesthesiologists score (ASA), baseline CA19-9 levels, and clinical tumor data (CT tumor size, EUS tumor size, and nodal involvement). Patients were classified at time of diagnosis into resectable, borderline-resectable, and locally advanced disease based on NCCN resectability guidelines.20
The decision of treating PDAC patients with NAT at our institution is undertaken by a multidisciplinary team that includes surgical, medical, and radiation oncologist. We attempt to treat most patients with NAT, even those with NCCN resectable disease. Certain patients may undergo a surgery first approach either due to patient preference and/or advanced age that limits receipt of multimodality therapy. Since systemic therapy for PDA has evolved over the study period, there was significant heterogeneity in the neoadjuvant regimens administered. NAC was classified as either gemcitabine-based (including gemcitabine monotherapy, gemcitabine-nab paclitaxel, and gemcitabine-capecitabine) or 5-fluorouracil (5-FU) based (including FOLFIRINOX, FOLFIRI, and FOLFOX). One NAC cycle is defined as 1 month of treatment (typically two treatments of 5FU-based therapy on days 1 and 14, or doublet or triplet gemcitabine-based therapy). The duration of NAC was defined as time between the first and last day of chemotherapy. Patients are typically evaluated every 2 months to assess response to treatment. In general, if radiographic and CA19-9 response is attained, surgery is recommended. NCRT is administered following NAC (typically completed 4–6 weeks prior to resection) and is generally reserved for threatened margins, particularly an arterial margin. RT was classified as CFRT (including IMRT and 3D conformal RT) or SBRT. SBRT, as a form of hypofractionated radiotherapy, was given at a dose of 30–36 Gray (Gy) over 3–5 fractions (6.6 Gy or more per fraction), while CFRT was delivered at a dose of 50–54 Gy over 25–28 fractions (1.8–2 Gy per fraction).
Changes in CA19-9 and CT tumor size before and after NAC and NCRT were recorded to assess biochemical and radiographic response. Pathological surrogates of efficacy included tumor size (T-size), lymph node (LN) positivity, LN ratio (LNR = positive LN/total LN harvest), lymphovascular invasion (LVI), perineural invasion (PNI), margin status, and pathological response. A positive margin (R1) was defined as the presence of invasive carcinoma within 1 mm of the inked margin, as per the 8th edition of AJCC.21 Pathological response was abstracted from pathology and classified as no response, partial response, near-complete response, or complete response based on the American College of Pathologists (CAP) grading system or the Evans grading system.22,23
Disease-free survival was calculated from date of diagnosis to date of recurrence or death, while overall survival was calculated from date of diagnosis to date of death. In both cases, survival times for patients who did not experience the event (recurrence or survival) were measured from date of diagnosis to date of last follow-up (right-censored).
Statistical Analysis
Continuous variables are reported as mean with standard deviation or as median with interquartile range. Categorical variables are reported as occurrence and percentage. To compare baseline characteristics of the two groups, the Wilcoxon rank-sum test was used for quantitative variables, and the likelihood-ratio Chi square test was used for categorical variables. To analyze the effect of NCRT on outcomes, univariate analysis was performed utilizing the outcome rank-sum test for continuous variables, and the likelihood ratio test for categorical variables. A case–control matched analysis using Mahalanobis distance nearest-neighbor matching was used to estimate the average treatment effect (ATE).24
Average treatment effect (ATE) is an estimate of the difference in the mean outcome if every patient received the treatment (NCRT) compared with the mean outcome if every patient did not receive the treatment (NAC). For the nonsurvival outcomes, we utilized a nearest neighbor matching algorithm in which variables related to receipt of the treatment, and variables related to the outcome were used as matching variables to compare treated (NCRT) versus nontreated (NAC) patients. The variables used for matching were selected based on other investigations to determine which variables were most significantly related to the treatment/outcome and included patient demographics, baseline lab values (such as pretreatment CA19-9), and disease characteristics (such as tumor size, EUS stage based on AJCC 8th edition, and tumor resectability based on NCCN criteria). For two patients to be matched, they do not have to be identical in every attribute—instead, they need only to be as similar as possible across all the matching variables. Unlike other matching algorithms where it is necessary to drop patients who do not have matches in the dataset, this method matches patients that are as similar as possible.
Survival analysis was performed initially using raw survival values calculated from date of diagnosis to date of recurrence (DFS) and date of death (OS). Survival was then censored to generate the estimated median survival (both DFS and OS) for both cohorts. Univariate analysis was performed using a log-rank test comparison. ATE was estimated using inverse probability weighted estimates (IPWE) for OS and DFS.25 For survival analyses, the nearest-neighbor matching approach is not sufficient since censoring of the survival times for patients who have not experienced the outcome must also be considered. We used inverse probability weighting to estimate the ATE for the survival outcomes. In this method, there is no matching of patients. Instead, there are two models that are fit together—one model that predicts treatment and another model that predicts survival time (taking the censoring into account). Variables for each of these models were selected based on other investigations to determine which variables were most significantly related to the treatment/outcome. Based on the outcomes of these models, weights are given to each patient to estimate the mean survival time if every patient received the treatment (NCRT) and the mean survival time if every patient did not receive the treatment. The differences in these estimated means is the depicted as the ATE.
