ABSTRACT
Introduction
Cervicogenic headache (CGH) may originate from the C1-C2 zygapophyseal joints. CGH is often associated with loss of range of motion (ROM), specific to this segment, and measurable by the cervical flexion-rotation test (CFRT). The main purposes of the study were: 1) to investigate the immediate effect of C1-C2 rotation SNAG mobilizations plus C1-C2 self-SNAG rotation exercise for patients with CGH and 2) to explore the link between the CFRT results and treatment response.
Methods
A prospective quasi-experimental single-arm design was used where patients with CGH received eight physical therapy treatments using a C1-C2 rotational SNAG technique combined with a C1-C2 self-SNAG rotation exercise over a four-week period. Outcome measures were pain intensity/frequency and duration, active cervical ROM, CFRT, neck-related and headache-related self-perceived physical function, fear-avoidance beliefs, pain catastrophizing and kinesiophobia.
Results
The intervention produced strong effects on pain intensity, CFRT, physical function and pain catastrophizing. Moderate improvement was noted on active cervical ROM and on fear-avoidance beliefs and kinesiophobia. No link was found between pre-intervention CFRT ROM and treatment response.
Conclusion
SNAG mobilization combined with a self-SNAG exercise resulted in favorable outcomes for the treatment of CGH on patient-important and biomechanical outcomes, as well as pain-related cognitive-affective factors.
KEYWORDS: Cervicogenic headache, snag, cervical flexion-rotation test, mulligan, mobilization with movement
Introduction
Headache is a highly prevalent painful symptom affecting up to 90% of the general population. Cervicogenic headache (CGH), a subtype of headache caused by a disorder involving any structure of the neck, including bony, muscular and other soft tissue elements [1], represents 15% to 20% of all chronic headaches [2] and 53% of persistent headaches following a whiplash injury [3]. Persons with chronic CGH may experience emotional distress, limitations in their daily activities, or restricted social participation [4].
Although the specific mechanisms of CGH remain uncertain, some evidence suggests that the associated symptoms might be linked to referred pain arising from dysfunction at the C1-C2 zygapophyseal joints [5, 6]. Moreover, CGH related to C1-C2 dysfunction is often associated with loss of range of motion at this specific cervical segment, which can be assessed by the cervical flexion-rotation test (CFRT) [7]. The CRFT is considered positive when showing a loss of rotation of 8º to 10º on one side. CFRT has been widely validated for the diagnosis of C1-C2-related CGH [8, 9, 10, 11–15].
Patients with CGH commonly seek physiotherapy care to alleviate their symptoms [16]. Different manual therapy techniques and exercises can be used to address C1-C2 range of motion deficits to relieve symptoms at the source [17, 18–24]. The use of Sustained Natural Apophyseal Glide (SNAG) is recommended in recent clinical practice guidelines [25]. It is a mobilization technique which consists in applying a direct force on the C1-C2 segment during active rotation of the neck by the patient The technique can be applied manually by the therapist (SNAG) and also by the patient (self-SNAG), using a strap or towel [26].
In that sense, different studies investigating the effect of C1-C2 SNAG or self-SNAG on CGH have demonstrated a beneficial effect on symptoms, on perceived physical function related to neck pain, on headache severity and on mechanical impairment of C1-C2 segments [18, , 21, 23, 24]. However, no study has verified the effect of the combination of SNAG mobilizations plus self-SNAG exercises on CGH (symptom intensity), or pain-related cognitive-affective factors (such as fear-avoidance beliefs, pain catastrophizing and kinesiophobia), nor has explored the association between the CFRT results and the treatment response to explore its prognostic ability.
The study’s main purposes were to:
1) investigate the immediate effect of treatments combining C1-C2 rotation SNAG mobilizations and a C1-C2 self-SNAG rotation exercise program on pain (headache frequency, duration and intensity), cervical mobility (active range of motion in flexion, extension, side flexion and rotation and passive C1-C2 rotation), neck-related and headache-related self-perceived disability, fear-avoidance beliefs, pain catastrophizing and kinesiophobia; and
2) explore the association between the results of the CFRT and treatment response on pain intensity and perceived disability.
Methods
Study design and participants
We employed a prospective quasi-experimental design (pre-post without control group comparison). Patients with CGH were recruited from advertisements and local physician referrals around the area of Drummondville (Quebec, Canada) from January to May 2018. Potential participants were first screened through phone interview. Thereafter, patients underwent a physical examination to further verify inclusion and exclusion criteria and confirm their eligibility. Inclusion criteria were: 1) age between 18 to 65 years old, 2) HA in temporal relation to the onset of the cervical disorder or appearance of the lesion, 3) HA aggravated or provoked by neck movements or postures, 4) HA associated with neck, shoulder and/or upper limb pain, 5) HA frequency ≥ 1 per week for ≥ 1 month, 5) average pain intensity ≥ 3/10 on VAS and 6) pain on palpation of paravertebral tissue of the cervical spine. Exclusion criteria were: 1) History of neck surgery, 2) Reported diagnosis of another type of HA that causes two or more episodes per month (ex: migraine), 3) Currently receiving another form of physical intervention, 4) Other chronic pain syndrome (ie: fibromyalgia, systematic inflammatory disease), 5) Inability to tolerate the flexion-rotation test, the SNAG mobilization, or the self-SNAG technique and 6) Presence of contraindications to cervical manual therapy. The initial assessment, including the physical examination, was performed by a registered PT (first author).
Inclusion criteria were based on the international classification of headache disorders by the Headache Classification Committee of the International Headache Society (IHS) (IHS, 2013) and the diagnostic criteria proposed by the Cervicogenic Headache International Study Group (CHISG). Contraindications were based on the International Framework for Examination of the Cervical Region for potential of Cervical Arterial Dysfunction prior to Orthopedic Manual Therapy Intervention by the International Federation of Orthopedic Manipulative Physical Therapists (IFOMPT) [27] (see Appendix 1).
Informed consent was obtained for all participants according to The Helsinki Accords. The study was approved by the Ethics Review Board of the Clinical Research Center of the Center intégré universitaire de santé et des services sociaux de l’Estrie – Center Hospitalier Universitaire de Sherbrooke (reference number: 2018–2642).
