Summary
The high prevalence of post-acute sequelae of SARS-CoV-2 infection (PASC) is a significant health concern. In particular, virus-specific immunity in patients who suffer from chronic neurologic symptoms after mild acute COVID remain poorly understood. Here, we report that neuro-PASC patients have a specific signature composed of humoral and cellular immune responses that are biased towards different structural proteins compared to healthy COVID convalescents. Interestingly, the severity of cognitive deficits or quality of life markers in neuro-PASC patients are associated with reduced effector molecule expressionn in memory T cells. Furthermore, we demonstrate that T cell responses to SARS-CoV-2 mRNA vaccines are aberrantly elevated in longitudinally sampled neuro-PASC patients compared with healthy COVID convalescents. These data provide a framework for the rational design of diagnostics and predictive biomarkers for long-COVID disease, as well as a blueprint for improved therapeutics.
Keywords: COVID-19 immunity, T cell memory, neuro-PASC, long-COVID, vaccine-induced immunity
Introduction
SARS-CoV-2 is the causative agent of a worldwide pandemic that started in Wuhan, China in December, 2019. There have been more than 200 million cases and over 4 million deaths globally attributable to COVID-19 disease (Center, 2021). Although highly effective vaccines are now used to prevent severe disease and death caused by SARS-CoV-2, long-term sequelae of SARS-CoV-2 infection are increasingly becoming an important medical concern.
SARS-CoV-2 infection is associated with presentations ranging from asymptomatic carriage to severe multi-organ dysfunction (Li et al., 2020; Liguori et al., 2020; Syed et al., 2020). Globally, the estimated fatality rate following SARS-CoV-2 infection is approximately 1–2%, but not all patients recover to their baseline states (Higgins et al., 2020). “Long COVID” affects an estimated 10–40% of all infected people and includes symptoms persisting more than 28 days after diagnosis of SARS-CoV-2 infection, termed “post-acute sequelae of SARS-CoV-2 infection” or PASC (Ladds et al., 2020; Vehar et al., 2021). Greater than two-thirds of hospitalized COVID-19 patients experience ongoing fatigue, breathlessness, and psychiatric issues such as post-traumatic stress disorder (PTSD) 4–8 weeks after discharge (Halpin et al., 2021). However, the vast majority of people with COVID experience mild disease not requiring hospitalization, and more than half of these have symptoms persisting more than 4 months after acute infection (Petersen et al., 2020). Many people who survived Middle-East respiratory virus (MERS) and the original SARS-CoV pandemic also experienced PTSD and neurological impairments up to 3.5 years after acute infection (Ahmed et al., 2020; Lam et al., 2009). More recent studies on recovered COVID-19 patients showed significant cognitive deficits in attention, working memory, and emotional processing months after acute infection had resolved (Hampshire, 2021). Here, we focus on a cohort of mostly non-hospitalized long-COVID patients presenting with neurological symptoms (“neuro-PASC”) who exhibit a reduction in quality of life with regards to cognitive and fatigue parameters (Graham et al., 2021).
Numerous studies suggest that a robust T cell response is necessary for viral clearance in infected individuals. In particular, CD4+ T cell responses directed against the Spike protein were found in 100% of healthy COVID convalescents, while 70% displayed Spike-specific CD8+ T cell responses (Grifoni et al., 2020). Another study found that memory T cells cross-reactive to SARS-CoV-2 nucleocapsid (N) protein persisted in people exposed to SARS-CoV up to 17 years after the epidemic of 2003 (Le Bert et al., 2020). Post-mortem autopsies of COVID-19 patients have shown that innate immune cells but not lymphocytes are enriched in lung infiltrate, and that these patients exhibit impaired germinal center formation in lung draining lymph nodes (Duan et al., 2020). Additional studies have shown that CD8+ T cell depletion after SARS-CoV-2 infection of rhesus macaques impairs anamnestic responses, suggesting a role for T cell memory in long-term immunity to SARS-CoV-2 infection (McMahan et al., 2021).
Most studies on T cell responses to SARS-CoV-2 have focused on acutely symptomatic individuals. However, adaptive immune responses in patients who remain chronically symptomatic remain largely unexplored. Our studies indicate that neuro-PASC patients (“CN”) had hypo-functional Ag-specific CD8+ T cell memory responses SARS-CoV-2 compared with healthy convalescents (“CC”) and enhanced reactivity to N and M proteins compared with CC subjects. The severity of cognitive deficits and quality of life markers were associated with enhanced polarization in cytolytic granule expression in memory T cell subsets. Finally, we demonstrate that T cell responses to SARS-CoV-2 vaccination are more robust and display aberrant kinetics in neuro-PASC patients compared with control groups. Together, these data demonstrate a wide-scale dysfunction in SARS-CoV-2 T cell memory in neuro-PASC long-hauler patients, with important implications for both appropriate diagnostic and vaccination strategies.
Results
Clinical characteristics of neuro-PASC patients
We enrolled a total of 111 participants prior to SARS-CoV-2 vaccination drawn from the neuro-COVID-19 outpatient clinic at Northwestern Memorial Hospital or from the surrounding Chicago area. This included 56 neuro-PASC “CN” patients (confirmed RT-PCR+ or anti-SARS-CoV-2 Spike IgG+) meeting Infectious Disease Society of America clinical criteria for COVID-19 starting after February 2020 and had neurologic symptoms lasting at least 6 weeks post-infection, as previously reported (Graham et al., 2021). Among those, 48 (86%) were never hospitalized for pneumonia or hypoxemia. We additionally recruited 24 healthy COVID convalescents without lasting symptoms (RT-PCR+ or seropositive for anti-SARS-CoV-2 Spike RBD IgG, “CC”); and 31 healthy controls who were RT-PCR- and seronegative for SARS-CoV-2 Spike-IgG (“HC”; study description in Fig. 1A).
Figure 1: Study design and clinical data.
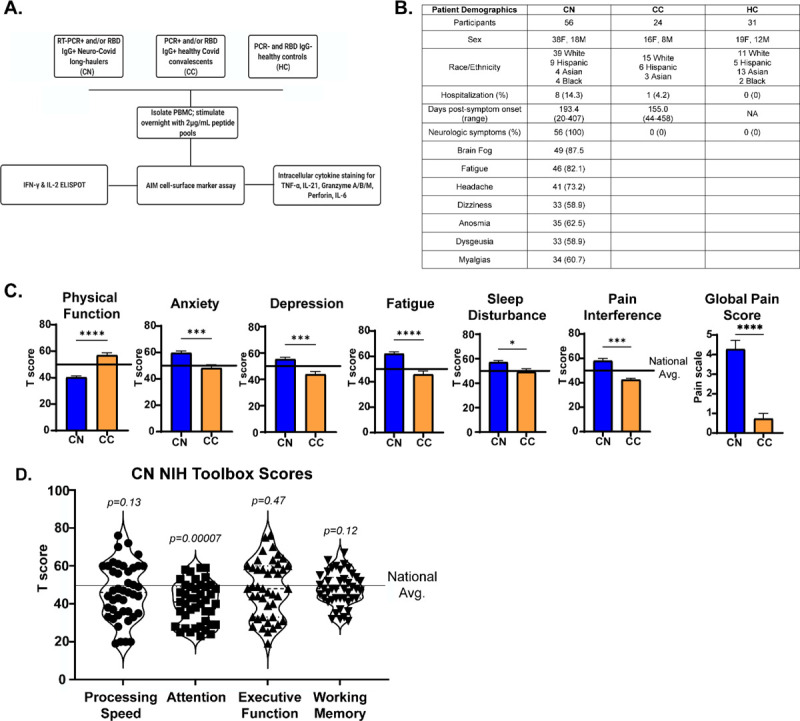
A.) Flow chart describing patient populations and experimental assays for each sample. B.) Table showing patient demographics and neurologic symptoms. C.) PROMIS-57 patient reported survey T scores for CN (n=36) and CC (n=13) groups. D.) NIH Toolbox cognitive T scores for CN (n=43). Horizontal black line represents the U.S. national average T score of 50; p values relative to US national average by one sample Wilcoxon signed rank test. *p<0.05, ***p<0.005, ****p<0.0001 by two-tailed Student’s t test.
CN patients displayed a constellation of neurological symptoms similar to those previously reported by our group and in other studies (Fig. 1B). In addition, we utilized standardized methods to quantify their quality of life and cognitive disturbances relative to healthy convalescents. Results from the patient reported outcomes information system (PROMIS-57) survey (Tang et al., 2019) showed that CN patients scored significantly lower on physical function and higher on anxiety, depression, pain and other metrics compared with CC subjects or the national average (Fig. 1C). NIH toolbox tests administered to CN patients to assess their cognitive function (Weintraub et al., 2013) showed CN patients had significantly lower T scores in the attention module, which was indicative of cognitive dysfunction relative to the national average (Fig. 1D).
Neuro-PASC is associated with a distinct immunodominance hierarchy.
