Supplemental Digital Content is available in the text.
Keywords: 4E1RCat, 4EGI-1, eIF4E, eIF4G, hepatocellular carcinoma, sorafenib
Abstract
The clinical efficacy of sorafenib in hepatocellular carcinoma (HCC) is disappointing due to its low response rate and high rates of adverse effects. The eukaryotic translation initiation factor 4F (eIF4F) complex, mainly consisting of eIF4E-eukaryotic translation initiation factor 4G (eIF4G) interaction, is involved in the induction of drug resistance. Herein, we aimed to demonstrate that eIF4E-eIF4G complex inhibition enhanced the effect of sorafenib. The antiproliferation effect of combined treatment was evaluated by MTT assay and colony formation assay. Flow cytometry was used to detect the early cell apoptosis and cell cycle. The specific mechanism was demonstrated using western blot and lentivirus transfection. The combination of sorafenib with eIF4E-eIF4G inhibitors 4E1RCat (structural) or 4EGI-1 (competitive) synergistically inhibited the cell viability and colony formation ability of HCC cells. Moreover, the combined treatment induced more early apoptosis than sorafenib alone through downregulating the Bcl-2 expression. Besides, the coadministration of sorafenib and 4E1RCat or 4EGI-1 synergistically inhibited the expressions of eIF4E, eIF4G and phospho-4E-BP1 in HCC cells while blocking the phosphorylation of 4E-BP1 with lentiviral transfection failed to increase the sensitivity of HCC cells to sorafenib treatment. PI3K-AKT-mTOR signaling was also inhibited by the combined treatment. In a word, eIF4E-eIF4G complex inhibition synergistically enhances the effect of sorafenib in HCC treatment.
Introduction
Liver cancer is the sixth commonly diagnosed cancer in both men and women worldwide and the fourth leading cause of cancer death in 2018 [1]. Hepatocellular carcinoma (HCC) contributes 75–85% of primary liver cancer cases [1]. Currently, effective treatments for HCC include liver resection, ablation, chemoembolization and systemic therapy [2]. For late-stage HCC, the multiple kinase inhibitor sorafenib is the first-line systemic treatment and is beneficial to prolong the survival of HCC patients [2–4]. Sorafenib inhibits cancer cells by targeting RAF1, BRAF, vascular endothelial growth factor receptor and platelet-derived growth factor receptor β (PDGFRβ) [5]. However, its low response rate (2–3%) and severe adverse effects are always challenging in HCC treatment [3,4].
The dysregulation of cap-dependent mRNA translation is crucial in the development of many human cancers [6,7], and the eukaryotic translation initiation factor 4F complex (eIF4F complex) is the key regulator of cap-dependent mRNA translation [8,9]. eIF4F complex is mainly composed of proteins eukaryotic translation initiation factor 4E (eIF4E) and eukaryotic translation initiation factor 4G (eIF4G) [10]. The formation of eIF4E-eIF4G interaction activates the biological function of eIF4F complex and initiates the cap-dependent mRNA translation. Furthermore, eIF4E-eIF4G interaction is also tightly regulated by eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1). The Thr37/46 phosphorylation of 4E-BP1 is activated by PI3K/AKT/mammalian target of rapamycin (mTOR) signaling and releases eIF4E from eIF4E-4E-BP1 interaction for eIF4E-eIF4G complex formation [7,11].
The PI3K/AKT/mTOR signaling pathway is involved in the acquired drug resistance of HCC cells after sorafenib treatment [12–14], and the eIF4F complex is an indicator of both innate and acquired drug resistance in human cancer [15]. Therefore, we hypothesized that interfering with eIF4E-eIF4G interaction could enhance the antitumor effects of sorafenib in HCC cells.
Materials and methods
Cell culture
HCC cell lines HepG2 and Huh7, which were authenticated by short tandem repeat analysis, were gifted from Cell Bank of the Chinese Academy of Science (Shanghai, China). The cells were cultured in Dulbecco’s Modified Eagle medium (DMEM, Hyclone, SH30022.01, Logan, Utah, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, 10099-141, Carlsbad, California, USA) and 1% Penicillin/Streptomycin (Beytime, C0222, Shanghai, China). Cells were maintained in a Series 8000 Direct-Heat CO2 Incubator (Thermo Fisher Scientific, Waltham, Massachusetts, USA) containing a temperature of 37°C and 5% CO2. The DMEM solution was exchanged every 2 days, and the cell lines were cultured no more than ten passages.