Intervention
The intervention took place in a private practice multidisciplinary clinic in Drummondville (Quebec, Canada) between March and October of 2018. All interventions were provided by the same licensed physical therapist, an experienced (8-years) clinician trained in Mulligan techniques with a fellowship in manipulative therapy (JPP, first author).
The intervention consisted of 8 physical therapy treatments (2x/week) given over a 4-week period. During each visit, all participants received a C1-C2 rotational SNAG technique, as described by Mulligan [28]. During the technique, the therapist placed both thumbs on the posterior arch of C1 (on the opposite side of the rotation) and pushed anteriorly. Then, the patient was asked to actively rotate, as far as possible, without pain, while the therapist kept pressure on C1 during the movement. The mobilization was maintained for 10 seconds at end-range, repeated 10 times per set for a total of 3 sets per treatment. The therapist applied overpressure at end-range if the participant could tolerate it (Figure 1). Each patient was also given a home exercise program which consisted of C1-C2 self-SNAG in rotation. The home-exercise was performed using a towel, which was used to apply and maintain a pressure on the posterior arch of C1 while they would actively rotate their cervical spine, mimicking the C1-C2 rotational SNAG technique. End-range position was maintained for 10 seconds, before returning to neutral position. Participants were instructed in the self-SNAG during the first appointment and the exercise was reviewed during subsequent visits [28]. The patients were asked to perform the exercise twice a day for 10 repetitions each time, for a total of 20 repetitions per day (Figure 1). Written instructions and a video of the exercise to be performed was sent to all participants to improve the quality and compliance of the home exercise. Adherence to the exercise program was monitored with a daily logbook that was completed by the patient. No further intervention was provided.
Figure 1.
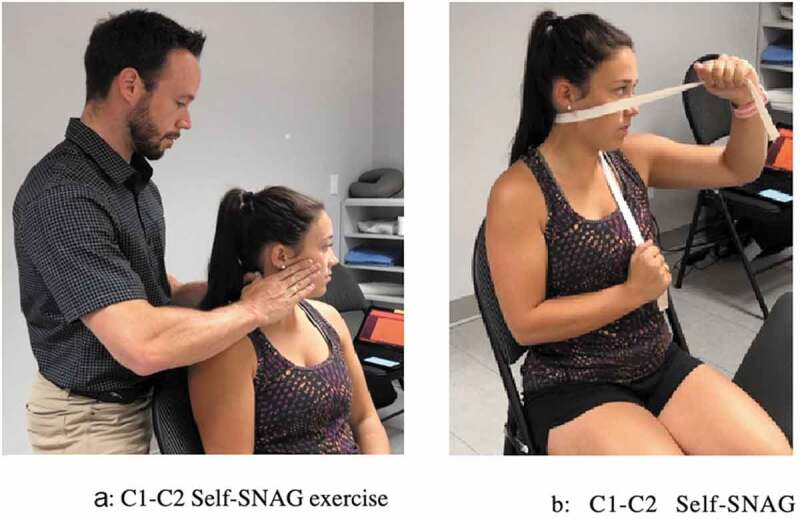
Main Outcome Measures
All outcome measures were assessed during the initial assessment (T0: baseline) and one month after completion of final treatment (T1: + 4 weeks). The primary outcome of this clinical design study was pain intensity (severity, duration, and frequency), while secondary outcomes were: ROM, perceived disability, and psychosocial factors (fear-avoidance beliefs, pain catastrophizing and kinesiophobia). All outcome measures are detailed below and synthesized in Table 1.
Table 1.
Description of outcome measures and definition of improvement
| Outcome measures | Measurement tools | When measurement was performed | Measurement method | Minimal Clinically Important Difference |
|---|---|---|---|---|
| Pain intensity | VAS | Pre- and post-intervention and daily | Direct questioning Logbook |
2.0 [29] |
| HA frequency | Daily | Logbook | ||
| HA duration | Daily | Logbook | ||
| Active neck ROM | CROM device | Pre- and post-intervention | In-clinic assessment | |
| CFRT | HALO goniometer | Pre- and post-intervention | In-clinic assessment | 4.7 to 7 degrees [9] |
| Neck-related disability | NDI (French Canadian version) | Pre- and post-intervention | Self-reported via questionnaire | 5.5 [38] |
| Headache-related disability | HDI (French Canadian version) | Pre- and post-intervention | Self-reported via questionnaire | 29 points |
| Fear-Avoidance beliefs | FABQ (French Canadian version) | Pre- and post-intervention | Self-reported via questionnaire | |
| Pain catastrophizing | PCS (French Canadian version) | Pre- and post-intervention | Self-reported via questionnaire | 38 to 44% [49] |
| Kinesophobia | TSK (French Canadian version) | Pre- and post-intervention | Self-reported via questionnaire | 5.6 points |
VAS: Visual Analogue Scale; ROM: Range of Motion; CFRT: Cervical Flexion-Rotation Test; CROM: Cervical Range of Motion device; NDI: Neck Disability Index; HDI: Headache Disability Inventory; TSK: Tampa Scale for Kinesiophobia; FABQ: modified Fear-Avoidance Beliefs Questionnaire; PCS: Pain Catastrophizing Scale
Pain severity
The authors measured overall average headache intensity (our main dependent variable) using a 10-cm visual analogue scale (VAS); the minimal clinical important difference (MCID) for the VAS is 2/10) [29]. Average episode frequency (number of episodes per day) and duration (in hours) were obtained through direct questioning at the first and final visits.
Pain severity was also measured daily via the logbook, where participants reported HA intensity (VAS) and duration (hours) for each episode. The frequency of episodes was obtained by dividing the number of episodes by the number of days for each week of measurement.
Active Range of Motion
Cervical active ROM was assessed pre-and post-intervention using the Cervical Range of Motion Device (CROM) while the patient was seated on a chair. Patients were advised to perform the neck movement with as much range as possible. Movement quality was visually monitored by the assessor. The CROM device showed moderate to good reliability and good validity in the assessment of active ROM [30–32].
