Cervical human papillomavirus testing led to more positive screening test results and diagnoses of high-grade cervical intraepithelial lesions than cytology.
Abstract
OBJECTIVE:
To compare the real-life screening outcomes after cytology was replaced by human papillomavirus (HPV) testing for women aged 60–64 years.
METHODS:
Using the Danish national pathology register, we compared screening outcomes during two consecutive calendar periods, one where women were screened with cytology and one where most women were screened with HPV testing. Our primary outcomes were the proportions of women with positive test results, high-grade cervical intraepithelial neoplasia (CIN 2 or worse), and cervical cancer.
RESULTS:
Women screened during the HPV testing period were more likely to have a positive screening test result than were women screened during the cytology period (relative proportion 2.80, 95% CI 2.65–2.96). The detection of CIN 2 or worse was also increased (relative proportion 1.54, 95% CI 1.31–1.80), whereas there was no increase in screen-detected cervical cancer diagnoses (relative proportion 1.27, 95% CI 0.76–2.12). Within the first 4 years after a negative screening test result, including 168,477 woman-years at risk after a negative screen result in the HPV period and 451,421 woman-years after a negative screen result in the cytology period, the risk of a cervical cancer diagnosis was approximately 4 per 100,000 woman-years and was similar for both screening tests (relative risk 0.99, 95% CI 0.41–2.35).
CONCLUSION:
Human papillomavirus testing led to more positive screening test results and diagnoses of high-grade CIN lesions. Few women were diagnosed with cervical cancer after a negative screening test result.
High-risk human papillomavirus (HPV) testing is replacing cytology in primary cervical cancer screening in several countries. This test is considered a superior alternative to cytology, because women with a negative HPV test result are less likely to develop progressive high-grade cervical intraepithelial neoplasia (CIN) and cervical cancer than women with negative cytology.1
European cervical screening programs first started preparing for national rollouts of HPV testing in the early 2010s. For example, the Dutch Health Council issued that advice in 2011, and a national rollout for women targeted by the program, aged 30–60 years, started in early 2017.2 The first Italian HPV pilots including, predominantly, women aged 35–64 commenced in 2012, and the Ministry of Health recommended a wider rollout in 2013.3 In England, a large pilot was started in 2013, and the national rollout for women aged 25–64 years was completed in December 2019. The Danish Health Authority recommended a switch from cytology to HPV-based screening in 2012.4 The advice at that time, however, applied only to women aged 60–64 years.
With approximately 30 cases of cervical cancer per 100,000 women (world age-standardized rate), Denmark used to have one of the highest incidence rates in Europe. This incidence rate decreased to the current level of approximately 10 per 100,000 after population-based screening had been introduced in the 1960s.5,6 The prevalence of HPV infections, however, remains high.7,8 This high prevalence was a major point in the discussions underpinning the 2012 screening recommendations and the reason why HPV testing, despite its superior negative predictive value, was not recommended for the entire screening age range.4 An immediate introduction of HPV testing was nevertheless considered appropriate for women in their 60s, given their lower HPV prevalence. It was also considered a priority, in that it could potentially offer a better protection from developing cervical cancer after the “exit” screen, that is, the last screening round before women are discharged from the program on account of their age.4 There were, as there are now, however, very few studies specifically in this age group that investigated HPV screening and the subsequent incidence of cervical cancer.9
Our aim was to report the outcomes of the first 4 years of routinely implemented HPV-based screening at ages 60–64 in Denmark, in comparison with cytology. We studied the proportions of women with inadequate and positive screening test results, with detected high-grade CIN, and a cervical cancer diagnosis.