Antibodies and reagents
The following antibodies were used: 4E-BP1 (CST, 9644, Boston, Massachusetts, USA), β-Actin (CST, 3700), AKT (CST, 9272), Bax (CST, 2772), Bcl-2 (CST, 3498), Caspase-3 (CST, 9662), Caspase-8 (CST, 4790), elF4E (CST, 2067), elF4G (CST, 2469), mTOR (Santa Cruz Biotechnology, sc-293089, Dallas, Texas, USA), Phospho-4E-BP1 (Thr37/46) (CST, 2855), Phospho-AKT (Ser473) (CST, 4060), Phospho-mTOR (Ser2448) (Santa Cruz Biotechnology, sc-293133), PI3K p85 (CST, 4257).
The following reagents were used: Sorafenib (MedChemExpress, HY-10201, San Francisco, California, USA), 4E1RCat (MedChemExpress, HY-14427), 4EGI-1 (MedChemExpress, HY-19831), the powders of these reagents were dissolved in a solvent according to the manufacturer’s instructions.
Lentivirus transfection
The pGY-lenti-CMV-eGFP lentivirus was constructed by Ecotop Scientific Co., Ltd. (Guangzhou, China) based on the plasmid from Addgene. The gene with 4E-BP1 overexpression was reconstructed based on the plasmid pcDNA3-TORCAR gifted from Jin Zhang (Addgene, 64927, Cambridge, Massachusetts, USA). The gene with the mutation of phosphorylation site Thr37/46 of 4E-BP1 was reconstructed based on the plasmid pcDNA3-TORCAR (T/A) gifted from Jin Zhang (Addgene, 64928). According to the manufacturer’s instructions, the lentivirus was transfected into Huh7 and HepG2 cells with polybrene (Solarbio, H8761, Beijing, China) after four hours of starvation. The pGY-lenti-CMV-eGFP lentivirus was used as a negative control. The effects of lentiviral transfection were evaluated by western blot analysis.
Western blot
Proteins lysate of HCC cells was extracted by the RIPA lysis buffer (Beytime, P0013B) supplemented with protease inhibitor cocktail (MedChemExpress, HY-K0010) and phosphatase inhibitor cocktail (MedChemExpress, HY-K0021). Protein concentrations were measured with an enhanced BCA protein assay kit (Beytime, P0010). Total proteins were separated using SDS–PAGE and electrotransferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, Massachusetts, USA). Then, after blocking with 5% BSA (Beyotime, ST023) in Tris-Buffered Saline Tween-20 (TBST) solution for one hour at room temperature, membranes were incubated with primary antibodies for 16 h at 4°C. Afterward, membranes were incubated with goat anti-mouse secondary antibody (ZSGB-BIO, ZDR-5307, Beijing, China) or goat anti-rabbit secondary antibody (ZSGB-BIO, ZDR-5306) for 1 h at room temperature. Finally, protein expression was detected with ECL-western blotting substrate (Solarbio, PE0010) using an automatic chemiluminescence analyzer (Tanon 6100, Shanghai, China).
Thiazolyl blue tetrazolium bromide (MTT) assay
Cells were seeded in a 96-well plate in a density of 3000 cells/100μl/well. Cells were treated with reagents the next day for 48 hours. Then, MTT (Solarbio, M8180) was added in the cell culture medium (5 mg/ml) and incubated for 4 h at 37°C. After that, the dimethyl sulfoxide (150 μl/well, Solarbio, D8370) was added in 96-well plate to dissolve formazan. The absorbance of optical density (OD)570–OD630 was set for viable cell detection, and the cell viability was calculated following the formula: cell viability = ODexperimental group/ODcontrol group.
Colony formation assay
HCC cells were seeded in six-well plates (1000 cells/well) and were treated with reagents the next day for 24 h. After cultured for 2 weeks, the cells were fixed with 4% paraformaldehyde (Beyotime, P0099) for 10 min and were subsequently stained with 0.1% (w/v) crystal violet (Solarbio, C8470) for 15 min. The colony quantity was counted with Image-J (NIH, Bethesda, Maryland, USA).