RESULTS
Patient and Treatment Characteristics
A total of 418 PDAC patients underwent NAT followed by resection (NAC = 327, NCRT = 91). The average age was 66.8 years, and 50.7% were women. Table 1 presents the baseline characteristics, disease-related variables, and treatment variables of both groups. There was a higher proportion of women in the NCRT group (63.7% vs. 47.1%, p = 0.005), but no other significant differences were observed in age, CCI, history of diabetes, history of cardiovascular disease, BMI, and ASA (all p > 0.05).
TABLE 1.
Patient demographics and treatment variables for PDAC patients receiving NAC and NCRT
| Variable | Overall (n = 418) | NCRT (n = 91) | NAC (n = 327) | p Value |
|---|---|---|---|---|
| Age | 66.8 ± 9.2 | 64.8 ± 9.3 | 66.7 ± 9.1 | 0.100 |
| Female sex | 212 (50.7%) | 58 (63.7%) | 154 (47.1%) | 0.005 |
| BMI | 26.9 ± 5.4 | 26.9 ± 5.9 | 27.0 ± 5.3 | 0.618 |
| DM | 115 (27.5%) | 24 (26.4%) | 91 (27.8%) | 0.783 |
| CVD | 191 (45.7%) | 35 (38.5%) | 156 (47.7%) | 0.116 |
| Prior abdominal surgery | 230 (55.0%) | 50 (54.9%) | 180 (55.0%) | 0.986 |
| ASA | 3.0 ± 0.4 | 2.9 ± 0.4 | 3.0 ± 0.4 | 0.452 |
| CCI age unadjusted | 2.7 ± 0.9 | 2.7 ± 1.1 | 2.6 ± 0.9 | 0.798 |
| CCI age adjusted | 4.8 ± 1.5 | 4.7 ± 1.7 | 4.8 ± 1.4 | 0.274 |
| Preop. albumin | 3.6 ± 0.4 | 3.6 ± 0.5 | 3.6 ± 0.4 | 0.360 |
| Preop. total bilirubin | 0.6 ± 0.6 | 0.7 ± 0.5 | 0.6 ± 0.6 | < 0.001 |
| Baseline CA19-9 | 161.9 (554.9) | 120.5 (470.3) | 180.2 (553.7) | 0.686 |
| CT tumor size at diagnosis, cm | 2.9 (1.4) | 2.9 (1.5) | 2.9 (1.4) | 0.634 |
| EUS tumor size, cm | 2.8 (1.0) | 3.0 (0.9) | 2.7 (1.0) | 0.276 |
| NAC duration, weeks | 9 ± 7.1 | 11.7 ± 9.7 | 8.9 ± 6.0 | < 0.001 |
| NAC cycles | 3.8 ± 2.1 | 4.6 ± 2.3 | 3.6 ± 2.0 | < 0.001 |
| Resectability | < 0.001 | |||
| Resectable | 111 (26.6%) | 15 (16.5%) | 96 (29.4%) | |
| Borderline | 252 (60.3%) | 54 (59.3%) | 198 (60.6%) | |
| Locally advanced | 55 (13.2%) | 22 (24.2%) | 33 (10.1%) | |
| NAC type | < 0.001 | |||
| Gemcitabine | 260 (62.7%) | 46 (50.5%) | 214 (66.0%) | |
| 5-Fluorouracil | 104 (25.1%) | 21 (23.1%) | 83 (25.6%) | |
| Both | 49 (11.8%) | 24 (26.4%) | 25 (7.7%) | |
| Other | 2 (0.5%) | 0 (0.0%) | 2 (0.6%) | |
| Surgery | < 0.001 | |||
| Robotic | 200 (47.8%) | 29 (31.9%) | 171 (52.3%) | |
| Open | 218 (52.2%) | 62 (68.1%) | 156 (47.7%) | |
| Vascular resection | 173 (41.4%) | 53 (58.2%) | 120 (36.7%) | < 0.001 |
| Receipt of adjuvant therapy* | 299 (72.5%) | 51 (57.3%) | 248 (76.8%) | < 0.001 |
| Time to adjuvant therapy | 9.54 ± 3.54 | 10.85 ± 3.77 | 9.27 ± 3.44 | 0.007 |
| Receipt of salvage therapy** | 185 (74.0%) | 39 (66.1%) | 146 (76.4%) | 0.121 |
Bold value denotes staistical significance p < 0.05
NAC neoadjuvant chemotherapy, NCRT neoadjuvant chemoradiotherapy, BMI body mass index, CCI Charlson comorbidity Index, CT computed tomography, EUS endoscopic ultrasound, CA19-9 carbohydrate antigen 19-9
Adjuvant data missing in five (1%) patients.
Salvage therapy not given to patients without radiographic recurrence (n = 135), and data missing in 33 patients
All quantitative variables presented as mean ± SD or median (IQR), and all qualitative variables as n (%). p Value < 0.05 considered significant
NCRT patients had higher rates of locally advanced disease (24.2% vs. 10.1%) and lower rates of resectable disease (16.5% vs. 29.4%) compared with NAC patients (p < 0.001). NCRT patients also had longer duration of chemotherapy (median 11.7 weeks vs. 8.9 weeks, p < 0.001), higher number of chemotherapy cycles (median 4.6 vs. 3.6, p < 0.001) and a higher proportion of patients who received both gemcitabine and 5-FU based regimens (26.4% vs. 7.7%, p < 0.001). The NCRT cohort also had higher rates of open (vs. minimally invasive) surgery (68.1% vs. 47.7%, p < 0.001) and vascular resection (58.2% vs. 36.7%, p < 0.001).