To determine whether neuro-PASC included alterations in the magnitude and/or specificity of Ag-specific T cell responses in CN patients, we performed ELISPOT for SARS-CoV-2-specific T cells. PBMC from each subject were cultured in the presence of peptide pools derived from spike (S), nucleocapsid (N), or membrane (M) proteins of SARS-CoV-2 (Fig. S1). The magnitude and specificity of both IFN-γ and IL-2 responses to S sub-pools were similar between CN and CC subjects (Fig. 2A–B). However, CN subjects produced high levels of IFN-γ against all sub-pools of N and M proteins, while CC subjects had a specificity for the N1 pool and lower responses against all M pools (Fig. 2C–D), indicative of different immunodominance hierarchies between groups. No significant differences were found in IL-2 production, and healthy controls exhibited some response to N pools likely caused by cross-reactivity with endemic coronaviruses (Fig. 2E–F). Antibody titers against the Spike receptor-binding domain (RBD) did not differ between CN and CC groups (Fig. 2G). Interestingly, high IgG titers did not correlate with the magnitude of the IFN-γ T cell response against S2 or S3 peptide pools comprising the 2 halves of the Spike RBD (Fig. S2).
Figure 2: Neuro-PASC long-haulers display altered reactivity to SARS-CoV-2 N and M peptides compared to healthy convalescents.
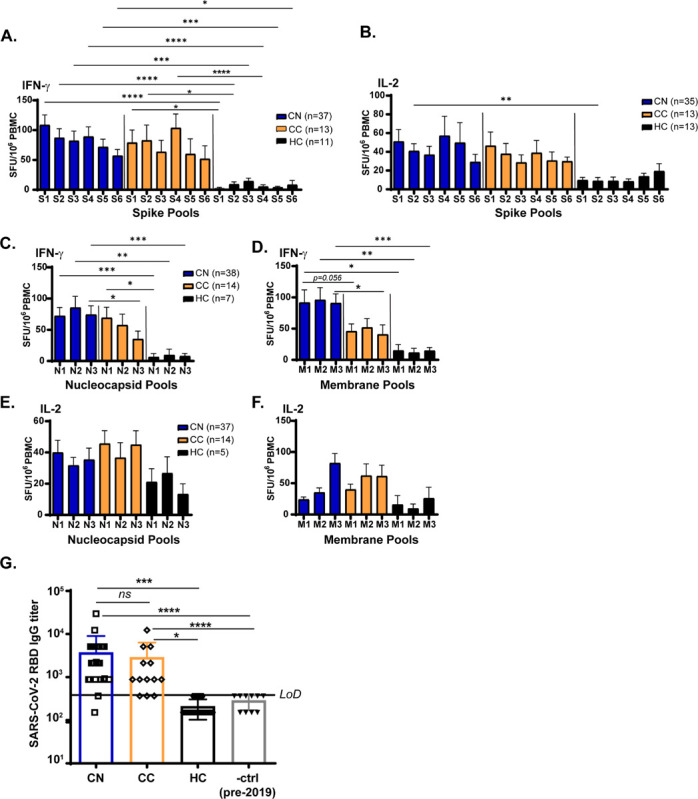
A-B.) CN (PCR+ or seropositive neuro-PASC) and CC (PCR+ or seropositive healthy COVID convalescent) groups display similar IFN-γ and IL-2 responses to peptides from SARS-CoV-2 Spike protein by ELISPOT. C-D.) CN samples show significantly enhanced IFN-γ responses to the N3 peptide pool (C) and to the M1 and M3 peptide pools (D) compared with CC or healthy controls. E-F.) N- and M- specific IL-2 production did not significantly differ between patient subgroups. G.) Spike RBD IgG endpoint titer quantification for CN, CC, and HC groups. LOD is limit of detection. Data representative of 10 experiments with all conditions plated in duplicate. *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001 by two-way ANOVA with Tukey’s posttest.
CD4+ Tfh cells display opposing reactivity to SARS-CoV-2 N- and M-proteins in neuro-PASC vs. healthy COVID convalescents
RNA-Seq analysis of CD4+ T cells from hospitalized and non-hospitalized COVID-19 patients showed that severe disease is associated with elevated CD4+ T follicular helper (Tfh) proportions relative to patients with mild disease (Meckiff et al., 2020). We sought to determine whether Tfh responses (gating in Fig. S3) that prime antibody production from plasma B cells could similarly differentiate neuro-PASC CN patients from CC individuals. Immunophenotyping showed that there were no differences in total percentages of T cell subsets, including Tfh cells, between groups (Fig. S4). The activation-induced marker (AIM) assay has been previously used to detect TCR-mediated activation of T cells (Grifoni et al., 2020) and we used this method to investigate Tfh cell activation (gating for Tfh cells in Fig. S3). N protein-specific CD134+CD137+ (AIM+) CD4 Tfh cells were significantly elevated in CN compared with CC subjects, while the opposite trend was observed in M protein-specific activation (Fig. 3A–B). No differences were seen in Tfh activation between CN and CC groups across Spike peptide pools (Fig. 3B). The magnitude of N- and M-specific Tfh cell activation did not correlate with the time since acute infection in either CN or CC patients (Fig. 3C–D) despite reports showing that antibody titers against N protein decrease rapidly post-infection (Van Elslande et al., 2021) which would presumably lead to decreased N-specific Tfh cell activation over time. N-specific IgG titers were significantly elevated in CN compared with CC subjects (Fig. 3E), consistent with the enhanced N-specific CD4+ Tfh cell activation shown in 3B. Similar to Tfh cells, there was no correlation between anti-N IgG titers and time post-symptom onset for either CN or CC subjects (Fig. S5A). Studies have shown that IgG titers against N protein decline to undetectable levels in 40% of COVID convalescents within 4 months (Muecksch et al., 2021). We largely did not observe this decline in CN patients despite collecting their blood samples an average of 193 days post-symptom onset (Fig. 1B).
Figure 3: N-specific CD4+ Tfh cells are more activated and S-specific CD4+ T cells display enhanced polyfunctional granzyme production in neuro-PASC long-haulers.
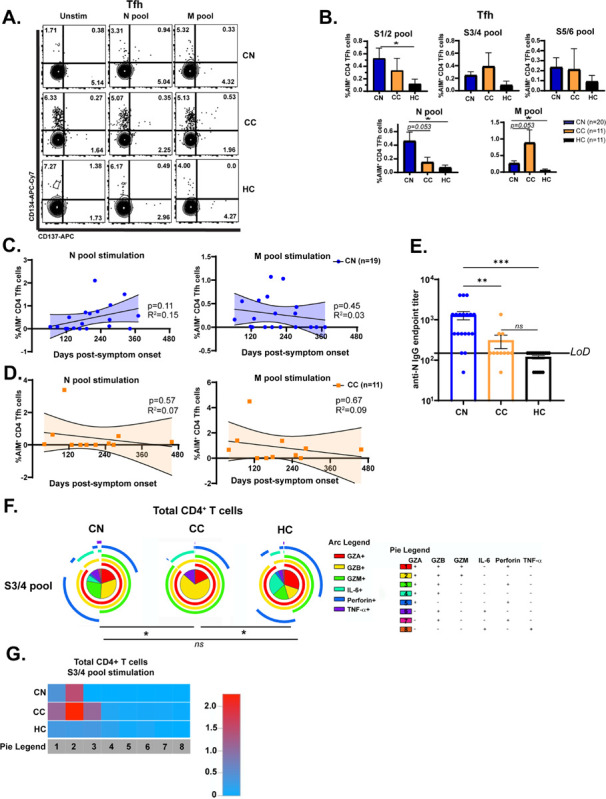
A.) FACS plots show that Ag-specific CD4 Tfh from CN patients are more highly activated in response to N peptide pool stimulation compared with CC or HC, but less activated by M peptides. B). Quantification of AIM+ Tfh cell activation. C-D.) N- and M-specific AIM+ Tfh cell percentages plotted by time sample obtained post-infection for CN and CC. No correlation was found between these parameters. E.) Anti-SARS-CoV-2 nucleocapsid IgG endpoint titers for CN, CC, and HC subjects shown in 3D. CN patients display significantly elevated anti-N IgG titers compared with CC subjects. F.) Pie graphs show CD4+ T cells from CC patients have enhanced production of granzymes A, B, & M compared with CN or HC subjects in response to SARS-CoV-2 Spike protein. G.) Heatmap quantifying polyfunctionality in different categories of cytokine production between groups. CC subjects produced 2-fold more granzymes A/B/M than CN patients. Data combined from 6 independent experiments with CN n=35, CC n=11, and HC n=9. *p<0.05 using one-way ANOVA with Bonferroni’s posttest (B) or a Permutation test (E). All pie graphs are showing data after subtracting background (unstimulated condition).
CD4+ T cell polyfunctionality to Spike protein differs in neuro-PASC vs. healthy COVID convalescents
Cytotoxic CD4+ T cells acquire the ability to produce cytolytic granules upon activation with cognate viral antigen (Goubard et al., 2015; Sledzinska et al., 2020). Viral infections specifically induce expansion of the memory CD4+ T effector memory cells re-expressing CD45RA (TEMRA) population that secrete copious amounts of cytolytic granules upon antigen encounter (Tian et al., 2017). We therefore investigated whether Ag-specific production of multiple granzymes and cytokines in CD4+ T cells were altered between groups. While CN and HC groups maintained polyfunctionality and polarization, CC subjects had significantly more polyfunctional CD4+ T cells producing granzymes A, B, and M after Spike pool stimulation (category 2 in yellow, Fig. 3F). A heatmap quantifying this effect showed that CC had a 2-fold elevation in category 2 effector molecules over CN subjects (Fig. 3G). This data suggests that cytotoxic responses to Spike protein in CD4+ T cells from healthy COVID-19 convalescents significantly differ from those experiencing chronic neurologic symptoms.
