Abstract
Background:
Intentional weight loss is associated with lower risk of heart failure (HF) and atherosclerotic cardiovascular disease (CVD) among patients with type 2 diabetes mellitus (T2DM). However, the contribution of baseline measures and longitudinal changes in fat mass (FM), lean mass (LM), and waist circumference (WC) to the risk of HF and myocardial infarction (MI) in T2DM is not well established.
Methods:
Adults from the Look AHEAD (Action for Health in Diabetes) trial without prevalent HF were included. FM and LM were predicted using validated equations and compared with dual-energy X-ray absorptiometry (DXA) measurements in a subgroup. Adjusted Cox models were used to evaluate the associations of baseline and longitudinal changes in FM, LM, and WC over 1- and 4-year follow-up with risk of overall HF, HF with preserved ejection fraction (EF) (HFpEF: EF ≥50%), HF with reduced EF (HFrEF: EF <50%), and MI.
Results:
Among 5,103 participants, there were 257 incident HF events over 12.4 years of follow-up. Predicted and measured FM / LM were highly correlated (R2=0.87-0.90) (n=1,369). FM and LM decreased over 4-year follow-up with greater declines in the intensive lifestyle intervention arm. In adjusted analysis, baseline body composition measures were not significantly associated with HF risk. Decline in FM and WC, but not LM, over 1-year were each significantly associated with lower risk of overall HF (aHR per 10% decrease in FM [95%CI] = 0.80 [0.68-0.95], aHR per 10% decrease in WC [95%CI] = 0.77 [0.62-0.95]). Decline in FM was significantly associated with lower risk of both HF subtypes. In contrast, decline in WC was significantly associated with lower risk of HFpEF but not HFrEF. Similar patterns of association were observed for 4-year changes in body composition and HF risk. Longitudinal changes in body composition were not significantly associated with risk of MI.
Conclusions:
In adults with T2DM, a lifestyle intervention is associated with significant loss of FM and LM. Decline in FM and WC, but not LM, were each significantly associated with lower risk of HF but not MI. Furthermore, decline in WC was significantly associated with lower risk of HFpEF but not HFrEF.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00017953.
Keywords: fat mass, heart failure, lean mass, myocardial infarction, type 2 diabetes mellitus, waist circumference
INTRODUCTION
The global burden of diabetes is increasing, and it is estimated to affect 700 million adults worldwide by 2045.1 The increasing prevalence of type 2 diabetes mellitus (T2DM) has been attributed to the growing burden of obesity and associated cardiometabolic dysfunction. T2DM is associated with 2-fold higher risk of adverse cardiovascular (CV) events such as myocardial infarction (MI) and heart failure (HF).2 Furthermore, while optimal control of traditional risk factors has been shown to mitigate the risk of MI, the risk of HF persists highlighting the need for novel approaches to its prevention.3
Besides contributing to the increasing burden of T2DM, obesity, commonly measured as high levels of body mass index (BMI), is also an important modifiable risk factor for CV disease (CVD), including HF. In the Look AHEAD (Action for Health in Diabetes) trial, reduction in BMI on follow-up was associated with lower risk of HF and atherosclerotic CVD among participants with T2DM who had overweight or obesity.4, 5 However, BMI is a heterogeneous composite measure of body size that does not differentiate metabolically distinct components such as fat mass (FM) and lean mass (LM). Differences exist in the cardiometabolic and CV phenotypes associated with specific adipose tissue depots, with visceral adiposity considered particularly detrimental for CV health.6-9 The associations of changes in different body composition parameters in response to weight loss interventions with risk of HF and MI are not well known. This represents an important knowledge gap to identify key targets for lifestyle interventions for prevention of HF and MI.
A major challenge to evaluating the contribution of changes in body composition towards the risk of HF and MI is the cumbersome nature of its assessment. However, recently, Lee et al. developed and validated a model to estimate FM and LM among adults using readily available clinical data.10 In the present study, we validated established anthropometric prediction equations using direct measures of FM and LM in a subset of participants from the Look AHEAD trial who underwent a dual-energy X-ray absorptiometry (DXA) scan. Additionally, we evaluated the associations of baseline and longitudinal changes in FM and LM, estimated from prediction equations, and waist circumference (WC) with the risk of HF, its subtypes (HF with preserved ejection fraction [HFpEF] and HF with reduced ejection fraction [HFrEF]), and MI. We hypothesized that reductions in FM and WC will be strongly associated with lower risk of HF and MI on follow-up.
METHODS
The data used for the present study will not be made available by the authors for the sole purpose of reproducing the study results.
Study design and participants
The study design and primary results of the Look AHEAD trial have been published previously.11, 12 In brief, the Look AHEAD trial was a randomized clinical trial that evaluated the effects of an intensive lifestyle intervention (ILI) focused on weight loss and increased physical activity versus diabetes support and education (DSE) among 5,145 adults with T2DM who had overweight or obesity. Additional details regarding eligibility criteria and trial interventions are described further in the Methods in the Supplement. The present study is a post-hoc analysis of the Look AHEAD trial and included participants (n = 5,103) without prevalent HF at baseline who had available baseline data necessary to estimate FM and LM based on prediction equations (demographics and anthropometric parameters), WC measurements, and had longitudinal follow-up to identify incident CV events (Figure I in the Supplement). Participants with missing data on left ventricular ejection fraction (LVEF) for an incident HF event on follow-up were excluded from the HF subtype analysis (n = 24 in baseline body composition measure analysis). Finally, participants with history of atherosclerotic CVD were also excluded from the analyses evaluating the associations of body composition measures with risk of incident MI. All participants provided written informed consent and the institutional review board of each site approved the study protocol and consent form.
Exposure variables and covariates
The primary exposure variables of interest were estimated FM, LM, and measured WC. Sex-specific anthropometric equations to estimate FM and LM were developed using clinical information and DXA results from the National Health and Nutrition Examination Survey.10 Prior studies have demonstrated that these anthropometric equations have high predictive ability (R2 for FM = 0.90-0.93; R2 for LM = 0.85-0.91) and estimated body composition measures have differential associations with risk of CVD death.10, 13 In the present study, these anthropometric equations used to estimate FM and LM were validated in the subgroup of participants from the Look AHEAD trial cohort who had direct measurements of FM and LM using DXA scans and then applied to estimate FM and LM in the overall study population.14, 15 The sex-specific anthropometric prediction equations used to estimate FM and LM incorporate age, sex, race/ethnicity, height, body weight, and WC as detailed in Table 1.10 Height was measured at study entry using a stadiometer.16 Weight and WC were assessed at baseline and during annual follow-up. Weight was measured in kilograms (kg) using a digital scale. WC was measured in centimeters (cm) with a Gulick II tape measure (model 67020) at the level of the iliac crest.17 Height, weight, and WC were each measured in duplicate and the average value was used for analysis. Other clinical covariates are described in detail in the Methods in the Supplement.
