Abstract
Antibodies to human leukocyte antigens (HLA) pose a significant barrier to transplantation and current strategies to reduce allosensitization are limited. We hypothesized that augmenting proteasome inhibition (PI) based desensitization with costimulation blockade (belatacept), to mitigate germinal center (GC) responses, might increase efficacy and prevent rebound. Four highly sensitized (cPRA class I and/or II >99%, CDC PRA+, C1q+) heart transplant candidates were treated with the combination of belatacept and PI therapy, which significantly reduced both class I and II HLA antibodies and increased the likelihood of identifying an acceptable donor. Three negative CDC crossmatches were achieved against 3, 6, and 8 DSA, including those that were historically C1q+ binding. Post-transplant, sustained suppression of 3/3, 4/6, and 8/8 DSA (cases 1–3) was achieved. Analysis of peripheral blood mononuclear cells before and after desensitization in one case revealed a decrease in naïve and memory B cells and a reduction in T follicular helper cells with a phenotype suggesting recent GC activity (CD38, PD1, and ICOS). Furthermore, a shift in the NK cell phenotype was observed with features suggestive of activation. Our findings support synergism between PI based desensitization and belatacept facilitating transplantation with a negative CDC crossmatch against historically strong, C1q binding antibodies.
1. Introduction:
Transplant candidates sensitized to HLA antigens wait longer for transplant, have increased waitlist mortality, and remain at risk of rejection after transplant.1,2 Contemporary desensitization strategies focus on eliminating antibodies (plasmapheresis and more recently Ides) and/or the precursor cells responsible for their production (proteasome inhibitors and anti-CD20 antibodies).3–6 While promising in select cases, their efficacy is variable and frequently complicated by rebound due to homeostatic proliferation (compensatory B cell proliferation in response to the depletion of antibody producing cells).7 These observations underscore the need for a more effective, durable ‘immune modulating’ strategy that reliably and sustainably reduces HLA antibodies before and after transplant.
Belatacept is a high affinity variant of CTLA4-Ig with increased potency in humans. Developed by Larsen et al., this fusion protein is comprised of the extracellular component of human CTLA-4, with a two amino acid substitution to increase avidity, and the Fc portion of human immunoglobulin G1, modified to limit effector functions.8,9 Belatacept binds to CD80 and CD86 on antigen presenting cells (APCs) thereby preventing CD28 mediated signaling critical for i) T cell activation and proliferation, ii) T follicular helper cell (Tfh) differentiation, and iii) cognate T/B cell interactions.10–12 Moreover, a CD28 dependent role in plasma cell survival signalling has been proposed.13 Approved for use in renal transplant recipients on the basis of two randomized controlled trials,14,15 belatacept has consistently been associated with a strikingly low incidence of de novo donor specific antibodies (DSA), and superiority in constraining pre-existing HLA antibody responses compared with CNI-based immunosuppression.16,17 Attesting to the mechanistic rationale for these observations, recent studies in mouse and nonhuman primate (NHP) models demonstrated the efficacy of belatacept in blunting germinal center (GC) homeostatic proliferation and show promise as a ‘dual targeting’ approach to desensitization in combination with proteasome inhibition.18–22 In this cohort of 4 very highly sensitized heart transplant candidates, belatacept was added to provide synergism with an intensive proteasome inhibitor based desensitization protocol.
2. Results:
2.1. Patient characteristics
Four highly sensitized heart transplant candidates (heart/liver, case 1) were prospectively selected for this study. All had class I and or II UNOS cPRAMFI>6000>99%, evidence of C1q binding, and cytotoxicity by complement-dependent cytotoxicity (CDC) assay. Attesting to their highly sensitized status, using the Canadian Transplant Registry cPRA to include DP antigens and an even more stringent cut-off (MFI>10,000), the combined class I and II cPRA was 97.59735% (Case 1), 98.62069% (Case 2), 100.00000% (Case 3), and 99.08865% (Case 4). All candidates were multiparous females with a history of blood transfusions. Three (75%) were supported on an LVAD during desensitization, and 3 (75%) had previously undergone attempts at desensitization (rituximab, IVIG, and/or bortezomib). Except in case 1, prior desensitization occurred at least 6 months before enrollment (Table 1).
Table 1.
Baseline Characteristics
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age | 38 | 68 | 60 | 54 |
| Female | Y | Y | Y | Y |
| Etiology | Rheumatic | Ischemic | Ischemic | Ischemic |
| Sensitization History | ||||
| Pregnancy | Y | Y | Y | Y |
| Blood Transfusions | Y | Y | Y | Y |
| Prior transplant | N | N | N | N |
| LVAD | N | Y | Y | Y |
| Prior desensitization | Y | Y | N | Y |
| Interval from prior desensitization to belatacept/PI | 1 month | 10 months | No prior desensitization | 6 months** |
| Class I (MFI) | ||||
| cPRA(2000) | 100 | 59 | 100 | 99 |
| cPRA(6000) | 100 | 42 | 73 | 97 |
| cPRA(10,000) | 96 | 27 | 54 | 96 |
| Class II (MFI) | ||||
| cPRA(2000) | 0 | 100 | 100 | 99 |
| cPRA(6000) | 0 | 100 | 100 | 99 |
| cPRA(10,000) | 0 | 100 | 100 | 93 |
| Canadian cPRA(10,000)* | 97.59735 | 98.62069 | 100.00000 | 99.08865 |
Canadian cPRA includes DP antigens. cPRA for class I and II combined.
received 4 doses (1 cycle) of bortezomib monotherapy at another center at least 6 months prior.