Cell apoptosis assay
HCC cells in six-well plates were treated with reagents for 48 h and then were collected for cell apoptosis assay. 5 μl FITC annexin V and 5 µl PI (BD Biosciences, 556547, New York, USA) were added into tubes with cells and reacted at room temperature for 15 min without light. Then cells were analyzed by CytoFLEX analysis of flow cytometry (Beckman Coulter, Brea, California, USA) within one hour.
Cell cycle assay
Cells were seeded in 60 mm dishes and were treated with reagents 24 h later. Before the administration of the reagents, all the cells were starved for 12 h to synchronize the cell cycle. After 48 h of exposure to reagents, cells were collected for cell cycle analysis, compared with the control without any treatment. First of all, the cells were fixed in 70% ethanol at 4°C overnight and washed twice with PBS. Then, they were stained with PI/RNase staining buffer (BD Biosciences, 550825) at room temperature for 15 min. Cell cycle analysis was conducted by CytoFLEX analysis of flow cytometry (Beckman Coulter, USA) within 1 h. After that, the results were analyzed further with software Flowjo 7.6. During this process, there are three gating choices in total that are FSC-A/SSC-A, PE-A/PE-H and PE-A/Count, respectively.
Statistical analysis
The data in this study were repeated at least three times independently and were analyzed using a one-way analysis of variance (ANOVA, Tukey’s multiple comparisons test) for multiple comparisons with Prism 6.0 software. P value <0.05 was set as a significant statistical difference.
Results