Outcomes of PDAC Patients Receiving NCRT Versus NAC
Table 2 presents unadjusted and adjusted (case matched by average treatment effect) outcomes of patients receiving NCRT compared with NAC. Before case–control matching, NCRT was associated with lower LNR (0.03 vs. 0.05), LN positive disease (51.6% vs. 70.3%), R1 resection (37.4% vs. 52.9%), LVI (57% vs. 76.7%), and PNI (70.3% vs. 85.1%). NCRT was also associated with a higher pathologic complete/near complete response rate (14.1% vs. 3.8%, p = 0.002). In the case–control matched analysis, NCRT remained associated with lower LN positivity [ATE = (−)0.24, p = 0.007] and LVI [ATE = (−)0.20, p = 0.020]; all other outcomes, including the near-complete/complete pathologic response rate were no longer significantly different between the cohorts.
TABLE 2.
Raw and adjusted (ATE) outcomes of PDAC patients receiving NAC versus NCRT
| Outcome | NCRT (N = 91) | NAC (N = 327) | p value | ATE* | p value |
|---|---|---|---|---|---|
| CA19-9 reduction | 71% (61%) | 73% (61%) | 0.486 | 5.0% (−15%, 24%) | 0.620 |
| CT tumor size reduction | 16% (41%) | 14% (35%) | 0.652 | −5.0% (−18%, 8%) | 0.471 |
| Tumor size, cm | 2.5 (1.4) | 2.5 (1.2) | 0.133 | −0.12 (−0.44, 0.20) | 0.460 |
| Lymph node ratio | 0.03 (0.12) | 0.05 (0.14) | 0.040 | −0.02 (−0.08, 0.03) | 0.381 |
| LN positive | 47 (51.6%) | 230 (70.3%) | 0.001 | −0.24 (−0.41, −0.07) | 0.007 |
| Margin (R1) | 34 (37.4%) | 173 (52.9%) | 0.008 | −0.11 (−0.28, 0.07) | 0.222 |
| Lymphovascular invasion | 49 (57%) | 240 (76.7%) | < 0.001 | −0.20 (−0.36, −0.03) | 0.020 |
| Perineural invasion | 64 (70.3%) | 274 (85.1%) | 0.002 | −0.09 (−0.24, 0.05) | 0.210 |
| Near/complete response* | 11 (14.1%) | 12 (3.8%) | 0.002 | 0.05 (−0.02, 0.12) | 0.134 |
Bold value denotes staistical significance p < 0.05
NAC neoadjuvant chemotherapy, NCRT neoadjuvant chemoradiotherapy, ATE average treatment effect, CA19-9 carbohydrate antigen 19-9, CT computed tomography, T size tumor size, LN lymph node, R1 positive margin
Response was recorded as nea-complete/complete based on the pathologist’s report which used the CAP grade/Evans grade
All values depicted as median (IQR) or n (%); p value < 0.05 considered significant
Impact of NCRT on Recurrence and Survival of Patients with PDAC
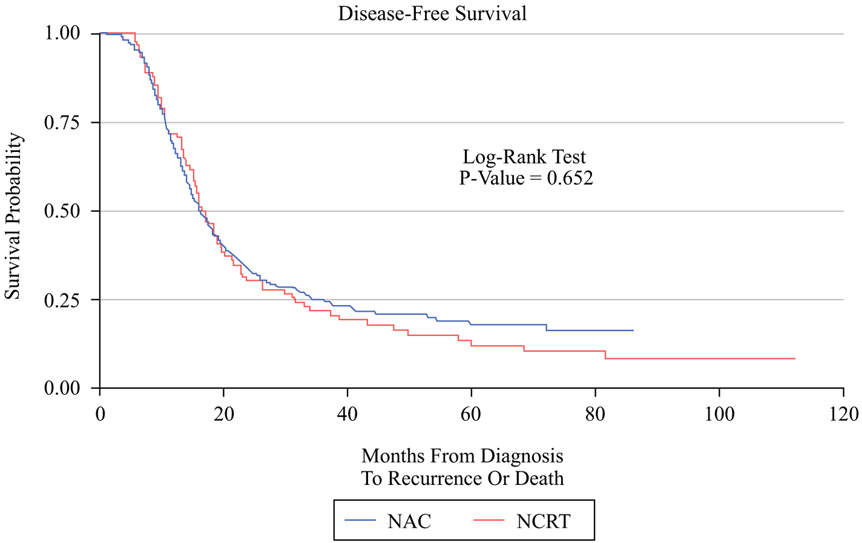
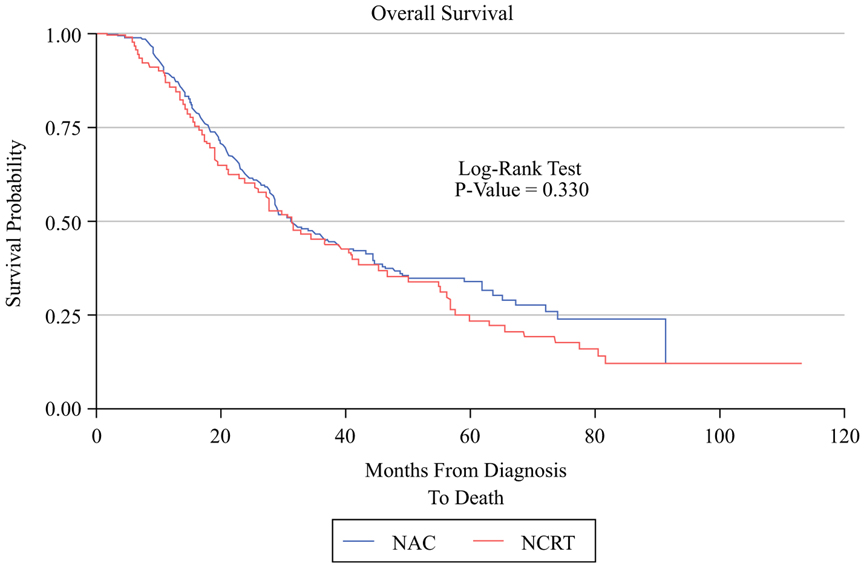
A lower percentage of patients who received NCRT ultimately received adjuvant therapy (57.3% vs. 76.8%, p < 0.001) and also had a significant delay in receiving adjuvant therapy (10.8 weeks vs. 9.3 weeks, p = 0.007). NCRT was associated with a significant delay in time to local recurrence (median 16.5 months vs. 14.1 months, p = 0.046), and a trend to delayed distal recurrence (median 15.6 months vs. 13.1 months, p = 0.062). Median survival times and IPWE-based ATE estimates are presented in Table 3. There was no significant difference