Cervical Flexion-Rotation Test
The CFRT was performed on all patients, as described by 33. This test demonstrated good construct and content validity in the measurement of C1-C2 passive rotation [33], good inter-rater reliability (92%) (8), high sensitivity (75% – 100%) [9, 13a] and specificity (85% – 94%) [11, 13] for the diagnosis of C1-C2-related CGH. Hence, it is considered as a routine diagnostic test by PTs in the assessment procedures for patients presenting with headache [34].
ROM (rotation) during the CFRT was measured by the HALO goniometer with the device fixed to the head with a Velcro strap. The HALO goniometer demonstrated good reliability and validity in the assessment of ROM at the shoulder [35,36]. It was also used in a previous study to measure ROM during the CFRT in subjects with temporomandibular disorders [37]. This device was used in replacement of the CROM for technical reasons: the position and fixation of the CROM device on the head made it impossible to execute the CFRT or could impair the test’s precision. The result of the CFRT test was interpreted as dichotomous data, considered positive when a side-to-side difference of 8º or more was present [14]. The MCID for this test is 4.7º for a right rotation and 7º for a left rotation [10].
Perceived Disability
Neck pain-related disability was measured with the French version of the Neck Disability Index questionnaire (NDI) (score ranging from 0–50). The questionnaire was validated for a CGH population, with a MCID of 5.5 points [38]. Headache-related disability was measured with the French version of the Henry Ford Hospital Headache Disability Inventory (HDI) (score ranging from 0–100, MCID: 29 points) [39].
Pain-Related Cognitive-Emotional Factors
We used three questionnaires to explore different aspects of cognitive-emotional drivers of pain: a modified version of the Fear-Avoidance Beliefs Questionnaire (FABQ), where « back » was replaced by « neck » (score ranging from 0–96), the Pain Catastrophizing Scale (PCS) (score ranging from 0–52) and the Tampa Scale for Kinesiophobia (TSK) (score ranging from 17–68). French versions were used for all questionnaires. Although these questionnaires have never been studied in a CGH population, they are widely used clinically for the prognosis of other chronic painful musculoskeletal conditions such as migraine and neck pain [40–42].
Compliance to self-SNAG exercises
Compliance was assessed by therapist reviewing the daily logbook. Each participant was asked to annotate the logbook with details on exercise parameters (time of day and number of reps).
Data analysis
Sample size calculation
Sample size estimates were based on pain intensity ratings (primary outcome), which was our main dependent variable. With power set to 80% to detect a difference in means of 20/100 on the VAS, assuming a standard deviation of differences of 12,7/100 and the 2-sided level of significance for alpha at 0.05 [based on a similar study design by [23], a minimum of 6 subjects was required.
Treatment Effect
All statistical analyses were performed with SPSS® statistical software (IBM SPSS Statistics for Windows, version 26.0; IBM, Chicago, IL) with an alpha level of.05. As all our main variables were not normally distributed (based on the Shapiro–Wilk test), non-parametric analysis were performed (a paired Wilcoxon test) to verify the pre-post effects. Accordingly, the median and interquartile range were reported for the main outcome measures.
Finally, the effect size (ES) was calculated based on the value of matched-pairs rank biserial r [43] to determine the magnitude of clinical changes after intervention following the equation below, with 95% of confidence intervals:
r = (Sf/S) – (Su/S)
where Sf is the sum of favorable ranks, S is the total sum ranks and Su is the sum of unfavorable ranks. The effect size was characterized by Cohen [44] as weak, moderate and strong effects, i.e., r = 0,2 is small, r = 0,5 medium and r = 0,8 large, respectively.
Association between CFRT and treatment response
Association between CFRT results at initial assessment (positive or negative) and significant improvement on pain intensity (>20/100 improvement) and neck-related disability (>5,5 improvement) were estimated using the Fisher exact test Figure 3.
Figure 3.
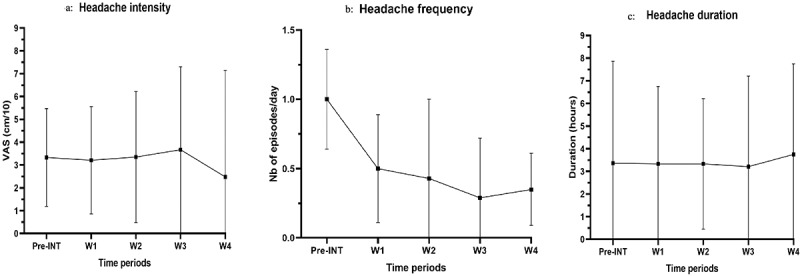
a. Pain intensity over time during the intervention period (expressed in median (IQR)), b. Headache frequency over time during the intervention period (expressed in median (IQR)), c. Headache duration over time during the intervention period (expressed in median (IQR))
Results
Demographics and baseline characteristics of participants
Baseline demographic information and clinical characteristics of participants are detailed in Table 2. A total of 38 potential participants contacted the main investigator for further information regarding the project. Sixteen (16) were excluded following the telephone screening and two (2) were excluded following initial clinical assessment, mainly because they were deemed to have another type of HA or other health conditions. Thus, our sample was comprised of 20 participants in pre- and post-intervention measurements. All 20 participants completed the study protocol (Figure 2).
Table 2.
Demographic information and baseline characteristics of participants
| Age (years) Gender (%) • Female • Male Headache history (years) Headache frequency (number of episodes/week) Pain intensity (/10) Pain duration (hours) Positive CFRT (%) Onset (%) • following neck trauma • following prolonged posture • unknown Comorbidity (%) • Other type of HA (ex: migraine) • Other MSK condition • Psychological condition (ex: depression) |
35.75 (SD: 11.48) 90 10 12.34 (SD: 10.52) 3.9 (SD: 2.26) 6.73 (SD:1.31) 15.34 (SD: 14.42) 75 25 30 45 15 10 5 |
CFRT: Cervical Flexion-Rotation Test; HA: Headache; MSK: Musculoskeletal; SD: Standard Deviation
Figure 2.