Table 1.
Sex-specific anthropometric prediction equations used to estimate fat mass and lean mass.
| Fat mass | |
|---|---|
| Women | Fat mass (kg) = 11.817 + 0.041*age (years) − 0.199*height (cm) + 0.610*weight (kg) + 0.044*waist circumference (cm) + 0.388*Mexican + 0.073*Hispanic − 1.187*Black + 0.325*Other |
| Men | Fat mass (kg) = −18.592 − 0.009*age (years) − 0.080*height (cm) + 0.226*weight (kg) + 0.387*waist circumference (cm) + 0.08*Mexican − 0.188*Hispanic − 0.483*Black + 1.05*Other |
| Lean mass | |
| Women | Lean mass (kg) = −10.683 − 0.039*age (years) + 0.186*height (cm) + 0.383*weight (kg) − 0.043*waist circumference (cm) − 0.359*Mexican − 0.059*Hispanic + 1.085*Black − 0.34*Other |
| Men | Lean mass (kg) = 19.363 + 0.001*age (years) + 0.064*height (cm) + 0.756*weight (kg) − 0.366*waist circumference (cm) − 0.066*Mexican + 0.231*Hispanic + 0.432*Black − 1.007*Other |
Prediction equations were derived using clinical data and dual-energy X-ray absorptiometry measurements from the National Health and Nutrition Examination Survey.10 For race, the reference group is white.
Study Endpoints: Incident HF and MI
The primary endpoint was incident HF hospitalization that was clinically adjudicated over 12.4 years of follow-up as described previously.4 HF cases were initially identified based on self-report and International Classification of Diseases, Ninth Revision codes from hospitalizations. Based on review of clinical data from hospitalization records, each event was classified as either definite or possible acute decompensated HF, chronic stable HF, HF unlikely, or unclassifiable. Definite or possible acute decompensated HF was considered an incident HF event using a previously established protocol.18 HF events with available data on LVEF at the time of the incident HF hospitalization were further subclassified as HFpEF (LVEF ≥50%) or HFrEF (LVEF <50%). Participants with missing data on LVEF for an incident HF event on follow-up were excluded from the HF subtype analysis (n = 16 in year 1 change in body composition analysis, n = 20 in year 4 change in body composition analysis). Secondary outcome of interest for the present study was incident MI event (fatal or non-fatal) during the follow-up period. Diagnosis of MI was based on a combination of history, electrocardiogram readings, and levels of cardiac biomarkers as previously described.12 Briefly, after review of the medical history, cardiac signs or symptoms were considered present or absent. Electrocardiogram tracings were reviewed by adjudicators and findings were classified as either evolving diagnostic, positive, nonspecific, or normal / other. Biomarker findings were classified based on the timing, levels, and pattern as either diagnostic, equivocal, missing, or normal. All clinical endpoints were adjudicated by reviewers blinded to randomized treatment group assignment.
Statistical analysis
Anthropometric prediction equations were validated among participants who had a DXA scan and available data to estimate body composition measures at baseline, 1- and 4-year follow-up. Predicted FM and LM were estimated for all study participants and the association between predicted and measured body composition parameters was assessed by the coefficient of determination (R2). Bland-Altman analysis was performed to evaluate agreement and assess for any systematic bias between predicted and measured body composition parameters. Across randomized treatment groups, differences in 1- and 4-year percent change in body composition measures were evaluated using generalized linear models.
Baseline characteristics were reported across tertiles of FM, LM, and WC as mean (standard deviation) for continuous variables and number (percentage) for categorical variables. Generalized linear models were used to compare baseline characteristics across groups. Multivariable-adjusted Cox proportional hazards analysis was performed to evaluate the independent associations of different body composition parameters (FM, LM, WC) with risk of HF. Separate models were constructed for overall HF, HFpEF, and HFrEF outcomes with mortality and end of follow-up as censoring events. For HFpEF and HFrEF models, the other HF subtype was also treated as a censoring event. Based on biological plausibility and prior epidemiological studies,4, 5 the following covariates were included in the sequentially adjusted models: Model 1 = demographics (age, sex, race/ethnicity, education, income), treatment group; Model 2 = Model 1 plus cardiorespiratory fitness (CRF); Model 3 = Model 2 plus traditional CV risk factors (history of hypertension, systolic blood pressure [BP], smoking status, alcohol use status, estimated glomerular filtration rate [eGFR], diabetes duration, insulin use, glycated hemoglobin [HbA1c]), history of CVD, FM, LM. Owing to collinearity, FM and LM were not included in Model 3 evaluating the association of WC with HF outcomes.
The associations of longitudinal changes in FM, LM, and WC from baseline to 1- and 4-year follow-up with risk of HF events were evaluated in landmark analyses restricted to the subset of participants without an interval HF event before the follow-up visit. Participants were stratified across data-derived tertiles of percent change (% Δ) in FM and LM to compare baseline and follow-up characteristics. The associations of 1- and 4-year % Δ in body composition parameters (FM, LM, and WC) with risk of HF outcomes were assessed using adjusted Cox models as detailed above with adjustment for the following covariates: Model 1 = demographics (age, sex, race/ethnicity, education, income), treatment group, CRF, traditional CV risk factors (history of hypertension, systolic BP, smoking status, alcohol use status, eGFR, diabetes duration, insulin use, HbA1c), history of CVD; Model 2 = Model 1 + % Δ in FM (except for WC model), % Δ in LM (except for WC model), % Δ in HbA1c, % Δ in systolic BP. Sensitivity analyses were performed to evaluate the association of baseline and short-term (1-year) longitudinal changes in body composition measures with risk of incident HFpEF and HFrEF hospitalization events among participants without a prior history of CVD.
Among participants with no prior history of CVD, the associations of baseline and longitudinal % Δ (1- and 4-year) in FM, LM, and WC with risk of incident MI were assessed using similar adjusted Cox models. Multiplicative interaction testing was performed to determine if the observed associations between body composition parameters and risk of CV outcomes were modified by sex and treatment arm.
All statistical tests were two-sided and p-value <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, United States).
RESULTS
Validation of anthropometric prediction equations
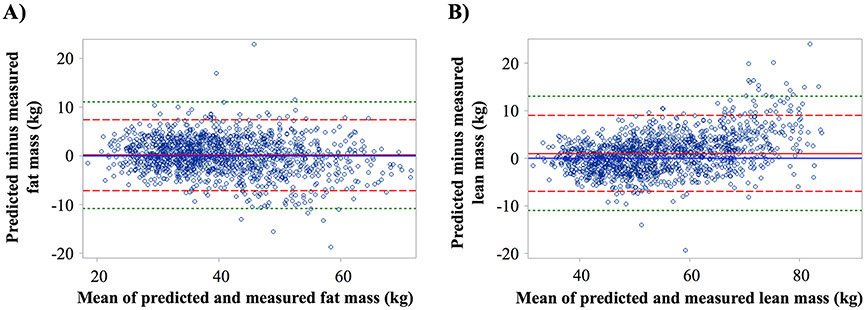
At baseline, 1,369 participants had predicted and DXA-based measures of FM and LM. Predicted and DXA-based FM were highly correlated (R2 = 0.87). The correlation between predicted and DXA-based LM was similarly high (R2 = 0.90). Agreement between predicted and DXA-based measures of FM and LM are shown in Bland-Altman plots (Figure 1). Bias (95% limits of agreement) for baseline predicted and DXA-based measures of FM and LM were 0.08 (−0.11 to 0.27) kg and 0.98 (0.77 to 1.19) kg, respectively. Similar findings were observed when examining year 1 and year 4 predicted and DXA-based measures of FM and LM (Figure II in the Supplement).
