Environmentally responsive hydrogels formed by peptide self-assembly lead to materials with defined structure and utility for a wide range of biomedical applications.[1] The formation of structurally defined materials through the complexation of metal ions by peptides has been of particular interest. In nature, interactions of metals with histidine, methionine, cysteine, aspartic acid, and glutamic acid residues are utilized to induce conformational changes in proteins to drive diverse biological reactions and to form supramolecular structures.[2] Peptides have been developed to take advantage of these naturally occurring ligands to enhance structural stability and promote self-assembly on binding to a variety of metal cations.[3] Additionally, non-natural metal-ligating residues have been synthesized to serve as triggers for higher-order assembly.[4]
Zinc is an essential cofactor in transcription factors and enzymes involved in cell replication, protein synthesis, and extracellular remodeling.[5,6] In wound repair, zinc promotes matrix metalloproteinase debridement and keratinocyte migration at the site of damaged tissue.[6,7] Topical administration of zinc results in the enhanced healing of both acute and chronic wounds.[8] In addition, zinc can inhibit the growth of several bacterial species, and thus can help to prevent infection in wound beds.[9] Therefore, the development of biomaterials incorporating zinc would be beneficial as wound dressings that help augment the healing process and lower the risk of infection.
Herein, we report the design of a metal-responsive peptide-based hydrogelation system, in which material formation is triggered by zinc binding (Figure 1a). In aqueous solution and in the absence of metal ions, the peptide is unfolded and soluble. The addition of Zn2+ ions results in chelation of the metal to a non-natural ligand on the peptide, which in turn triggers peptide folding and subsequent self-assembly into a β-sheet-rich fibrillar hydrogel. The peptide, designated ZnBHP (zinc-binding hairpin peptide, Figure 1b), is a 20-residue β-hairpin peptide[10] composed of two amphiphilic β strands that have alternating hydrophilic lysine and hydrophobic valine and isoleucine residues. The strands are connected by a type II’ β turn (-VDPPT-), which contains four residues. To confer metal responsiveness to the peptide, a negatively charged unnatural metal-binding amino acid, 3-amidoethoxyaminodiacetoxy-2-aminopropionic acid (1, Figure 1c) was placed at the C terminus at position 20.[4] In the apo state (no zinc), this residue is designed to inhibit peptide folding by significantly raising the energy barrier that must be overcome to initiate folding. For peptide folding to occur in the absence of metal, the negatively charged side chain (2−) of residue 1 would need to be desolvated and incorporated within the hydrophobic environment of the folded state, an energetically costly proposition. However, 1 is a strong chelator of divalent metal ions that form electroneutral multivalent complexes. As will be shown, when residue 1 binds Zn2+ ions, its side chain becomes neutral, the energy barrier en route to the folded state is decreased, and β-hairpin formation is enabled. Once folded, ZnBHP is designed to fold and self-assemble into a β-sheet-rich network of fibrils, in which each fibril is composed of a bilayer of folded hairpins that is hydrogen-bonded along the fibril long axis (Figure 1). A control peptide with a valine at position 20 was also prepared to investigate the ability of residue 1 to prevent folding of ZnBHP in its apo state (Figure 1b).
Figure 1.