between NAC and NCRT in unadjusted DFS (17.02 vs. 16.16 months, p = 0.652, Fig. 1) or OS (31.44 vs. 31.21 months, p = 0.33, Fig. 2). IPWE-based ATE demonstrated a significant improvement in DFS for NCRT by 9.5 months (p = 0.006) after using gender, resectability, NAC regimen, and NAC cycles to predict receipt of treatment while using baseline CA19-9, receipt of adjuvant chemotherapy, and adjuvant RT to model the censoring. Regarding overall survival, IPWE-based ATE did not demonstrate a significant OS benefit for NCRT over NAC (ATE = 5.4 months, p = 0.32) after using gender, resectability at diagnosis, NAC regimen, and number of NAC cycles to predict receipt of therapy, while using age, BMI, diabetes, preoperative albumin, mode of surgery, receipt of adjuvant chemotherapy, adjuvant radiotherapy, and recurrence to model the censoring.
TABLE 3.
IPWE-based ATE depicting the impact of NCRT on survival of patients with PDAC
| Median survival (95%CI) | p value (log-rank sum test) | ATE (NCRT) (95%CI) | p value (ATE) | |
|---|---|---|---|---|
| NCRT (N =91) | NAC (N = 327) | |||
| DFS | ||||
| 17.02 (15.15, 19.52) | 16.16 (14.82, 18.14) | 0.652 | 9.5 (2.8, 16.2) * | 0.006 |
| OS | ||||
| 31.44 (23.85, 42.02) | 31.21 (28.42, 38.64) | 0.330 | 5.4 (−5.2, 16.1)** | 0.318 |
Bold value denotes staistical significance p < 0.05
NAC neoadjuvant chemotherapy, NCRT neoadjuvant chemoradiotherapy, ATE average treatment effect, 95%o CI 95% confidence interval, DFS disease-free survival, OS overall survival
DFS analysis after matching for: gender, resectability, baseline CA19-9, NAC regimen, NAC cycles, receipt of adjuvant chemotherapy, and adjuvant RT.
OS analysis after matching for: age, gender, BMI, diabetes, resectability at diagnosis, NAC regimen, number of NAC cycles, preoperative albumin, mode of surgery, receipt of adjuvant chemotherapy and radiotherapy, and recurrence
FIG. 1.
Kapla–Meier graph comparing DFS in the NCRT group versus the NAC group
FIG. 2.
Kaplan–Meier graph comparing OS in the NCRT group versus the NAC group
Impact of SBRT Versus CFRT on Outcomes
In the NCRT group, SBRT was utilized in 43 patients (47.25%) and CFRT in 38 (41.8%), while 10 patients (10.9%) had missing data on type of RT received and were excluded from this analysis. Table 3 depicts a subgroup analysis comparing outcomes of NCRT to NAC utilizing SBRT or CFRT. Compared with NAC alone, NCRT utilizing SBRT significantly reduced the risk of LVI on case-control matched analysis [ATE = (−)0.26, p = 0.042], while NCRT utilizing CFRT was associated with a significant reduction in LN-positive disease [ATE = (−)0.27, p = 0.011]. Although SBRT was associated with significantly improved DFS compared with NAC alone with an average treatment effect of 14.4 months (p = 0.027), there was no significant effect on OS with NCRT using either type of RT when compared to NAC (Table 4).
TABLE 4.
