Vacuum-induced tamponade appears to be an easy-to-use, promising method for the treatment of postpartum hemorrhage, particularly in women with uterine atony.
Abstract
BACKGROUND:
Postpartum hemorrhage is a main cause of maternal mortality worldwide, with rising incidence, thus demanding new treatment approaches. Intrauterine balloon systems with application of intrauterine vacuum are a promising new method.
METHOD:
All women treated with vacuum-induced tamponade using a modified balloon system were included in this single-center study. Aiming to reduce uterine size for control of postpartum hemorrhage, the intrauterine balloon was filled to 50–100 mL and connected to a vacuum device. Success rate of vacuum-induced tamponade, defined as no need for additional interventional treatment, was analyzed by etiology of postpartum hemorrhage and time period of use.
EXPERIENCE:
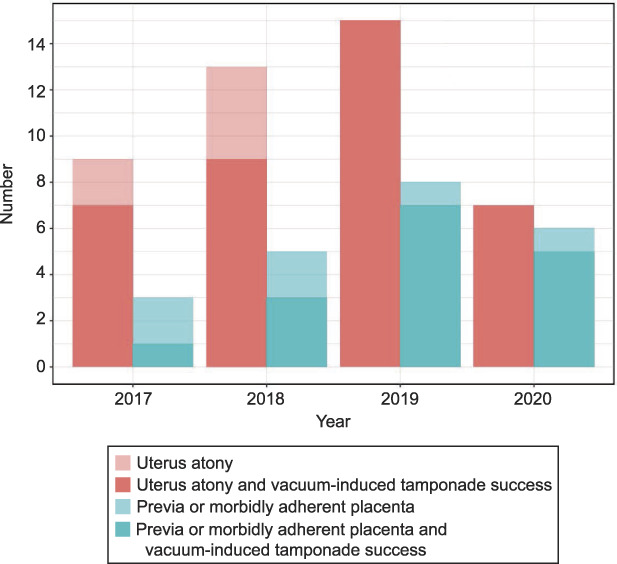
Vacuum-induced tamponade was applied in 66 women. Success rate was 86% in women with uterine atony (n=44) and 73% in women with postpartum hemorrhage due to placental pathology (n=22). Success rate improved over the study period, culminating in a success rate of 100% in women with postpartum hemorrhage due to uterine atony in the second half of the observation period (n=22).
CONCLUSION:
This observational study supports our pathophysiologic understanding of uterine atony: to treat an atonic uterus, uterine volume must be reduced, leading to coiling of the uterine spiral arteries and, hence, reduced blood loss.
Postpartum hemorrhage, one of the main causes of maternal morbidity and mortality, has a rising incidence,1–6 mainly driven by an increase in uterine atony. Therefore, new strategies in the treatment of postpartum hemorrhage are urgently needed. In 2016, Purwosunu et al7 published an intriguing proof-of-concept investigation of 10 women treated with an intrauterine balloon device that, in contrast to the established balloon devices, was not inflated but used instead to induce negative pressure in the uterine cavity. Their rationale for creating negative pressure was that the uterus should become smaller (ie, contract) to impede bleeding due to uterine atony. We decided to test this concept in our patients with uterine atony and tested vacuum-induced tamponade using a modified Bakri balloon system.
METHOD
This was a single-center, observational cohort study at the Department of Obstetrics, University Hospital Zurich, Switzerland. All women with primary postpartum hemorrhage who were treated with vacuum-induced tamponade between March 2017 and June 2020, irrespective of the etiology of postpartum hemorrhage, were included in this study. The study had ethical approval from the ethics review board of Zurich (reference number KEK-ZH-Nr. 2019-00,907).