CD8+ memory T cell functionality in neuro-PASC patients
CD8+ memory T cells are crucial for protection from recurrent viral infections (Mockus et al., 2019). Studies have shown that memory CD8+ cells can persist for several years after patient exposure to SARS-CoV (Chen et al., 2005) and that acute mild disease induces stronger Ag-specific CD8+ memory responses than severe disease (Peng et al., 2020). However, little is known about whether CD8+ memory differs in neuro-PASC long-haulers vs. healthy COVID convalescents months after acute infection resolves. To address this, we probed the dynamics of CD8+ TEM, TCM, and TEMRA activation in CN, CC, and HC subjects. CD8+ TEM were significantly activated by various S, N, and M peptide pools in CC subjects while remaining relatively quiescent after antigen stimulation in CN patients (Fig. 4A–B). CD8+ TEMRA are terminally differentiated memory cells with distinct transcriptional programs that respond quickly to viral infections (Tian et al., 2019). Total percentages of CD8+ TEMRA cells were significantly elevated in CN compared with CC and HC groups (Fig. 4C), but despite their increased numbers, CD8+ TEMRA cells were less activated by the S3/4 peptide pool in CN patients and showed a trend towards decreased activation after N pool stimulation compared with CC subjects (Fig. 4D–E).
Figure 4: Neuro-PASC long-haulers have decreased CD8+ memory T cell activation and function compared with healthy convalescents.
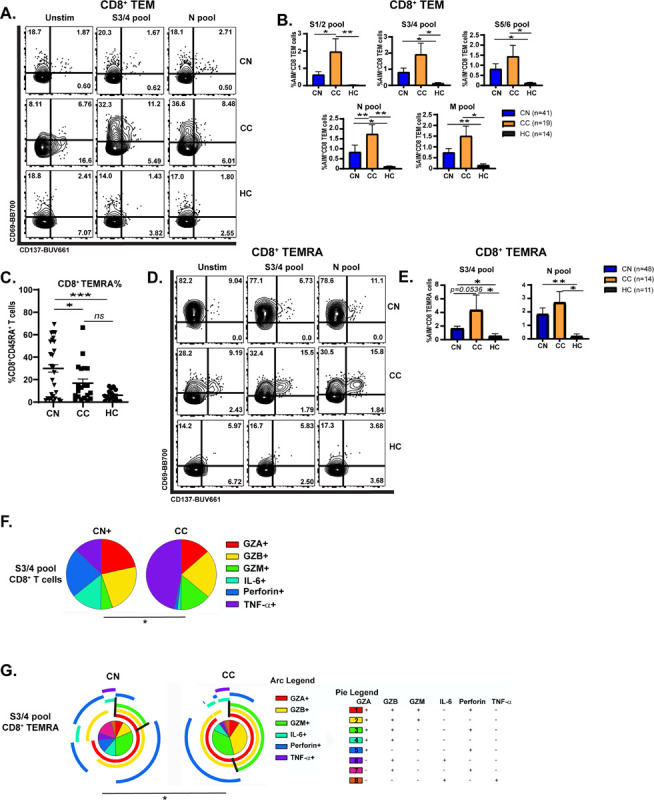
A.) Selected FACS plots showing CD8 TEM cells are less activated in CN vs. CC groups after S and N peptide pool stimulation. B.) Quantification of AIM+ CD8 TEM cells after SARS-CoV-2 peptide stimulation. C.) CD8+ TEMRA cells accumulate significantly in PBMC from CN subjects compared with CC or HC. D.) CD8+ TEMRA cells from CN patients are less activated by S3/4 and N pools compared with those from CC subjects. E.) Quantification of AIM+ CD8+ TEMRA cells from D. F.) Pie charts demonstrating that S3/4-specific CD8+ TEM are polarized to produce more TNF-α in CC group while those from CN patients produce significantly more IL-6. G.) CD8+ TEMRA from CC patients are polarized towards category 2 cytokine production in response to S3/4 stimulation compared with CN subjects. Black lines demonstrate boundaries of category 2 cytokine production in each group. Data combined from 5 independent experiments with n=35 CN, n=11 CC. *p<0.05, **p<0.01 using two-tailed Student’s t test with Welch’s correction (B, C, E) or permutation test (F, G). All pie graphs are showing data after subtracting background (unstimulated condition).
Similarly, antigen stimulation resulted in altered cytokine polarization in CD8+ T cell subsets in CN and CC groups. Effector molecule production in CD8+ T cells was similar across groups in the unstimulated condition, despite showing some statistically significant differences in perforin production (Fig. S6B). However, stimulation with the S3/4 pool induced greater TNF-α production in the CC group while CN patients had enhanced IL-6 production to the same stimulus (Fig. 4F). S pool stimulation also induced CD8+ TEMRA cells from the CC group to produce elevated levels of granzymes, which was not seen in CN or HC groups (Fig. 4G, black lines), demonstrating that CD8+ T cell subsets display decreased functionality in CN vs. CC patients after stimulation.
Impaired cognition and decreased quality of life metrics correlate with distinct patterns of polyfunctionality in memory T cell subsets
Having shown that Tfh and T cell memory responses differed between patient groups, we next sought to probe whether within-group differences in T cell responses correlated with various clinical measures in CN patients. We found a significant positive correlation between the magnitude of IFN-γ production to N protein and higher pain interference scores (Fig. 5A). There was also a trend towards a positive correlation between high scores for depression and the magnitude of the N-specific T cell response (Fig. 5B). To look at associations between clinical parameters and T cell activation, we separated T scores from NIH Toolbox or PROMIS-57 measurements (Fig. 1C–D) into quartiles and only the lowest and highest groups (Q1 vs. Q4) were used for analysis (Fig. S7A, red boxes). CN subjects reporting high (Q4) pain interference scores produced significantly less granzyme A or B and more IL-6 than those with low scores (Fig. 5C–D) from CD8+ T cells after S pool stimulation. Further, patients reporting low depression scores had highly polyfunctional Spike-specific CD8+ TEM, while those reporting high levels of depression had TEM producing ~3-fold higher granzymes A, B, and M alone (category 2; Fig. 5E, F). The severity of cognitive impairment could also be significantly correlated with T cell responses. Q1 patients scoring low on executive function tests had CD8+ TCM polarized towards granzymes A, B, M and perforin production (category 1), while those in Q4 were biased to produce granzymes A, B, and perforin (Fig. 5G–H). Similar analyses were performed for other CD8+ and CD4+ memory T cell subsets, and significant differences by quartile were also found in correlations with depression, processing speed, working memory, and global pain (Fig. S7B–K). These data show that the severity of cognitive deficits or quality of life measures can be significantly correlated with differences in SARS-CoV-2-specific memory T cell function.
Figure 5: Clinical measures of cognitive dysfunction and depression correlate with altered CD8+ memory T cell function in neuro-PASC long-haulers.
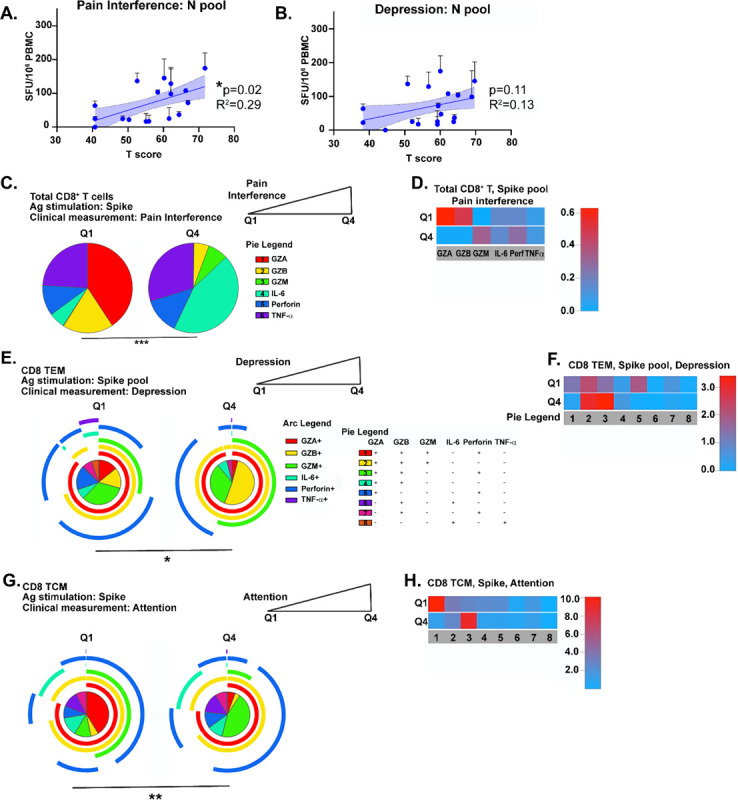
A.) Higher T cell responses to N pool stimulation are positively correlated with high pain scores in CN patients. B.) A positive trend exists between elevated N-specific IFN-γ production and higher depression scores in CN patients. C.) CN patients scoring high on pain interference have Spike-specific CD8+ T cells producing more IL-6 than those scoring low. D.) Heatmap showing CN with low pain scores have Spike-specific CD8+ T cells producing significantly more granzymes A or B than those reporting high pain levels. E.) Pie graph to demonstrate that CN patients in Q4 for depression have significantly enhanced production of granzymes A/B/M compared with patients with low depression scores. F.) Heatmap demonstrating that CN patients with high depression scores had Spike-specific CD8+ TEM producing 3-fold higher levels of granzymes A, B, and M or perforin than those scoring low on depression. G.) Spike-specific CD8+ TCM from CN patients with high scores for executive function are more polarized to produce granzymes A/B/M compared with CN patients from Q1. H.) Heatmap showing Spike-specific CD8+ TCM in Q1 CN patients are more polyfunctional and produce 8–10x more granzymes A/B/M and perforin than those in Q4. Data representative of 5 independent experiments with n=8–9/quartile. All pie graphs showing Ag-specific cytokine production are background subtracted (unstimulated conditions). *p<0.05, **p<0.01, ****p<0.001 using Permutation tests.