Figure 1. Bland-Altman plot of predicted versus measured fat mass (Panel A) and lean mass (Panel B) at baseline.
Solid red line is the mean of difference (dashed red line is +/− 2 standard deviations and dashed green is +/− 3 standard deviations).
Changes in lean mass and fat mass with a lifestyle intervention
Participants randomly assigned to the ILI treatment group had greater 1- and 4-year reductions in FM and LM compared with those assigned to the DSE (p<0.001 for all) (Figure 2). The median reductions in FM and LM in the ILI group were −4.7 kg (IQR: −7.7 to −2.2 kg) and −2.7 kg (IQR: −4.7 to −1.1 kg), respectively, over 1-year follow-up, and −2.0 kg (IQR: −5.1 to 0.7 kg) and −1.8 kg (IQR: −3.9 to −0.1 kg), respectively, over the 4-year follow-up period.
Figure 2. Predicted fat mass (Panel A) and lean mass (Panel B) at baseline, 1- and 4-year follow-up stratified by treatment group.
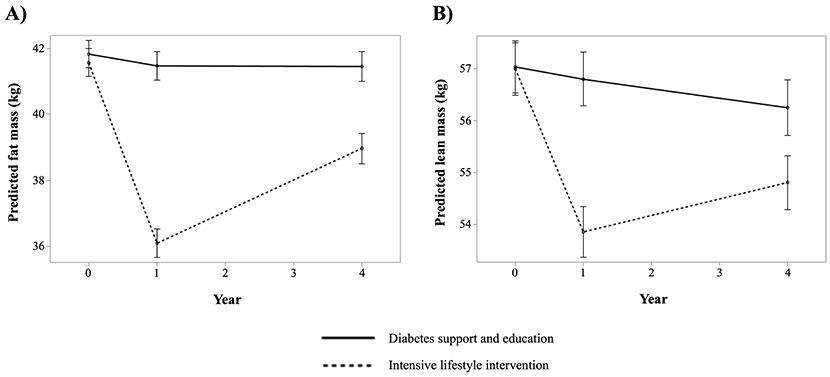
Predicted fat mass (Panel A) and lean mass (Panel B) among participants randomized to the intensive lifestyle intervention and diabetes support and education groups are shown. The means and 95% confidence intervals are shown.
Baseline characteristics of participants across body composition measures
Baseline characteristics of participants across tertiles of FM, LM, and WC are shown in Table 2 and Table I in the Supplement. Participants with higher FM were more commonly women, white, and had higher burden of cardiometabolic risk factors such as hypertension, higher HbA1c, higher low-density lipoprotein cholesterol (LDL-C) but lower prevalence of established CVD. At enrollment, participants with higher LM and WC at baseline were more commonly men, white, and had higher burden of traditional risk factors including hypertension and prevalent CVD.
Table 2.
Baseline characteristics stratified by baseline predicted fat and lean mass tertiles
| Predicted fat mass | Predicted lean mass | |||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1 18.6 to 35.8 kg (n=1,701) |
Tertile 2 35.9 to 45.3 kg (n=1,701) |
Tertile 3 45.4 to 89.6 kg (n=1,701) |
Tertile 1 31.7 to 49.3 kg (n=1,701) |
Tertile 2 49.4 to 61.7 kg (n=1,701) |
Tertile 3 61.8 to 112.2 kg (n=1,701) |
P-value for FM tertiles |
P-value for LM tertiles |
|
| Predicted fat mass, kg | 30.8 (3.6) | 40.3 (2.7) | 54.1 (7.5) | 37.4 (6.2) | 44.9 (11.8) | 42.8 (12.0) | <0.001 | <0.001 |
| Predicted lean mass, kg | 53.9 (11.6) | 55.8 (12.9) | 61.3 (13.4) | 44.0 (3.6) | 54.8 (3.6) | 72.3 (8.7) | <0.001 | <0.001 |
| Waist circumference, cm | 103.5 (9.1) | 112.9 (10.1) | 125.2 (12.8) | 103.9 (10.7) | 115.0 (11.2) | 122.8 (12.8) | <0.001 | <0.001 |
| Age, years | 60.0 (6.9) | 59.0 (6.7) | 57.1 (6.5) | 59.1 (6.9) | 58.3 (6.7) | 58.7 (6.8) | <0.001 | 0.004 |
| Female, n (%) | 707 (41.6) | 1,058 (62.2) | 1,279 (75.2) | 1,679 (98.7) | 1,208 (71.0) | 157 (9.2) | <0.001 | <0.001 |
| White, n (%) | 1,020 (60.0) | 1,095 (64.4) | 1,114 (65.5) | 866 (50.9) | 1,030 (60.6) | 1,333 (78.4) | <0.001 | <0.001 |
| Education, n (%) | ||||||||
| <13 years | 354 (20.8) | 340 (20.0) | 317 (18.6) | 496 (29.2) | 333 (19.6) | 182 (10.7) | <0.001 | <0.001 |
| 13-16 years | 563 (33.1) | 639 (37.6) | 701 (41.2) | 654 (38.5) | 628 (36.9) | 621 (36.5) | ||
| >16 years | 750 (44.1) | 679 (39.9) | 646 (38.0) | 507 (29.8) | 697 (41.0) | 871 (51.2) | ||
| Missing | 34 (2.0) | 43 (2.5) | 37 (2.2) | 44 (2.6) | 43 (2.5) | 27 (1.6) | ||
| Income, n (%) | ||||||||
| <$20,000 | 207 (12.2) | 179 (10.5) | 196 (11.5) | 287 (16.9) | 200 (11.8) | 95 (5.6) | <0.001 | <0.001 |
| $20,000-$39,999 | 314 (18.5) | 324 (19.1) | 341 (20.1) | 420 (24.7) | 330 (19.4) | 229 (13.5) | ||
| $40,000-$59,999 | 282 (16.6) | 324 (19.1) | 340 (20.0) | 329 (19.3) | 328 (19.3) | 289 (17.0) | ||
| $60,000-$79,999 | 218 (12.8) | 244 (14.3) | 281 (16.5) | 187 (11.0) | 273 (16.1) | 283 (16.6) | ||
| ≥$80,000 | 513 (30.2) | 455 (26.8) | 384 (22.6) | 292 (17.2) | 391 (23.0) | 669 (39.3) | ||