4E1RCat or 4EGI-1 enhanced the effect of sorafenib in hepatocellular carcinoma cell lines
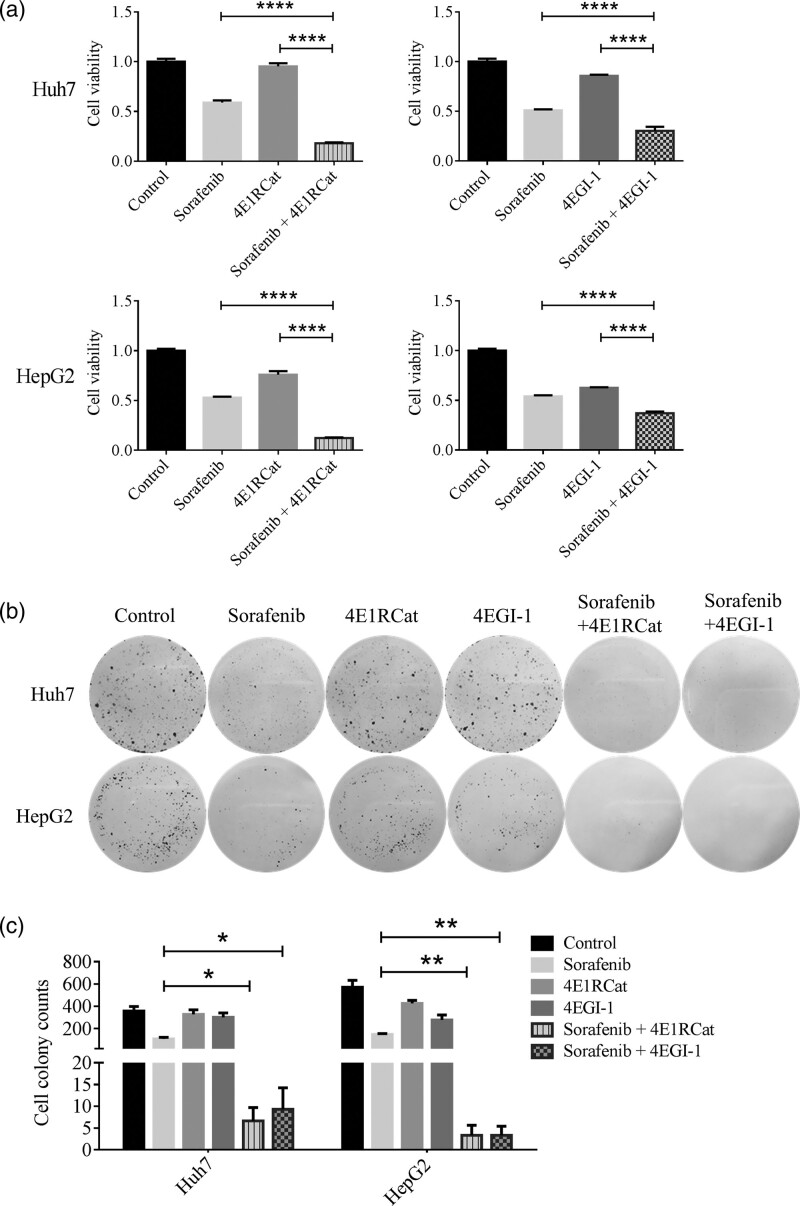
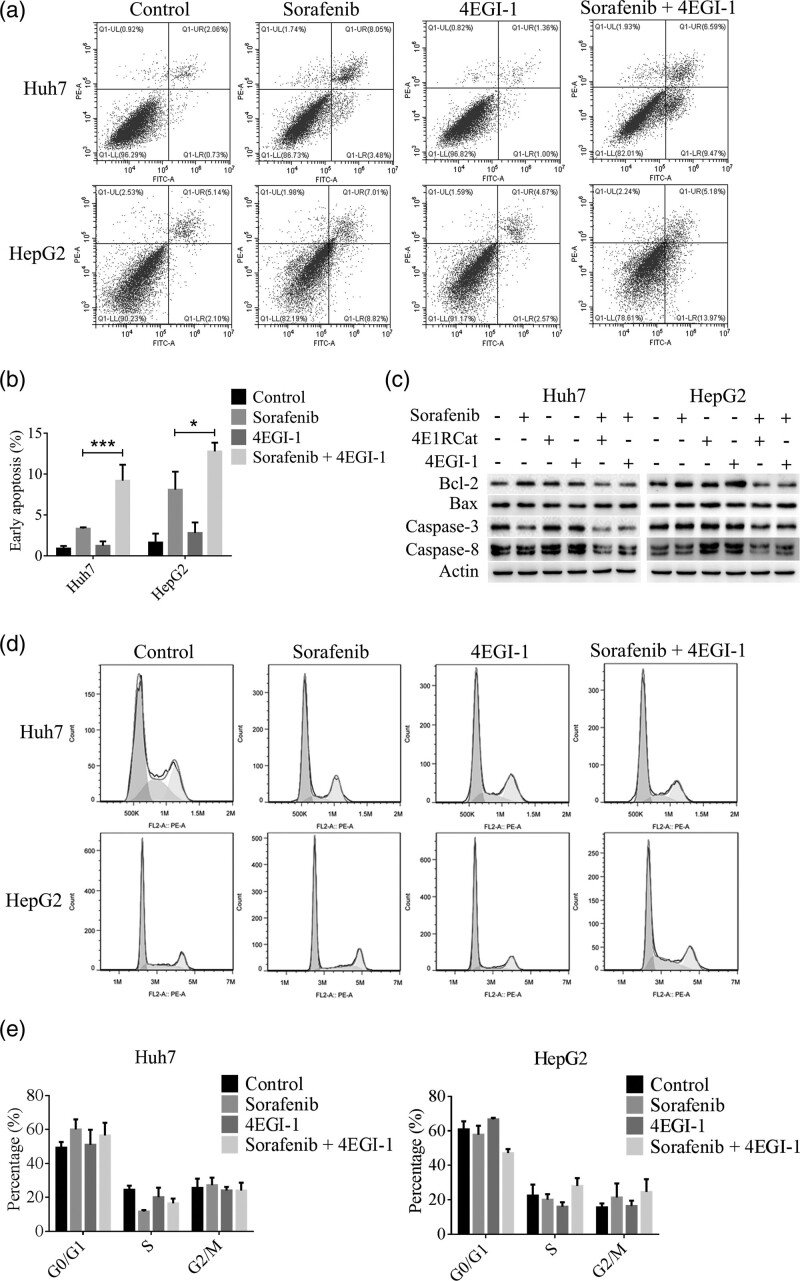
To detect the effect of sorafenib in HCC cells when combined with inhibitors of eIF4E-eIF4G interaction, both structural inhibitor (4E1RCat) and competitive inhibitor (4EGI-1) were used. The MTT assay showed that the combination of sorafenib with 4E1RCat or 4EGI-1 significantly decreased the cell viability than sorafenib, 4E1RCat or 4EGI-1 alone (all P < 0.0001, Fig. 1a, Supplementary Figure S1, Supplemental digital content 1, http://links.lww.com/ACD/A380). After combined with 4E1RCat or 4EGI-1, sorafenib significantly inhibited the colony formation ability than single drug administration in Huh7 (sorafenib vs. sorafenib+4EIRCat, P = 0.0119; sorafenib vs. sorafenib+4EGI-1, P = 0.0143) and HepG2 (sorafenib vs. sorafenib+4EIRCat, P = 0.002; sorafenib vs. sorafenib+4EGI-1, P = 0.002) cell lines (Fig. 1b,c). Furthermore, the combination of sorafenib with 4EGI-1 significantly induced early apoptosis of Huh7 (P = 0.0005) and HepG2 (P = 0.0205) cells (Fig. 2a,b). The expression of Bcl-2 was downregulated by the combined treatment of sorafenib and inhibitors of eIF4E-eIF4G interaction (Fig. 2c). However, there was no significant difference in the cell cycle process after combination treatment (Fig. 2d,e).
Fig. 1.