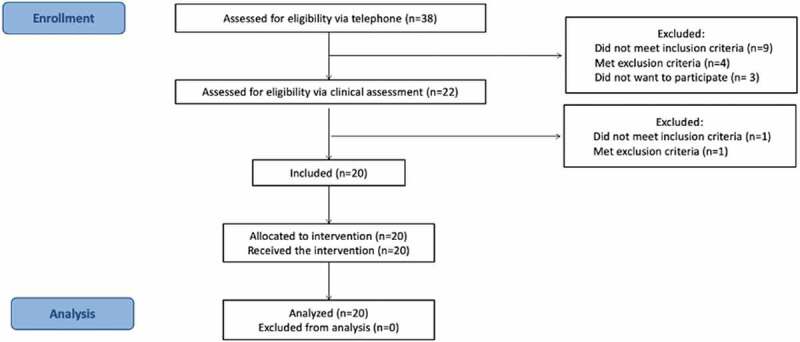
Flowchart
Treatment Effect
Overall pain intensity: The average pain intensity during HA episodes obtained through subjective perception significantly improved after intervention, as measured with the VAS (p < 0.00, Z = −3.763) (Table 3). A strong ES on pain reduction was observed after the treatment (ES r = 0.957), with a meaningful difference of 66% between times (baseline versus 4 weeks of intervention).
Table 3.
Median values and quartiles 1 and 3 of pain intensity, self-perceived physical function and pain-related cognitive-affective factors at baseline and post-intervention
| Pre-intervention (T0: baseline) |
Post-intervention (T1: +4 weeks) |
Paired Wilcoxon test |
Effect-size |
||
|---|---|---|---|---|---|
| Outcome | Median (Q1;Q3) | Median (Q1;Q3) | Z | p | Matched-paired rank-biserial r |
| Overall pain intensity | 6.80 (6.10;7.25) | 2.30 (1.10;3.60) | −3.763 | .000 | 0.957 |
| Neck-related perceived physical function | 16.00 (11.00;18.00) | 6.50 (4.75;9.00) | −3.724 | .000 | 0.974 |
| Headache-related perceived physical function | 41.00 (29.00;48.50) | 16.00 (9.00;26.00) | −3.772 | .000 | 0.962 |
| Fear-Avoidance beliefs | 23.00 (16.00;32.00) | 18.00 (9.00;25.50) | −1.420 | .156 | 0.362 |
| Pain catastrophizing | 25.00 (14.00; 31.50) | 5.00 (3.00; 12.00) | −3.865 | .000 | 0.986 |
| Kinesiophobia | 30.00 (28.00; 39.00) | 28.00 (26.50; 32.50) | −2.785 | .005 | 0.726 |
| Active neck ROM: flexion | 49.00 (41.50; 54.00) | 52.00 (45.75; 56.50) | −2.420 | 0.016 | 0.649 |
| Active neck ROM: extension | 50.50 (47.25; 60.5) | 58.00 (51.50; 66.25) | −2.462 | 0.014 | 0.661 |
| Active neck ROM: right rotation | 60.00 (56.50; 62.25) | 65.00 (61.50; 70.50) | −2.564 | 0.010 | 0.706 |
| Active neck ROM: left rotation | 62.00 (57.50; 66.50) | 68.00 (62.00; 78.00) | −3.141 | 0.002 | 0.800 |
| Active neck ROM: right side bending | 30.00 (25.50; 32.00) | 31.00 (27.50; 38.00) | −2.205 | 0.027 | 0.608 |
| Active neck ROM: left side bending | 32.00 (26.25; 38.00) | 36.00 (30.00; 40.50) | −2.728 | 0.006 | 0.800 |
| CFRT ROM: Affected side | 28.50 (24.25; 31.25) | 38.00 (26.00; 40.25) | −3.462 | 0.001 | 0.905 |
| CFRT ROM: Non-affected side | 41.50 (36.50; 46.00) | 39.00 (35.50; 41.25) | −1.615 | 0.106 | 0.421 |
ROM: Range Of Motion device; CFRT: Cervical Flexion-Rotation Test; Q1: Quartile 1; Q3: Quartile 3
Active range of motion and CFRT: Active range of motion in the neck significantly improved for all directions of movements after the 4 weeks of intervention (Table 3), with ES varying from moderate to strong for clinical changes across time (r = 0.608 to r = 0.800).
In addition, ROM measured during the CFRT significantly increased (p < 0.01) from T0 to T1 (+25% on affected side), with 15 participants showing a positive test before the intervention and only one participant still showing a positive test after the intervention. The magnitude of this change was strong across time (ES r = 0.905). The median side-to-side difference in patients showing a positive test was 12.00 degrees before the intervention and decreased to 1.00 degree after the intervention.
Perceived disability: The NDI score significantly improved post-intervention (p < 0.00, Z = −3.73) (Table 3), with 13 participants (65% of the sample) reaching minimal clinical improvement. The HDI score also significantly improved (p < 0.00, Z = −3.77) (Table 3) and 7 participants (35%) reached minimal clinical important change cutoff. These improvements were supported by clinical changes observed with the strong ES between pre- and post-intervention (r = 0.962 to 0.974 across questionnaires used).
Pain-related cognitive-emotional factors: PCS scores significantly decreased post-intervention (p < 0.00, Z = −3,865) (Table 3), as well as TSK scores (p < 0.01, Z = −2,785) (Table 3). Five (5) participants (25%) reached the minimal clinical improvement for PCS. However, the FABQ scores did not significantly change following the intervention (p = 0.16, Z = −1,420) (Table 3). The ES was strong for PCS (r = 0.986), while moderate for TSK (r = 0.726) on time factor differences (baseline versus 4 weeks of intervention).
Daily Logbook Measures
Median values of pain intensity and HA episode duration reported in the logbook during the pre-intervention period were similar to those reported during week 4 (p = 0,709, Z = −0,373 and p = 0,629, Z = −0,483 respectively) (Table 3). The paired Wilcoxon test revealed a statistically significant improvement in HA frequency (p < 0,000, Z = −3,641) (Table 3). This variable improved every week during the intervention period, with participants reporting a median of 1,000 episodes per day pre-intervention and 0.345 episode per day during week 4, which represents a decrease of more than 65%.