| Missing | 167 (9.8) | 175 (10.3) | 159 (9.4) | 186 (10.9) | 179 (10.5) | 136 (8.0) | ||
| Weight, kg | 86.6 (12.6) | 97.9 (13.8) | 117.5 (16.7) | 82.9 (9.1) | 101.7 (12.0) | 117.4 (16.9) | <0.001 | <0.001 |
| BMI, kg/m2 | 30.7 (2.5) | 34.9 (2.8) | 42.2 (4.7) | 32.9 (3.9) | 37.1 (5.9) | 37.8 (6.4) | <0.001 | <0.001 |
| Systolic BP, mm Hg | 126 (17) | 129 (17) | 131 (17) | 128 (17) | 129 (17) | 130 (17) | <0.001 | <0.001 |
| Diastolic BP, mm Hg | 71 (10) | 70 (10) | 69 (9) | 68 (9) | 70 (10) | 73 (9) | <0.001 | <0.001 |
| History of hypertension, n (%) | 1,340 (78.8) | 1,414 (83.1) | 1,487 (87.4) | 1,358 (79.8) | 1,431 (84.1) | 1,452 (85.4) | <0.001 | <0.001 |
| History of CVD, n (%) | 254 (14.9) | 252 (14.8) | 170 (10.0) | 163 (9.6) | 210 (12.4) | 303 (17.8) | <0.001 | <0.001 |
| Diabetes duration, years | 7.1 (7.0) | 6.7 (6.3%) | 6.5 (6.2) | 6.8 (7.0) | 6.7 (6.5) | 6.8 (6.0) | 0.05 | 0.95 |
| Insulin use, n (%) | 220 (13.4) | 252 (15.3) | 311 (19.0) | 225 (13.7) | 279 (17.1) | 279 (17.0) | <0.001 | 0.01 |
| Smoking, n (%) | 0.30 | <0.001 | ||||||
| Never | 822 (48.4) | 854 (50.2) | 883 (52.1) | 1,035 (60.9) | 886 (52.2) | 638 (37.7) | ||
| Past | 797 (47.0) | 769 (45.2) | 742 (43.8) | 592 (34.8) | 731 (43.0) | 985 (58.2) | ||
| Present | 78 (4.6) | 78 (4.6) | 69 (4.1) | 72 (4.2) | 82 (4.8) | 71 (4.2) | ||
| Alcohol, n (%) | <0.001 | <0.001 | ||||||
| None/week | 1,056 (62.2) | 1,144 (67.4) | 1,249 (73.8) | 1,323 (78.0) | 1,194 (70.5) | 932 (54.9) | ||
| 1-3/week | 329 (19.4) | 349 (20.6) | 309 (18.3) | 269 (15.9) | 319 (18.8) | 399 (23.5) | ||
| 4+/week | 312 (18.4) | 204 (12.0) | 134 (7.9) | 104 (6.1) | 180 (10.6) | 366 (21.6) | ||
| HbA1c, % | 7.2 (1.2) | 7.3 (1.2) | 7.4 (1.2) | 7.3 (1.2) | 7.3 (1.2) | 7.3 (1.2) | 0.006 | 0.45 |
| GFR, mL/min per 1.73 m2 | 88.4 (15.1) | 89.5 (16.0) | 91.2 (16.7) | 90.6 (16.2) | 89.8 (16.7) | 88.7 (15.0) | <0.001 | 0.002 |
| LDL-C, mg/dL | 111 (33) | 111 (32) | 114 (32) | 115 (33) | 114 (33) | 107 (30) | 0.04 | <0.001 |
| Estimated CRF, METs | 8.3 (2.1) | 7.2 (1.7) | 6.1 (1.4) | 7.1 (1.8) | 6.9 (1.9) | 7.6 (2.1) | <0.001 | <0.001 |
| ILI treatment group, n (%) | 879 (51.7) | 830 (48.8) | 837 (49.2) | 841 (49.4) | 865 (50.9) | 840 (49.4) | 0.19 | 0.62 |
Categorical data presented as n (percentage) and continuous data presented as mean (standard deviation). Comparison across groups performed using generalized linear models.
Abbreviations: BMI = body mass index; BP = blood pressure; CRF = cardiorespiratory fitness; CVD = cardiovascular disease; FM = fat mass; GFR = glomerular filtration rate; HbA1c = glycated hemoglobin; ILI = intensive lifestyle intervention; LDL-C = low-density lipoprotein cholesterol; LM = lean mass; METs = metabolic equivalents; WC = waist circumference
Association of baseline body composition measures and risk of heart failure
Over 12.4 years of follow-up, an incident HF hospitalization event was observed in 257 participants (129 HFpEF, 104 HFrEF, 24 missing LVEF). In adjusted analysis, higher baseline FM, LM, and WC were each significantly associated with higher risk of overall HF after adjustment for demographics and treatment assignment (HR [95% CI] per 10 kg higher FM = 1.29 [1.14 to 1.46], per 10 kg higher LM = 1.32 [1.15 to 1.52], per 10 cm higher WC = 1.26 [1.16 to 1.38]) (Table 3, model 1). However, these associations were attenuated and no longer significant after additional adjustment for CRF (Table 3, model 2) and further adjustment for traditional CV risk factors and other body composition measures (Table 3, models 3). The association between baseline measures of body composition and risk of HF was not modified by sex or treatment arm (p-interaction >0.05 for all). Among HF subtypes, baseline measures of FM, LM, and WC, were not significantly associated with risk of HFpEF or HFrEF in the most adjusted model (Table 3, model 3). A similar pattern of association was observed in a sensitivity analysis excluding participants with a history of CVD (Figure III in the Supplement).
Table 3.