The coadministration of eIF4E-eIF4G inhibitors and sorafenib inhibited the proliferation and colony formation of HCC cell lines. (a) The coadministration of sorafenib with 4E1RCat or 4EGI-1 significantly decreased the viability in HCC cell lines. (b,c) The combined treatment of 4E1RCat or 4EGI-1 with sorafenib significantly decreased the colony formation ability in HCC cell lines. The concentrations of sorafenib, 4E1RCat and 4EGI-1 were 8, 25 and 25 µM, respectively. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. All data were displayed as mean ± SD. eIF4F, eukaryotic translation initiation factor 4E; eIF4G, eukaryotic translation initiation factor 4G; HCC, hepatocellular carcinoma.
Fig. 2.
4EGI-1 enhanced the effect of sorafenib in apoptosis induction in HCC cell lines. (a,b) The combined administration of sorafenib and 4EGI-1 induced more early apoptosis than sorafenib or 4EGI-1 alone. (c) The expressions of apoptosis-related proteins were detected by western blot 48 h later after the coadministration of sorafenib and 4E1RCat or 4EGI-1. (d,e) The cell cycle was not retarded significantly after the combined treatment. The concentrations of sorafenib, 4E1RCat and 4EGI-1 were 8, 25 and 25 µM, respectively. *P < 0.05, **P < 0.01 and ***P < 0.001. All data were displayed as mean ± SD. HCC, hepatocellular carcinoma.
4E1RCat or 4EGI-1 synergistically enhanced the effect of sorafenib by inhibiting eIF4E and eIF4G in hepatocellular carcinoma cell lines
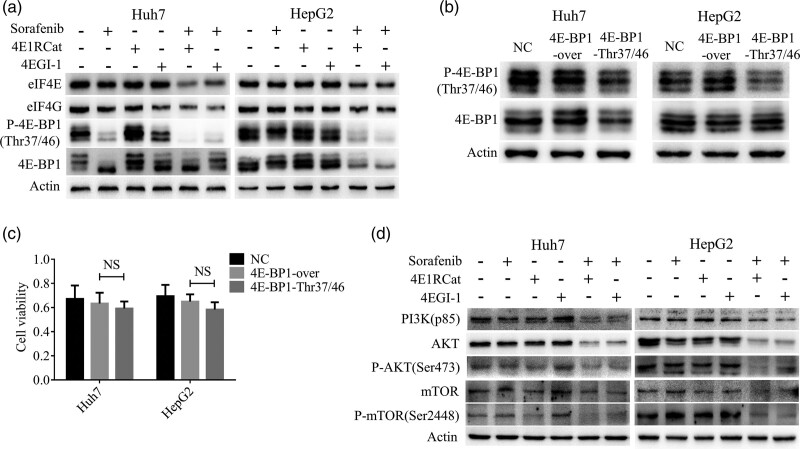
Immunoblotting results showed that the expressions of eIF4E and eIF4G were downregulated after the combined treatment of sorafenib with 4E1RCat or 4EGI-1 in HepG2 and Huh7 cell lines (Fig. 3a). Besides, the phosphorylation of 4E-BP1(Thr37/46) was deactivated as well (Fig. 3a).