METHODS
In Denmark, organized (call-recall) cervical screening started on a regional basis in the 1960s and became national in the second half of the 1990s. Personal invitations are sent electronically or by letter to all resident women who fulfill the age eligibility and screening history criteria for the program (see below), are not registered as having had a hysterectomy, and did not actively opt out of the program. These invitations play the role of safety net for women who do not obtain regular screening on their own, that is, when they do not have a screening sample registered in the national pathology database (Patobank) in the past 3 years (if aged 23–49) or in the past 5 years (if aged 50–64). Screening samples of women that were taken without an invitation are usually referred to as “opportunistic,” although they equally contribute to the national screening coverage as do samples taken after an invitation.10 Most women in the target population participate in screening, as the age-appropriate coverage has been approximately 73% in the recent years.11 Corrected for hysterectomies, furthermore, the 5.5-year coverage in women aged 60–64 was estimated at approximately 77% in 2010.12 Historical data for birth cohorts included in our analysis suggest that the majority of women included in the present analysis must have participated in screening throughout their entire lives.6
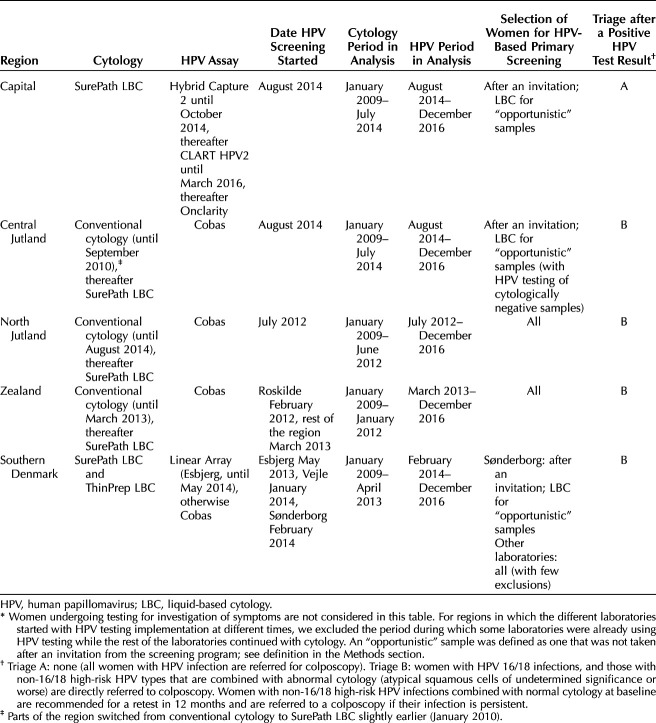
Screening is organized by administrative region. Each regionally appointed screening laboratory has been free to procure the cytology and HPV testing systems. When switching over to HPV testing, most regions extended this to all samples, with minor exceptions, predominantly in cases of nonscreening samples. Laboratories in three regions, however, continued to use cytology for “opportunistic” screening samples (see above for a definition). The regional differences in the transition from cytology-based to HPV-based screening for women aged 60–64 years are described in Table 1.
Table 1.
Regional Policies for Human Papillomavirus Testing in Primary Screening of Women Aged 60–64 Years Between 2009 and 2016*
With cytology as the primary test, women screened in their 60s were referred to colposcopy if they had atypical squamous cells of undetermined significance (ASC-US) or low-grade squamous intraepithelial lesions (LSIL) combined with a positive HPV test result, or any worse cytologic abnormality regardless of the HPV infection. Those with negative cytology, and those with ASC-US or LSIL and a negative HPV triage test result, were discharged from the screening program. In regions that did not use HPV triage for LSIL cytology, the guidelines recommended a new test in 6 months and a colposcopy in case of persistent cytologic abnormalities.
For women with a positive HPV primary screening test result, the national guidelines recommended that those with abnormal triage cytology, or an HPV 16 or 18 infection, are referred to colposcopy, and that other women be retested in 12 months. This policy was followed in all regions but one, where all women with HPV infection were referred to colposcopy (Table 1). Women with a negative HPV test result are discharged from the program.
In the Patobank, samples are recorded regardless of whether they were taken in general practice or elsewhere, and regardless of whether they were taken for screening or for other purposes. This way, the women's screening histories can be reconstructed in full even if women change their (Danish) address.13 The Patobank can be considered satisfactorily complete for cervical cytology since 2005 and for cervical histology since 2009.14 Colposcopies are not registered in the Patobank. Because the national guidelines recommended taking blind biopsies if no lesion could be visualized colposcopically, a record of a biopsy was used as an indicator that a colposcopy took place. The Danish unique personal identification numbers (pseudonymized for the purpose of this analysis by the Danish Health Data Authority) were used for linkage between Patobank records. We retrieved samples with T8X2* or T8X3* (cervical cytology), T83* (cervical histology), or T82000 (uterine histology, not otherwise specified) codes following the Danish version of the SNOMED (Systematized Nomenclature of Medicine) classification. The retrieved samples were recorded between January 1, 2007, and December 31, 2018, in women born between January 1, 1944, and December 31, 1956.