Multivariable adjusted associations of baseline body composition measures with risk of HF events
| Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Person- years | Event rate per 1,000 person years |
HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | |
| Per 10 kg higher predicted fat mass | ||||||||
| Overall HF | 58,018 | 4.43 | 1.29 (1.14, 1.46) |
<0.001 | 0.98 (0.86, 1.13) |
0.83 | 0.94 (0.76, 1.16) |
0.56 |
| HFpEF | 57,956 | 2.23 | 1.35 (1.14, 1.60) |
<0.001 | 1.02 (0.84, 1.24) |
0.83 | 0.98 (0.73, 1.31) |
0.88 |
| HFrEF | 57,956 | 1.79 | 1.15 (0.94, 1.40) |
0.19 | 0.94 (0.75, 1.18) |
0.60 | 0.85 (0.60, 1.21) |
0.37 |
| Per 10 kg higher predicted lean mass | ||||||||
| Overall HF | 58,018 | 4.43 | 1.32 (1.15, 1.52) |
<0.001 | 1.05 (0.90, 1.22) |
0.53 | 1.12 (0.90, 1.40) |
0.33 |
| HFpEF | 57,956 | 2.23 | 1.35 (1.11, 1.64) |
0.003 | 1.05 (0.86, 1.30) |
0.63 | 1.08 (0.79, 1.47) |
0.64 |
| HFrEF | 57,956 | 1.79 | 1.25 (1.00, 1.57) |
0.05 | 1.08 (0.85, 1.37) |
0.54 | 1.26 (0.89, 1.80) |
0.20 |
| Per 10 cm higher waist circumference | ||||||||
| Overall HF | 58,018 | 4.43 | 1.26 (1.16, 1.38) |
<0.001 | 1.08 (0.97, 1.19) |
0.16 | 1.10 (0.98, 1.22) |
0.10 |
| HFpEF | 57,956 | 2.23 | 1.27 (1.13, 1.43) |
<0.001 | 1.08 (0.94, 1.24) |
0.30 | 1.08 (0.93, 1.26) |
0.31 |
| HFrEF | 57,956 | 1.79 | 1.20 (1.05, 1.38) |
0.01 | 1.08 (0.92, 1.27) |
0.34 | 1.12 (0.95, 1.34) |
0.19 |
Multivariable adjusted Cox proportional hazard models were constructed for each continuous measure of body composition and for each HF outcome with sequential adjustment for potential confounders
Model 1 included age, sex, race/ethnicity, education, income, treatment group
Model 2 included Model 1 covariates plus baseline CRF
Model 3 included Model 2 covariates plus history of hypertension, systolic BP, smoking status, alcohol use status, estimated GFR, diabetes duration, insulin use, HbA1c, history of CVD, predicted fat mass (except for WC model), predicted lean mass (except for WC model)
Abbreviations: BP = blood pressure; CI = confidence interval; CRF = cardiorespiratory fitness; GFR = glomerular filtration rate; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio; WC = waist circumference
Longitudinal changes in body composition measures and risk of HF
Baseline and follow-up characteristics of study participants across strata of short-term (1-year) and intermediate-term (4-year) change in FM and LM are shown in Tables II-III in the Supplement. Participants with greater decline in FM and LM over follow-up were more commonly white, randomized to the ILI treatment group, and had fewer CVD risk factors including lower LDL-C, lower HbA1c, and less insulin use. Additionally, participants with greater decline in FM and LM during follow-up had significantly greater reductions in other body composition and cardiometabolic parameters such as WC, systolic BP, and HbA1c.
In adjusted analysis, greater short-term (1-year) reduction in FM was significantly associated with lower risk of HF after adjustment for baseline demographic characteristics, treatment group, CV risk factors, and CRF (HR [95% CI] per 10% decrease in FM = 0.74 [0.64 to 0.86]) (Table 4, model 1). This association between greater reduction in FM and lower risk of HF remained significant after further adjustment for other changes in body composition and cardiometabolic parameters (HR [95% CI] per 10% decrease in FM = 0.80 [0.68 to 0.95]) (Table 4, model 2). Similar patterns of association were noted between short-term changes in FM and risk of HF subtypes such that a 10% decrease in FM from baseline to 1-year follow-up was significantly associated with 22% lower risk of HFpEF and 24% lower risk of HFrEF.
Table 4.
Multivariable adjusted associations of changes in body composition measures from baseline to 1-year follow-up with risk of HF events
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Person- years |
Event rate per 1,000 person years |
HR (95% CI) |
P value | HR (95% CI) |
P value | |
| Per 10% decrease in predicted fat mass from baseline to 1-year follow-up | ||||||
| Overall HF | 55,847 | 4.19 | 0.74 (0.64, 0.86) |
<0.001 | 0.80 (0.68, 0.95) |
0.01 |
| HFpEF | 55,715 | 2.08 | 0.72 (0.58, 0.89) |
0.002 | 0.78 (0.61, 0.996) |
0.046 |
| HFrEF | 55,715 | 1.72 | 0.72 (0.57, 0.90) |
0.004 | 0.76 (0.59, 0.97) |
0.03 |
| Per 10% decrease in predicted lean mass from baseline to 1-year follow-up | ||||||
| Overall HF | 55,847 | 4.19 | 0.64 (0.50, 0.81) |
<0.001 | 0.75 (0.55, 1.02) |
0.06 |
| HFpEF | 55,715 | 2.08 | 0.57 (0.40, 0.81) |
0.002 | 0.68 (0.44, 1.06) |
0.09 |
| HFrEF | 55,715 | 1.72 | 0.72 (0.49, 1.04) |
0.08 | 0.88 (0.54, 1.42) |
0.60 |
| Per 10% decrease in waist circumference from baseline to 1-year follow-up | ||||||
| Overall HF | 55,845 | 4.19 | 0.75 (0.61, 0.93) |
0.007 | 0.77 (0.62, 0.95) |
0.02 |
| HFpEF | 55,713 | 2.08 | 0.62 (0.46, 0.83) |
0.002 | 0.61 (0.44, 0.83) |
0.002 |
| HFrEF | 55,713 | 1.72 | 0.86 (0.62, 1.18) |
0.34 | 0.89 (0.64, 1.24) |
0.49 |
Multivariable adjusted Cox proportional hazard models were constructed for each continuous measure of body composition and for each HF outcome with sequential adjustment for potential confounders
Model 1 included age, sex, race/ethnicity, education, income, treatment group, baseline CRF, history of hypertension, systolic BP, smoking status, alcohol use status, estimated GFR, diabetes duration, insulin use, HbA1c, history of CVD
Model 2 included Model 1 covariates plus year 1 percent change in predicted fat mass (except for WC model), year 1 percent change in predicted lean mass (except for WC model), year 1 percent change in HbA1c, year 1 percent change in systolic BP
Abbreviations: BP = blood pressure; CI = confidence interval; CRF = cardiorespiratory fitness; CVD = cardiovascular disease; GFR = glomerular filtration rate; HbA1c = glycated hemoglobin; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio; WC = waist circumference
Similarly, short-term (1-year) change in WC was significantly associated with risk of overall HF independent of baseline characteristics, changes in other body composition measures, and changes in cardiometabolic parameters (HR [95% CI] per 10% decrease in WC = 0.77 [0.62 to 0.95]) (Table 4, model 2). Among HF subtypes, short-term (1-year) decline in WC was significantly associated with lower risk of HFpEF (HR [95% CI] = 0.61 [0.44 to 0.83]) but not HFrEF. In contrast, short-term (1-year) change in LM was not significantly associated with risk of HF in the most adjusted model (Table 4, model 2). In a sensitivity analysis restricted to participants with no prior history of CVD, the magnitude and direction of associations between these covariates and risk of HFpEF and HFrEF were similar to that observed in the primary analysis (Figure IV in the Supplement).
The association between changes in FM, WC, and LM over intermediate-term (4-years) follow-up and risk of HF was consistent with that observed for 1-year changes in these parameters (Table IV in the Supplement).