Subgroup analysis based on the type of RT used in NCRT
| Outcome | NCRT (SBRT) (n = 43) | NAC (n = 327) | p value (UVA) | ATE | p value (ATE) |
|---|---|---|---|---|---|
| CA19-9 reduction | 88% (45%) | 73% (61%) | 0.019 | 10% (−23%, 43%) | 0.557 |
| CT T-size reduction | 15% (37%) | 14% (35%) | 0.518 | 13% (−10%, 35%) | 0.269 |
| T size, cm | 2.40 (2.00) | 2.5 (1.2) | 0.081 | 0.07 (−0.52, 0.65) | 0.827 |
| Lymph node ratio | 0.03 (0.19) | 0.05 (0.14) | 0.205 | −0.002 (−0.16, 0.15) | 0.972 |
| LN positive | 24 (55.8%) | 230 (70.3%) | 0.060 | 0.05 (−0.13, 0.23) | 0.605 |
| Margin (R1: 1 mm) | 21 (48.8%) | 173 (52.9%) | 0.616 | −0.03 (−0.37, 0.31) | 0.851 |
| Lymphovascular invasion | 24 (60.0%) | 240 (76.7%) | 0.028 | −0.26 (−0.52, −0.01) | 0.042 |
| Perineural invasion | 29 (67.4%) | 274 (85.1%) | 0.007 | −0.22 (−0.51, 0.06) | 0.123 |
| Near/complete response | 9 (22.5%) | 12 (3.8%) | < 0.001 | −0.03 (−0.20, 0.13) | 0.719 |
| Disease-free survival, months | 18.83 (15.74–23.00) | 16.16 (14.82–18.14) | 0.551 | 14.41 (1.60, 27.21) | 0.027 |
| Overall survival, months | 9.03 (25.36–56.15) | 31.21 (28.42–38.64) | 0.759 | 7.53 (−10.38, 25.45) | 0.410 |
| Outcome | NCRT (CFRT) (n = 38) | NAC (n = 327) | p value (UVA) | ATE | p value (ATE) |
| CA19-9 reduction | 64% (74%) | 73% (61%) | 0.338 | 7% (−26%, 41%) | 0.663 |
| CT T-size reduction | 17% (34%) | 14% (35%) | 0.846 | −4% (−21%, 12%) | 0.615 |
| T size, cm | 2.50 (1.40) | 2.5 (1.2) | 0.468 | −0.15 (−0.47, 0.18) | 0.376 |
| Lymph node ratio | 0.00 (0.12) | 0.05 (0.14) | 0.109 | 0.01 (−0.07, 0.10) | 0.759 |
| LN positive | 17 (44.7%) | 230 (70.3%) | 0.002 | −0.27 (−0.47, −0.06) | 0.011 |
| Margin (R1: 1 mm) | 12 (31.6%) | 173 (52.9%) | 0.012 | −0.03 (−0.25, 0.19) | 0.764 |
| Lymphovascular invasion | 20 (54.1%) | 240 (76.7%) | 0.005 | −0.10 (−0.34, 0.15) | 0.444 |
| Perineural invasion | 28 (73.7%) | 274 (85.1%) | 0.088 | −0.06 (−0.21, 0.10) | 0.490 |
| Near/complete response | 2 (6.9%) | 12 (3.8%) | 0.452 | 0.06 (−0.03, 0.15) | 0.201 |
| Disease-free survival, months | 15.28 (10.51–17.28) | 16.16 (14.82–18.14) | 0.203 | 10.01 (−1.99, 22.01) | 0.102 |
| Overall survival, months | 27.63 (17.31–40.61) | 31.21 (28.42–38.64) | 0.343 | −3.06 (−17.61, 11.50) | 0.680 |
Bold value denotes staistical significance p < 0.05
NAC neoadjuvant chemotherapy, NCRT neoadjuvant chemoradiotherapy, ATE average treatment effect, SBRT stereotactic body radiotherapy, CFRT conventionally fractionated radiotherapy, CA19-9 carbohydrate antigen 19-9, CT computed tomography, T size tumor size, LN lymph node
All values depicted as median (IQR) or n (%). Survival depicted as median (95% confidence interval); p value < 0.05 considered significant
Effect of NAC Versus NCRT by Baseline Resectability Status
Due to the selection bias of treating locally advanced tumors with NCRT at our institution (NCRT patients with locally advanced disease 24.2% versus 10.1% in NAC, p = < 0.001), we conducted a subgroup analysis of the impact of NCRT on patients with resectable/borderline-resectable versus locally advanced disease, as defined by NCCN resectability criteria at diagnosis (Table 5). NCRT was associated with a significant reduction in LN-positive disease [ATE = (−)0.26, p = 0.009] and improved DFS (ATE = 6.44 months, p = 0.041) for patients with resectable/borderline-resectable disease but no improvement in OS (ATE = 7.19 months, p = 0.232) of this subgroup. For locally advanced tumors, NCRT was associated with a significant reduction in LVI [ATE = (−)0.45, p = 0.002] but no difference in DFS (ATE = 6.27 months, p = 0.412) or OS (ATE = 0.40 months, p = 0.969).
TABLE 5.