We classified SNOMED codes for squamous and glandular lesions into diagnostic categories based on previous work.15 One of the authors (R.R.S.) checked this categorization and added any recently introduced codes.
The pathology register reflects the daily production in pathology laboratories. Inconclusive malignancy codes will sometimes be used, in which case additional clinical workup is recommended before a definite diagnosis can be determined. Consequently, the numbers of apparent cases of cervical cancer reported in this register tend to overestimate the numbers from the official national cancer statistics. For our study, a pathologist who reports to the pathology register on a daily basis (R.R.S.) and a gynecologist who is a daily pathology register user (J.B.S.) corrected the counts using the information available in the SNOMED string for each case. The record was counted as a diagnosis of cervical cancer if the string suggested that cervix uteri was the primary location.
We included the first primary screening sample for each woman if it was taken at age 60–64 years between January 1, 2009, and December 31, 2016. Age was determined from the woman's date of birth and the date the sample arrived in a laboratory.
Because the reason for taking a sample is not reliably registered in the Patobank, we defined a primary screening sample as cervical cytology or HPV testing that was not preceded by another cervical testing record in the Patobank in 2 years. In line with the definitions used for the national program monitoring scheme undertaken by the Danish Quality Database for Cervical Screening,11 we additionally excluded samples that were described as consultation material, marked for various types of special (nonstandard) testing, or taken for research purposes.
We compared all primary screening samples during the HPV screening period to those during the cytology screening period. During the HPV screening period (Table 1), we included women who were still being screened with cytology. This is because in both observed periods, women who chose to be screened without receiving an invitation may have had a different risk profile than women who waited for their invitation and excluding them from the analysis could introduce bias. Because each regional laboratory switched from cytology to HPV testing at different times between February 2012 and August 2014 (Table 1), we defined periods of screening with cytology and periods of screening with HPV testing depending on the region. In regions with several screening laboratories, the end of the cytology period was defined as the date the first of the laboratories switched to HPV testing, and the beginning of the HPV period was defined as the date the last of the laboratories switched to HPV testing. Samples were assigned to a particular region based on the laboratory code. Owing to data protection restrictions, we could not report exact counts when those were lower than or equal to five.
We determined the proportions of women with an inadequate or positive screening test result, and, within 2 years of a positive screening test result, with at least one follow-up sample, colposcopy, and CIN 2 or worse, CIN 3 or worse, or cervical cancer as the worst diagnosis. We considered these events to be a consequence of screening. Among women undergoing a colposcopy, we determined the positive predictive value for CIN 2 or worse. A positive screening test result was defined as atypical squamous cells of undetermined significance (ASC-US) or worse for cytology, and a positive signal on an HPV test for HPV-based screening. The 95% CIs for all relative frequencies were calculated assuming that their logarithms were approximately normally distributed.
Woman-years at risk to determine the incidence of cervical cancer after a negative screen result were counted from the date of screening until 4 years later, December 31, 2018, the date of a CIN 2 or a CIN 3 diagnosis, or the date of a cancer diagnosis, whichever came first. The 95% CI for a relative risk was calculated by using Wald's normal approximation, with help from the “epitools” package in R version 4.02. We used a nonparametric survival function to draw a Kaplan-Meier plot.
The project was reviewed by the Knowledge Centre on Data Protection Compliance on behalf of the Danish Capital Region (reference number: VD-2019-95) and did not require patient consent. No contact was made to patients, their relatives, or treating physicians. Data were pseudonymized by the Danish Health Data Authority before they were made available for analysis.