To establish vacuum-induced tamponade, a modified Bakri balloon system was used. When indicated (ie, when standard uterotonic postpartum hemorrhage treatment consisting of the administration of oxytocin followed by prostaglandins failed, or when bleeding continued despite removal of the placenta or retained placental tissue), the Bakri balloon was inserted under ultrasonographic surveillance. The following steps differed from the usual approach, representing off-label use: the balloon was inflated with only 50–100 mL of physiologic saline solution; the catheter was connected with a nonsterile tube to a vacuum device, and intrauterine vacuum was applied with 60–70 kPa (Fig. 1). The correct position of the vacuum-induced tamponade system as well as the condition of the uterine cavity were assessed regularly by ultrasonography (Fig. 2) to rule out accumulation of blood. The vacuum-induced tamponade system remained in situ for at least an hour. If the bleeding stopped (ie, there was no further accumulation of blood either in the system or in the uterus and the patient was stable), the vacuum was paused (but the system remained in the uterus). After an additional hour without increased bleeding, the balloon was deflated. If a persistently stable situation was then observed, the balloon was removed. The balloon remained in utero for a maximum of 24 hours. No additional antibiotics or analgesia were administered.
Fig. 1. Vacuum-induced tamponade system used in our center.
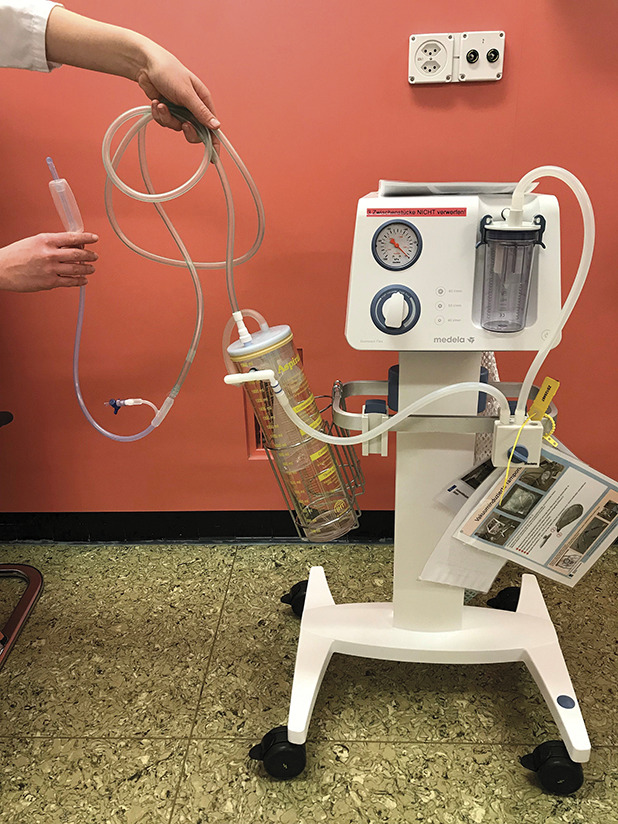
Haslinger. Vacuum-Indued Tamponade for Postpartum Hemorrhage. Obstet Gynecol 2021.
Fig. 2. Vacuum-induced tamponade in utero. Uterine fundus (red arrow); tip of catheter (yellow arrow); balloon filled with 50 mL physiologic saline solution (NaCl 0.9%) in a woman with postpartum hemorrhage (blue arrow).
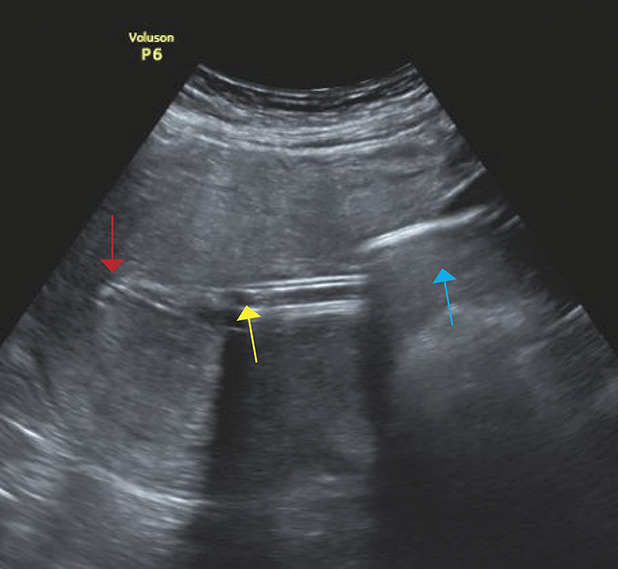
Haslinger. Vacuum-Indued Tamponade for Postpartum Hemorrhage. Obstet Gynecol 2021.