2.2. Desensitization Strategy
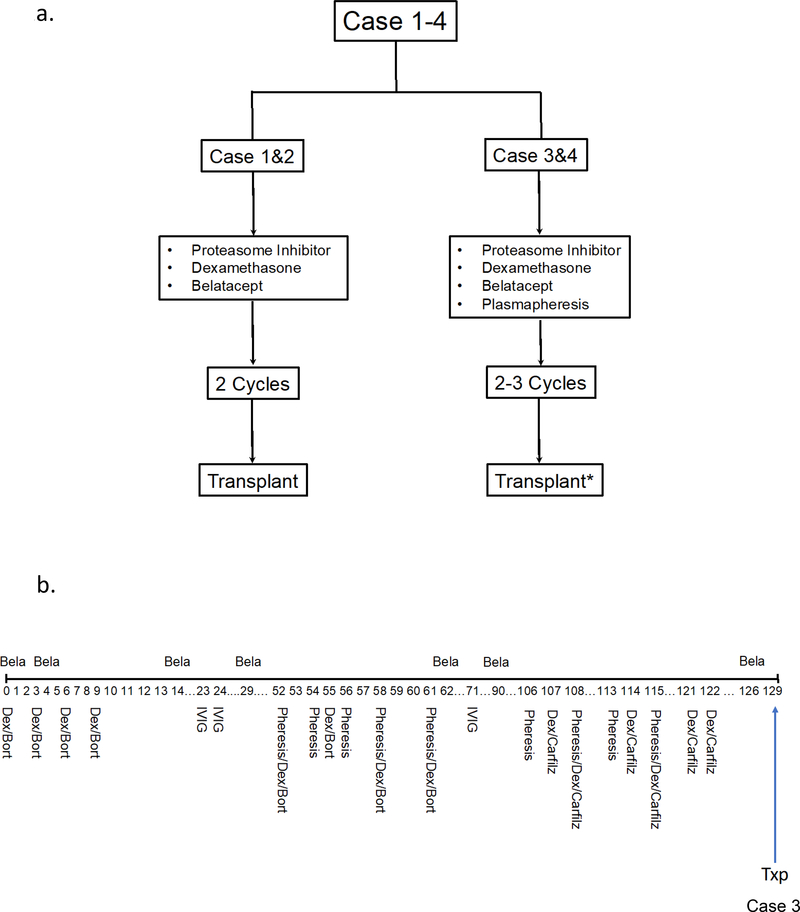
The overarching approach combined proteasome inhibition (PI) with upfront belatacept (Figure 1a). Bortezomib was used as first-line PI (two cycles) then switched to carfilzomib given the potentially more potent effects on proteasome inhibition (see discussion). To more rapidly remove antibody, and possibly ‘augment’ PI effects by stimulating protein production, plasmapheresis was added in cases 3 (cycles 2,3) and 4. Figure 1b illustrates the protocol for case 3 (other cases in Table S1). Time from the last dose of PI to transplant was 14, 203, and 7 days in cases 1–3 respectively (Figure 1b, Table S1).
Figure 1.
Approach to desensitization. a) All candidates (n=4) were treated with multiple cycles of PI therapy and belatacept. Plasmapheresis was added in cases 3 (cycle 2, 3) and 4. (b) Detailed protocol for a representative case (case 3). Bela, belatacept; Bort, bortezomib; Carfilz, carfilzomib; Dex, dexamethasone; IVIG, intravenous immunoglobulin; Pheresis, plasmapheresis; Txp, transplant.
*Case 4 not transplanted
2.3. Synergistic desensitization results in clinically relevant reductions in HLA antibodies
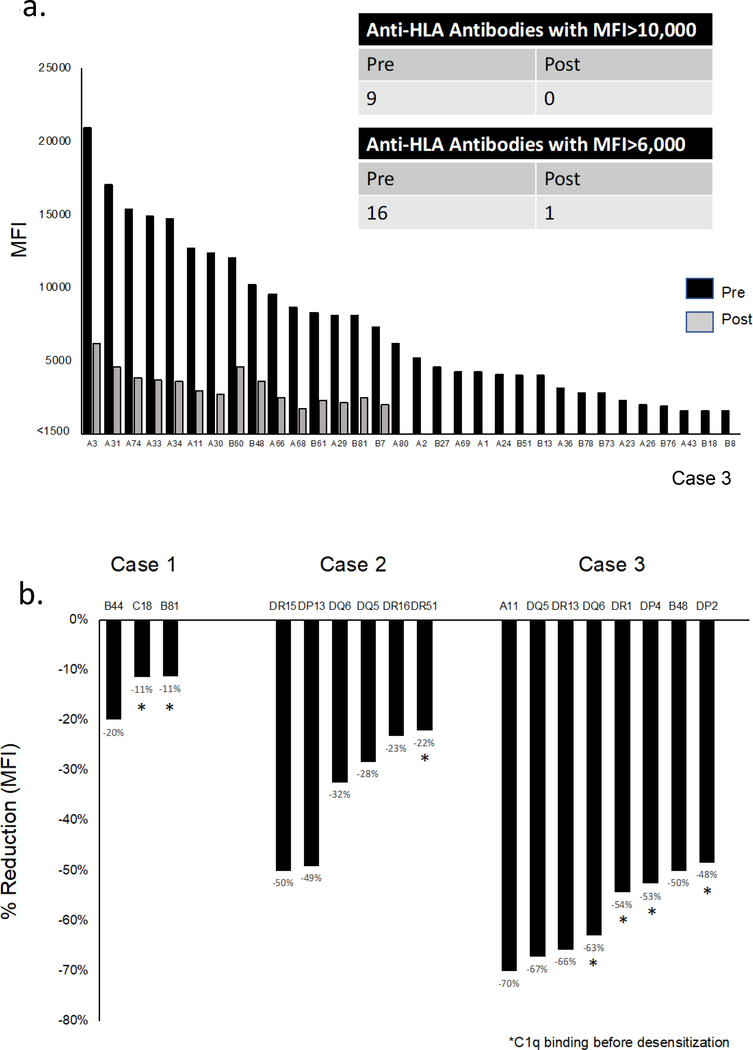
Desensitization markedly decreased the average MFI of class I and II antibodies in all 4 cases (Figure 2a,b). Notably, even antibodies with the highest baseline MFIs responded to sequential cycles of therapy (Figure 2c,d, Figure S1). Importantly, the response was sustained between cycles and after cessation of PI therapy in most, but not all cases (Figure S1).