RESULTS
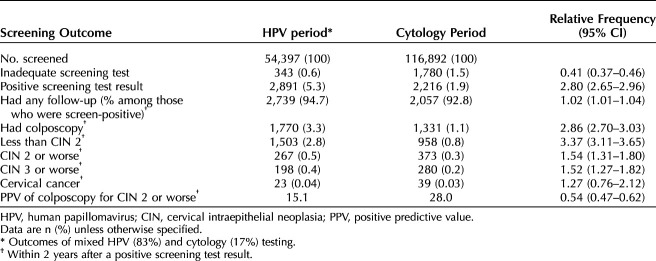
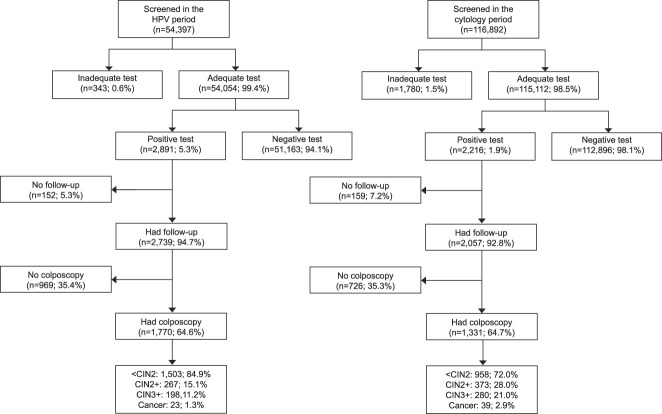
In total, 116,892 women aged 60–64 years born between 1944 and 1956 were screened in the cytology period and 54,397 were screened in the HPV period (Table 2 and Fig. 1). The average ages of the women were 62.7 (SD 1.4) and 62.5 (SD 1.4) years, respectively. During the HPV period, 9,072 (17%) of the women were screened with cytology, mostly due to a deliberate policy for “opportunistic” samples in some of the regions (Table 1).
Table 2.
Screening Outcomes During the Human Papillomavirus Testing Period Compared With the Cytology Period
Fig. 1. Flowchart for the study. HPV, human papillomavirus; CIN, cervical intraepithelial neoplasia.
Schroll. Human Papillomavirus Testing in the Last Screening Round. Obstet Gynecol 2021.
Women screened with HPV testing were more likely to have an adequate test, and the proportion of those with an inadequate test decreased from 1.5% with cytology to 0.6% with HPV testing (Table 2 and Fig. 1). Almost three times as many women had a positive screening test result (5.3% vs 1.9%) after HPV testing than after cytology. The proportion of women followed up after a positive screening test result did not differ substantially between the screening tests; about 95% were followed up, and approximately 65% of those had a colposcopy. In total, 3.3% of women screened with HPV testing underwent a colposcopy, compared with 1.1% of women screened with cytology.
Among 1,770 women undergoing a colposcopy after a positive test result during the HPV testing period, CIN 2 or worse was diagnosed in 267 (15.1%). Among 1,331 women undergoing a colposcopy after abnormal cytology, 373 (28.0%) had a CIN 2 or worse diagnosis. The detection of CIN 2 or worse, measured as the proportion of all screened women, increased from 0.3% with cytology to 0.5% with HPV testing (relative frequency 1.54, 95% CI 1.31–1.80). For CIN 3 or worse, the detection increased from 0.2% to 0.4% (relative frequency 1.52, 95% CI 1.27–1.82). An increase in the detection of CIN 2 or worse could be observed in each region (data not shown). An additional 73 women had a diagnosis of CIN not otherwise specified during the HPV screening period and 70 during the cytology period (not tabulated). In the main analysis presented in Table 2, diagnoses of CIN not otherwise specified were combined with less than CIN 2. Even if all of these diagnoses represented high-grade CIN (CIN 2 or worse), the relative frequency for detection with HPV testing compared with cytology would remain similarly elevated, 1.65 (95% CI 1.43–1.90).
An increase in the detection of cervical cancer in 2 years after a positive HPV test result of about 27% was not statistically significant (Table 2). For both screening tests, the majority of cases were diagnosed within the first year of screening taking place and few (five or fewer) were diagnosed in the second year when some surveillance might still have been taking place (data not tabulated). Very few additional cervical cancer diagnoses (five or fewer) were made later than 2 years after screening in women with HPV infection who were not treated for CIN 2 or CIN 3 during the first 2 years (data not tabulated owing to small numbers).