Data were recorded prospectively immediately after delivery. Primary outcome was success of vacuum-induced tamponade, defined as no need for additional interventional treatment such as surgical intervention or embolization of the pelvic arteries. Secondary outcomes related to postpartum hemorrhage were analyzed as well. Blood loss measurement at our institution follows a strict, previously described protocol that includes the use of a blood-loss collection bag with a quantitative scale.8
Success rate was calculated 1) overall, 2) according to etiology (uterine atony vs placental pathology), 3) according to institutional experience with the new device (period under observation A [2017–2018] vs period under observation B [2019–2020]), and 4) according to etiology in the two observation periods, A and B.
EXPERIENCE
From March 2017 until June 2020, 66 women were treated with vacuum-induced tamponade (Tables 1 and 2, Fig. 3). Two thirds of the women (44/66) were treated with vacuum-induced tamponade due to uterine atony and one third (22/66) due to placental pathology. Vacuum was applied for a median duration of 2.5 hours (interquartile range 1–4 hours). Adequate vacuum was achieved by the balloon in all cases. No adverse events directly related to vacuum-induced tamponade, such as perforation of the uterus, anaphylaxis, or endomyometritis were observed.
Table 1.
Patient Characteristics Overall and in Observation Periods A and B
Table 2.
Primary and Secondary Outcomes Overall and in Observation Periods A and B
Fig. 3. Number of women treated with vacuum-induced tamponade per year, etiology of postpartum hemorrhage, and success rate per group. Previa or morbidly adherent placenta refers to the placental pathology group.
Haslinger. Vacuum-Indued Tamponade for Postpartum Hemorrhage. Obstet Gynecol 2021.
DISCUSSION
In this single-center, observational cohort study, we evaluated the use of vacuum-induced tamponade in women with postpartum hemorrhage and failed first-line therapy. We found, first, that vacuum-induced tamponade was an easy-to-use technique, and second, that vacuum-induced tamponade was associated with the highest success rate in women with uterine atony. During observation period A, obstetricians opted for embolization of the pelvic arteries even in cases in which bleeding had stopped after the application of vacuum-induced tamponade. With increasing confidence in the method, such “overtreatment” embolizations were no longer performed during observation period B (2019–2020), and the vacuum-induced tamponade success rate was 100% in women with uterine atony. We assume that, with increasing training and confidence in this new method, caregivers possibly tended to apply vacuum-induced tamponade at somewhat earlier stages of postpartum hemorrhage.
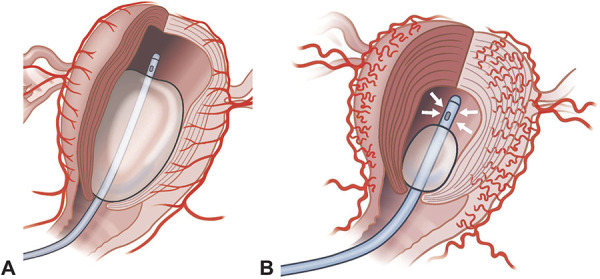
The rationale behind vacuum-induced tamponade is as simple as it is logical: to stop postpartum bleeding due to uterine atony, the uterus should become smaller (ie, contract) to allow the spiral arteries to take a curled form (like a corkscrew), leading to reduced blood flow. The traditional Bakri balloon system does the opposite: it enlarges the uterus (Fig. 4). The concept of vacuum-induced tamponade was first introduced in 2016 by Purwosunu et al7 with their intriguing proof-of-concept investigation of 10 women using a new device. A subsequent multicenter study of 106 women with uterine atony treated with this device reported a treatment success rate of 94%.9 The findings of our observational study are in line with these results, showing a success rate of 86% in women with uterine atony in the overall study period and even 100% in women with uterine atony in the latter 2 years. However, in contrast to the latter study, we did not use vacuum-induced tamponade exclusively in women with atony. Nonetheless, we postulated that, in cases of postpartum hemorrhage due to placental pathologies, vacuum-induced tamponade might back up the further necessary treatment steps.