Figure 2.
Effect of desensitization on class I & II HLA antibodies.
(a) Mean MFI for all class I HLA antibodies before and after desensitization in each case (n=4). Mean MFI was calculated as the average of the MFIs of all antibodies against HLA class I. Diluted serum was used when MFI was >15,000 to keep values within the linear portion of the curve. HLA antibodies were reassessed at >1 month after PI therapy except in case 3 (n=3 days due to anticipated transplant). (b) Mean MFI for all class II HLA antibodies before and after desensitization in each case. Case 1 did not have class II antibodies (N/A). (c) Heatmap illustrating the response of class I HLA antibodies to desensitization with sequential cycles of treatment (case 3). Cycle 1: bortezomib/belatacept (no plasmapheresis); Cycle 2: bortezomib/plasmapheresis, belatacept continued; Cycle 3: carfilzomib/plasmapheresis, belatacept continued. (d) Heatmap illustrating the response of class II HLA antibodies to desensitization with sequential cycles of treatment as above (case 3; results shown in 1:8 dilution). MFI, mean fluorescence intensity.
Using the cPRA (MFI>10,000) to determine likelihood ratios,23 the chances of finding a donor to whom the recipient did not have high MFI antibodies increased markedly after desensitization (Table 2). Even in case 3 (baseline cPRA 100.00000%), finding such a donor became possible. Figure 3a highlights the clinically relevant reduction in class I HLA antibodies for this case. Figure 3b, shows the reduction in donor specific antibodies crossed at the time of a negative CDC crossmatch (cases 1–3). This included historically C1q binding antibodies in all cases. Importantly in case 2, the reduction in DSA was maintained with belatacept and IVIG during the 189 days between the last cycle of PI and transplant (Figure S2).
Table 2.
Likelihood of identifying an acceptable donor
| Before desensitization | After desensitization | |
|---|---|---|
| Case 1 | 1:42 | 1:12 |
| Case 2 | 1:73 | 1:26 |
| Case 3 | 0 | 1:105 |
| Case 4 | 1:110 | 1:17 |
Likelihood = 1 in 1/(1-cPRA), calculated using Canadian cPRA calculator to 5 decimal places.
Figure 3.
Desensitization with proteasome inhibition and belatacept results in a clinically relevant response. (a) Strong class I antibodies prior to desensitization were reduced to low levels or suppressed below the threshold for positivity after desensitization (case 3). Desensitization reduced the MFI of all class I HLA antibodies to <6000 (except A3, MFI 6200). (b) Reduction in MFI with treatment of HLA antibodies specific to the donor in cases 1–3 (CDC crossmatch negative in all cases). Reduction calculated using (MFIpost – MFIpre)/MFIpre. Pre, before desensitization; post, at transplant.
2.4. Costimulation blockade & proteasome inhibition is well tolerated with acceptable early post-transplant outcomes
Considering the baseline risk and frequency of anticipated complications in this cohort, desensitization was well tolerated. Table 3 lists complications during desensitization. Two confirmed infections occurred. In both cases, desensitization was continued after a course of antimicrobial therapy without recurrence. There were no cases of CMV or EBV viremia. Low grade thrombocytopenia with carfilzomib led to dose reduction, but did not delay treatment.
Table 3.
Adverse events before transplant
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| CMV | None | None | None | None |
| EBV | None | None | None | None |
| Infections (other) | C. Difficile | E. Coli UTI and bacteremia | None | None |
| Thrombocytopenia | None | Grade 1 | Grade 2 | Grade 2 |
| Peripheral neuropathy | None | None | None | None |
| Serious bleeding events | None | None | None | None |
| Thrombotic event | None | None | None | Device thrombosis* |
CMV, cytomegalovirus tested by PCR; EBV, Epstein-barr virus DNA tested by PCR; UTI, urinary tract infection; thrombocytopenia, CTCAE grading; peripheral neuropathy (self-reported); serious bleeding events (any event requiring transfusion, any overt GI bleeding, any intracranial bleed); thrombotic event, any peripheral or central thrombosis.
the patient developed LVAD thrombosis (HeartMate II) complicated by a stroke and expired.
This occurred 5 months after the last cycle of PI therapy.