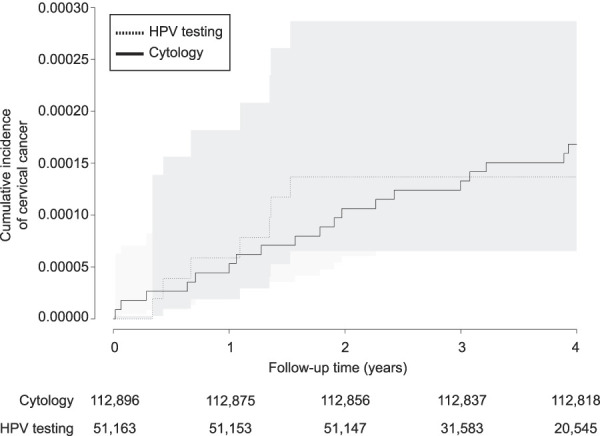
In these early data, the risk of cervical cancer after a negative screening test result was approximately 4 per 100,000 woman-years for both HPV testing and cytology. The relative risk for the HPV period compared with the cytology period was 0.99 (95% CI 0.41–2.35), based on seven cases in the HPV period (168,477 woman-years at risk) and 19 cases in the cytology period (451,421 woman-years at risk, Fig. 2).
Fig. 2. Cumulative incidence of cervical cancer within 4 years after a negative screening test result, for the period with human papillomavirus (HPV) testing compared with the period with cytology as the primary screening test. Log rank P=.90.
Schroll. Human Papillomavirus Testing in the Last Screening Round. Obstet Gynecol 2021.
DISCUSSION
In the “exit” screening round among Danish women aged 60–64, HPV testing detected about 50% more CIN 2 or worse and CIN 3 or worse than cytology but necessitated three times as many colposcopies. In the first 4 years after screening with a negative test result, the risk of cervical cancer remained low.
A strength of our analysis is that the data were retrieved from the national pathology register. This register is the most complete record of cervical screening in Denmark. The analysis includes all women who attended screening; it is, therefore, fully representative of an unselected older population that was previously screened with cytology.
There are also some limitations to our analysis. We did not have data available for analysis with which to adjust for any sociodemographic differences between women screened during those two periods. However, these factors16 were likely unchanged between the two periods, judging by how constant screening coverage has been over time and between regions.17,18 It is also unlikely that the underlying risk of cervical cancer differed between the two cohorts, given the short periods for inclusion of samples in the two chronologically adjacent periods. Although cytology was the recommended primary screening test, laboratories used both conventional and liquid-based technologies; during the HPV period, all laboratories used liquid-based technologies but different HPV assays. Hence, the analyses should be understood as comparing routine implementation of two distinct types of screening tests rather than a comparison of specific testing technologies or even brands.
Our results with cancer as the endpoint should still be interpreted with some caution.
In this initial phase of a national implementation of HPV testing in the “exit” round, the data do not yet show a decrease in the incidence of cervical cancer after a negative screening test result compared with cytology-based screening. This was not because of continued use of cytology for a proportion of women, as hardly any cancer was diagnosed in the HPV screening period in women with “opportunistic” samples analyzed with cytology (the exact number cannot be reported owing to restrictions when the number of cases was five or fewer). Likewise, we could not find an indication of a specific, poorly performing HPV testing cluster, as the seven observed cases were reported from four of five regions and in five different laboratories.