Fig. 3.
4E1RCat or 4EGI-1 potentiated the effect of sorafenib by inhibiting eIF4E and eIF4G in HCC cell lines. (a) The western blot showed that eIF4E, eIF4G and phospho-4E-BP1 (Thr37/46) were downregulated after the combined administration for 48 h. (b) The transfection effectiveness of 4EBP1-Thr37/46 mutation was verified using western blot. (c) The MTT showed that the inhibition of phospho-4EBP1(Thr37/46) did not enhance the effect of sorafenib. (d) The PI3K-AKT-mTOR signaling pathway was inhibited after the coadministration of drugs for 48 h. The concentrations of sorafenib, 4E1RCat and 4EGI-1 were 8, 25 and 25 µM, respectively. All data were displayed as mean ± SD. 4E-BP1-over, 4E-BP1 overexpression; 4E-BP1-Thr37/46, 4E-BP1 Thr37/46 mutation; HCC, hepatocellular carcinoma; mTOR, mammalian target of rapamycin; NC, negative control.
Gene mutation of the Thr37/46 phosphorylation site of 4E-BP1 failed to enhance the effect of sorafenib in hepatocellular carcinoma cell lines
The effectiveness of lentivirus transfection was confirmed by western blot in Huh7 and HepG2 cell lines (Fig. 3b). Huh7 and HepG2 cells with 4E-BP1 overexpression and 4E-BP1 Thr37/46 mutation were treated with sorafenib, but there was no significant difference in the cell viability between 4E-BP1 overexpression and 4E-BP1 Thr37/46 mutation HCC cells (Fig. 3c).
The combination of sorafenib and 4E1RCat or 4EGI-1 synergistically inhibited the PI3K-AKT-mTOR signaling pathway in hepatocellular carcinoma cell lines
When combined with 4E1RCat or 4EGI-1, sorafenib downregulated the expression of PI3K (p85) in Huh7 and HepG2 cells (Fig. 3d). Furthermore, the expression of AKT was inhibited and the phosphorylation of AKT (Ser473) was deactivated (Fig. 3d). Moreover, the downstream target of AKT, mTOR, was downregulated and deactivated at the phosphorylation site Ser2448 by the combined treatment in HCC cells (Fig. 3d).
Discussion
The drug response of sorafenib in HCC patients is about 2.0–3.3%, and the adverse effects of sorafenib limit its widespread clinical application [3,4]. Herein, we found a novel way to enhance the antitumor effect of sorafenib in HCC cells by inhibiting the eIF4E-eIF4G complex.
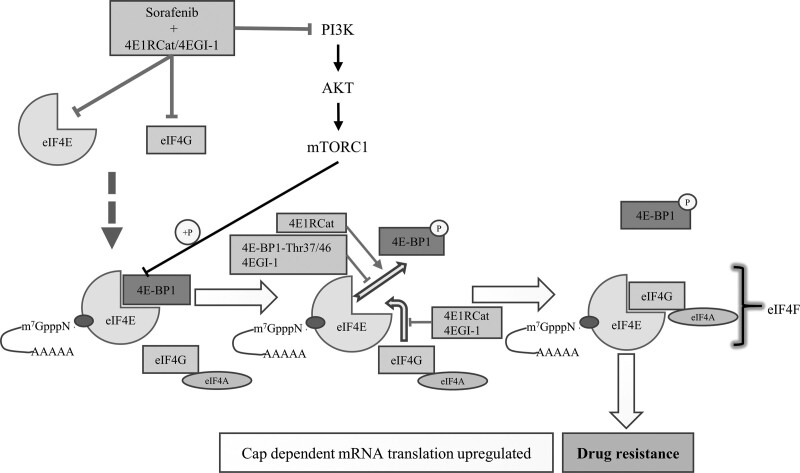
It is well known that 4E-BP1 and eIF4G competitively bind to the same site of eIF4E, and eIF4E-eIF4G interaction activates the initiation phase of cap-dependent mRNA translation. 4E1RCat is the structural inhibitor and 4EGI-1 is the competitive inhibitor for eIF4E-eIF4G interaction. 4E1RCat interferes with eIF4E-eIF4G interaction by overlapping the binding site of eIF4E. As a result, both eIF4E-eIF4G and eIF4E-4EBP1 interactions are inhibited by 4E1RCat [16]. On the contrast, the competitive inhibitor 4EGI-1 displaces eIF4G from eIF4E-eIF4G interaction and empties the docking site for 4EBP1 to promote eIF4E-4EBP1 interaction [17,18]. In this study, both the structural inhibitor 4E1RCat and the competitive inhibitor 4EGI-1 synergistically enhanced the antitumor effect of sorafenib in HCC cells (Fig. 4).