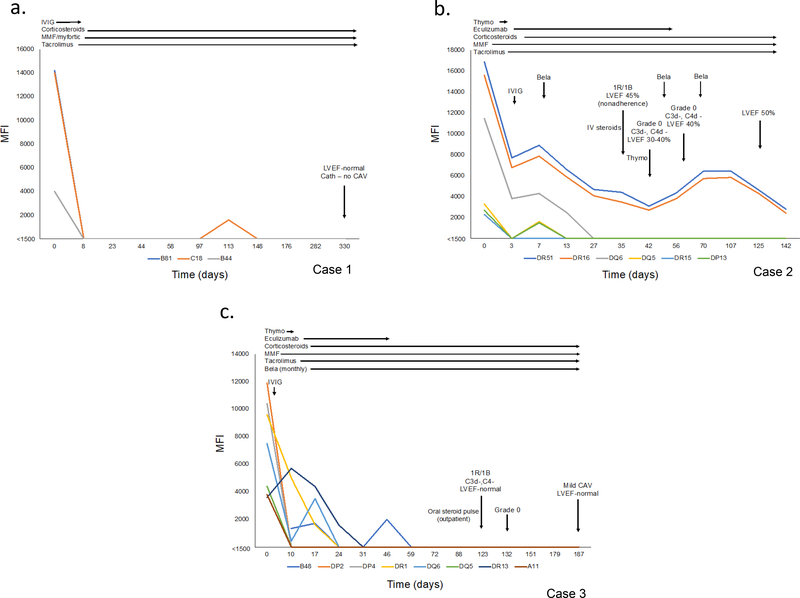
Antibody kinetics, immunosuppression, and clinical course for the 3 transplanted patients are presented in Figure 4 (Table S2). Early graft dysfunction (case 3) prompted the administration of eculizumab which was subsequently used pre-emptively in case 2 given the number of DSA crossed at the time of transplant. Staining for C3d and C4d, which are not affected by eculizumab, has been negative for AMR and no cellular rejection > 2R/3A has occurred (Table 4). DSA remain suppressed at 11 months (3/3), 4 months (4/6), and 6 months (8/8). Case 1 and 3 continue to do well clinically. Mild cardiac allograft vasculopathy was observed on surveillance angiography (6 months) in case 3. Graft function remains normal. Case 2 developed graft dysfunction in the setting of medication nonadherence. Biopsy showed grade 1R/1B cellular rejection without evidence of AMR (histology, C3d/C4d staining negative). Augmented immunosuppression for graft dysfunction resulted in leukopenia (belatacept held) and multiple viral infections requiring inpatient treatment (Figure 4b, Table 4). The left ventricular ejection fraction (LVEF) improved to 50% and the MFI of the 2 remaining DSA are below 5000. Case 4 developed LVAD (HeartMate II) related complications (device thrombosis and stroke) and expired while waiting for transplant. This occurred 5 months after the last cycle of PI/plasmapheresis.
Figure 4.
Post-transplant course. (a) Case 1 underwent heart/liver transplant (DSA=3, class I). Uncomplicated post-transplant course with no rejection and no DSA at last follow-up. (b) Case 2 underwent heart transplant (DSA=6, class II). Belatacept continued post-transplant. DSA (4/6) suppressed. Graft dysfunction (grade 1R/1B ACR, no AMR) in the setting of medication nonadherence. Treated with IV corticosteroids and thymoglobulin. Developed several viral infections. LVEF improved to 50% and the remaining 2/6 DSA have MFI< 5000 at last follow-up. (c) Case 3 underwent heart transplant (DSA=8, class I and II). Belatacept was continued post-transplant. All 8 DSA suppressed. Mild (grade 1R/1B) ACR treated with outpatient oral prednisone pulse (normal graft function, no AMR). Mild cardiac allograft vasculopathy on 6-month surveillance angiography. Graft function remains normal (>55%).
ACR, acute cellular rejection; AMR, antibody mediated rejection.
Table 4.
Post-transplant Course
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Index hospitalization | Urinary tract infection | S. epidermidis bacteremia/mediastinal infection | Early graft dysfunction Pericardial effusion |
| Biopsies (n) | 9 | 9 | 12 |
| ACR>2R/3A | 0 | 0 | 0 |
| ACR 1R/1B | 0 | 1 | 1 |
| AMR* | 0 | 0 | 0 |
| Other | Mild neutropenia (resolved) |
Graft dysfunction DVT/PE Leukopenia |
CAV – mild, normal graft function |
| Last follow-up | Doing well, at home, normal graft function | Hospitalized for infections | Doing well, at home, normal graft function |
CAV, cardiac allograft vasculopathy; DVT, deep vein thrombosis; PE, pulmonary embolism
AMR assessed by histology and immunohistochemistry (C3d, C4d)
2.5. Desensitization reduces B cells and germinal center (GC) related T-follicular helper (Tfh) cells
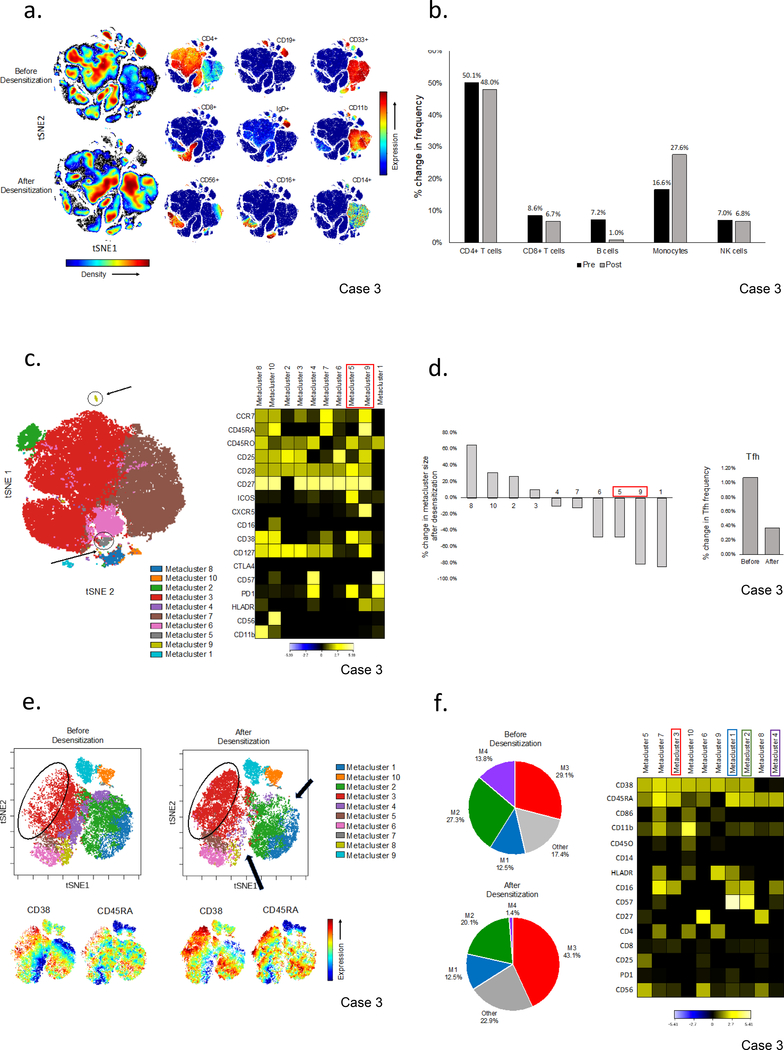
To begin investigating underlying mechanisms responsible for the observed reduction in HLA antibodies, we used high-parameter mass cytometry (CyTOF) to interrogate the peripheral blood immune cell repertoire before and after 4 months of desensitization in case 3. The density plots in Figure 5a provide an overview of differences in peripheral blood immune cell subsets before and after desensitization. Quantitative analysis confirmed a reduction in CD19+ B cells (7.2% vs. 1.0%) including both naïve (CD27-IgD+) and memory (CD27+,IgD-) B cells (Figure 5b, S3a). CD19+B cells were independently quantified by flow cytometry during treatment confirming this reduction (Figure S3b). To begin unravelling responses at the level of the germinal center (GC), we interrogated the circulating Tfh (cTfh) pool focusing on subsets reflecting recent GC activity and identified 2 small populations (PD1+ICOS+CD4+ and CXCR5+PD1+CD38+ICOS+) which were markedly reduced after desensitization (Figure S4a,b). This finding was independently demonstrated using unsupervised cluster analysis (FlowSOM) which defined 2 small subsets bearing an activated Tfh phenotype. These were amongst the subsets most reduced after desensitization (Figure 5c,d).
Figure 5.