SARS-CoV-2 vaccination induces sustained elevation in Spike-specific IFN-γ responses in neuro-PASC but not healthy COVID convalescents
SARS-CoV-2 mRNA vaccines have been proven to be highly effective against contracting infection in the short-term in both clinical trials and in real-world settings (Haas et al., 2021; Polack et al., 2020). However, neither clinical trials nor field studies specifically characterized the vaccine-induced immune response in long-COVID patients, and it is similarly unknown whether vaccination can ameliorate long-haul symptoms. One prospective study showed that vaccination did not significantly worsen symptoms in 22 patients with long-COVID (Arnold et al., 2021), but also did not provide evidence to show any decrease in symptoms. To address how vaccine-induced immunity evolves over time in neuro-PASC long-haulers, we conducted a longitudinal vaccine study assessing Spike-specific antibody and T cell responses over the course of 6 months post-vaccination (study design and patient demographics in Fig. 6A–B). While this study is ongoing, we report data from the first 4 months comparing responses in CN, CC, and HC subjects (n for each group per study visit shown in Fig. S8). Vaccination with either Pfizer or Moderna mRNA vaccines induced robust SARS-CoV-2 Spike RBD IgG titers in all groups tested by 3 weeks post-2nd dose, with the highest titers found in CN patients. Antibody titers were at similar levels in all groups by 3 months post-1st dose, but trended higher in CN than in HC subjects at visit (V)4 (Fig. 6C). In parallel, we measured Spike-specific T cell responses in study subjects. The magnitude and kinetics of the T cell response in CC and HC subjects were similar and peaked at V3. However, CN patients had significantly elevated Spike-specific T cell responses compared with either HC or CC groups at V2 after the vaccination boost (Fig. 6D; individual pool data shown in Fig. S9). IFN-γ-specific T cell responses remained high at 4 months post-vaccination in CN patients while not significantly differing from pre-vaccination levels in both CC and HC subjects. To our knowledge, these are the first data that longitudinally compare the T cell response to vaccination in neuro-PASC patients with healthy COVID convalescents and unexposed individuals.
Figure 6: Spike-specific T cell responses wane within 4 months after vaccination in CC and HC subjects while remaining high in CN patients over time.
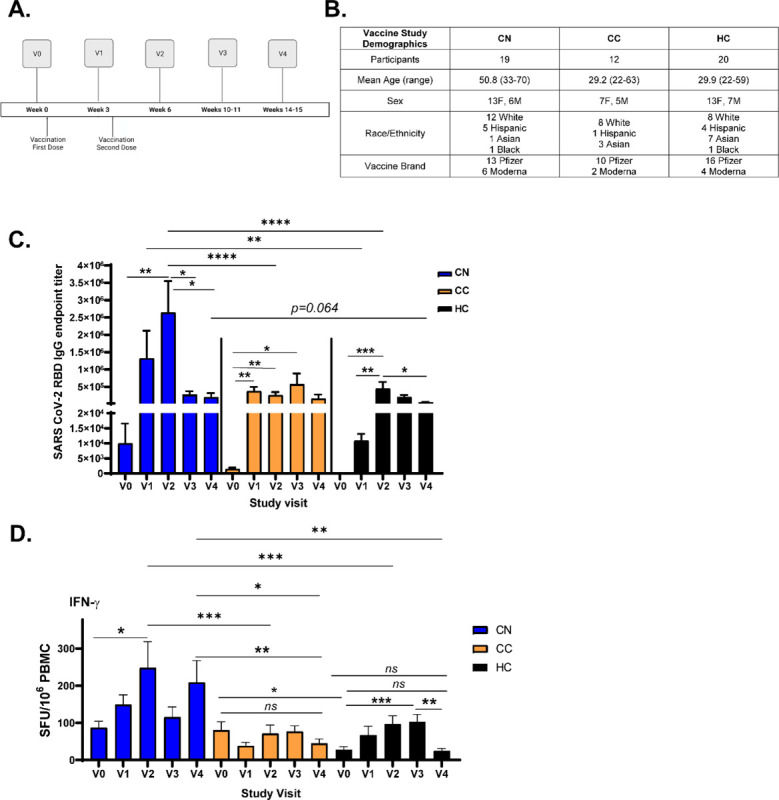
A.) Vaccine study visit timeline. V0 was obtained before the first dose of either Pfizer or Moderna mRNA vaccines. V1 and V2 were conducted 3 weeks after the first and second doses, respectively. B.) Vaccine study subject demographics. C.) Longitudinal anti-Spike RBD IgG responses from V0–V4 across groups. Antibody titers are highest in CN patients and wane most quickly in HC subjects. D.) IFN-γ production from Spike-specific T cells do not significantly increase in CC and HC groups while remaining high in CN up to V4 post-vaccination. Total S-specific IFN-γ SFU calculated by averaging responses from each sub-pool for each participant (data in Fig. S9). Data combined from 10 individual experiments with all ELISA conditions done in triplicate and all ELISPOT conditions plated in duplicate. *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001 by two-way ANOVA with Tukey’s posttest or by two-tailed Student’s t test.
Overall, our study demonstrates that neuro-PASC patients have elevated IFN-γ responses to N and M proteins, enhanced Ag-specific activation of Tfh cells, sustained anti-N IgG production, and deficient activation of multiple CD8+ memory T cell subsets compared with healthy COVID-19 convalescents. There were also correlations between the severity of cognitive deficits or quality of life impairments and Ag-specific cytokine signatures (Fig. 5, S7). Importantly, vaccination resulted in sustained enhancements in the magnitude of Spike-specific T cell responses in neuro-PASC patients vs. all other groups, regardless of prior COVID exposure. Together, we show that CN patients exhibit a large-scale dysfunction in multiple aspects of the memory T cell response which may inform treatment and/or vaccination options down the line.
Discussion
COVID-19 is well-recognized as a multi-organ disease with long-term sequelae associated with neurological disease. PASC has been reported in up to 87% of those hospitalized with SARS-CoV-2 pneumonia and in up to 40% of those with mild disease who do not require hospitalization (Havervall et al., 2021; Hirschtick et al., 2021; Mahase, 2020). SARS-CoV-2 vaccines have decreased the rates of SARS-CoV-2 infection and mortality in unexposed individuals, but it is not clear that vaccines help to ameliorate long-COVID symptoms in previously infected individuals (Arnold et al., 2021) and thus further study is needed to inform treatment options. Long-term sequelae after other coronavirus infections can persist for years (Ahmed et al., 2020); therefore, it is important to specifically characterize SARS-CoV-2-specific immune responses in long-COVID patients. Most studies on effector and memory T cell responses to SARS-CoV-2 have focused on acute infection or healthy convalescents as opposed to those with long-COVID (Rodda et al., 2021; Sekine et al., 2020; Weiskopf et al., 2020). We aimed to fill this knowledge gap and determine whether and how T cell phenotype and function differ in patients with neuro-PASC and healthy COVID-19 convalescents.
Clinically, neuro-PASC resembles myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), which many patients report as a post-viral infectious complication (Rasa et al., 2018). The causes of ME/CFS remain elusive, and the underlying mechanisms of neuro-PASC remain similarly unknown. It is possible that direct infection of the brain is responsible for neuro-PASC symptoms. It is believed that SARS-CoV-2 gains entry into the central nervous system through the olfactory bulb and has been shown to infect neurons in vitro, which is supported by findings of viral protein expression in cortical neurons from post-mortem autopsies (Klingenstein et al., 2020; Song et al., 2021). However, another study was unable to find any evidence of SARS-CoV-2 in the brains of 4 patients with neurological symptoms during acute infection (Kantonen et al., 2020), suggesting that infection of the nervous system may be transient. Another study of acutely infected neuro-COVID patients did not find any SARS-CoV-2 RNA present in the CSF, though they did identify enhanced presence of exhausted T cells and dedifferentiated monocytes (Heming et al., 2021). However, all of these prior studies were conducted on patients with acute SARS-CoV-2 infection as opposed to neuro-PASC, and thus mechanisms for neurological dysfunction may differ. As lumbar punctures or brain biopsies are not indicated in neuro-PASC outpatients, reproducing the above study results in outpatient populations will not be possible. Additional hypotheses for neuro-PASC pathogenesis include a contribution of autoimmune mechanisms which is suggested by the skewed ratio of females to males affected, similar to that seen in rheumatoid arthritis or systemic lupus erythematosus (Chakravarty et al., 2007; Myasoedova et al., 2010), as well as the possibility of persistent SARS-CoV-2 infection in the periphery (Al-Aly et al., 2021). Much of our findings on the SARS-CoV-2 T cell response in neuro-PASC patients supports this latter hypothesis.