Fig. 4.
The schematic molecular mechanism by which 4E1RCat or 4EGI-1 enhanced the antitumor effect of sorafenib in HCC cell lines. As the eIF4E-eIF4G interaction inhibitors, both 4E1RCat and 4EGI-1 block the binding of eIF4E and eIF4G and inhibit the formation of eIF4F complex that participates acquired drug resistance. However, Thr37/46 site mutation of 4E-BP1, which promotes the binding of 4E-BP1 and eIF4E and then inhibits the formation of the eIF4F complex, failed to enhance the effect of sorafenib. Besides, the coadministration of sorafenib and 4E1RCat or 4EGI-1 downregulated the expression of eIF4E and eIF4G, as well as inhibiting the PI3K-AKT signaling pathway. Overall, these indicate that the synergetic relationship between drugs might be due to the inhibition of eIF4E, eIF4G, or PI3K/AKT signaling pathway. 4E-BP1-Thr37/46: 4E-BP1 Thr37/46 mutation. HCC, hepatocellular carcinoma.
eIF4E-4EBP1 interaction can be promoted by 4EGI-1 and the dephosphorylation of 4EBP1 (Thr37/46). In this study, the Thr37/46 phosphorylation of 4EBP1 was deactivated after specific lentivirus transfection in HCC cells and the results showed that the mutation Thr37/46 sites did not affect the drug response of sorafenib to HCC cells. These results enhanced the evidence that it was eIF4E-eIF4G interaction that mediated the synergistic effect of sorafenib combined with 4EGI-1 or 4E1RCat, while eIF4E-4EBP1 interaction had no significant influence in this synergistic phenomenon (Fig. 4).
The PI3K/AKT signaling is also a crucial mechanism mediating the resistance of HCC cells to sorafenib treatment, and AKT inhibition by GDC0068 can enhance the chemosensitivity to sorafenib in advanced HCC [13,19]. Furthermore, the antiapoptotic protein Bcl-2 is upregulated in acquired resistant HCC cells, and inhibiting the Bcl-2 by microRNA-34a potentiates the effect of sorafenib [19,20]. Besides, the knockdown of eIF4E enhances the chemoresistance of cancer cells through Bax/Bcl-2 regulation [21]. Therefore, these findings support that the synergetic efficacy of sorafenib and eIF4E-eIF4G interaction inhibitors might result from the inhibition of PI3K/AKT signaling, eIF4E or eIF4G (Fig. 4).
In conclusion, eIF4E-eIF4G complex inhibitors, 4E1RCat and 4EGI-1, synergistically enhanced the effect of sorafenib in HCC cell lines. These findings provide a potential strategy to increase the response rate of sorafenib in HCC patients. Therefore, the clinical studies of sorafenib and eIF4E-eIF4G complex inhibitors in HCC patients are in plan in our group. Also, the more detailed biological mechanism of this synergistic effect will be explored according to our plan.
Acknowledgements
The authors thank Prof. Peter Schemmer for the research supporting. This project is the subsequent work of the previous research of Prof. Peter Schemmer who focused on the research of the biological function of mRNA translation in human cancers. The authors thank the Center for Scientific Research of Anhui Medical University for valuable help in our experiment.