Effect of desensitization on the peripheral blood immune cell repertoire in case 3. (a) Analysis of PBMCs from case 3 before and after desensitization. Individual tSNE plots of the lineage defining surface markers are shown to complement the overall density plots which provide a visual overview of the changes in major immune cell subsets after desensitization. Analyses were performed using equal sampling (n=100,000). (b) Quantification of major PBMC subsets before and after treatment. Peripheral blood CD19+ B cells were reduced (7.2% to 1.0%) and CD14+ monocytes increased (16.6% to 27.6%) with treatment. (c) Unsupervised cluster analysis of CD4+ T cells using self-organizing maps (flowSOM) and consensus hierarchical clustering identified two metaclusters with an activated T follicular helper cell (Tfh) phenotype (M5, ICOS+CD38+PD1+CXCR5dim and M9, CXCR5brightCD38+ICOSdim). Median surface marker expression is normalized to the cluster with the minimum expression in each row. (d) Changes in the frequency of CD4+ T cells in each metacluster. Tfh (M5 and M9) were amongst the populations most reduced after desensitization. (e) Analysis of CD3-CD19-CD14-CD56+NK cells before and after desensitization. Marked shifts in the size of major metaclusters (top) are seen which visually correspond to increased CD38 and CD45RA expression (bottom). (f) Pie charts illustrating the changes in metacluster frequency (left) and a heatmap providing an overview of the surface markers defining each metacluster (right). Metaclusters are arranged from left to right in order of the change in their frequency after desensitization (M5,7,3,10,6,9 increased; M1, no change; M2,8,4 decreased). Median surface marker expression is normalized to the cluster with the minimum expression in each row.
Unexpectedly, a shift in the NK cell phenotype was observed with expansion of CD38+ and CD45RA+ clusters (Figure 5e). Although our panel was not optimized for NK cell analysis, the heatmap (arranged from left to right by percent change in cluster size) suggests an increase in potentially activated, cytokine producing subsets (CD38+, CD16+, CD11b+, HLADR+) and a decrease in the CD57+CD16+CD56dim signature characteristic of terminally differentiated NK cells (Figure 5f).
3. Discussion:
Proteasome inhibitors (PIs) induce apoptosis in response to the accumulation of misfolded proteins underlying their efficacy in targeting antibody producing plasma cells.24 Despite reducing bone marrow plasma cells (BMPCs), PIs have had variable efficacy in decreasing HLA antibodies and are plagued by rebound, tempering enthusiasm for their use in desensitization.6,7,25 Robust germinal center (GC) homeostatic proliferation may underlie this finding calling for approaches that mitigate upstream responses.7 Belatacept is a promising therapeutic option which has been shown to target the GC reaction.18,20 These observations led to a series of nonhuman primate (NHP) studies demonstrating synergism between PI and belatacept in reducing DSA and ultimately prolonging post-transplant survival.20–22 The present study extends these findings to a cohort of 4 highly sensitized heart transplant candidates. The salient findings include i) a marked reduction in class I and II HLA antibodies, including those with high MFI and C1q binding capability, and ii) a benefit from multiple cycles of treatment resulting in a clinically relevant response with negative CDC crossmatches across previously strong, C1q binding antibodies. Moreover, we provide encouraging preliminary evidence that these effects can be sustained both before and after transplant.
How one defines ‘successful’ desensitization continues to stimulate debate. Indeed, one can argue that the ultimate measure of ‘success’ is transplantation itself with acceptable allograft outcomes and patient survival. It is well known that highly sensitized patients wait longer for transplant, have increased waitlist mortality, and may not be offered a transplant altogether.2 Herein we therefore chose to use two strategies to demonstrate success. Firstly, by using a comprehensive cPRA calculation, we complemented our objective findings by Luminex, with a marked increase in the likelihood of finding a donor to whom the recipient no longer had high MFI DSA. Secondly, we demonstrated the feasibility of transplanting 3 highly sensitized heart transplant candidates across multiple DSA, some with high MFI, but all of which had been reduced by desensitization. With respect to the latter, it is important to consider the tenuous balance between risking clinical progression and finding a more optimal donor, the reality of which was brought to light in case 4.