Baseline and longitudinal changes in body composition measures and risk of MI
There were 351 incident MI events over follow-up (event rate per 1,000-person years = 5.43). In multivariable adjusted Cox models, higher baseline FM and WC were each significantly associated with paradoxically lower risk of MI (HR [95% CI] per 10 kg higher FM = 0.74 [0.59 to 0.92]; HR [95% CI] per 10 cm higher WC = 0.88 [0.79 to 0.99]) (Table 5). In contrast, baseline LM was not significantly associated with risk of MI. The association between baseline body composition measures and risk of MI was not different across sex-based groups (p-interaction by sex for each body composition parameter >0.05 for all). In the subset of participants with repeat assessment of body composition parameters and no history of CVD prior to 1- (n = 4,413) and 4-year follow-up (n = 4,376), short- (1-year) and intermediate-term (4-year) changes in body composition measures were not significantly associated with risk of MI (Table 5).
Table 5.
Multivariable adjusted associations of baseline and changes in body composition measures from baseline to 1- and 4-year follow-up with risk of myocardial infarction among patients with no history of cardiovascular disease
| Per 10 kg higher baseline predicted FM/LM or Per 10 cm higher baseline WC |
Per 10% decrease from baseline to 1-year follow-up |
Per 10% decrease from baseline to 4-year follow-up |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | |
| Predicted fat mass | 0.74 (0.59, 0.92) |
0.007 | 0.86 (0.72, 1.03) |
0.10 | 0.91 (0.78, 1.05) |
0.21 |
| Predicted lean mass | 0.97 (0.76, 1.23) |
0.79 | 1.29 (0.90, 1.84) |
0.16 | 1.19 (0.87, 1.63) |
0.28 |
| Waist circumference | 0.88 (0.79, 0.99) |
0.03 | 0.86 (0.69, 1.08) |
0.19 | 0.95 (0.76, 1.17) |
0.61 |
Multivariable adjusted Cox proportional hazard models were constructed for the MI outcome with adjustment for potential confounders
Baseline model included age, sex, race/ethnicity, education, income, treatment group, baseline CRF, history of hypertension, systolic BP, smoking status, alcohol use status, estimated GFR, diabetes duration, insulin use, HbA1c, predicted fat mass (except for WC model), predicted lean mass (except for WC model)
Baseline to 1- and 4-year follow-up models included age, sex, race/ethnicity, education, income, treatment group, baseline CRF, history of hypertension, systolic BP, smoking status, alcohol use status, estimated GFR, diabetes duration, insulin use, HbA1c, year 1 or 4 percent change in predicted fat mass (except for WC model), year 1 or 4 percent change in predicted lean mass (except for WC model), year 1 or 4 percent change in HbA1c, year 1 or 4 percent change in systolic BP
Abbreviations: BP = blood pressure; CI = confidence interval; CRF = cardiorespiratory fitness; GFR = glomerular filtration rate; HbA1c = glycated hemoglobin; HR = hazard ratio; WC = waist circumference
DISCUSSION
This study has several important findings. First, among overweight or obese adults with T2DM, FM and LM could be estimated using previously validated prediction equations with good overall agreement with DXA measures. Second, higher levels of baseline measures of FM, LM, and WC were significantly associated with higher risk of HF. However, these associations were largely related to differences in CRF. In contrast, there was a paradoxical association of higher FM and WC with lower risk of MI among the study participants independent of potential confounders. Third, reduction in FM over longitudinal follow-up was significantly associated with lower risk of incident HF hospitalization with consistently lower risk for both HF subtypes. Finally, reduction in central adiposity, as measured by WC, was also significantly associated with lower risk of HF, which was driven by reduction in risk of HFpEF but not HFrEF. Taken together, our study findings suggest that loss of FM and central adiposity may be important modifiable targets for prevention of HF, particularly HFpEF, in patients with T2DM.
In the Look AHEAD trial, participants assigned to the ILI treatment group had significant reductions in DXA-based measures of FM and LM during follow-up.15 However, direct evaluation of FM and LM was restricted to approximately 20% of the Look AHEAD cohort who underwent DXA testing at select study sites, limiting the evaluation of the prognostic importance of changes in body composition measures. In the present study, we estimated FM and LM in nearly the entire cohort (>99%) of Look AHEAD participants using routinely measured parameters and established, previously validated anthropometric prediction equations.10 We demonstrated good overall agreement between anthropometric prediction equations and DXA-based measures of FM and LM. Furthermore, similar to the DXA substudy, we observed significant reductions in both FM and LM in the ILI treatment group.15 Taken together, our study findings highlight the potential role of these anthropometric prediction equations among patients with T2DM to estimate and track body composition measures longitudinally.
We observed higher risk of HF among participants with higher FM, LM, and WC at baseline. However, the associations were largely driven by differences in CRF levels. In contrast with our study findings, previous studies have demonstrated significant associations of these baseline body composition measures with risk of HF independent of risk factors.19, 20 Several factors may underlie these differences. First, prior studies have not accounted for differences in CRF. This is particularly relevant as prior works have shown that low CRF may account for up to 47% of obesity-associated risk of HF.21 Second, higher amounts of adiposity contribute to development of HF through downstream metabolic dysregulation. Our study included participants with prevalent T2DM and overweight or obesity who have greater baseline metabolic dysfunction and associated risk of HF. Thus, the variability in body composition parameters in our study population at baseline may not contribute to meaningful differences in the downstream burden of cardiometabolic dysfunction.
In contrast with baseline measures, we observed that longitudinal changes in FM and WC were significantly associated with risk of HF independent of other baseline confounders and changes in cardiometabolic parameters. Prior studies have demonstrated that intentional weight loss and reductions in BMI are associated with lower risk of HF.4 Our study extends these observations and suggests that longitudinal reductions in adiposity parameters, but not LM, may lower risk of HF. Furthermore, we observed that reduction in central adiposity, measured by WC, is more strongly associated with lower risk of HFpEF but not HFrEF. Prior studies have demonstrated distinct contributions of regional fat depots, particularly visceral adipose tissue, towards risk of HFpEF vs. HFrEF.8 Specifically, higher amounts of VAT have been associated with greater risk of HFpEF but not HFrEF. It is plausible that loss of central adiposity, assessed by changes in WC, are largely driven by reduction in visceral adiposity. Future studies are needed to determine if lifestyle interventions aimed at preferential reduction of FM or central adiposity may be effective in preventing HF, particularly HFpEF.