Ag-specific IFN-γ and IL-2 production was similar between CN and CC groups in response to stimulation by different SARS-CoV-2 Spike peptide pools (Fig. 2A–B). However, T cells from CN patients retained high IFN-γ responses to all N- and M-peptide pools tested, while CC subjects had a reactivity limited to N1 and N2 peptide pools with little IFN-γ production in response to M pools (Fig. 2C–D). The presence of an immunodominance hierarchy in CC subjects is the expected outcome of an effective memory response to SARS-CoV-2 infection. Studies have shown that while a primary CD4+ or CD8+ T cell response to influenza A or LCMV infection is highly diverse and contains T cells reactive to many viral proteins and epitopes, the memory response preferentially contains T cells responding to specific immunodominant viral antigens (Sant et al., 2018; Tebo et al., 2005). This effect is more pronounced in the CD8+ T cell compartment where memory responses are dependent on the ability of dendritic cells to present viral antigen (Crowe et al., 2003) or on antigen availability (Henrickson et al., 2013). The evident lack of a narrow and targeted IFN-γ response to N- and M-peptides in CN patients thus suggests that they are unable to effectively generate a memory response to SARS-CoV-2. Though there were no differences observed in the magnitude or specificity of the IFN-γ response to SARS-CoV-2 Spike peptides, this is somewhat expected as memory T cell responses to Spike protein remain diverse after both infection (Grifoni et al., 2020) and vaccination (Alter et al., 2021). It is additionally not surprising that differences in reactivity were observed for IFN-γ and not IL-2 T cell responses between CN and CC groups (Fig. 2B, E–F). Studies have demonstrated that while Ag-specific T cells produce IL-2 as a proliferation factor, IFN-γ production is largely confined to the memory T cell population (Anthony et al., 2012), which is largely where we see differences in activation and reactivity between groups (Fig. 4).
Antibody production is a key outcome measure after SARS-CoV-2 infection, Tfh cell activation can inform the effectiveness of an antibody response. Stimulation with SARS-CoV-2 N peptides activated Tfh cells in CN but not CC subjects, while the opposite trend was found with M pool stimulation (Fig. 3A–B). Tfh cell activation is crucial to the establishment of germinal centers in secondary lymphoid organs, ultimately resulting in B cell maturation into long-lived plasma cells that can continuously produce class-switched, high-affinity antibodies (Good-Jacobson and Shlomchik, 2010). Studies have demonstrated that the magnitude of the Tfh cell response is dependent on the amount of antigen available and directly correlates with the magnitude of the B cell response (Baumjohann et al., 2013). Indeed, the same CN patients with high N-specific Tfh cell activation also displayed anti-N IgG titers significantly greater than CC subjects (Fig. 3E) despite the fact that we obtained their samples more than 6 months on average after acute infection. Altogether, these findings are consistent with the presence of a persisting nucleocapsid antigen reservoir resulting in enhanced N-specific Tfh cell activation in CN patients. Clinical reports have identified cases of persistent SARS-CoV-2 infection in the nasopharynx lasting up to 63 days (Bennasrallah et al., 2020) and many patients re-tested RT-PCR+ for SARS-CoV-2 up to 38 days post-discharge (Dao et al., 2021). Though many of our CN patients have not re-tested RT-PCR+ by nasal swab (data not shown), others have yet to be tested. The nasopharynx is not the only possible testing site, however, as there are numerous studies showing that infectious SARS-CoV-2 particles can be detected in fecal matter and can persist for up to 70 days post-symptom onset (Tian et al., 2020; van Doorn et al., 2020). Gastrointestinal symptoms also did not have to be present in order to test RT-PCR+ for SARS-CoV-2 in stool samples (Chen et al., 2020). Thus, it is possible that the gut may also act as a reservoir for persistent SARS-CoV-2 infection which leads to aberrant T cell responses and the development of neuro-PASC.
Effective generation of T cell memory responses is crucial to protect against future infections with the same pathogen. CD8+ TEM cells from CC subjects retained high levels of activation to all S, N, and M pools assayed while CN patients displayed very little TEM activation (Fig. 4A–B), suggestive of aberrant memory T cell function. Studies on convalescents from the original SARS-CoV epidemic of 2002–03 found that the majority of Ag-specific CD8+ memory T cells were TEM, and these persisted up to 4 years after infection (Channappanavar et al., 2014). Sterilizing immunity can be a precondition for the development and maintenance of memory T cells because high levels of foreign antigen favor the generation of short-lived effector cells (Mueller et al., 2013), which is why chronic infections often limit the formation and/or function of memory T cells (Wherry, 2011). In fact, chronic LCMV infection in mice induced aberrantly functioning Ag-specific memory CD8+ T cells requiring the presence of viral peptide rather than simply the homeostatic cytokines IL-7 and IL-15 to proliferate (Shin et al., 2007; Wherry et al., 2004). The inability of CD8+ TEM to become activated by antigenic stimulation in CN patients is thus suggestive of a chronic infection state wherein viral antigen can persist but limits the formation of CD8+ T cell memory. It is also possible that chronic SARS-CoV-2 infection in CN patients leads to memory T cell exhaustion, as shown in chronic HIV and HBV infections in humans (McLane et al., 2019). However, we did not find any differences in the inhibitory marker PD-1 expression on CD8+ memory T cell subsets between CN and CC patients (data not shown).
Further providing support to the chronic infection hypothesis in neuro-PASC patients, there was a significant elevation in the CD8+ TEMRA cell population in CN patients over CC or HC groups (Fig. 4C). CD8+ TEMRA cells are a terminally differentiated memory subset that do not traffic through secondary lymphoid organs, and their induction during viral infection can be protective (Tian et al., 2019). Yet, they have also been shown to accumulate during persistent viral infections and contribute to immunosenescence (Derhovanessian et al., 2011). CD8+ TEMRA cell reactivity to SARS-CoV-2 peptides was also decreased in CN patients over CC subjects. Functionally, the polarization in granzyme production in S3/4-specific CD8+ TEMRA cells from CC patients (Fig. 4G) suggests higher cytotoxic capacity compared with CN patients and coincides with their higher activation state in Fig. 4E. Ag-specific CD8+ TEMRA cells are expanded and functionally active in people with significant anti-Dengue virus immunity, and this phenotype is seen as a goal for vaccine-induced protection (Tian et al., 2019). We propose that CD8+ TEMRA cells are more expanded but less functionally active in CN subjects over CC as a consequence of inappropriate CD8+ T cell memory formation, which is lacking in multiple T cell subsets for neuro-PASC patients.
We also showed that CD8+ T cells from CC subjects skewed towards TNF-α production after S pool stimulation compared with CN patients. Comparatively, CN patients displayed significantly more IL-6 production from S-specific CD8+ T cells (Fig. 4F). Vaccination against the intracellular pathogen Shigella flexneri showed that polyfunctional TNF-α-producing CD8+ TEM were an important correlate of protection (Toapanta et al., 2018). Despite multiple reports indicating that enhanced TNF-α production is correlated with worse COVID-19 outcomes (Karki et al., 2020, 2021; Pedersen and Ho, 2020), we speculate that Ag-specific TNF-α production is protective in our model system because we are looking at chronically and not acutely infected CN patients. In contrast, IL-6 is known to suppress TH1 differentiation (Diehl et al., 2000) and can promote pathogen survival and exacerbate clinical disease during the original SARS-CoV infection (Channappanavar and Perlman, 2017). Indeed, studies in severely ill, hospitalized COVID-19 patients demonstrated that high serum levels of IL-6 significantly correlated with poor clinical outcome (Weiskopf et al., 2020). These data suggest a role for enhanced IL-6 production by CD8+ T cells in the pathogenesis of neuro-PASC, and open new avenues of research for the treatment of long-COVID through limiting IL-6 activity.
Clinically, neuro-PASC patients reported significantly elevated levels of anxiety, depression, fatigue, sleep disturbance and pain as well as decreased physical function compared with healthy convalescents (Fig. 1C). The severity of these deficits was highly correlated with Ag-specific enhancements in polyfunctionality and decreases in polarization of various memory T cell subsets (Fig. 5, S7). It is possible that T cell function contributes to the genesis and persistence of some of these symptoms. Studies in rodents have shown that T cell activation and function can affect the severity of pain and analgesia (Rosen et al., 2019; Sorge et al., 2015). Indeed, pain is a common hallmark of chronic viral infection (Addis et al., 2020) and recognized among post-COVID sequelae (Kemp et al., 2020); it might follow then that aberrant T cell activation can associate with high pain scores. Additionally, reports have shown that transcriptional programs in immunity and inflammation were differentially regulated in CD4+ T cells from patients with depression compared with healthy controls (Wang et al., 2015). Treg cells may also decrease depressive behavior through negative regulation of inflammation (Miller, 2010), and CN patients do display elevated TH1-type cytokine production to S pool stimulation (Fig. 2, 6) while not displaying any compensatory upregulation in Treg total numbers or function (data not shown). T cell-derived cytokines can also impact learning and memory. Studies in mouse models of West Nile and Zika viral encephalitis have demonstrated that IFN-γ production from CD8+ T cells in the brain is responsible for neuronal apoptosis and spatial learning deficits (Garber et al., 2019). Thus, there is a precedent for correlating T cell function with cognitive deficits, pain, or depression. Ag-specific cytokine signatures associated with the severity of cognitive and quality of life deficits in neuro-PASC patients may therefore provide some predictive value in terms of clinical outcomes.