In Denmark, cytology screening has been effective in keeping the incidence of cervical cancer at levels comparable with other European countries with screening programs, despite a higher background risk of the disease.19 The program has appointed regional heads who are responsible for quality control and assurance, and the screening process is monitored nationally through a standard set of indicators and an audit of cervical cancer cases. The low risk of cervical cancer after negative “exit” cytology that was observed in our study is consistent with the findings from the English and Welsh cervical screening audit. There, the cumulative 20-year risk was estimated at 8 per 10,000 in women with consecutive negative screening cytology at 50–64 years, and this risk was particularly low in the first few years after screening had ended (measured at age 65–69 years).20 If HPV testing is to improve on this, it is likely that this will become apparent only after a longer follow-up, as was also the case in the meta-analysis of four randomized controlled trials.1
Danish screening guidelines recommend that women aged 60 years or more with an HPV infection but without a CIN lesion requiring treatment, provided that their colposcopy-directed biopsies are representative of the transformation zone, can be discharged from the screening program. Women with HPV infection whose transformation zone could not be sampled adequately, in contrast, often continue to be re-tested yearly. There is no national guideline to determine when the re-testing should stop, so these decisions are often made at a local level. Whether continued surveillance should be advocated continues to be one of the most prominent questions that screening programs are trying to solve. In the Danish data reported here, the detection of CIN 2 or CIN 3 (83/1,000 women with HPV infection) and of cancer (8/1,000 women with HPV infection) was the highest during the first 2 years after a positive HPV test result. This was expected because of the recommended timing of the follow-up and diagnostic tests. After those 2 years had passed, the residual cumulative risk of cancer in the subsequent 2 years was much lower, between 1 and 2 cases per 1,000 (estimated from five or fewer cases in the entire study in years 3 and 4, combined). The risk thereafter, when women with HPV infection reach the age of their late 60s, could not yet be observed in our study. A one-time national offer of a single round of HPV screening to all female Danish residents older than age 68 years (born any time before 1948) in 201721 may provide some information on this risk. In that one-time offer, 4,479 women aged 69 to older than 90 years were positive for HPV infection, and 37 (8/1,000) had a screen-detected cancer.22 These data suggest that, in women with persistent HPV infection, the risk of residual cancer is not zero but is still low enough to not necessitate an intensive (eg, yearly) continued follow-up beyond the early recall 12 months after screening that is already in place. Continued follow-up at longer intervals might turn out to be beneficial, but observational data that could support such an intervention have yet to accrue.
Replacing cytology with HPV testing in women in their early 60s, most of whom are postmenopausal, will have an important effect on screening services such as primary care, pathology, and colposcopy. First, the Danish screening protocol requires the use of cytology only in triage of women with non-16 or 18 high-risk HPV infections. In our data, this led to a more than 95% reduction in the cytopathology workload in this age group. Not only the volume, also the nature of the remaining cytology work will change. Owing to lower levels of estrogen, postmenopausal cervical tissue is often atrophic.23 This was a challenge for cytopathology, because atrophy is itself not a risk factor for cervical cancer but presents with cellular changes that are difficult to differentiate from true dysplasia. With HPV testing, the work of cytopathologists will be supported by confirming the presence of an HPV infection beforehand; this should decrease the overcalling of benign changes. Second, tissue atrophy is also associated with a higher proportion of sample inadequacy for cytologic interpretation.23 Sample inadequacy is lower (more than halved) with HPV testing, reducing the need for repeated screening visits in primary care. Finally, the postmenopausal transformation zone becomes less easily visible as it becomes located higher in the endocervical canal. Consequently, it is more difficult to perform a colposcopy and take representative biopsies.9 The Danish CIN treatment guidelines for women with HPV infection in their 60s discuss an increased use of surgical procedures to reduce the problem of under-diagnosis of progressive lesions.24 Given that HPV testing requires more women to undergo a colposcopy (tripling in our study) with a lower positive predictive value for CIN 2 or worse (about halved), the guidelines require that the decision to proceed with surgical procedures is to be made in agreement with the patient, out of concern for potential overtreatment that this recommendation would almost certainly lead to.24
In fact, the extent of overdiagnosis and overtreatment of both CIN 2 or worse and cervical cancer in this population should continue to be monitored. With cytology screening, between six and eight women underwent CIN treatment at any age for every case of cervical cancer saved.25 This relationship is still largely unknown for HPV testing, in particular for older women in whom fewer life-years are to be gained by prevention of cancer. Nevertheless, women's remaining life expectancy is increasing. In Denmark, it is currently almost 22 years for women aged 64 years, whereas just 10 years ago it was approximately 20 years.26 This period is long enough even for newly developed CIN 2 or CIN 3 to develop into cervical cancer.27,28 Hence, the extent of overdiagnosis in the “exit” round is becoming smaller in younger birth cohorts compared with what must have been the case not so long ago.
Footnotes
Matejka Rebolj was supported by Cancer Research UK (reference: C8162/A27047). Data retrieval was funded by the Department of Pathology, Copenhagen University Hospital Amager and Hvidovre.
Financial Disclosure Jeppe Bennekou Schroll is a member of the Danish National Steering Group for Cervical Screening. Reza Rafiolsadat Serizawa is head of the cervical screening program in the Capital Region, member of the Danish Quality Database for Cervical Screening. Matejka Rebolj has received funding from Public Health England for the epidemiological evaluation of the HPV primary screening pilot, where she has been the principal investigator; attended meetings with various HPV assay manufacturers; received a fee for lecture from Hologic paid to employer (2018); and she has been member of various groups convened by Public Health England providing advice to the English Cervical Screening Program.