Several potential mechanisms may underlie the observed associations between reduction in FM and WC and lower risk of HF events. First, it may be related to a direct favorable effect of loss of FM and central adiposity on cardiac structure and function.7, 22 Furthermore, reduction in adiposity measures have been associated with favorable changes in cardiac remodeling patterns.23 Second, favorable changes in inflammation-, adipokine-, and neurohormonal-related pathways with reductions in adipose tissue mass, particularly central adiposity, may also contribute to the lower downstream risk of HF, especially HFpEF.24
Patterns of association between baseline and longitudinal changes in body composition parameters with risk of MI were markedly distinct from that observed for HF. We observed a paradoxically lower risk of MI among individuals with higher baseline FM or WC. This is consistent with prior observations among patients with long-standing T2DM and may be related to greater metabolic reserve in obese individuals, reverse epidemiology, and residual confounding by age (younger individuals with higher adiposity).25
Our study findings have important clinical implications. Randomized controlled trials evaluating intentional weight loss interventions, such as intensive lifestyle interventions and weight loss pharmacotherapies have not been associated with significant reductions in risk of HF.4, 12, 26 Our study findings suggest that the beneficial effects of weight loss vary across the different components of body composition parameters. Thus, loss of FM and central adiposity, but not LM, are associated with reduction in downstream risk of HF. Future studies are needed to evaluate if interventions preferentially targeting large, sustained losses of FM and central adiposity may be more effective in lowering the risk of HF. This is particularly noteworthy because lifestyle-based weight loss interventions, such as those used in the Look AHEAD trial, led to mild reductions in FM followed by regain and, thus, may not be effective in lowering the risk of HF.15 In contrast, observational studies among patients undergoing bariatric surgery have demonstrated large declines in FM that may be durable.27 This long-term effect on FM may contribute to the lower downstream risk of HF in patients undergoing bariatric surgery.28 Furthermore, among FM depots, reductions in central adiposity may be particularly useful to lower the risk of HFpEF.29 This is particularly relevant considering the growing burden of HFpEF and lack of effective therapies against this disease.24
Several limitations to our study are noteworthy. First, direct measures of FM and LM were not available in all study participants, and anthropometric prediction equations were used to estimate FM and LM in the entire study population. We observed that predicted and DXA-based measures of FM / LM were highly correlated and there was excellent agreement between the two values highlighting the validity of the estimated body composition parameters used in this study. Second, the present study included participants enrolled in the Look AHEAD trial who had T2DM and overweight or obesity and were able to complete a maximal exercise treadmill test. Thus, there is potential for selection bias and study findings may not be generalizable. Third, we do not have measures of regional adipose tissue depots, such as visceral adipose tissue, subcutaneous adipose tissue, and lower body fat, or measures of muscle strength, each of which could affect downstream risk of HF differently.30, 31 Additionally, CRF was estimated based on speed and grade achieved during a standardized treadmill-based exercise test protocol rather than direct measurement of peak oxygen consumption. However, estimated and direct measures of CRF are highly correlated.32, 33 Finally, the primary outcome in the present study was incident HF hospitalization and outpatient diagnoses of HF may have been missed. However, incident HF hospitalization is an objective endpoint that captures clinically meaningful events and this outcome is consistent with that used in prior analyses.4
In conclusion, among participants with T2DM who had overweight or obesity from the Look AHEAD trial, anthropometric prediction equations estimated FM and LM with minimal bias. Longitudinal decline in FM and WC is associated with lower risk of HF, particularly HFpEF, but not MI. Future studies are needed to confirm these findings and investigate whether interventions targeting preferential reductions in FM and central adiposity modify the risk of HF among patients with T2DM.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
Among patients with type 2 diabetes mellitus and overweight or obesity, fat mass and lean mass can be estimated using anthropometric equations with good overall agreement compared with dual-energy X-ray absorptiometry measures.
Large reductions in fat mass and waist circumference over 1- and 4-year follow-up were significantly associated with lower risk of heart failure with preserved ejection fraction in type 2 diabetes mellitus.
What are the clinical implications?
Readily available clinical information can be incorporated into validated anthropometric prediction equations to estimate and track fat mass longitudinally to assess risk of heart failure among patients who have type 2 diabetes mellitus and overweight or obesity.
Fat mass and waist circumference may represent key, modifiable targets for lifestyle interventions to reduce the risk of heart failure with preserved ejection fraction in type 2 diabetes mellitus.
Acknowledgements:
The authors thank the Look AHEAD study participants, staff, and investigators.
Funding and Support:
Dr. Patel is supported by the National Heart, Lung, and Blood Institute T32 postdoctoral training grant (5T32HL125247-03). Dr. Pandey is supported by the Texas Health Resources Clinical Scholars Program. Dr. Kitzman is supported by: R01AG18915, R01AG045551, P30AG21331.
The Look AHEAD Trial was funded by the National Institutes of Health through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. This work was partially supported by a NORC Center Grant P30DK072476 and Louisiana Clinical and Translational Science Center grant U54 GM104940. The Indian Health Service provided personnel, medical oversight, and use of facilities. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the Indian Health Service or other funding sources. Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the Harvard Clinical and Translational Science Center (RR025758-04); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (M01RR000056), the Clinical Translational Research Center funded by the Clinical & Translational Science Award (UL1 RR 024153) and a National Institutes of Health grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346). The following organizations have committed to make major contributions to Look AHEAD: FedEx Corp; Health Management Resources; LifeScan, Inc, a Johnson & Johnson Company; OPTIFAST of Nestle HealthCare Nutrition, Inc; Hoffmann-La Roche Inc; Abbott Nutrition; and Slim-Fast Brand of Unilever North America. Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Non-standardized Abbreviations and Acronyms
- FM
fat mass
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- ILI
intensive lifestyle intervention
- LM
lean mass
- MI
myocardial infarction
- T2DM
type 2 diabetes mellitus
- WC
waist circumference
Footnotes
Disclosures: Dr. Kitzman reported receiving honoraria outside the present study as a consultant for AbbVie, Bayer, Merck, Medtronic, Relypsa, Corvia Medical, Boehringer-Ingelheim, NovoNordisk, Astra Zeneca, and Novartis, and grant funding outside the present study from Novartis, Bayer, and Astra Zeneca, and stock ownership in Gilead Sciences.
Dr Berry received research funding from Abbott. Dr. Pandey has served on the advisory board of Roche Diagnostics. Other authors report no relevant disclosures.