Adherence with Self-SNAG Exercise
All participants reported performing the self-SNAG home exercise. However, the mean number of sets performed was 1.85/day, but participants reported completing the prescribed number of repetitions for each set. No adverse effect was reported during the technique or home exercise.
Association Between CFRT and Treatment Effect
Fifteen (15) participants had a positive CFRT before the intervention. All 15 participants significantly improved (surpassed the MCID) on pain intensity ratings as measured pre-and post-intervention, and 13 significantly improved (surpassed the MCID) on NDI. The Fisher exact test failed to demonstrate an association between CFRT results (positive) and significant improvement (MCID reached) for both pain intensity (p = 0.634) and neck-related physical function (p = 0.594).
Participants with a negative CFRT pre-intervention showed significant improvement on different outcomes: four (4) participants significantly improved on overall pain intensity, three (3) significantly improved on CFRT range of motion (on the less mobile side) while one significantly worsened, two (2) significantly improved on self-perceived physical function (NDI and HDI scores) and one (1) improved on pain-related cognitive-affective factors (FABQ-PA subscale, PCS and TSK) (see Table 4).
Table 4.
Contingency tables for Fisher’s tests to explore the association between CFRT results on pain and physical function response
| Significant improvement on pain intensity (MCID reached) |
||||
|---|---|---|---|---|
| Yes | No | Total | ||
| Positive CFRT pre-intervention | Yes | 11 | 4 | 15 |
| No | 4 | 1 | 5 | |
| Total | 15 | 5 | 20 | |
| Significant improvement on NDI score (MCID reached) | ||||
| Yes | No | Total | ||
| Positive CFRT pre-intervention | Yes | 10 | 4 | 15 |
| No | 3 | 2 | 5 | |
| Total | 13 | 7 | 20 | |
CFRT: Cervical Flexion-Rotation Test; NDI: Neck Disability Index; MCID: Minimal Clinical Important Difference
Discussion
Main findings
The main purpose of this study was to assess the immediate effect of treatments combining C1-C2 rotation SNAG mobilizations and a C1-C2 self-SNAG rotation exercise program on pain (headache frequency, duration and intensity), cervical mobility, neck and headache-related disability, fear-avoidance beliefs, pain catastrophizing and kinesiophobia variables. Significant clinical benefits were found after treatment for our primary outcome related to pain measurement (clinical change ES r = 0.957), with 66% of reduction on VAS scale. This positive improvement (4 points on scale from differences reported between pre- and post-intervention was higher when compared to minimal clinical important difference (MCID: 2/10) [29]. These results were further supported by strong ES on clinical changes with the treatment, supporting the use of this type of intervention for this population.
There is some dissonance when extracting data from the logbook regarding pain intensity, duration, and frequency. When analyzing data on a weekly basis, only HA frequency decreased, while pain intensity and duration remained stable. This discrepancy might be explained by an information bias. The VAS measures pain severity at a very specific time (ie: when exercises are performed and pain intensity is likely to be heightened). Thus, when we try to extrapolate changes in pain severity ratings to overall clinical change (improvement), we may observe a biased result. Hence, it may have been more relevant to use a specific tool that measures global improvement, such as the Patient Global Rating of Change scale.
Significant benefits were also reported on biomechanical outcomes, such as active neck ROM, but more importantly, on neck rotation during the CFRT in the affected side (an increase of 25% after intervention). This positive improvement (9.50° between pre- and post-intervention was higher when compared to the minimal clinical important difference (MCID: 7°) [10]. Moderate ES was found for active range of motion in the neck, but a strong ES (r = 0.905) was shown for CFRT change. The magnitude of change on this test supports the use of the Mulligan approach to restore expected mobility at the C1-C2 segment. Our results on biomechanical outcomes are consistent with the pathophysiology of CGH and may support the idea that addressing C1-C2 ROM deficits is important to attenuate CGH symptoms.
Important improvements were also noted on self-perceived physical function in relation to neck pain and headache. Strong ES were obtained for the NDI score (r = 0.974) and for the HDI score (r = 0.962), which supports a significant clinical change with the treatment for self-perceived physical function in relation to neck pain and HA.
Overall, the change was also significant for pain catastrophizing (80% improvement on the PCS) and kinesiophobia (0.07% improvement on the TSK). The ES was strong for PCS (r = 0.986), while moderate for TSK (r = 0.726) on time factor differences (baseline versus 4 weeks of intervention). Fear-Avoidance beliefs did not significantly change after the intervention. However, questionnaires used to measure pain-related cognitive-affective factors did not show significant fear-avoidance beliefs, kinesiophobia and pain catastrophizing in our sample before the intervention. This should be taken into consideration when interpreting the results, since these factors might not have been an issue at first.
The results of the present study are consistent with current knowledge on manual therapy mechanisms, supporting that a mechanical force from a manual intervention is most likely related to biomechanical and systemic neurophysiological responses leading to pain inhibition; the reduction in pain intensity might be explained by a biomechanical effect (supporting the idea that restoring C1-C2 mobility would eliminate the cause of CGH), as well as nonspecific response of hands-on treatment modalities, which are known to have effects on the psychological areas of pain [45–47].
Many participants showing a positive CFRT test pre-intervention, as well as participants showing a negative response to this same test, significantly improved post-intervention on patient-important outcomes as well as biomechanical outcomes. In addition, no association was found between CFRT results as a dichotomous data (positive versus negative) and significant improvement in pain intensity and function in relation to neck pain, pre-and post-intervention. The low and unbalanced number of participants in the two subgroups did not allow sufficient statistical power to adequately explore the question. Future studies are needed to examine the possible utility of CFRT as a predictive factor for treatment response.
Comparison of Findings with Previous Studies
Our results on overall pain and neck-related self-perceived physical function are consistent with other studies investigating the effect of the SNAG manual technique or self-SNAG exercise to improve C1-C2 rotation. Significant improvement on pain intensity (VAS) was observed when using the technique alone in 3 studies [23,24] and disability due to neck pain (NDI) was observed when using the technique alone in 4 studies [22–24]. HA episode duration was also measured and significantly improved in one of these studies [24].