Preliminary reports showed that the Pfizer mRNA vaccine could elicit a T cell response 7 days after completion of the full prime-boost protocol (Sahin et al., 2020), but until our studies there has been no data on longitudinal T cell responses primed by vaccination and how these vary between groups with different types of prior SARS-CoV-2 exposure. Our results demonstrate for the first time that vaccine-elicited immune responses are significantly divergent in neuro-PASC versus healthy COVID convalescents (Fig. 6C–D). CN patients consistently had higher antibody titers after receiving the second dose of the vaccine compared with CC and HC groups, though titers were similar in all groups at 11–15 weeks post-1st dose (Fig. 6C). In contrast, the magnitude of Spike-specific IFN-γ production by T cells remained high in CN patients out to 4 months post-vaccination while not significantly higher than pre-vaccination levels in CC and HC subjects along the same timeline (Fig. 6D). These data suggest that the mRNA vaccines do not induce robust long-term T cell responses in many individuals, regardless of prior COVID exposure, if they are not neuro-PASC patients. Yet, despite vaccines enhancing Spike-specific IFN-γ production in CN T cells, the fact that these responses continue to increase at 15 weeks post-vaccination suggests that CN patients may still have an active SARS-CoV-2 infection or a persistent antigen reservoir rather than developing a robust T cell memory response. These results suggest that alternate SARS-CoV-2 vaccines that induce long-lasting memory T cell responses in previously unexposed individuals as well as healthy COVID convalescents are needed in order to mediate long-term protection from infection. Conversely, vaccination may not be indicated for long-COVID patients who might have a persistent infection as it may be ineffective in the absence of viral clearance. Indeed, current clinical guidance from the CDC recommends that vaccination be delayed until 3 months after acute infection in unvaccinated COVID convalescents. Thus, vaccination strategies that include the memory T cell response as a marker for efficacy should be carefully considered. Together, these data show that irregular Tfh responses, broad scale dysfunction in CD8+ T cell memory generation and aberrant T cell responses to vaccination are hallmarks of neuro-PASC and require further study to inform treatment and vaccination strategies across the population.
Limitations of study
One limitation of our study is the relatively small sample size of unvaccinated neuro-PASC patients. This was due to the wide implementation of SARS-CoV-2 vaccines in the Chicago area soon after beginning study enrollment. Another limitation was not being able to control for time of sample collection with respect to date of COVID-19 symptom onset. As it is possible that neuro-PASC could be the result of a persistent infection, further investigations would require testing of potential cryptic reservoirs, including stool samples from CN patients.
Materials and Methods
Ethics Statement
This study was approved by the Northwestern University Institutional Review Board (Koralnik Lab, IRB STU00212583). Informed consent was obtained from all enrolled participants. Samples were de-identified before banking.
Study participants, NIH Toolbox, and PROMIS-57 data collection
We enrolled consenting unvaccinated adult outpatients seen in the Neuro-PASC-19 clinic at Northwestern Memorial Hospital from September 2020-June 2021, including 56 neuro-PASC “long-hauler” patients with documented PCR+ or seropositive IgG results for SARS-CoV-2 (CN). In parallel, we recruited 24 unvaccinated healthy COVID convalescents from the surrounding community who tested PCR+ for SARS-CoV-2 but had no lingering neurological symptoms (CC) and 31 healthy controls who tested PCR- for SARS-CoV-2 and were also seronegative for IgG against SARS-CoV-2 Spike RBD. All study subjects remained living throughout the period of observation. Heparinized blood samples were collected one time from each subject at an average of 155–315 days post-symptom onset (as in Fig. 1B). Other demographic information is contained in Fig. 1B. CN patients completed a cognitive function evaluation in the clinic coincident or near the date of their blood sample acquisition with the National Institutes of Health (NIH) Toolbox v2.1 instrument, including assessments of: processing speed (pattern comparison processing speed test); attention and executive memory (inhibitory control and attention test); executive function (dimensional change card sort test); and working memory (list sorting working memory test) (Lai et al., 2011; Weintraub et al., 2013). PROMIS-57 was administered to CN and CC patients an average of 72 days post-sample collection. Both PROMIS-57 and NIH Toolbox results are expressed as T-scores adjusted for age, education, gender, and race/ethnicity with a score of 50 representing the normative mean/median of the US reference population with a standard deviation of 10. Lower cognition T-scores indicate worse performance while higher fatigue, depression, anxiety, or pain interference T-scores indicate greater symptom severity.
PBMC and plasma collection
30mL of venous blood from study volunteers was collected in blood collection tubes containing sodium heparin from BD Biosciences. Whole blood was layered on top of 15mL of Histopaque 1077 (Sigma-Aldrich) in 50mL Leucosep blood separation tubes (Greiner Bio-One) and spun at 1000g for 18min at RT. Plasma was collected and stored at −80°C. The PBMC layer was collected and washed 2x in sterile PBS before red blood cell lysis with ACK buffer (Quality Biologicals). PBMCs were used in assays either immediately or frozen down for use in the near term.
SARS-CoV-2 peptide antigens
All S, N and M peptide arrays used in ELISPOT and flow cytometry studies were obtained from BEI Resources, NIAID, NIH: Peptide Array, SARS-Related Coronavirus 2 Spike (S) Protein; NR-52402, Nucleocapsid (N) Protein, NR-52404; Membrane (M) Protein, NR-52403. The S peptide array consisted of 181 peptides of 13–17aa in length and split into 6 sub-pools (S1–S6) containing 30–31 peptides each. The N peptide array consisted of 59 peptides of 13–17aa each split into 3 sub-pools containing 29–30 peptides each. The M peptide array consisted of 31 peptides of 12–17aa and split into 3 sub-pools of 10–11 peptides each (Fig. S1). All peptides were dissolved in either sterile H2O or 50% sterile H2O-DMSO up to 1mL for a universal 1mg/mL stock concentration. Peptides were used at a final concentration at 2μg/mL in all assays.
IgG Spike RBD and Nucleocapsid ELISA
Antigen-specific total antibody titers were measured by ELISA as described previously (Dangi et al., 2020; Palacio et al., 2020). In brief, 96-well flat-bottom MaxiSorp plates (Thermo Scientific) were coated with 1 μg/ml of Spike RBD for 48 hr at 4°C. Plates were washed three times with wash buffer (PBS + 0.05% Tween 20). Blocking was performed with blocking solution (PBS + 0.05% Tween 20 + 2% bovine serum albumin), for 4 hr at room temperature. 6 μl of sera was added to 144 μl of blocking solution in the first column of the plate, 1:3 serial dilutions were performed until row 12 for each sample, and plates were incubated for 60 min at room temperature. Plates were washed three times with wash buffer followed by addition of secondary antibody conjugated to horseradish peroxidase, goat anti-human IgG (H + L) (Jackson ImmunoResearch) diluted in blocking solution (1:1000) and 100 μl/well was added and incubated for 60 min at room temperature. After washing plates three times with wash buffer, 100 μl/well of Sure Blue substrate (SeraCare) was added for 1 min. Reaction was stopped using 100 μl/well of KPL TMB Stop Solution (SeraCare). Absorbance was measured at 450 nm using a Spectramax Plus 384 (Molecular Devices). SARS-CoV-2 RBD and N proteins used for ELISA were produced at the Northwestern Recombinant Protein Production Core by Dr. Sergii Pshenychnyi using plasmids that were produced under HHSN272201400008C and obtained from BEI Resources, NIAID, NIH: Vector pCAGGS containing the SARS-related coronavirus 2, Wuhan-Hu-1 spike glycoprotein gene (soluble, stabilized), NR-52394 and receptor binding domain (RBD), NR-52309, nucleocapsid gene NR-53507.
Cell stimulation and IFN-γ/IL-2 ELISPOT
Multiscreen-IP plates (Millipore-Sigma) were coated overnight at 4°C with 2μg/mL anti-IFN-γ (clone 1-D1K, Mabtech) or 5μg/mL anti-IL-2 (clone MT2A91/2C95, Mabtech), washed with sterile PBS, and blocked with complete RPMI-10% FBS. PBMC isolated from CN, CC, and HC subjects were used either freshly isolated or after thawing and resting overnight in media containing 10ng/μL recombinant human IL-15 (Peprotech) at 37°C, 5% CO2. Cells were then plated at a concentration of 2.5×105 cells/well in 100μL of media and stimulated with the indicated antigen mixtures from SARS-CoV-2 at a concentration of 2μg/mL in complete RPMI medium containing 5% human AB serum (Sigma-Aldrich) and 5ng/mL IL-15. Plates were incubated at 37°C, 5% CO2 for 20 h and washed 5x with dH2O and PBS-0.05% Tween-20 (PBS-T). 2μg/mL biotinylated IFN-γ (clone 7-B6–1, Mabtech) or 5μg/mL IL-2 (clone MT8G10, Mabtech) diluted in PBS-10% FBS (PBS-F) was added to the respective wells and plates were incubated for 1.5h at RT. Plates were subsequently incubated for 40 minutes at RT in streptavidin-alkaline phosphatase in PBS-F (Jackson ImmunoResearch) was added after washing plates 5x in PBS-T. ELISPOT plates were developed using an Alkaline Phosphatase Conjugate Substrate Kit according to manufacturer’s instructions (Bio-Rad Laboratories, Carlsbad, CA). IFN-γ or IL-2-producing cells were quantified using an ImmunoSpot reader (Cellular Technologies, Ltd., Shaker Heights, OH).