This work was supported by Research funding of Anhui Medical University (No.2017xkj034) and Research funding of the Education Agency in Henan Province (No.16A320007).
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
These authors contributed equally to the writing of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.anti-cancerdrugs.com
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018; 391:1301–1314. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. ; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018; 15:599–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiegering A, Uthe FW, Jamieson T, Ruoss Y, Hüttenrauch M, Küspert M, et al. Targeting translation initiation bypasses signaling crosstalk mechanisms that maintain high MYC levels in colorectal cancer. Cancer Discov. 2015; 5:768–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I. Targeting the translation machinery in cancer. Nat Rev Drug Discov. 2015; 14:261–278. [DOI] [PubMed] [Google Scholar]
- 8.Qin X, Jiang B, Zhang Y. 4E-BP1, a multifactor regulated multifunctional protein. Cell Cycle. 2016; 15:781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malka-Mahieu H, Newman M, Désaubry L, Robert C, Vagner S. Molecular pathways: the eIF4F translation initiation complex-new opportunities for cancer treatment. Clin Cancer Res. 2017; 23:21–25. [DOI] [PubMed] [Google Scholar]
- 10.Grüner S, Peter D, Weber R, Wohlbold L, Chung MY, Weichenrieder O, et al. The structures of eIF4E-eIF4G complexes reveal an extended interface to regulate translation initiation. Mol Cell. 2016; 64:467–479. [DOI] [PubMed] [Google Scholar]
- 11.Peter D, Igreja C, Weber R, Wohlbold L, Weiler C, Ebertsch L, et al. Molecular architecture of 4E-BP translational inhibitors bound to eIF4E. Mol Cell. 2015; 57:1074–1087. [DOI] [PubMed] [Google Scholar]
- 12.Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH, Chen PJ, Cheng AL. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2011; 337:155–161. [DOI] [PubMed] [Google Scholar]
- 13.Niu L, Liu L, Yang S, Ren J, Lai PBS, Chen GG. New insights into sorafenib resistance in hepatocellular carcinoma: responsible mechanisms and promising strategies. Biochim Biophys Acta Rev Cancer. 2017; 1868:564–570. [DOI] [PubMed] [Google Scholar]
- 14.Zhu YJ, Zheng B, Wang HY, Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017; 38:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boussemart L, Malka-Mahieu H, Girault I, Allard D, Hemmingsson O, Tomasic G, et al. eIF4F is a nexus of resistance to anti-BRAF and anti-MEK cancer therapies. Nature. 2014; 513:105–109. [DOI] [PubMed] [Google Scholar]
- 16.Cencic R, Hall DR, Robert F, Du Y, Min J, Li L, et al. Reversing chemoresistance by small molecule inhibition of the translation initiation complex eIF4F. Proc Natl Acad Sci U S A. 2011; 108:1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moerke NJ, Aktas H, Chen H, Cantel S, Reibarkh MY, Fahmy A, et al. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell. 2007; 128:257–267. [DOI] [PubMed] [Google Scholar]
- 18.Sekiyama N, Arthanari H, Papadopoulos E, Rodriguez-Mias RA, Wagner G, Léger-Abraham M. Molecular mechanism of the dual activity of 4EGI-1: dissociating eIF4G from eIF4E but stabilizing the binding of unphosphorylated 4E-BP1. Proc Natl Acad Sci U S A. 2015; 112:E4036–E4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai B, Hu F, Jiang X, Xu J, Zhao D, Liu B, et al. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther. 2014; 13:1589–1598. [DOI] [PubMed] [Google Scholar]
- 20.Yang F, Li QJ, Gong ZB, Zhou L, You N, Wang S, et al. MicroRNA-34a targets Bcl-2 and sensitizes human hepatocellular carcinoma cells to sorafenib treatment. Technol Cancer Res Treat. 2014; 13:77–86. [DOI] [PubMed] [Google Scholar]
- 21.Zhou FF, Yan M, Guo GF, Wang F, Qiu HJ, Zheng FM, et al. Knockdown of eIF4E suppresses cell growth and migration, enhances chemosensitivity and correlates with increase in Bax/Bcl-2 ratio in triple-negative breast cancer cells. Med Oncol. 2011; 28:1302–1307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.