Other authors assessed physical function in headache patients with the Headache Impact Test (HIT-6) [21,22] and the Headache Activities of Daily Living Index (HADLI) [21] to measure the effectiveness of the C1-C2 SNAG mobilization technique. In these studies, SNAG mobilizations demonstrated significant improvement on both the HIT-6 and HADLI. Finally, one study reported significant improvements in HA severity, including pain intensity, frequency and duration as well as neck-perceived physical function with the sole use of self-SNAG exercise [18].
Biomechanical impairments were less commonly reported in previous studies. Significant improvement in neck active range of motion with the SNAG manual technique was reported by 23, and 22. Significant improvement on CFRT [restricted side) with the self-SNAG exercise was reported by 18. The CFRT was also measured pre-and post-intervention by 21, and showed an improvement of 13º, but no statistical analysis was provided.
Strengths and Limitations
Considering previous studies, the current authors used the Mulligan approach as practiced in clinical settings, including in-clinical manual treatment and a home exercise program to maintain progress and self-sufficiency. The authors also used a specific exercise dosage (intervention procedures], which is easily reproducible in a clinical setting or in future studies.
The authors included outcomes measuring different aspects of pain, as well as biomechanical features related to CGH. The variety of measurement tools used in our study allowed us to appreciate the involvement of biomechanics as well as pain, physical function, and cognitive-affective factors. This is an important strength of our study when considering the actual knowledge on CGH pathophysiology, manual therapy mechanisms and chronic pain development. The use of a manual approach combining a hands-on intervention with a home-exercise program targeting C1-C2 segment seems beneficial for multiple aspects of pain and disability.
Several limitations should be considered when interpreting the results of this study. First, no conclusion can be brought forth on the efficacy of the intervention, due to the absence of a control group. Also, the benefits noted in the current study were only assessed immediately post-intervention and no long-term follow-up was performed. Patient sample size is another important limitation. Although the sample size required to determine the effect of our intervention on overall pain was respected, normality of data could not be reached. This sample size calculation did not include other important outcomes such as CFRT, self-perceived physical function and pain-related cognitive-affective factors. Therefore, generalization of these results is not indicated. Another limitation lies in the fact that many steps of the project were entrusted to the same person, including selection of participants, pre-and post-intervention measurements, as well as performing the intervention. This could lead to potential bias in patient response as well as data collection, and might influence the results.
Implications for practice
The results of the current study contribute to support the use of Mulligan’s SNAG and self-SNAG in the management of patients with CGH by showing the potential benefits of different aspects of pain and physical impairments.
Implications for research
Self-reported outcome measures used for pain-related cognitive-affective factors were not specifically validated for the CGH population and failed to demonstrate the presence of these factors in our sample. However, pain catastrophizing may be involved in CGH and would therefore warrant further studies. The current results suggest that the C1-C2 SNAG technique, combined with self-SNAG exercises, might have a positive effect on pain catastrophizing and kinesiophobia. Therefore, such measurement tools could be validated with the CGH population and be included in interventional studies.
The association between the C1-C2 rotation range of motion during the CFRT and treatment response should be further explored, as this study’s sample size was not large enough to reach a level of statistical power that could adequately answer our research question. Another study with a larger sample size and a precise methodology might be able to add additional information, which could lead to a better understanding of the CFRT’s value.
Conclusion
SNAG mobilization combined with a self-SNAG home exercise resulted in favorable outcomes for the treatment of cervicogenic headache. Beneficial effects were noted on patient-important outcomes, such as pain intensity and self-perceived physical function, as well as biomechanical outcomes, including active range of motion in the neck with CFRT. Additional results showed a potential benefit on pain-related cognitive-affective factors, namely on pain catastrophizing. Future studies are required to strengthen knowledge on the benefits of physical therapy in CGH patients for all aspects of pain [48,49].
Acknowledgments
We thank Clinique Physio-Santé for their infrastructures and all the participants to this study
Biographies
Jean-Philippe Paquin, PT, is a clinical professor at Health Sciences Department (Physical Therapy Program) of Université du Québec à Chicoutimi, Qc, Canada. Jean-Philippe’s fields of interest include manual therapy, therapeutic exercise, headache, neck disorders and neurodynamics.
Yannick Tousignant-Laflamme, PT, PhD, is Professor and director of the Physical Therapy Program (School of Rehabilitation) of the Université de Sherbrooke, Qc, Canada. Yannick’s fields of interest include pain science/management, low back pain, and orthopedic physical rehabilitation.
Jean-Pierre Dumas, PT, PhD, is an Associate Professor at the School of Rehabilitation (Physical Therapy Program) of the Université de Sherbrooke, Qc, Canada. Jean-Pierre’s fields of interest include neck disorders, cervicogenic headache and clinical reasoning.
Appendices.
Appendix 1: List of contraindications to cervical manipulative therapy
uncontrolled cardiovascular disease (such as hypertension, hypercholesterolemia, etc.)