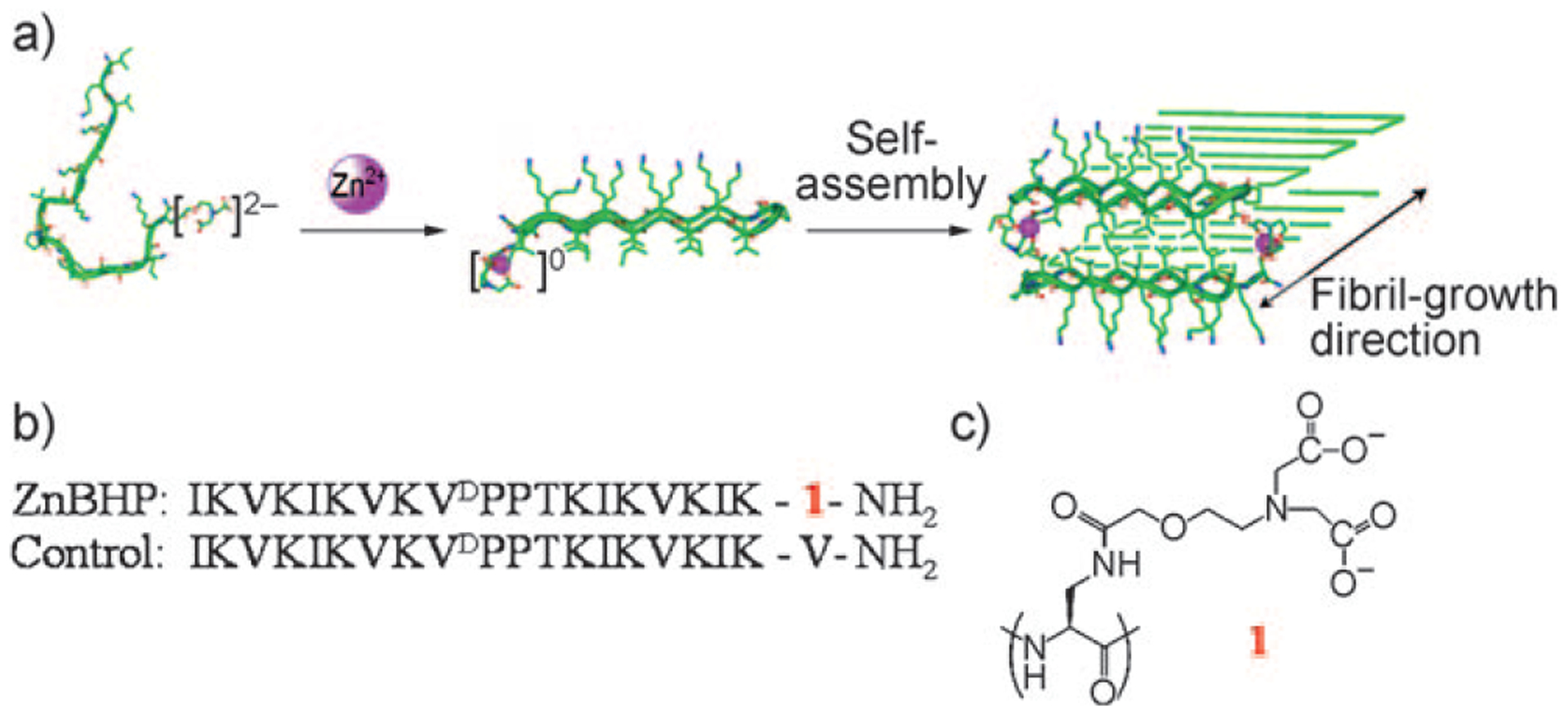
a) Proposed mechanism of metal-triggered folding and self-assembly of ZnBHP. b) Primary sequences of ZnBHP and the control peptide. c) Structure of the non-natural amino acid 1 (3-amidoethox-yaminodiacetoxy-2-aminopropionic acid).
Circular dichroism (CD) spectroscopy was used to follow the folding and subsequent self-assembly of ZnBHP and the control peptide at pH 7.4 (50 mM bis-tris propane (BTP), 50 mM NaCl) and 30 °C. Figure 2a shows CD spectra of 0.5 wt% ZnBHP and the control peptide under apo conditions. As expected, under these conditions ZnBHP adopts a random-coil conformation, which is caused by the net negative charge of 1. The control peptide is capable of folding and shows a spectrum characteristic of a β-sheet structure with a minimum at 216 nm. This result demonstrates that the metal-free residue 1 is capable of keeping the peptide in its unfolded state. When ZnCl2 is added to the peptide solution, the evolution of the β-sheet structure commences (Figure 2b). As more Zn2+ ions are added, the signal intensity at 216 nm increases until a maximal β-sheet signal is reached at a stoichiometry of 1 equivalent of Zn2+ ions. Further addition of Zn2+ ions does not influence the observed CD signal. This observation supports the existence of a 1:1 metal/peptide stoichiometry, as was intended by the peptide design. FTIR spectroscopy also showed β-sheet evolution as a consequence of zinc binding (see the Supporting Information). Zinc chelation and the 1:1 binding stoichiometry of ZnBHP was confirmed by ESI mass spectrometry (m/z: calcd for [M + 2H + Zn]2+: 1276.4, found: 1276.7; see the Supporting Information). Taken together, this data is consistent with zinc-triggered folding and self-assembly of ZnBHP.