In the present study, we used 2–3 cycles of desensitization demonstrating sequential reductions in both class I and II HLA antibodies. This observation is consistent with a mouse model where repeat courses of PI and sustained CTLA4-Ig were required to reverse established DSA responses.19 Importantly, our findings suggest that antibody suppression can be maintained both before and after transplant. While IVIG may have contributed in the pre-transplant setting, the reduction in activated cTfh in case 3 after desensitization is consistent with a mechanism by which belatacept constrains the humoral immune response.20–22 Moreover, the observation that many highly sensitized renal transplant candidates experience a greater reduction in non-DSA when belatacept is used as part of post-transplant immunosuppression, argues for its ability to mitigate HLA antibody responses amongst a similarly sensitized cohort.17 While adjunctive therapies may contribute to DSA suppression after transplant, the observation that 8/8 DSA remain suppressed at 6-months (case 3) is consistent with findings in albeit more mildly sensitized renal transplant recipients treated with belatacept.16 Given the slight rebound in class II antibodies (case 4), it is interesting to consider whether a ‘threshold’ reduction in BMPCs is necessary. Moreover, whether there are intrinsic differences between BMPCs producing class I versus II antibodies requires further investigation.
Our findings raise critical questions regarding desensitization and the bone marrow plasma cell environment. Firstly, although both bortezomib and carfilzomib reduce BMPCs in patients with HLA antibodies, superiority of carfilzomib has been suggested based on irreversible proteasome inhibition.6,26 Alternatively, recent evidence implicates greater β5/β2 co-inhibition, an effect that is dose dependent.27 Indeed, clinical trials of carfilzomib in multiple myeloma used doses up to 56 – 70mg/m2. Interestingly, in case 2, cycle 1 (carfilzomib 20mg/m2) failed to reduce antibodies while a robust response was seen in cycle 2 (carfilzomib 36mg/m2). Nonetheless, the risk of cardiotoxicity (e.g. right ventricular failure) and consequences of hypertension for patients supported on an LVAD are noteworthy. Thus, a protocol ‘escalating’ from bortezomib, which was also effective in our hands, to carfilzomib may be reasonable. Moreover, while plasmapheresis has been put forth as a means of ‘enhancing’ PI toxicity by stimulating antibody production3, the established reduction in BMPCs with PI therapy alone and successful DSA reduction in the NHP model without plasmapheresis along with our preliminary findings in cases 1–3, raise questions about this approach.6,20–22 Whether plasmapheresis hastens antibody reduction in a sustainable fashion in conjunction with desensitization using belatacept/PI, requires further study. Importantly, the observation that CD28 expression on a subset of BMPCs mediates survival signalling, suggests a direct role for belatacept in the bone marrow microenvironment.13 While we were not able to directly interrogate these cells, it is noteworthy that the highest frequency of CD28+ BMPCs may reside in the long-lived CD19-CD38hiCD138+ subset which was found to harbor specificities for remote antigen exposures, drawing an intriguing parallel to pregnancy induced allosensitization.28 The implications for remote allosensitization versus sensitization by a failed renal allograft in situ, remain to be determined.
Interestingly, a marked shift in the NK cell phenotype occurred after desensitization with expansion of clusters suggestive of an activated phenotype. Thus, it is possible that ‘off target’ consequences of desensitization itself may contribute to graft injury such as the vasculopathy observed in case 3. While DSA may play a role in NK cell mediated damage, the early and sustained suppression raises the possibility of an antibody independent contribution as previously described in a mouse model devoid of donor reactive T and B cells.29 Recently, Koenig et al., provided evidence supporting a DSA-independent contribution of activated NK cells to microvascular injury in human renal allografts.30 Although preliminary in nature, we raise the possibility that during PI-based desensitization, the loss of ‘self-recognition’ in the setting of impaired class I peptide loading may contribute to NK cell priming and activation.
The present study is limited by its small size. However, it is amongst the first to report the outcomes of combining costimulation blockade and PI in a clinical pre-transplant setting. Moreover, despite selecting only the most highly sensitized candidates with active, complement binding antibodies, we nonetheless demonstrate clinically meaningful responses. While recent rituximab administration could have potentially confounded the interpretation of our results in case 1, notably case 3, the most highly sensitized heart transplant candidate, had no prior history of desensitization, a striking reduction in class I and II antibodies, and sustained suppression of 8 DSA after transplant. Finally, while disentangling the role of belatacept amongst multiple therapies is challenging, the minimal rebound during desensitization, sustained DSA suppression post-transplant, and reduction in Tfh reflecting recent GC activity (case 3) are consistent with proposed mechanisms by which belatacept constrains the humoral response.18,20,22
In conclusion, the present study provides preliminary evidence substantiating the efficacy of combining proteasome inhibition with belatacept as part of a pre-transplant desensitization regimen. The reduction in class I and II HLA antibodies was complemented by negative CDC crossmatches against multiple previously high-level, complement binding antibodies. The regimen was well tolerated with acceptable early outcomes considering the number of DSA crossed at the time of transplant. Finally, with the panoply of promising novel therapeutics, these findings lend support for further studies complemented by phenotypic investigations to decipher underlying immune mechanisms.
4. Concise materials and methods:
4.1. Study population & protocol:
Four highly sensitized heart transplant candidates were selected for this study. The regimen in each case is outlined in Figure 1 and Table S1. Additional details are available in the Online Supplementary Methods. All candidates provided informed consent and were included in our highly sensitized study protocol approved by the Columbia University IRB (IRB-AAAS2339).