multi-level nerve root pathology
worsening neurological function
unremitting, severe, non-mechanical pain
unremitting night pain
recent trauma (less than 6 weeks)
upper motor neuron lesions
spinal cord damage
signs and symptoms of vertebrobasilar insufficiency (dizziness, diplopia, dysarthria, dysphagia, drop attacks, nausea, nystagmus, facial numbness, ataxia, vomiting, hoarseness, loss of short-term memory, vagueness, hypotonia/limb weakness, anhidrosis, hearing disturbances, malaise, perioral dysesthesia, photophobia, papillary changes, clumsiness, agitation)
signs and symptoms of internal carotid insufficiency (carotidynia, ptosis, transient retinal dysfunction, transient ischemic attacks, cerebrovascular accidents)
cranial nerve dysfunction
hindbrain stroke (e.g. Wallenberg’s syndrome, locked-in syndrome)
signs and symptoms of upper cervical instability (bilateral foot and hand dysesthesia, feeling of a lump in the throat, metallic taste in the mouth, arm and leg weakness, lack of coordination bilaterally, positive instability tests)11
Osteoporosis
Hyperlaxity Syndrome (ex: Marfan syndrome)
Down’s Syndrome
Local infection
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Olesen J.The International Classification of Headache Disorders, 3rd edition. Cephalagia. 2013;33(9):629‑808. [DOI] [PubMed] [Google Scholar]
- [2].Nilsson N. The prevalence of cervicogenic headache in a random population sample of 20-59 year olds. Spine (Phila Pa 1976). 1995;20(17):1884‑1888. [DOI] [PubMed] [Google Scholar]
- [3].Lord SM, Barnsley L, Wallis BJ, et al. Third occipital nerve headache : A prevalence study. J Neurol Neurosurg Psychiatry. 1994;57(10):1187‑1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Diener I. The impact of cervicogenic headache on patients attending a private physiotherapy practice in Cape Town. S Afr J Physiother. 2001;57(1):35‑39. [Google Scholar]
- [5].Cooper G, Bailey B, Bogduk N. Cervical Zygapophysial Joint Pain Maps. Pain medecine. 2007;8(4):344‑353. [DOI] [PubMed] [Google Scholar]
- [6].Zito G, Jull G, Story I. Clinical tests of musculoskeletal dysfunction in the diagnosis of cervicogenic headache. Manual Ther. 2006;11(2):118‑129. [DOI] [PubMed] [Google Scholar]
- [7].Dvořák J, Dvořák V, Drobný T, et al. Manual medicine : diagnostics. Thieme; 1984. [Google Scholar]
- [8].Hall T, Briffa K, Hopper D. Clinical Evaluation of Cervicogenic Headache : A Clinical Perspective. J Man Manip Ther. 2008;16(2):73‑80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hall T, Briffa K, Hopper D. The influence of lower cervical joint pain on range of motion and interpretation of the flexion-rotation test. J Man Manip Ther. 2010;18(3):126‑131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hall T, Briffa K, Hopper D, et al. Long-term stability and minimal detectable change of the cervical flexion-rotation test. J Orthop Sports Phys Ther. 2010;40(4):225–229. [DOI] [PubMed] [Google Scholar]
- [11].Hall TM, Briffa K, Hopper D, et al. The relationship between cervicogenic headache and impairment determined by the flexion-rotation test. J Manipulative Physiol Ther. 2010b;33(9):666‑671. [DOI] [PubMed] [Google Scholar]
- [12].Hall TM, Briffa K, Hopper D, et al. The relationship between cervicogenic headache and impairment determined by the flexion-rotation test. J Manipulative Physiol Ther. 2010c;33(9):666–671. [DOI] [PubMed] [Google Scholar]
- [13].Hall TM, Briffa K, Hopper D, et al. Comparative analysis and diagnostic accuracy of the cervical flexion-rotation test. J Headache Pain. 2010a;11(5):391‑397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ogince M, Hall T, Robinson K, et al. The diagnostic validity of the cervical flexion-rotation test in C1/2-related cervicogenic headache. Manual Ther. 2007;12(3):256‑262. [DOI] [PubMed] [Google Scholar]
- [15].Schäfer A, Lüdtke K, Breuel F, et al. Validity of eyeball estimation for range of motion during the cervical flexion rotation test compared to an ultrasound-based movement analysis system. Physiother Theory Pract. 2018;8:1‑7. doi: 10.1080/09593985.2017.1423523 [DOI] [PubMed] [Google Scholar]
- [16].Nicholson GG, Gaston J. Cervical Headache. J Orthop Sports Phys Ther. 2001;31(4):184‑193. [DOI] [PubMed] [Google Scholar]
- [17].Dunning JR, Butts R, Mourad F, et al. Upper cervical and upper thoracic manipulation versus mobilization and exercise in patients with cervicogenic headache : A multi-center randomized clinical trial. BMC Musculoskelet Disord. 2016;17(1):64‑64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hall T, Chan HT, Christensen L, et al. Efficacy of a C1-C2 self-sustained natural apophyseal glide (SNAG) in the management of cervicogenic headache. J Orthop Sports Phys Ther. 2007;37(3):100‑107. [DOI] [PubMed] [Google Scholar]
- [19].Jull G, Trott P, Potter H, et al. A randomized controlled trial of exercise and manipulative therapy for cervicogenic headache. discussion 1843Spine (Phila Pa 1976). 2002;2717:1835‑1843. [DOI] [PubMed] [Google Scholar]
- [20].Mohamed AA, Shendy WS, Semary M, et al. Combined use of cervical headache snag and cervical snag half rotation techniques in the treatment of cervicogenic headache. J Phys Ther Sci. 2019;31(4):376‑381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Put M, Huber J, Pieniążek M, Gądek-Michalska A, Szczygieł A. A Randomized Clinical Trial of Multimodal Therapy and Mulligan’s Concept of Manual Therapy for Patients with Chronic Pain Syndrome Caused by Upper Cervical Spine Disorders. Int J Orthop Rehabil. 2016. doi: 10.12974/2313-0954.2016.03.01.6 [DOI] [Google Scholar]
- [22].Shin E-J, Lee B-H. The effect of sustained natural apophyseal glides on headache, duration and cervical function in women with cervicogenic headache. J Exerc Rehabil. 2014;10(2):131‑135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Blanpied PR, Gross AR, Elliott JM, et al. Neck Pain : revision 2017. J Orthop Sports Phys Ther. 2017;47(7):A1‑A83. [DOI] [PubMed] [Google Scholar]