Figure 2.

CD spectra of peptides in pH 7.4 buffer (50 mM BTP) with 50 mM NaCl at 30 °C: a) 0.5 wt% apo ZnBHP (▲) and control peptide (□). b) 0.5 wt% ZnBHP in the presence of 0 equiv (▲), 0.25 equiv (○), 0.5 equiv (□), 1.0 equiv (◆), and 2.0 equiv (▲) of ZnCl2. c) 0.5 wt% ZnBHP apo (▲) and with 1.0 equiv of Zn2+ ions (◆), Ca2+ ions (◯), Cu2+ ions(□), and Hg2+ ions(▲).
A representative selection of other metal ions (2 + charge) was also investigated by using CD spectroscopy to probe the selectivity of metal binding. Figure 2c shows CD spectra of ZnBHP with 1 equivalent of Ca2+ ions, Cu2+ ions, and Hg2+ ions. All metals were introduced as their chloride salts. The samples containing Ca2+ ions and Cu2+ ions showed spectra that were consistent with a random coil conformation. The addition of Hg2+ ions led to precipitation of the peptide and attenuation of the CD signal. Of the four metals, Zn2+ ions were the only metal ions capable of initiating folding, self-assembly, and ultimate gelation of ZnBHP; the basis of this selectivity is not entirely apparent. Chelation studies of amino polycarboxylate ligands, such as N-methoxyethyl-iminodiacetic acid, show a clear preference of the chelating ligands for Zn2+ ions over either Ca2+ or Hg2+ ions.[11] However, residue 1 with up to at least 5 possible ligating atoms should accommodate the known four- and five-coordinate binding geometries of Cu2+ ions.[12] Therefore, additional factors that include Lewis acidity and ionic radius may be contributing to selectivity.
Material properties of the resulting ZnBHP–Zn2+ hydrogel were characterized using oscillatory sheer rheology. Figure 3a shows a dynamic time sweep experiment that monitors the evolution of the storage modulus (G’) after hydrogelation has been triggered in the presence of 1 equivalent of Zn2+ ions. A G’ of (157 ± 43) Pa was realized after 2 hours at 30 °C. For these gels, G′ is one order of magnitude larger than the loss modulus (G“), thus being indicative of a moderately rigid viscoelastic material. Frequency and strain sweep experiments were also performed (see the Supporting Information). In the frequency sweep, G′ and G″ show a gradual slope upwards with increasing frequency. This behavior is observed for soft cross-linked elastomers as opposed to viscoelastic liquids and is consistent with the moderately rigid character of the peptide gel.[13] The strain sweep data show that ZnBHP–Zn2+ hydrogels have a yielding strain of approximately 80%. The control peptide has a G′ of (86±9) Pa (see the Supporting Information), which is slightly less than the metal-binding peptide in the presence of zinc. A representative bulk ZnBHP–Zn2+ hydrogel is shown in Figure 3b; the gel is optically clear and self-supporting, whereas the apo sample remains a freely flowing liquid.
Figure 3.

a) Oscillatory rheological dynamic time sweep of 0.5 wt% ZnBHP in pH 7.4 buffer (50 mM BTP) with 50 mM NaCl at 30 °C.G′ (■) and G″ (□) are the storage modulus and loss modulus, respectively. b) Image of 0.5 wt% ZnBHP bulk samples in pH 7.4 buffer (50 mM BTP) with 50 mM NaCl at 30 °C in the presence and absence of 1.0 equivalent of ZnCl2.
The local morphology of the ZnBHP hydrogel network was examined using TEM (Figure 4). The gel is composed of fibrils with widths of approximately 3 nm, a distance that is consistent with the length of a folded ZnBHP hairpin in the self-assembled state (Figure 1). The fibrils are entangled, thus forming physical cross-links that presumably contribute to the mechanical properties of the hydrogel. Higher-order self-assembly is also observed in the form of fibrillar lamination, where fibrils can be seen to stack next to each other or gently twisting around each other.
Figure 4.

TEM image of 0.5 wt% ZnBHP in pH 7.4 buffer (50 mM BTP, 50 mM NaCl) with 1.0 equivalent of ZnCl2.