Subgroup analysis of the impact of NCRT by resectability status at diagnosis
| Variables | NCRT | NAC | p value (UVA) | ATE | p value (ATE) |
|---|---|---|---|---|---|
| Resectable/borderline tumors | n = 69 | n = 294 | |||
| CA19-9 reduction | 71% (56%) | 69% (64%) | 0.532 | < 1% (−25%, 26%) | 0.973 |
| CT T-size reduction | 14% (29%) | 12% (35%) | 0.612 | −5% (−20%, 9%) | 0.481 |
| T size, cm | 2.50 (1.00) | 2.50 (1.20) | 0.352 | −0.09 (−0.44, 0.27) | 0.640 |
| Lymph node ratio | 0.03 (0.12) | 0.05 (0.14) | 0.123 | −0.02 (−0.08, 0.04) | 0.466 |
| LN positive | 37 (53.6%) | 206 (70.1%) | 0.010 | −0.26 (−0.46, −0.07) | 0.009 |
| Margin (R1: 1 mm) | 25 (36.2%) | 148 (50.3%) | 0.034 | −0.09 (−0.28, 0.11) | 0.380 |
| Lymphovascular invasion | 39 (60.9%) | 213 (75.8%) | 0.019 | −0.14 (−0.32, 0.04) | 0.138 |
| Perineural invasion | 51 (73.9%) | 247 (85.2%) | 0.032 | −0.08 (−0.25, 0.09) | 0.357 |
| Near/complete response | 5 (8.8%) | 10 (3.5%) | 0.104 | 0.03 (−0.04, 0.10) | 0.347 |
| Disease-free survival, months | 16.07 (14.69–19.52) | 16.03 (14.62–17.94) | 0.539 | 6.44 (0.27, 12.61) | 0.041 |
| Overall survival, months | 29.63 (20.90–41.00) | 30.72 (28.16–37.03) | 0.362 | 7.19 (−4.59, 18.96) | 0.232 |
| Locally advanced tumors | n = 22 | n = 33 | |||
| CA19-9 reduction | 77% (69%) | 84% (33%) | 0.792 | 36% (−18%, 90%) | 0.189 |
| CT T-size reduction | 35% (38%) | 22% (34%) | 0.339 | −16% (−40%, 8%) | 0.194 |
| T size, cm | 2.05 (3.00) | 2.50 (1.20) | 0.249 | −0.84 (−1.86, 0.18) | 0.105 |
| Lymph node ratio | 0.00 (0.18) | 0.05 (0.10) | 0.137 | −0.02 (−0.10, 0.05) | 0.534 |
| LN positive | 10 (45.5%) | 24 (72.7%) | 0.042 | −0.20 (−0.51, 0.11) | 0.211 |
| Margin (R1: 1 mm) | 9 (40.9%) | 25 (75.8%) | 0.009 | −0.25 (−0.56, 0.07) | 0.126 |
| Lymphovascular invasion | 10 (45.5%) | 27 (84.4%) | 0.002 | −0.45 (−0.72, −0.17) | 0.002 |
| Perineural invasion | 13 (59.1%) | 27 (84.4%) | 0.038 | −0.06 (−0.37, 0.25) | 0.698 |
| Near/complete response | 6 (28.6%) | 2 (6.5%) | 0.030 | 0.19 (−0.05, 0.42) | 0.120 |
| Disease-free survival, months | 18.46 (10.58–31.21) | 18.14 (12.19–20.37) | 0.983 | 6.27 (−8.70, 21.23) | 0.412 |
| Overall survival, months | 36.63 (13.9–56.15) | 47.67 (19.78–91.27) | 0.279 | 0.40 (−19.43, 20.23) | 0.969 |
Bold value denotes staistical significance p < 0.05
NAC neoadjuvant chemotherapy, NCRT neoadjuvant chemoradiotherapy, ATE average treatment effect, CA19-9 carbohydrate antigen 19-9, CT computed tomography, T size tumor size, LN lymph node
All values depicted as median (IQR) or n (%). Survival depicted as median (95% confidence interval); p value < 0.05 considered significant
DISCUSSION
In this analysis, we determined whether NCRT was associated with differences in pathologic and survival outcomes compared with NAC alone in patients with resected PDAC. The NCRT group had a higher proportion of LA disease, received longer duration and more cycles of NAC, and underwent more open surgery and vascular resection compared with NAC. After matching using IPWE, patients who received NCRT had significantly lower rates of LN-positive disease, LVI, and improved disease-free survival. However, NCRT was not associated with a significant improvement in overall survival.
NAT is now commonly accepted as an important component of multimodality therapy for patients with borderline-resectable and locally advanced disease.3,4 Due to the systemic nature of PDAC, it may also benefit patients with resectable disease. While the survival benefit of NAT has not been firmly established,5,6 it is associated with smaller tumor size at resection, LN sterilization, and higher rates of margin negative resection; potential surrogates for improved survival.7,8,26 Complete pathologic response to NAT—albeit uncommon—has also been associated with prolonged DFS and OS.27
Although radiotherapy is used for both neoadjuvant and adjuvant treatment of patients with PDAC, its impact on outcomes is controversial. In the adjuvant setting, the ESPAC-1 trial demonstrated no survival benefit of RT over chemotherapy alone, however recent large scale national studies suggest a potential benefit in patients with positive margins and/or LN positive disease.11,28,29 In the neoadjuvant setting, NCRT is used to improve local disease control.30-33 Studies comparing the use of NCRT to a surgery first approach demonstrate improved margin-negative resection rates, LN sterilization, and disease-free survival, but no benefit in overall survival.3,34 Our analysis supports those findings, demonstrating a decrease in LN positivity and LVI rates, as well as improved disease-free survival in patients who received NCRT compared with NAC after case–control matching. We also demonstrate a delay in local recurrence with NCRT, confirming findings in earlier studies.35 Our data, however, suggests no improvement in overall survival for NCRT over NAC after matching. Although this may be viewed as a negative finding, the comparable survival between both cohorts may suggest a benefit to NCRT, particularly in view of the advanced nature of disease in this cohort, as evidenced by increased need for vascular resections and longer duration of neoadjuvant chemotherapy.