Fig. 4. Schematic representation of a balloon filled with 500 mL of solution (A) and of a balloon filled with 70 mL of solution (B) and applied intrauterine vacuum. We postulate that vacuum-induced tamponade helps to suck blood from the uterine cavity and supports uterine contraction, leading to a coiling of the spiral arteries and hence reduced blood loss. © C. Haslinger. Used with permission.
Haslinger. Vacuum-Indued Tamponade for Postpartum Hemorrhage. Obstet Gynecol 2021.
The main limitation of this study is that we do not have data on women with postpartum hemorrhage who were not treated with this new method and that, as in the aforementioned multicenter study, our study lacks a control group.
Even though our perception of this innovative method is extremely positive, we must still be aware that our results need to be interpreted cautiously. Our observations reflect association, not causation. Even though the increased vacuum-induced tamponade success rate in the latter observation period was associated with reduced measured blood loss and associated adverse outcome parameters, we cannot exclude that this success could be due to overall improved postpartum hemorrhage management at our center or fewer women at risk for severe postpartum hemorrhage. Although both occurrences seem unlikely, because postpartum hemorrhage management protocols remained unchanged at our institution and patient characteristics did not differ between observation periods A and B, a more conclusive evaluation of this promising method is necessary.
Footnotes
Financial Disclosure The authors did not report any potential conflicts of interest.
Presented at the Congress of the Swiss Society for Gynecology and Obstetrics, June 24–26, 2021, Interlaken, Switzerland.
In order to establish vacuum-induced tamponade, a modified Bakri® balloon system (CookMedical LLC, Bloomington, Indiana) was used. When indicated, the Bakri® balloon was inserted as customary. The following steps differed from the usual approach, representing off-label use: The balloon, which has a maximum volume of 500 mL, was inflated with only 50–100 mL physiological saline solution in order to ensure the correct placement and proper tightness of the vacuum system afterwards. The catheter was connected via a nonsterile tube to a vacuum device and intrauterine vacuum was applied with 60–70 kPa.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C380.
Contributor Information
Kathrin Weber, Email: kathrin.weber@uzh.ch.
Roland Zimmermann, Email: roland.zimmermann@usz.ch.
Figure.
No available caption
REFERENCES
- 1.World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Accessed February 13, 2019. http://www.ncbi.nlm.nih.gov/books/NBK131942/ [PubMed] [Google Scholar]
- 2.Say L, Chou D, Gemmill A, Tunҫalp Ӧ, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2:e323–33. doi: 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 3.GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015 [published erratum appears in Lancet 2017;389:e1]. Lancet 2016;388:1775–812. doi: 10.1016/S0140-6736(16)31470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reale SC, Easter SR, Xu X, Bateman BT, Farber MK. Trends in postpartum hemorrhage in the United States from 2010 to 2014. Anesth Analg 2020;130:e119–22. doi: 10.1213/ANE.0000000000004424 [DOI] [PubMed] [Google Scholar]
- 5.Weeks A. The prevention and treatment of postpartum haemorrhage: what do we know, and where do we go to next? BJOG 2015;122:202–10. doi: 10.1111/1471-0528.13098 [DOI] [PubMed] [Google Scholar]
- 6.Kramer MS, Berg C, Abenhaim H, Dahhou M, Rouleau J, Mehrabadi A, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol 2013;209:449.e1–7. doi: 10.1016/j.ajog.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 7.Purwosunu Y, Sarkoen W, Arulkumaran S, Segnitz J. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol 2016;128:33–6. doi: 10.1097/AOG.0000000000001473 [DOI] [PubMed] [Google Scholar]
- 8.Kahr MK, Brun R, Zimmermann R, Franke D, Haslinger C. Validation of a quantitative system for real-time measurement of postpartum blood loss. Arch Gynecol Obstet 2018;298:1071–7. doi: 10.1007/s00404-018-4896-0 [DOI] [PubMed] [Google Scholar]
- 9.D'Alton ME, Rood KM, Smid MC, Simhan HN, Skupski DW, Subramaniam A, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol 2020;136:882–91. doi: 10.1097/AOG.0000000000004138 [DOI] [PMC free article] [PubMed] [Google Scholar]