4.2. HLA and crossmatch testing:
HLA Antibodies were quantified using single antigen beads (LABScreen®, One Lambda, Canoga Park, CA) run on the Luminex (Austin, TX) platform. Results were reported as mean fluorescence intensity (MFI), normalized to background, with dilutions performed (1:8) to maintain MFI in the linear range. The C1q Screen™ (One Lambda, Canoga Park, CA) was performed on the Luminex platform (MFI>500 considered positive). Complement-dependent cytotoxicity (CDC) PRA was performed according to standard methods with a panel of 40 cells representing all known HLA Class I and II antigens. Positive CDC PRA was defined as reactivity to >10% of the reference panel. Prospective CDC crossmatches were performed in all 3 cases using magnetically sorted donor B and T cells and current (within 24 h) recipient sera treated with DTT.
4.3. Statistical analysis (HLA):
Continuous data (MFI) was evaluated by mean MFI for class I and II antibodies respectively with significance determined using the student’s t-test. Dilution were used to maintain MFI within the linear portion of the standard curve. Net MFI reduction was calculated as:
Likelihood ratios were calculated according to the equation:23
4.4. Mass cytometry:
Peripheral blood mononuclear cells (PBMCs) were thawed and stained with a panel of 28 antibody-metal conjugates (Table S3). Sample preparation, antibody staining, CyTOF acquisition, and data analysis are detailed in the Online Supplementary Methods.
Supplementary Material
Supplementary Figure 2. Sustained reduction in donor specific antibodies (DSA) while waiting for transplant in case 2. MFI of DSA after cycle 2 and at transplant (HLA antibody analysis performed 189 days apart). Monthly belatacept was continued along with IVIG.
Supplementary Figure 1. Antibody kinetics with sequential cycles of desensitization. (a) Response to sequential cycles of desensitization for 5 high MFI class I antibodies (neat serum). (b) Response to sequential cycles of desensitization for 5 high MFI class II antibodies (1:8 dilution in cases 2 and 3). Case 2, day 256 not available in dilution. Protocol details in Table S2 and Figure 1b.
Supplementary Figure 3. Peripheral blood CD19+ B cells decreased with desensitization in case 3. (a) Scatter plots of CD19+ B cells. Both naïve (CD27-IgD+) and memory (CD27+IgD-) B cells were reduced after desensitization. (b) CD19+ B cells were followed longitudinally by flow cytometry during desensitization. After a transient rise, there was a steep and rapid reduction in peripheral blood B cells that was sustained over time without evidence of rebound.
Supplementary Figure 4. Reduction in the frequency of circulating T follicular helper (cTfh) cell subsets suggestive of recent germinal center activity in case 3. (a) ICOS+PD1+ cTfh in the peripheral blood before and after desensitization (gated on CD4+ T cells). (b) CD38+ICOS+ cTfh (gated on CXCR5+PD1+ cTfh) before and after desensitization.
5. Acknowledgements:
The authors thank the Human Immune Monitoring Core (HIMC) at Columbia University Medical Center (RRID:SCR_016740) for advice and support using the CyTOF Helios mass cytometry system; the CyTOF instrument was funded by NIH S10 grant 1S10OD025218.
List of Abbreviations:
- APC
antigen presenting cell
- BMPC
bone marrow plasma cell
- CDC
complement dependent cytotoxicity
- cPRA
calculated panel reactive antibodies
- CTLA4
cytotoxic T-lymphocyte-associated protein 4
- HLA
human leukocyte antigen
- ICOS
inducible T-cell costimulator
- Ides
IgG-degrading enzyme derived from Streptococcus pyogenes
- IgD
Immunoglobulin D isotype
- IgG
Immunoglobulin G isotype
- ISHLT
International Society for Heart and Lung Transplantation
- IVIG
Intravenous immunoglobulin
- LVAD
left ventricular assist device
- MFI
mean fluorescence intensity
- PRA
panel reactive antibodies
- PD1
Programmed cell death protein 1
- SAB
single antigen bead
Footnotes
6. Disclosures:
The authors of this manuscript do not have conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information:
Additional Supporting Information may be found online in the supporting information tab for this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
5. References:
- 1.Colvin MM, Cook JL, Chang PP, et al. Sensitization in Heart Transplantation: Emerging Knowledge: A Scientific Statement From the American Heart Association. Circulation. 2019;139(12): e553–e578. [DOI] [PubMed] [Google Scholar]
- 2.Kransdorf EP, Kittleson MM, Patel JK, Pando MJ, Steidley DE, Kobashigawa JA. Calculated panel-reactive antibody predicts outcomes on the heart transplant waiting list. J Heart Lung Transplant. 2017;36(7): 787–796. [DOI] [PubMed] [Google Scholar]
- 3.Patel J, Everly M, Chang D, Kittleson M, Reed E, Kobashigawa J. Reduction of alloantibodies via proteasome inhibition in cardiac transplantation. J Heart Lung Transplant. 2011;30(12): 1320–1326. [DOI] [PubMed] [Google Scholar]
- 4.Jordan SC, Lorant T, Choi J, et al. IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. N Engl J Med. 2017;377(5): 442–453. [DOI] [PubMed] [Google Scholar]
- 5.Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359(3): 242–251. [DOI] [PubMed] [Google Scholar]
- 6.Tremblay S, Driscoll JJ, Rike-Shields A, et al. A prospective, iterative, adaptive trial of carfilzomib-based desensitization. Am J Transplant. 2020;20(2): 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwun J, Burghuber C, Manook M, et al. Humoral Compensation after Bortezomib Treatment of Allosensitized Recipients. J Am Soc Nephrol. 2017;28(7): 1991–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5(3): 443–453. [DOI] [PubMed] [Google Scholar]
- 9.Wekerle T, Grinyo JM. Belatacept: from rational design to clinical application. Transpl Int. 2012;25(2): 139–150. [DOI] [PubMed] [Google Scholar]