Antibodies and Flow Cytometry
Fresh or frozen PBMCs isolated from the indicated patient groups were stimulated with antigen mixtures as above for 20–22h at 37°C, 5% CO2. For intracellular staining and cytokine detection, the Brefeldin-A Golgi plug (Biolegend) was added at a 1:1000 concentration 2 hours after antigenic stimulation commenced. Cells were washed with PBS-1% BSA after incubation and incubated with the indicated antibodies for surface phenotyping by AIM assay or for intracellular cytokine staining (ICS). Cells from each subject were left unstimulated in medium containing 5ng/mL IL-15 (“background”) or stimulated in the presence of the indicated antigens. Fixation and permeabilization was performed using Cytofix/Cytoperm (BD Biosciences). Surface staining was done in the dark at 4°C for 30 minutes, while ICS was done in the dark at RT for 45 minutes. Flow cytometry was conducted on 2–5×105 cells per condition. Data was acquired on a BD FACSymphony Spectral analyzer and analyzed using FlowJo v10 (BD Biosciences) and SPICE-Pestle (Roederer et al., 2011).
Quantification and Statistical Analysis
Statistical tests to determine significance are described in figure legends and conducted largely in Prism (GraphPad). For pie graphs or heatmaps generated using SPICE analysis, statistics were determined by Permutation test following unstimulated background subtraction, with additional thresholding of 0.03% to account for noise, using SPICE-Pestle. P-values lower than 0.05 were considered statistically significant. Quantile stratification was performed within group for CN cohort. Clinical data were collected and managed using REDCap electronic data capture tools hosted at Northwestern University Feinberg School of Medicine (Harris et al., 2009). REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing 1) an intuitive interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources.
Supplementary Material
Acknowledgements
We would like to thank the Flow Cytometry Core Facility at the Robert H. Lurie Comprehensive Cancer Center at Northwestern University supported by Cancer Center Support Grant (NCI CA060553) for their assistance in optimizing antibody panels and help with flow cytometry instrumentation. L.V. was supported by a T32 grant T32AR007611 from the Department of Rheumatology, Northwestern University Feinberg School of Medicine. P.P.M. is supported by a grant from the Emerging and Re-Emerging Pathogens Program (EREPP) at Northwestern University, and a grant from the National Institute on Drug Abuse (NIDA, DP2DA051912).
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- Addis D.R., DeBerry J.J., and Aggarwal S. (2020). Chronic Pain in HIV. Mol Pain 16, 1744806920927276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H., Patel K., Greenwood D.C., Halpin S., Lewthwaite P., Salawu A., Eyre L., Breen A., O’Connor R., Jones A., et al. (2020). Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med 52, jrm00063. [DOI] [PubMed] [Google Scholar]
- Al-Aly Z., Xie Y., and Bowe B. (2021). High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264. [DOI] [PubMed] [Google Scholar]
- Alter G., Yu J., Liu J., Chandrashekar A., Borducchi E.N., Tostanoski L.H., McMahan K., Jacob-Dolan C., Martinez D.R., Chang A., et al. (2021). Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony D.D., Milkovich K.A., Zhang W., Rodriguez B., Yonkers N.L., Tary-Lehmann M., and Lehmann P.V. (2012). Dissecting the T Cell Response: Proliferation Assays vs. Cytokine Signatures by ELISPOT. Cells 1, 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D.T., Milne A., Samms E., Stadon L., Maskell N.A., and Hamilton F.W. (2021). Symptoms After COVID-19 Vaccination in Patients With Persistent Symptoms After Acute Infection: A Case Series. Ann Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumjohann D., Preite S., Reboldi A., Ronchi F., Ansel K.M., Lanzavecchia A., and Sallusto F. (2013). Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 38, 596–605. [DOI] [PubMed] [Google Scholar]
- Bennasrallah C., Bannour R., Jlassi O., Kacem M., Fredj M.B., Abroug H., Zemni I., Garrach B., Bahri R., Charfeddine N., et al. (2020). Three COVID-19 cases with a long-term viral shedding period in Tunisia. Pan Afr Med J 35, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center J.C.R. (2021). Cumulative worldwide Covid-19 cases (Baltimore, MD: Johns Hopkins University of Medicine; ). [Google Scholar]
- Chakravarty E.F., Bush T.M., Manzi S., Clarke A.E., and Ward M.M. (2007). Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum 56, 2092–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., and Perlman S. (2017). Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 39, 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Zhao J., and Perlman S. (2014). T cell-mediated immune response to respiratory coronaviruses. Immunol Res 59, 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Hou J., Jiang X., Ma S., Meng M., Wang B., Zhang M., Zhang M., Tang X., Zhang F., et al. (2005). Response of memory CD8+ T cells to severe acute respiratory syndrome (SARS) coronavirus in recovered SARS patients and healthy individuals. J Immunol 175, 591–598. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L., Yang Y., Liu B., Wang W., Wei C., et al. (2020). The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol 92, 833–840. [DOI] [PubMed] [Google Scholar]
- Crowe S.R., Turner S.J., Miller S.C., Roberts A.D., Rappolo R.A., Doherty P.C., Ely K.H., and Woodland D.L. (2003). Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections. J Exp Med 198, 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao T.L., Hoang V.T., and Gautret P. (2021). Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: a narrative review. Eur J Clin Microbiol Infect Dis 40, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derhovanessian E., Maier A.B., Hahnel K., Beck R., de Craen A.J.M., Slagboom E.P., Westendorp R.G.J., and Pawelec G. (2011). Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol 92, 2746–2756. [DOI] [PubMed] [Google Scholar]
- Diehl S., Anguita J., Hoffmeyer A., Zapton T., Ihle J.N., Fikrig E., and Rincon M. (2000). Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity 13, 805–815. [DOI] [PubMed] [Google Scholar]
- Duan Y.Q., Xia M.H., Ren L., Zhang Y.F., Ao Q.L., Xu S.P., Kuang D., Liu Q., Yan B., Zhou Y.W., et al. (2020). Deficiency of Tfh Cells and Germinal Center in Deceased COVID-19 Patients. Curr Med Sci 40, 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber C., Soung A., Vollmer L.L., Kanmogne M., Last A., Brown J., and Klein R.S. (2019). T cells promote microglia-mediated synaptic elimination and cognitive dysfunction during recovery from neuropathogenic flaviviruses. Nat Neurosci 22, 1276–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good-Jacobson K.L., and Shlomchik M.J. (2010). Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol 185, 3117–3125. [DOI] [PubMed] [Google Scholar]
- Goubard A., Loiez C., Abe J., Fichel C., Herwegh S., Faveeuw C., Porte R., Cayet D., Sebbane F., Penet S., et al. (2015). Superantigenic Yersinia pseudotuberculosis induces the expression of granzymes and perforin by CD4+ T cells. Infect Immun 83, 2053–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham E.L., Clark J.R., Orban Z.S., Lim P.H., Szymanski A.L., Taylor C., DiBiase R.M., Jia D.T., Balabanov R., Ho S.U., et al. (2021). Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol 8, 1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. (2020). Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 181, 1489–1501 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., Brooks N., Smaja M., Mircus G., Pan K., et al. (2021). Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 397, 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., Walshaw C., Kemp S., Corrado J., Singh R., et al. (2021). Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol 93, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Hampshire A., Trender W., Chamberlain SR, Jolly AE, Grant JE, Patrick F, Mazibuko N, Williams S, Barnaby JM, Hellyer H, Mehta MA. (2021). Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., and Conde J.G. (2009). Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havervall S., Rosell A., Phillipson M., Mangsbo S.M., Nilsson P., Hober S., and Thalin C. (2021). Symptoms and Functional Impairment Assessed 8 Months After Mild COVID-19 Among Health Care Workers. JAMA 325, 2015–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heming M., Li X., Rauber S., Mausberg A.K., Borsch A.L., Hartlehnert M., Singhal A., Lu I.N., Fleischer M., Szepanowski F., et al. (2021). Neurological Manifestations of COVID-19 Feature T Cell Exhaustion and Dedifferentiated Monocytes in Cerebrospinal Fluid. Immunity 54, 164–175 e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrickson S.E., Perro M., Loughhead S.M., Senman B., Stutte S., Quigley M., Alexe G., Iannacone M., Flynn M.P., Omid S., et al. (2013). Antigen availability determines CD8(+) T cell-dendritic cell interaction kinetics and memory fate decisions. Immunity 39, 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins V., Sohaei D., Diamandis E.P., and Prassas I. (2020). COVID-19: from an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci, 1–23. [DOI] [PubMed] [Google Scholar]
- Hirschtick J.L., Titus A.R., Slocum E., Power L.E., Hirschtick R.E., Elliott M.R., McKane P., and Fleischer N.L. (2021). Population-based estimates of post-acute sequelae of SARS-CoV-2 infection (PASC) prevalence and characteristics. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantonen J., Mahzabin S., Mayranpaa M.I., Tynninen O., Paetau A., Andersson N., Sajantila A., Vapalahti O., Carpen O., Kekalainen E., et al. (2020). Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol 30, 1012–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.K.S., et al. (2020). COVID-19 cytokines and the hyperactive immune response: Synergism of TNF-alpha and IFN-gamma in triggering inflammation, tissue damage, and death. bioRxiv. [Google Scholar]
- Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.K.S., et al. (2021). Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 184, 149–168 e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp H.I., Corner E., and Colvin L.A. (2020). Chronic pain after COVID-19: implications for rehabilitation. Br J Anaesth 125, 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenstein M., Klingenstein S., Neckel P.H., Mack A.F., Wagner A.P., Kleger A., Liebau S., and Milazzo A. (2020). Evidence of SARS-CoV2 Entry Protein ACE2 in the Human Nose and Olfactory Bulb. Cells Tissues Organs 209, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladds E., Rushforth A., Wieringa S., Taylor S., Rayner C., Husain L., and Greenhalgh T. (2020). Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res 20, 1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J.S., Cella D., Choi S., Junghaenel D.U., Christodoulou C., Gershon R., and Stone A. (2011). How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch Phys Med Rehabil 92, S20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M.H., Wing Y.K., Yu M.W., Leung C.M., Ma R.C., Kong A.P., So W.Y., Fong S.Y., and Lam S.P. (2009). Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med 169, 2142–2147. [DOI] [PubMed] [Google Scholar]
- Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., Chng M.H.Y., Lin M., Tan N., Linster M., et al. (2020). SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584, 457–462. [DOI] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., and Hashikawa T. (2020). The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 92, 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C., Pierantozzi M., Spanetta M., Sarmati L., Cesta N., Iannetta M., Ora J., Mina G.G., Puxeddu E., Balbi O., et al. (2020). Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun 88, 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. (2020). Covid-19: What do we know about “long covid”? BMJ 370, m2815. [DOI] [PubMed] [Google Scholar]
- McLane L.M., Abdel-Hakeem M.S., and Wherry E.J. (2019). CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu Rev Immunol 37, 457–495. [DOI] [PubMed] [Google Scholar]
- McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., Liu J., Peter L., Atyeo C., Zhu A., et al. (2021). Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 590, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckiff B.J., Ramirez-Suastegui C., Fajardo V., Chee S.J., Kusnadi A., Simon H., Eschweiler S., Grifoni A., Pelosi E., Weiskopf D., et al. (2020). Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4(+) T Cells in COVID-19. Cell 183, 1340–1353 e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H. (2010). Depression and immunity: a role for T cells? Brain Behav Immun 24, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockus T.E., Ren H.M., Shwetank, and Lukacher A.E. (2019). To Go or Stay: The Development, Benefit, and Detriment of Tissue-Resident Memory CD8 T Cells during Central Nervous System Viral Infections. Viruses 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muecksch F., Wise H., Batchelor B., Squires M., Semple E., Richardson C., McGuire J., Clearly S., Furrie E., Greig N., et al. (2021). Longitudinal Serological Analysis and Neutralizing Antibody Levels in Coronavirus Disease 2019 Convalescent Patients. J Infect Dis 223, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.N., Gebhardt T., Carbone F.R., and Heath W.R. (2013). Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol 31, 137–161. [DOI] [PubMed] [Google Scholar]
- Myasoedova E., Crowson C.S., Kremers H.M., Therneau T.M., and Gabriel S.E. (2010). Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum 62, 1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S.F., and Ho Y.C. (2020). SARS-CoV-2: a storm is raging. J Clin Invest 130, 2202–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C., et al. (2020). Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol 21, 1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.S., Kristiansen M.F., Hanusson K.D., Danielsen M.E., B A.S., Gaini S., Strom M., and Weihe P. (2020). Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C., et al. (2020). Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 383, 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasa S., Nora-Krukle Z., Henning N., Eliassen E., Shikova E., Harrer T., Scheibenbogen C., Murovska M., Prusty B.K., and European Network on M.C. (2018). Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med 16, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda L.B., Netland J., Shehata L., Pruner K.B., Morawski P.A., Thouvenel C.D., Takehara K.K., Eggenberger J., Hemann E.A., Waterman H.R., et al. (2021). Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell 184, 169–183 e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M., Nozzi J.L., and Nason M.C. (2011). SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 79, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S.F., Ham B., Haichin M., Walters I.C., Tohyama S., Sotocinal S.G., and Mogil J.S. (2019). Increased pain sensitivity and decreased opioid analgesia in T-cell-deficient mice and implications for sex differences. Pain 160, 358–366. [DOI] [PubMed] [Google Scholar]
- Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., et al. (2020). COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586, 594–599. [DOI] [PubMed] [Google Scholar]
- Sant A.J., DiPiazza A.T., Nayak J.L., Rattan A., and Richards K.A. (2018). CD4 T cells in protection from influenza virus: Viral antigen specificity and functional potential. Immunol Rev 284, 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T., Perez-Potti A., Rivera-Ballesteros O., Stralin K., Gorin J.B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., et al. (2020). Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 183, 158–168 e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H., Blackburn S.D., Blattman J.N., and Wherry E.J. (2007). Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med 204, 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledzinska A., Vila de Mucha M., Bergerhoff K., Hotblack A., Demane D.F., Ghorani E., Akarca A.U., Marzolini M.A.V., Solomon I., Vargas F.A., et al. (2020). Regulatory T Cells Restrain Interleukin-2- and Blimp-1-Dependent Acquisition of Cytotoxic Function by CD4(+) T Cells. Immunity 52, 151–166 e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., Lu P., Weizman O.E., Liu F., Dai Y., et al. (2021). Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge R.E., Mapplebeck J.C., Rosen S., Beggs S., Taves S., Alexander J.K., Martin L.J., Austin J.S., Sotocinal S.G., Chen D., et al. (2015). Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 18, 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed A., Khan A., Gosai F., Asif A., and Dhillon S. (2020). Gastrointestinal pathophysiology of SARS-CoV2 - a literature review. J Community Hosp Intern Med Perspect 10, 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang E., Ekundayo O., Peipert J.D., Edwards N., Bansal A., Richardson C., Bartlett S.J., Howell D., Li M., Cella D., et al. (2019). Validation of the Patient-Reported Outcomes Measurement Information System (PROMIS)-57 and −29 item short forms among kidney transplant recipients. Qual Life Res 28, 815–827. [DOI] [PubMed] [Google Scholar]
- Tebo A.E., Fuller M.J., Gaddis D.E., Kojima K., Rehani K., and Zajac A.J. (2005). Rapid recruitment of virus-specific CD8 T cells restructures immunodominance during protective secondary responses. J Virol 79, 12703–12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Babor M., Lane J., Schulten V., Patil V.S., Seumois G., Rosales S.L., Fu Z., Picarda G., Burel J., et al. (2017). Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat Commun 8, 1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Babor M., Lane J., Seumois G., Liang S., Goonawardhana N.D.S., De Silva A.D., Phillips E.J., Mallal S.A., da Silva Antunes R., et al. (2019). Dengue-specific CD8+ T cell subsets display specialized transcriptomic and TCR profiles. J Clin Invest 129, 1727–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Rong L., Nian W., and He Y. (2020). Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther 51, 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toapanta F.R., Bernal P.J., Kotloff K.L., Levine M.M., and Sztein M.B. (2018). T cell mediated immunity induced by the live-attenuated Shigella flexneri 2a vaccine candidate CVD 1208S in humans. J Transl Med 16, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn A.S., Meijer B., Frampton C.M.A., Barclay M.L., and de Boer N.K.H. (2020). Systematic review with meta-analysis: SARS-CoV-2 stool testing and the potential for faecal-oral transmission. Aliment Pharmacol Ther 52, 1276–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elslande J., Oyaert M., Ailliet S., Van Ranst M., Lorent N., Vande Weygaerde Y., Andre E., Lagrou K., Vandendriessche S., and Vermeersch P. (2021). Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J Clin Virol 136, 104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehar S., Boushra M., Ntiamoah P., and Biehl M. (2021). Post-acute sequelae of SARS-CoV-2 infection: Caring for the ‘long-haulers’. Cleve Clin J Med 88, 267–272. [DOI] [PubMed] [Google Scholar]
- Wang T., Ji Y.L., Yang Y.Y., Xiong X.Y., Wang I.M., Sandford A.J., Liang Z.A., and He J.Q. (2015). Transcriptomic profiling of peripheral blood CD4(+) T-cells in asthmatics with and without depression. Gene 565, 282–287. [DOI] [PubMed] [Google Scholar]
- Weintraub S., Dikmen S.S., Heaton R.K., Tulsky D.S., Zelazo P.D., Bauer P.J., Carlozzi N.E., Slotkin J., Blitz D., Wallner-Allen K., et al. (2013). Cognition assessment using the NIH Toolbox. Neurology 80, S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D., Schmitz K.S., Raadsen M.P., Grifoni A., Okba N.M.A., Endeman H., van den Akker J.P.C., Molenkamp R., Koopmans M.P.G., van Gorp E.C.M., et al. (2020). Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry E.J. (2011). T cell exhaustion. Nat Immunol 12, 492–499. [DOI] [PubMed] [Google Scholar]
- Wherry E.J., Barber D.L., Kaech S.M., Blattman J.N., and Ahmed R. (2004). Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A 101, 16004–16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