In summary, we describe a selective, zinc-induced formation of a peptide hydrogel by triggered amphiphilic β-hairpin folding and subsequent self-assembly. Peptide folding is possible through the chelation of a divalent zinc ion to the negatively charged unnatural amino acid 1. This binding lowers the activation energy of folding and ultimately permits gelation by self-assembly of folded ZnBHP. CD and FTIR spectroscopy, as well as mass spectrometry, confirm that the peptide binds Zn2+ ions with a 1:1 metal/peptide stoichiometry. Rheology and TEM demonstrate that ZnBHP forms a moderately rigid, fibrillar hydrogel in the presence of Zn2+ ions. Interestingly, the amount of zinc present in the hydrogel network is controlled by the amount of self-assembling peptide used to form the hydrogel. Thus, a precise and controllable concentration of Zn2+ ions is deliverable to a wound bed to promote healing and decrease the potential for bacterial infection. Further investigations as to the potential application of this gel are underway.
Supplementary Material
Acknowledgments
This work was supported in part by the National Science Foundation (CHE 0348323, JPS) and the Arnold and Mabel Beckman Foundation.
Contributor Information
Monica C. Branco, National Cancer Institute—National Institutes of Health, Frederick, MD 21701 (USA)
Joel P. Schneider, National Cancer Institute—National Institutes of Health, Frederick, MD 21701 (USA).
References
- [1].a) Chung HJ, Park TG, Nano Today 2009, 4, 429; [Google Scholar]; b) Kopecek J, Yang J, Acta Biomater. 2009, 5, 805; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lu S, Chen P, Front. Mater. Sci. China 2010, 4, 145; [Google Scholar]; d) Mart RJ, Osborne RD, Stevens MM, Ulijn RV, Soft Matter 2006, 2, 822; [DOI] [PubMed] [Google Scholar]; e) Tsitsilianis C, Soft Matter 2010, 6, 2372; [Google Scholar]; f) Williams RJ, Mart RJ, Ulijn RV, Pept. Sci 2010, 94, 107; [DOI] [PubMed] [Google Scholar]; g) Zhang S, Marini DM, Hwang W, Santoso S, Curr. Opin. Chem. Biol 2002, 6, 865; [DOI] [PubMed] [Google Scholar]; h) Smith AM, Williams RJ, Tang C, Coppo P, Collins RF, Turner ML, Saiani A, Ulijn RV, Adv. Mater 2008, 20, 37; [Google Scholar]; i) Ulijn RV, J. Mater. Chem 2006, 16, 2217; [Google Scholar]; j) Adams DJ, Topham PD, Soft Matter 2010, 6, 3707; [Google Scholar]; k) Naskar J, Palui G, Banerjee A, J. Phys. Chem. B 2009, 113, 11787; [DOI] [PubMed] [Google Scholar]; l) Adhikari B, Palui G, Banerjee A, Soft Matter 2009, 5, 3452; [Google Scholar]; m) Banwell EF, Abelardo ES, Adams DJ, Birchall MA, Corrigan A, Donald AM, Kirkland M, Serpell LC, Butler MF, Woolfson DN, Nat. Mater 2009, 8, 596; [DOI] [PMC free article] [PubMed] [Google Scholar]; n) Panda JJ, Mishra A, Basu A, Chauhan VS, Biomacromolecules 2008, 9, 2244; [DOI] [PubMed] [Google Scholar]; o) Ma ML, Kuang Y, Gao Y, Zhang Y, Gao P, Xu B, J. Am. Chem. Soc 2010, 132, 2719; [DOI] [PubMed] [Google Scholar]; p) Yang Z, Liang G, Xu B, Acc. Chem. Res 2008, 41, 315; [DOI] [PubMed] [Google Scholar]; q) Rughani RV, Schneider JP, MRS Bull. 2008, 33, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Berthon G, Pure Appl. Chem 1995, 67, 1117; [Google Scholar]; b) Lenz GR, Martell AE, Biochemistry 1964, 3, 745. [DOI] [PubMed] [Google Scholar]