Presented at the Eurogin Conference, December 4–7, 2019, Monte Carlo, Monaco.
The authors thank Rikke Holst Andersen, Susanne Merete Nielsen, Helle Suurballe Lanner, Dorthe Ejersbo, Sanne Christiansen, and Marianne Waldstrøm for providing information on regional cervical screening policies.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C382.
Contributor Information
Reza Rafiolsadat Serizawa, Email: reza.serizawa@regionh.dk.
Matejka Rebolj, Email: matejka.rebolj@kcl.ac.uk.
Figure.
No available caption
REFERENCES
- 1.Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 2014;383:524–32. doi: 10.1016/S0140-6736(13)62218-7 [DOI] [PubMed] [Google Scholar]
- 2.Aitken CA, van Agt HME, Siebers AG, van Kemenade FJ, Niesters HGM, Melchers WJG, et al. Introduction of primary screening using high-risk HPV DNA detection in the Dutch cervical cancer screening programme: a population-based cohort study. BMC Med 2019;17:228. doi: 10.1186/s12916-019-1460-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronco G, Giorgi Rossi P, Giubilato P, Del Mistro A, Zappa M, Carozzi F. A first survey of HPV-based screening in routine cervical cancer screening in Italy. Epidemiol Prev 2015;39:77–83. [PubMed] [Google Scholar]
- 4.Sundhedsstyrelsen. Screening for livmoderhalskræft—anbefalinger. Accessed November 18, 2020. https://www.sst.dk/∼/media/B1211EAFEDFB47C5822E883205F99B79.ashx
- 5.Danckert B, Ferlay J, Engholm G, Hansen HL, Johannesen TB, Khan S, et al. NORDCAN: cancer incidence, mortality, prevalence and survival in the Nordic countries. Version 8.2. (26.3.2019). Association of the Nordic Cancer Registries. Danish Cancer Society. Accessed November 18, 2020. http://www.ancr.nu/ [Google Scholar]
- 6.Lynge E, Clausen LB, Guignard R, Poll P. What happens when organization of cervical cancer screening is delayed or stopped? J Med Screen 2006;13:41–6. doi: 10.1258/096914106776179773 [DOI] [PubMed] [Google Scholar]
- 7.Rebolj M, Bonde J, Ejegod D, Preisler S, Rygaard C, Lynge E. A daunting challenge: human papillomavirus assays and cytology in primary cervical screening of women below age 30 years. Eur J Cancer 2015;51:1456–66. doi: 10.1016/j.ejca.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 8.Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 Years and above. PLoS One 2016;11:e0147326. doi: 10.1371/journal.pone.0147326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gravitt PE, Landy R, Schiffman M. How confident can we be in the current guidelines for exiting cervical screening? Prev Med 2018;114:188–92. doi: 10.1016/j.ypmed.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 10.Tranberg M, Larsen MB, Mikkelsen EM, Svanholm H, Andersen B. Impact of opportunistic testing in a systematic cervical cancer screening program: a nationwide registry study. BMC Public Health 2015;15:681. doi: 10.1186/s12889-015-2039-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Styregruppen for DKLS. Årsrapport DKLS 2019: dansk Kvalitetsdatabase for Livmoderhalskræftscreening. Accessed November 18, 2020. https://www.sundhed.dk/content/cms/82/4682_dkls_aarsrapport_2019_off_version.pdf
- 12.Lam JU, Lynge E, Njor SH, Rebolj M. Hysterectomy and its impact on the calculated incidence of cervical cancer and screening coverage in Denmark. Acta Oncol 2015;54:1136–43. doi: 10.3109/0284186X.2015.1016625 [DOI] [PubMed] [Google Scholar]
- 13.Bjerregaard B, Larsen OB. The Danish pathology register. Scand J Public Health 2011;39:72–4. doi: 10.1177/1403494810393563 [DOI] [PubMed] [Google Scholar]
- 14.Rebolj M, Njor S, Lynge E, Preisler S, Ejegod D, Rygaard C, et al. Referral population studies underestimate differences between human papillomavirus assays in primary cervical screening. Cytopathology 2017;28:419–28. doi: 10.1111/cyt.12451 [DOI] [PubMed] [Google Scholar]