The use of neoadjuvant SBRT has steadily increased over the last two decades, driven by encouraging data on its efficacy and favorable safety profile.36,37 Since our practice patterns have evolved to incorporate more SBRT in treating pancreatic cancer, we performed a subgroup analysis comparing NCRT utilizing SBRT or CFRT to NAC. We demonstrate that SBRT-based NCRT was associated with reduced LVI and significantly prolonged DFS compared with patients receiving NAC. This was not seen in patients who received CFRT-based NCRT. Similar analyses on specific subgroups of patients with PDAC have shown an advantage of SBRT over CFRT in resectable and locally advanced PDAC.38,39 Although one study suggested a benefit in overall survival with SBRT-based NCRT,38 our study failed to show similar results. Clinical trials assessing the effect of SBRT on PDAC are currently underway and may help in establishing a more definite role for this modality.40,41
Since selection bias favors the administration of NCRT to locally advanced tumors, we also performed a subgroup analysis to examine the effect of NCRT on a subset of patients with locally advanced disease compared with resectable and borderline tumors. Studies of patients with LA disease demonstrate this group to have inferior survival,42 based on a high probability of positive margins, larger tumor size, and aggressive disease biology. Although the current study demonstrates no benefit for NCRT over NAC in LA patients, the comparable survival between LA and resectable tumors may again suggest a benefit to NCRT in this cohort.
Despite using case–control matching to control for confounders, our study has several limitations. First, our cohort was restricted to patients who underwent resection, and does not account for the larger denominator of patients who started NAC or NCRT but failed to undergo resection, therefore our analysis was not performed on an intent to treat basis. This important selection bias could not be accounted for, but we assume the rate of progression or occult metastatic disease during NAT to be somewhat similar for both groups. Second, although we employed IPWE to alleviate selection bias—including resectability, baseline CA19-9 levels, type of NAC regimen, NAC cycles, and use of adjuvant therapy—other confounders, such as dose modifications and quality assurance of RT, were not accounted for in this analysis. The latter factor is particularly important, since SBRT was delivered centrally at our main surgical oncology campus, whereas CFRT was often administered at satellite hospitals, some of which were outside our hospital system. Third, although the overall sample size for the study enabled us to employ IPWE, our subgroup analysis of SBRT and CFRT groups was limited by small numbers.
CONCLUSION
This case–control matched analysis demonstrates that neoadjuvant chemoradiation is associated with improvement in several pathological outcomes compared with neoadjuvant chemotherapy alone. Despite this improvement in local control and disease-free survival, there was no overall survival benefit for NCRT over NAC alone. The role of neoadjuvant radiation as a component of multimodality therapy in localized pancreatic cancer remains to be determined by prospective studies.
FUNDING
Melissa Hogg—Grant money from SAGES and Intuitive Surgical. No funding.
REFERENCES
- 1.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10–27. 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2016, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. [Google Scholar]
- 3.Nagakawa Y, Sahara Y, Hosokawa Y et al. Clinical impact of neoadjuvant chemotherapy and chemoradiotherapy in borderline resectable pancreatic cancer: analysis of 884 patients at facilities specializing in pancreatic surgery. Ann Surg Oncol. 2019;26(6):1629–36. 10.1245/s10434-018-07131-8. [DOI] [PubMed] [Google Scholar]
- 4.Youngwirth LM, Nussbaum DP, Thomas S et al. Nationwide trends and outcomes associated with neoadjuvant therapy in pancreatic cancer: an analysis of 18243 patients. J Surg Oncol. 2017;116(2):127–32. 10.1002/jso.24630. [DOI] [PubMed] [Google Scholar]
- 5.Zhan HX, Xu JW, Wu D et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med. 2017;6(6):1201–19. 10.1002/cam4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Versteijne E, Suker M, Groothuis K et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;27:JCO1902274. 10.1200/jco.19.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macedo FI, Ryon E, Maithel SK et al. Survival outcomes associated with clinical and pathological response following neoadjuvant FOLFIRINOX or Gemcitabine/Nab-Paclitaxel chemotherapy in resected pancreatic cancer. Ann Surg. 2019;270(3):400–13. 10.1097/sla.0000000000003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Abbas AI, Zenati M, Reiser CJ et al. Serum CA19-9 Response to neoadjuvant therapy predicts tumor size reduction and survival in pancreatic adenocarcinoma. Ann Surg Oncol. 2020. 10.1245/s10434-019-08156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tempero MA, Malafa MP, Chiorean EG et al. Pancreatic adenocarcinoma, Version 1.2019. J Natl Compr Cancer Netw. 2019;17(3):202–10. 10.6004/jnccn.2019.0014. [DOI] [PubMed] [Google Scholar]
- 10.Landau E, Kalnicki S. The evolving role of radiation in pancreatic cancer. Surg Clin North Am. 2018;98(1):113–25. 10.1016/j.suc.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Neoptolemos JP, Dunn JA, Stocken DD et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358(9293):1576–85. [DOI] [PubMed] [Google Scholar]
- 12.Neoptolemos JP, Stocken DD, Friess H et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. . 2004;350(12):1200–10. [DOI] [PubMed] [Google Scholar]
- 13.Regine WF, Winter KA, Abrams RA et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299(9):1019–26. 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 14.Neoptolemos JP, Palmer DH, Ghaneh P, et al. European study group for pancreatic cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–24. 10.1016/s0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 15.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473–81. [DOI] [PubMed] [Google Scholar]
- 16.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O’Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB. Canadian cancer trials group and the unicancer-GI-PRODIGE. Group FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–406. [DOI] [PubMed] [Google Scholar]
- 17.Regine WF, Winter KA, Abrams R et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the US Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L, Zhou Y, Fan Y et al. Consolidative chemoradiotherapy after induced chemotherapy is an optimal regimen for locally advanced pancreatic cancer. Front Oncol. 2020;21(9):1543. 10.3389/fonc.2019.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen-Zhao X, Hernando O, López M et al. A prospective observational study of the clinical and pathological impact of stereotactic body radiotherapy (SBRT) as a neoadjuvant strategy of chemoradiation in pancreatic cancer. Clin Transl Oncol. 2020. 10.1007/s12094-020-02287-w. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. Pancreatic cancer (Version 1.2020). https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 26 Feb 2020.