- 10.Thompson CB, Lindsten T, Ledbetter JA, et al. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989;86(4): 1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CJ, Heuts F, Ovcinnikovs V, et al. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci U S A. 2015;112(2): 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leibler C, Thiolat A, Henique C, et al. Control of Humoral Response in Renal Transplantation by Belatacept Depends on a Direct Effect on B Cells and Impaired T Follicular Helper-B Cell Crosstalk. J Am Soc Nephrol. 2018;29(3): 1049–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozanski CH, Utley A, Carlson LM, et al. CD28 Promotes Plasma Cell Survival, Sustained Antibody Responses, and BLIMP-1 Upregulation through Its Distal PYAP Proline Motif. J Immunol. 2015;194(10): 4717–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10(3): 535–546. [DOI] [PubMed] [Google Scholar]
- 15.Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant. 2010;10(3): 547–557. [DOI] [PubMed] [Google Scholar]
- 16.Bray RA, Gebel HM, Townsend R, et al. Posttransplant reduction in preexisting donor-specific antibody levels after belatacept- versus cyclosporine-based immunosuppression: Post hoc analyses of BENEFIT and BENEFIT-EXT. Am J Transplant. 2018;18(7): 1774–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsons RF, Zahid A, Bumb S, et al. The impact of belatacept on third-party HLA alloantibodies in highly sensitized kidney transplant recipients. Am J Transplant. 2020;20(2): 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Yin H, Xu J, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. Am J Transplant. 2013;13(9): 2280–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain D, Rajab A, Young JS, et al. Reversing donor-specific antibody responses and antibody-mediated rejection with bortezomib and belatacept in mice and kidney transplant recipients. Am J Transplant. 2020.doi: 10.1111/ajt.15881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwun J, Burghuber C, Manook M, et al. Successful desensitization with proteasome inhibition and costimulation blockade in sensitized nonhuman primates. Blood Adv. 2017;1(24): 2115–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burghuber CK, Manook M, Ezekian B, et al. Dual targeting: Combining costimulation blockade and bortezomib to permit kidney transplantation in sensitized recipients. Am J Transplant. 2019;19(3): 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezekian B, Schroder PM, Mulvihill MS, et al. Pretransplant Desensitization with Costimulation Blockade and Proteasome Inhibitor Reduces DSA and Delays Antibody-Mediated Rejection in Highly Sensitized Nonhuman Primate Kidney Transplant Recipients. J Am Soc Nephrol. 2019;30(12): 2399–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kransdorf EP, Pando MJ. Calculated panel reactive antibody with decimals: A refined metric of access to transplantation for highly sensitized candidates. Hum Immunol. 2017;78(3): 252–256. [DOI] [PubMed] [Google Scholar]
- 24.Meister S, Schubert U, Neubert K, et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67(4): 1783–1792. [DOI] [PubMed] [Google Scholar]
- 25.Eskandary F, Regele H, Baumann L, et al. A Randomized Trial of Bortezomib in Late Antibody-Mediated Kidney Transplant Rejection. J Am Soc Nephrol. 2018;29(2): 591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry DK, Burns JM, Pollinger HS, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9(1): 201–209. [DOI] [PubMed] [Google Scholar]
- 27.Besse A, Besse L, Kraus M, et al. Proteasome Inhibition in Multiple Myeloma: Head-to-Head Comparison of Currently Available Proteasome Inhibitors. Cell Chem Biol. 2019;26(3): 340–351 e343. [DOI] [PubMed] [Google Scholar]
- 28.Halliley JL, Tipton CM, Liesveld J, et al. Long-Lived Plasma Cells Are Contained within the CD19(−)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity. 2015;43(1): 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uehara S, Chase CM, Kitchens WH, et al. NK Cells Can Trigger Allograft Vasculopathy: The Role of Hybrid Resistance in Solid Organ Allografts. The Journal of Immunology. 2005;175(5): 3424–3430. [DOI] [PubMed] [Google Scholar]
- 30.Koenig A, Chen CC, Marcais A, et al. Missing self triggers NK cell-mediated chronic vascular rejection of solid organ transplants. Nat Commun. 2019;10(1): 5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 2. Sustained reduction in donor specific antibodies (DSA) while waiting for transplant in case 2. MFI of DSA after cycle 2 and at transplant (HLA antibody analysis performed 189 days apart). Monthly belatacept was continued along with IVIG.
Supplementary Figure 1. Antibody kinetics with sequential cycles of desensitization. (a) Response to sequential cycles of desensitization for 5 high MFI class I antibodies (neat serum). (b) Response to sequential cycles of desensitization for 5 high MFI class II antibodies (1:8 dilution in cases 2 and 3). Case 2, day 256 not available in dilution. Protocol details in Table S2 and Figure 1b.
Supplementary Figure 3. Peripheral blood CD19+ B cells decreased with desensitization in case 3. (a) Scatter plots of CD19+ B cells. Both naïve (CD27-IgD+) and memory (CD27+IgD-) B cells were reduced after desensitization. (b) CD19+ B cells were followed longitudinally by flow cytometry during desensitization. After a transient rise, there was a steep and rapid reduction in peripheral blood B cells that was sustained over time without evidence of rebound.
Supplementary Figure 4. Reduction in the frequency of circulating T follicular helper (cTfh) cell subsets suggestive of recent germinal center activity in case 3. (a) ICOS+PD1+ cTfh in the peripheral blood before and after desensitization (gated on CD4+ T cells). (b) CD38+ICOS+ cTfh (gated on CXCR5+PD1+ cTfh) before and after desensitization.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.