- 15.Rebolj M, Rask J, van Ballegooijen M, Kirschner B, Rozemeijer K, Bonde J, et al. Cervical histology after routine ThinPrep or SurePath liquid-based cytology and computer-assisted reading in Denmark. Br J Cancer 2015;113:1259–74. doi: 10.1038/bjc.2015.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristensson JH, Sander BB, von Euler-Chelpin M, Lynge E. Predictors of non-participation in cervical screening in Denmark. Cancer Epidemiol 2014;38:174–80. doi: 10.1016/j.canep.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 17.Styregruppen for DKLS. Årsrapport DKLS 2010: dansk Kvalitetsdatabase for Livmoderhalskræftscreening. Accessed November 18, 2020. https://www.sundhed.dk/content/cms/80/1880_aarsrapport-2010-livmoderhalskraeftscreening.pdf
- 18.Styregruppen for DKLS. Årsrapport DKLS 2016: dansk Kvalitetsdatabase for Livmoderhalskræftscreening. Accessed November 18, 2020. https://www.sundhed.dk/content/cms/82/4682_dkls_%C3%A5rsrapport2016_offentligversion.pdf
- 19.Vaccarella S, Franceschi S, Engholm G, Lönnberg S, Khan S, Bray F. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer 2014;111:965–9. doi: 10.1038/bjc.2014.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castañón A, Landy R, Cuzick J, Sasieni P. Cervical screening at age 50-64 years and the risk of cervical cancer at age 65 years and older: population-based case control study. PLoS Med 2014;11:e1001585. doi: 10.1371/journal.pmed.1001585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen B, Christensen BS, Christensen J, Ejersbo D, Heje HN, Jochumsen KM, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol 2019;154:118–23. doi: 10.1016/j.ygyno.2019.04.680 [DOI] [PubMed] [Google Scholar]
- 22.Dansk Kvalitetsdatabase for Livmoderhalskræftscreenings styregruppe og RKKPs Videnscenter. Resultater fra Engangstilbuddet: Livmoderhalskræftscreening blandt danske kvinder født før 1948: 2. delrapport. Accessed November 18, 2020. https://www.sundhed.dk/content/cms/82/4682_2_del_engangstilbuddet_off_version.pdf
- 23.Elit L. Role of cervical screening in older women. Maturitas 2014;79:413–20. doi: 10.1016/j.maturitas.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 24.Petersen LK. National klininsk retningslinje for celleforandringer på livmoderhalsen. Udredning, behandling og opfølgning med fokus på kvinder over 60 år. Accessed November 18, 2020. https://www.sst.dk/-/media/Opgaver/Patientforl%C3%B8b-og-kvalitet/NKR/Puljefinansierede-NKR/pdf-version-af-published_guideline_2633.ashx?la=da&hash=0809DD9A773B341B3D08AE73C9361E2AB84029E8
- 25.Barken SS, Rebolj M, Andersen ES, Lynge E. Frequency of cervical intraepithelial neoplasia treatment in a well-screened population. Int J Cancer 2012;130:2438–44. doi: 10.1002/ijc.26248 [DOI] [PubMed] [Google Scholar]
- 26.Danmarks Statistik. HISB9: average life expectancy. Life table (5 years table) by life table, sex, age and time. Accessed November 18, 2020. https://www.statistikbanken.dk/HISB9
- 27.Vink MA, Bogaards JA, van Kemenade FJ, de Melker HE, Meijer CJ, Berkhof J. Clinical progression of high-grade cervical intraepithelial neoplasia: estimating the time to preclinical cervical cancer from doubly censored national registry data. Am J Epidemiol 2013;178:1161–9. doi: 10.1093/aje/kwt077 [DOI] [PubMed] [Google Scholar]
- 28.van den Akker-van Marie ME, van Ballegooijen M, Rozendaal L, Meijer CJ, Habbema JD. Extended duration of the detectable stage by adding HPV test in cervical cancer screening. Br J Cancer 2003;89:1830–3. doi: 10.1038/sj.bjc.6601355 [DOI] [PMC free article] [PubMed] [Google Scholar]