- [24].Vicenzino B. Mobilisation with movement : the art and the science. Chatswood, N.S.W.: Churchill Livingstone/Elsevier; 2011. [Google Scholar]
- [25].Rushton A, Rivett D, Carlesso L, Flynn T, Hing W, Kerry R. International framework for examination of the cervical region for potential of Cervical Arterial Dysfunction prior to Orthopaedic Manual Therapy intervention. Man Ther. 2014 Jun;19(3):222‑8. doi: 10.1016/j.math.2013.11.005. Epub 2013 Nov 23. PMID: 24378471 [DOI] [PubMed] [Google Scholar]
- [26].Mulligan BR. Manual Therapy : nags, Snags, Mwms, Etc. 6th ed.) ed. Wellington, New Zealand: Bateson Publishing Ltd; 2010. [Google Scholar]
- [27].MacDowall A, Skeppholm M, Robinson Y, et al. Validation of the visual analog scale in the cervical spine. J Neurosurg Spine. 2018;28(3):227‑235. [DOI] [PubMed] [Google Scholar]
- [28].Audette I, Dumas J-P, Côté JN, et al. Validity and between-day reliability of the cervical range of motion (CROM) device. J Orthop Sports Phys Ther. 2010;40(5):318‑323. [DOI] [PubMed] [Google Scholar]
- [29].Inokuchi H, Tojima M, Mano H, et al. Neck range of motion measurements using a new three-dimensional motion analysis system : validity and repeatability. Eur Spine J. 2015;24(12):2807‑2815. [DOI] [PubMed] [Google Scholar]
- [30].Williams MA, McCarthy CJ, Chorti A, et al. A Systematic Review of Reliability and Validity Studies of Methods for Measuring Active andPassive Cervical Range of Motion. J Manipulative Physiol Ther. 2010;33(2):138‑155. [DOI] [PubMed] [Google Scholar]
- [31].Takasaki H, Hall T, Oshiro S, et al. Normal kinematics of the upper cervical spine during the Flexion-Rotation Test—In vivo measurements using magnetic resonance imaging. Manual Ther. 2011;16(2):167‑171. [DOI] [PubMed] [Google Scholar]
- [32].Luedtke K, Boissonnault W, Caspersen N, et al. International consensus on the most useful physical examination tests used by physiotherapists for patients with headache : A Delphi study. Manual Ther. 2016;23:17‑24. [DOI] [PubMed] [Google Scholar]
- [33].Correll S, Field J, Hutchinson H, et al. Reliability and validity of the HALO digital goniometer for shoulder range of motion in healthy subjects. Int J Sports Phys Ther. 2018;13(4):707‑714. [PMC free article] [PubMed] [Google Scholar]
- [34].Furness J, Johnstone S, Hing W, et al. Assessment of shoulder active range of motion in prone versus supine : A reliability and concurrent validity study. Physiother Theory Pract. 2015;31(7):489‑495. [DOI] [PubMed] [Google Scholar]
- [35].von Piekartz H, Pudelko A, Danzeisen M, et al. Do subjects with acute/subacute temporomandibular disorder have associated cervical impairments : A cross-sectional study. Manual Ther. 2016;26:208‑215. [DOI] [PubMed] [Google Scholar]
- [36].Young IA, Dunning J, Butts R, et al. Psychometric properties of the Numeric Pain Rating Scale and Neck Disability Index in patients with cervicogenic headache. Cephalalgia. 2019;39(1):44‑51. [DOI] [PubMed] [Google Scholar]
- [37].Jacobson GP, Ramadan NM, Aggarwal SK, et al. The Henry Ford Hospital Headache Disability Inventory (HDI). Neurology. 1994;44(5):837‑842. [DOI] [PubMed] [Google Scholar]
- [38].Benatto MT, Bevilaqua-Grossi D, Carvalho GF, et al. Kinesiophobia Is Associated with Migraine. Pain Med. 2019;20(4):846‑851. [DOI] [PubMed] [Google Scholar]
- [39].Landers MR, Creger RV, Baker CV, et al. The use of fear-avoidance beliefs and nonorganic signs in predicting prolonged disability in patients with neck pain. Manual Ther. 2008;13(3):239‑248. [DOI] [PubMed] [Google Scholar]
- [40].Mortazavi Nasiri FS, Pakdaman S, Dehghani M, et al. The Relationship between Pain Catastrophizing and Headache-Related Disability : the Mediating Role of Pain Intensity. Japanese Psychological Research. 2017;59(4):266‑274. . [Google Scholar]
- [41].Kerby DS. The Simple Difference Formula: An Approach to Teaching Nonparametric Correlation. Comprehensive Psychology. January 2014. Doi: 10.2466/11.IT.3.1 [DOI] [Google Scholar]
- [42].Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Routledge. New York; 10.4324/9780203771587 [DOI] [Google Scholar]
- [43].Bialosky JE, Beneciuk JM, Bishop MD, et al. Unraveling the Mechanisms of Manual Therapy : modeling an Approach. J Orthop Sports Phys Ther. 2017;8(1):1‑31. [DOI] [PubMed] [Google Scholar]
- [44].Bialosky JE, Bishop MD, Price DD, et al. The mechanisms of manual therapy in the treatment of musculoskeletal pain : A comprehensive model. Manual Ther. 2009;14(5):531‑538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bishop MD, Torres-Cueco R, Gay CW, et al. What effect can manual therapy have on a patient’s pain experience? Pain Manag. 2015;5(6):455‑464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Aprill C, Axinn MJ, Bogduk N. Occipital headaches stemming from the lateral atlanto-axial (C1-2) joint. Cephalalgia. 2002 Feb;22(1):15-22. Doi: 10.1046/j.1468-2982.2002.00293.x. PMID: 11993608 [DOI] [PubMed]
- [47].Hall TM, Robinson KW, Fujinawa O, et al. Intertester Reliability and Diagnostic Validity of the Cervical Flexion-Rotation Test. J Manipulative Physiol Ther. 2008;31(4):293‑300. [DOI] [PubMed] [Google Scholar]
- [48].Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013. Jul;33(9):629–808. doi: 10.1177/0333102413485658. PMID: 23771276. [DOI] [PubMed] [Google Scholar]
- [49].Scott W, Wideman TH, Sullivan MJL. Clinically meaningful scores on pain catastrophizing before and after multidisciplinary rehabilitation: a prospective study of individuals with subacute pain after whiplash injury. Clin J Pain. 2014;30(3):183–190. doi: 10.1097/AJP.0b013e31828eee6c [DOI] [PubMed] [Google Scholar]