REFERENCES
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 2.Rawshani A, Rawshani A, Franzen S, Eliasson B, Svensson AM, Miftaraj M, McGuire DK, Sattar N, Rosengren A and Gudbjornsdottir S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N Engl J Med. 2017;376:1407–1418. [DOI] [PubMed] [Google Scholar]
- 3.Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2018;379:633–644. [DOI] [PubMed] [Google Scholar]
- 4.Pandey A, Patel KV, Bahnson JL, Gaussoin SA, Martin CK, Balasubramanyam A, Johnson KC, McGuire DK, Bertoni AG, Kitzman D, et al. Association of Intensive Lifestyle Intervention, Fitness, and Body Mass Index With Risk of Heart Failure in Overweight or Obese Adults With Type 2 Diabetes Mellitus: An Analysis From the Look AHEAD Trial. Circulation. 2020;141:1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Look ARG, Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, Clark JM, Coday M, Curtis JM, Egan C, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR, Berry JD, Khera A, McGuire DK, Vega GL, et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging. 2013;6:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey A, Kondamudi N, Patel KV, Ayers C, Simek S, Hall ME, Musani SK, Blackshear C, Mentz RJ, Khan H, et al. Association Between Regional Adipose Tissue Distribution and Risk of Heart Failure Among Blacks. Circ Heart Fail. 2018;11:e005629. [DOI] [PubMed] [Google Scholar]
- 8.Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH, Cushman M, Blumenthal RS and Michos ED. Adiposity and Incident Heart Failure and its Subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC Heart Fail. 2018;6:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neeland IJ, Yokoo T, Leinhard OD and Lavie CJ. Twenty-First Century Advances in Multimodality Imaging of Obesity for Care of the Cardiovascular Patient. JACC Cardiovasc Imaging. 2020. doi: 10.1016/j.jcmg.2020.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Sun Q, Willett WC and Giovannucci EL. Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999-2006. Br J Nutr. 2017;118:858–866. [DOI] [PubMed] [Google Scholar]
- 11.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ and Look ARG. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. [DOI] [PubMed] [Google Scholar]
- 12.Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Willett WC and Giovannucci EL. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ. 2018;362:k2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heshka S, Ruggiero A, Bray GA, Foreyt J, Kahn SE, Lewis CE, Saad M, Schwartz AV and Look ARG. Altered body composition in type 2 diabetes mellitus. Int J Obes (Lond). 2008;32:780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pownall HJ, Bray GA, Wagenknecht LE, Walkup MP, Heshka S, Hubbard VS, Hill J, Kahn SE, Nathan DM, Schwartz AV, et al. Changes in body composition over 8 years in a randomized trial of a lifestyle intervention: the look AHEAD study. Obesity (Silver Spring). 2015;23:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Look ARG and Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribisl PM, Lang W, Jaramillo SA, Jakicic JM, Stewart KJ, Bahnson J, Bright R, Curtis JF, Crow RS, Soberman JE, et al. Exercise capacity and cardiovascular/metabolic characteristics of overweight and obese individuals with type 2 diabetes: the Look AHEAD clinical trial. Diabetes Care. 2007;30:2679–2684. [DOI] [PubMed] [Google Scholar]
- 18.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G and Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S and Vatten LJ. Body Mass Index, Abdominal Fatness, and Heart Failure Incidence and Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Circulation. 2016;133:639–649. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Bartz TM, Murthy V, Santanasto A, Shah R, Djousse L, Mukamal K, Forman D, Hirsch C, Newman A, et al. Relationship of body composition to incident heart failure in two population-based cohorts of older adults. J Am Coll Cardiol. 2019;73 (Issue 9 Supp 1). [Google Scholar]
- 21.Pandey A, Cornwell WK 3rd, Willis B, Neeland IJ, Gao A, Leonard D, DeFina L and Berry JD. Body Mass Index and Cardiorespiratory Fitness in Mid-Life and Risk of Heart Failure Hospitalization in Older Age: Findings From the Cooper Center Longitudinal Study. JACC Heart Fail. 2017;5:367–374. [DOI] [PubMed] [Google Scholar]
- 22.Corden B, de Marvao A, Dawes TJ, Shi W, Rueckert D, Cook SA and O'Regan DP. Relationship between body composition and left ventricular geometry using three dimensional cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2016;18:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilner B, Garg S, Ayers CR, Maroules CD, McColl R, Matulevicius SA, de Lemos JA, Drazner MH, Peshock R and Neeland IJ. Dynamic Relation of Changes in Weight and Indices of Fat Distribution With Cardiac Structure and Function: The Dallas Heart Study. J Am Heart Assoc. 2017;6: e005897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD and Lavie CJ. Physical Activity, Fitness, and Obesity in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6:975–982. [DOI] [PubMed] [Google Scholar]
- 25.Xing Z, Pei J, Huang J, Peng X, Chen P and Hu X. Relationship of Obesity to Adverse Events Among Patients With Mean 10-Year History of Type 2 Diabetes Mellitus: Results of the ACCORD Study. J Am Heart Assoc. 2018;7:e010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohula EA, Wiviott SD, McGuire DK, Inzucchi SE, Kuder J, Im K, Fanola CL, Qamar A, Brown C, Budaj A, et al. Cardiovascular Safety of Lorcaserin in Overweight or Obese Patients. N Engl J Med. 2018;379:1107–1117. [DOI] [PubMed] [Google Scholar]
- 27.Zalesin KC, Franklin BA, Lillystone MA, Shamoun T, Krause KR, Chengelis DL, Mucci SJ, Shaheen KW and McCullough PA. Differential loss of fat and lean mass in the morbidly obese after bariatric surgery. Metab Syndr Relat Disord. 2010;8:15–20. [DOI] [PubMed] [Google Scholar]
- 28.Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, Kattan MW and Nissen SE. Association of Metabolic Surgery With Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes and Obesity. Jama. 2019;322:1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitzman DW and Nicklas BJ. Pivotal Role of Excess Intra-Abdominal Adipose in the Pathogenesis of Metabolic/Obese HFpEF. JACC Heart Fail. 2018;6:1008–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carbone S, Kirkman DL, Garten RS, Rodriguez-Miguelez P, Artero EG, Lee D-c and Lavie CJ. Muscular Strength and Cardiovascular Disease: AN UPDATED STATE-OF-THE-ART NARRATIVE REVIEW. Journal of Cardiopulmonary Rehabilitation and Prevention. 2020;40:302–309. [DOI] [PubMed] [Google Scholar]
- 31.Haykowsky MJ, Nicklas BJ, Brubaker PH, Hundley WG, Brinkley TE, Upadhya B, Becton JT, Nelson MD, Chen H and Kitzman DW. Regional Adipose Distribution and its Relationship to Exercise Intolerance in Older Obese Patients Who Have Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward A, White SR and Linnerud AC. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. [DOI] [PubMed] [Google Scholar]
- 33.Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC and Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982;103:363–373. [DOI] [PubMed] [Google Scholar]
- 34.Schoeller DA, Tylavsky FA, Baer DJ, Chumlea WC, Earthman CP, Fuerst T, Harris TB, Heymsfield SB, Horlick M, Lohman TG, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81:1018–1025. [DOI] [PubMed] [Google Scholar]
- 35.Galgani JE, Smith SR and Ravussin E. Assessment of EchoMRI-AH versus dual-energy X-ray absorptiometry to measure human body composition. Int J Obes (Lond). 2011;35:1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Look Ahead Research G, Bray G, Gregg E, Haffner S, Pi-Sunyer XF, WagenKnecht LE, Walkup M and Wing R. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3:202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Look ARG, Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Look ARG. Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ACSM’s Guidelines for Exercise Testing and Prescription 8th Edition: Lippincott Williams & Wilkins; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.