- [3].a) Albrecht M, Stortz P, Nolting R, Synthesis 2003, 1307; [Google Scholar]; b) Dong J, Canfield JM, Mehta AK, Shokes JE, Tian B, Childers WS, Simmons JA, Mao Z, Scott RA, Warncke K, Lynn DG, Proc. Natl. Acad. Sci. USA 2007, 104, 13313; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Dublin SN, Conticello VP, J. Am. Chem. Soc 2007, 130, 49; [DOI] [PubMed] [Google Scholar]; d) Emmanouil K, Estelle M, Lihi A-A, Edward PM, Forsyth VT, Ehud G, Anna M, Pept. Sci 2009, 92, 164; [Google Scholar]; e) Matsumura S, Uemura S, Mihara H, Supramol. Chem 2006, 18, 397; [Google Scholar]; f) Pires MM, Chmielewski J, J. Am. Chem. Soc 2009, 131, 2706; [DOI] [PubMed] [Google Scholar]; g) Przybyla DE, Chmielewski J, J. Am. Chem. Soc 2008, 130, 12610; [DOI] [PubMed] [Google Scholar]; h) Yang H, Pritzker M, Fung SY, Sheng Y, Wang W, Chen P, Langmuir 2006, 22, 8553; [DOI] [PubMed] [Google Scholar]; i) Pires M, Przybyla D, Chmielewski J, Angew. Chem 2009, 121, 7953; Angew. Chem. Int. Ed. 2009, 48, 7813. [DOI] [PubMed] [Google Scholar]
- [4].Micklitsch CM, Yu Q, Schneider JP, Tetrahedron Lett. 2006, 47, 6277. [Google Scholar]
- [5].Beyersmann D, Haase H, BioMetals 2001, 14, 331. [DOI] [PubMed] [Google Scholar]
- [6].Tenaud I, Leroy S, Chebassier N, Dreno B, Exp. Dermatol 2000, 9, 407. [DOI] [PubMed] [Google Scholar]
- [7].Jones PW, Williams DR, in Metal Ions in Biological Systems, Vol. 41: Metal Ions and Their Complexes in Medication, Marcel Dekker, New York, 2004, p. 139. [PubMed] [Google Scholar]
- [8].a) Lansdown ABG, Mirastschijski U, Stubbs N, Scanlon E, Ågren MS, Wound Repair Regen. 2007, 15, 2; [DOI] [PubMed] [Google Scholar]; b) Lansdown ABG, Lancet 1996, 347, 706; [DOI] [PubMed] [Google Scholar]; c) Ågren MS, Arch. Dermatol 1999, 135, 1273. [DOI] [PubMed] [Google Scholar]
- [9].a) Ågren MS, Soderberg TA, Reuterving CO, Hallmans G, Tengrup I, Acta Chir. 1991, 157, 97; [PubMed] [Google Scholar]; b) Soderberg TA, Scand. J. Plast. Reconstr. Surg. Hand Surg 1990, 1; [DOI] [PubMed] [Google Scholar]; c) Soderberg TA, Sunzel B, Holm S, Elmros T, Hallmans G, Sjoberg S, Scand. J. Plast. Reconstr. Surg. Hand Surg 1990, 24, 193. [DOI] [PubMed] [Google Scholar]
- [10].a) Ozbas B, Kretsinger J, Rajagopal K, Schneider JP, Pochan DJ, Macromolecules 2004, 37, 7331; [Google Scholar]; b) Pochan DJ, Schneider JP, Kretsinger J, Ozbas B, Rajagopal K, Haines L, J. Am. Chem. Soc 2003, 125, 11802; [DOI] [PubMed] [Google Scholar]; c) Schneider JP, Pochan DJ, Ozbas B, Rajagopal K, Pakstis L, Kretsinger J, J. Am. Chem. Soc 2002, 124, 15030. [DOI] [PubMed] [Google Scholar]
- [11].Schwarzenbach G, Anderegg G, Schneider W, Senn H, Helv. Chim. Acta 1955, 38, 1147. [Google Scholar]
- [12].a) Dudev M, Wang J, Dudev T, Lim C, J. Phys. Chem. B 2006, 110, 1889; [DOI] [PubMed] [Google Scholar]; b) Dudev T, Lin YL, Dudev M, Lim C, J. Am. Chem. Soc 2003, 125, 3168. [DOI] [PubMed] [Google Scholar]
- [13].Mezger TG, The Rheology Handbook, 2nd ed., Vincentz Network, Hannover, Germany, 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