- 21.Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25(4):845–7. [DOI] [PubMed] [Google Scholar]
- 22.Kakar S, Shi C, Adsay V et al. Protocol for the examination of specimens from patients with carcinoma of the pancreas, [Internet], version Pancreas Exocrine 4.0.0.1. CAP, 2017. https://documents.cap.org/protocols/cp-gihepatobiliary-pancreas-exocrine17protocol4001.pdf. [Google Scholar]
- 23.Evans DB, Rich TA, Byrd DR et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127(11):1335–9. [DOI] [PubMed] [Google Scholar]
- 24.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–55. 10.1002/sim.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schorn S, Demir IE, Reyes CM et al. The impact of neoadjuvant therapy on the histopathological features of pancreatic ductal adenocarcinoma—a systematic review and meta-analysis. Cancer Treat Rev. 2017;55:96–106. 10.1016/j.ctrv.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee D, Katz MH, Rashid A et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118(12):3182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinde A, Verma V, Li R et al. The role of sequential radiation following adjuvant chemotherapy in resected pancreatic cancer. J Gastrointest Oncol. 2019;10(3):462–73. 10.21037/jgo.2019.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutter CE, Park HS, Corso CD et al. Addition of radiotherapy to adjuvant chemotherapy is associated with improved overall survival in resected pancreatic adenocarcinoma: An analysis of the National Cancer Data Base. Cancer. 2015;121(23):4141–9. 10.1002/cncr.29652. [DOI] [PubMed] [Google Scholar]
- 30.Khattab A, Patruni S, Abel S et al. Long-term outcomes by response to neoadjuvant chemotherapy or chemoradiation in patients with resected pancreatic adenocarcinoma. J Gastrointest Oncol. 2019;10(5):918–27. 10.21037/jgo.2019.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.di Sebastiano P, Grottola T, di Mola FF. Borderline resectable pancreatic cancer and the role of neoadjuvant chemoradiotherapy. Updates Surg. 2016;68(3):235–9. [DOI] [PubMed] [Google Scholar]
- 32.Reames BN, Blair AB, Krell RW et al. Management of locally advanced pancreatic cancer: results of an international survey of current practice. Ann Surg. 2019. 10.1097/sla.0000000000003568. [DOI] [PubMed] [Google Scholar]
- 33.Cloyd JM, Chen HC, Wang X et al. Chemotherapy versus chemoradiation as preoperative therapy for resectable pancreatic ductal adenocarcinoma: a propensity score adjusted analysis. Pancreas.2019;48(2):216–22. 10.1097/mpa.0000000000001231. [DOI] [PubMed] [Google Scholar]
- 34.Versteijne E, Vogel JA, Besselink MG et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105(8):946–58. 10.1002/bjs.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murata Y, Mizuno S, Kishiwada M et al. Impact of histological response after neoadjuvant chemoradiotherapy on recurrence-free survival in UICC-T3 pancreatic adenocarcinoma but not in UICC-T4. Pancreas. 2012;41(1):130–6. 10.1097/mpa.0b013e3182236442. [DOI] [PubMed] [Google Scholar]
- 36.Jiang W, Haque W, Verma V, Butler EB, Teh BS. Neoadjuvant stereotactic body radiation therapy for nonmetastatic pancreatic adenocarcinoma. Acta Oncol. 2019;58(9):1259–66. 10.1080/0284186x.2019.1631472. [DOI] [PubMed] [Google Scholar]
- 37.Mellon EA, Strom TJ, Hoffe SE et al. Favorable perioperative outcomes after resection of borderline resectable pancreatic cancer treated with neoadjuvant stereotactic radiation and chemotherapy compared with upfront pancreatectomy for resectable cancer. J Gastrointest Oncol. 2016;7(4):547–55. 10.21037/jgo.2016.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang M, Heestand GM, Chang DT, Pollom EL. Neoadjuvant treatment strategies for resectable pancreas cancer: A propensity-matched analysis of the National Cancer Database. Radiother Oncol. 2020;143:101–7. 10.1016/j.radonc.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Tchelebi LT, Lehrer EJ, Trifiletti DM et al. Conventionally fractionated radiation therapy versus stereotactic body radiation therapy for locally advanced pancreatic cancer (CRiSP): an international systematic review and meta-analysis. Cancer. 2020. 10.1002/cncr.32756. [DOI] [PubMed] [Google Scholar]
- 40.Gao S, Zhu X, Shi X et al. Comparisons of different neoadjuvant chemotherapy regimens with or without stereotactic body radiation therapy for borderline resectable pancreatic cancer: study protocol of a prospective, randomized phase II trial (BRPCNCC-1). Radiat Oncol. 2019;14(1):52. 10.1186/s13014-019-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holyoake DL, Ward E, Grose D et al. A phase-I trial of preoperative, margin intensive, stereotactic body radiation therapy for pancreatic cancer: the ‘SPARC’ trial protocol. BMC Cancer. 2016;16(1):728. 10.1186/s12885-016-2765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Lei, Jansen Lina, Balavarca Yesilda et al. Stratified survival of resected and overall pancreatic cancer patients in europe and the USA in the early twenty-first century: a large, international population-based study. BMC Med. 2018;16(1):125. 10.1186/s12916-018-1120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]