Abstract
Background
The urine lipoarabinomannan (LAM) antigen test is a tuberculosis (TB) diagnostic test with highest sensitivity in individuals with advanced human immunodeficiency virus (HIV). Its role in TB diagnostic algorithms for HIV-positive outpatients remains unclear.
Methods
The AIDS Clinical Trials Group (ACTG) A5274 trial demonstrated that empiric TB therapy did not improve 24-week survival compared to isoniazid preventive therapy (IPT) in TB screen–negative HIV-positive adults initiating antiretroviral therapy with CD4 counts <50 cells/µL. Retrospective LAM testing was performed on stored urine obtained at baseline. We determined the proportion of LAM-positive participants and conducted modified intent-to-treat analysis excluding LAM-positive participants to determine the effect on 24-week survival, TB incidence, and time to TB using Kaplan-Meier method.
Results
A5274 enrolled 850 participants; 53% were male and the median CD4 count was 18 (interquartile range, 9–32) cells/µL. Of the 850, 566 (67%) had LAM testing (283 per arm); 28 (5%) were positive (21 [7%] and 7 [2%] in the empiric and IPT arms, respectively). Of those LAM-positive, 1 participant in each arm died and 5 of 21 and 0 of 7 in empiric and IPT arms, respectively, developed TB. After excluding these 28 cases, there were 19 and 21 deaths in the empiric and IPT arms, respectively (P = .88). TB incidence remained higher (4.6% vs 2%, P = .04) and time to TB remained faster in the empiric arm (P = .04).
Conclusions
Among outpatients with advanced HIV who screened negative for TB by clinical symptoms, microscopy, and Xpert testing, LAM testing identified an additional 5% of individuals with TB. Positive LAM results did not change mortality or TB incidence.
Keywords: urine LAM, tuberculosis, advanced HIV disease, empiric TB therapy, isoniazid preventive therapy
The role of urine lipoarabinomannan antigen test in tuberculosis diagnostic algorithms for outpatients with advanced Human Immunodeficiency Virus (HIV) disease remains unclear. Urine lipoarabinomannan testing yielded more additional positive tuberculosis tests among tuberculosis -prescreened outpatients with advanced HIV.
(See the Editorial Commentary by Burke and Gupta Wright on pages e878–9.)
Tuberculosis (TB) is one of the top 10 leading causes of deaths worldwide and the leading cause of death among people living with human immunodeficiency virus (HIV). In 2018, the World Health Organization (WHO) estimated 10 million cases of TB and 1.5 million deaths with the largest burden in low- and middle-income countries (LMICs). In the same year, there were about 862 000 cases of TB among HIV-positive individuals and 251 000 HIV-associated TB deaths [1]. The global TB incidence is declining at about 2% per year. However, more effort is required to achieve the WHO End TB Strategy targets—to end TB as a global epidemic by reducing TB deaths by 95% and the number of new TB cases by 90% by 2035 [2].
Improving TB diagnosis and treatment is imperative to achieving global targets for ending the TB epidemic. Between 2000 and 2017, approximately 54 million deaths were averted through TB diagnosis and treatment [1]. However, diagnosis of TB remains a challenge in LMICs, especially in HIV-positive individuals [3]. Since 2010, TB diagnosis has expanded from relying largely on sputum smear microscopy that detects only about half of TB cases, to use of rapid molecular diagnostic tools such as GeneXpert (Xpert) MTB/RIF assay and Xpert Ultra assay, and biomarker-based test such as urine lipoarabinomannan (LAM) antigen test [1]. Xpert has been scaled up in many countries but its use and availability is still limited in LMICs, particularly in peripheral health facilities [3].
The Alere Determine TB LAM antigen lateral flow strip is a WHO-recommended rapid, point-of-care, lateral flow immunochromatographic test for qualitative detection of LAM antigen of mycobacteria in urine. It is recommended as an adjunct test to assist in TB diagnosis among HIV-positive adults, adolescents, and children with signs and symptoms of TB or with advanced HIV disease/seriously ill or those with a CD4 count <200 cells/µL irrespective of signs and symptoms [4]. Its sensitivity increases with lower CD4 counts in inpatient settings and in high-TB-prevalence settings, making it potentially useful in LMICs, where the HIV-TB coinfection burden is highest [3] and where a significant proportion of patients present late with severe immunosuppression [5]. In addition, urine LAM testing has potential mortality benefit in some subgroups of inpatients including those with CD4 count <100 cells/µL or low hemoglobin and those suspected to have TB [6]. However, its broader application to TB diagnostic algorithms for HIV-positive individuals in outpatient settings remains unclear.
We sought to determine (1) the diagnostic yield of urine LAM in advanced HIV-positive outpatient adults (CD4 count <50 cells/µL) who remained without a TB diagnosis after TB screening using routine diagnostic tools during evaluation for enrollment into a trial of empiric TB therapy; (2) the clinical outcomes in participants who were urine LAM positive at baseline; and (3) the effect of urine LAM testing on mortality, TB incidence, and time to probable/confirmed TB when LAM-positive participants were excluded.
METHODS
Study Design and Setting
Details of the AIDS Clinical Trials Group (ACTG) A5274 methods have been published elsewhere [7]. In brief, the A5274 study, also known as Reducing Early Mortality and Morbidity by Empiric TB Treatment (REMEMBER), was an international, open-label, randomized clinical trial. It demonstrated that a 4-drug empiric TB therapy regimen did not improve 24-week survival and was associated with an increased incidence of TB during 24 weeks of follow-up compared to isoniazid preventive therapy (IPT) in HIV-positive adults initiating efavirenz-based antiretroviral therapy (ART) with CD4 counts <50 cells/µL [7]. The study was conducted at 18 sites in 10 countries (Malawi, South Africa, Haiti, Kenya, Zambia, India, Brazil, Zimbabwe, Peru, and Uganda), with most participants from sub-Saharan Africa. To be included, sites had to have a TB incidence >100 per 100 000 person-years and national ART programs with documented high early mortality rates (>10–20 per 100 person-years) among outpatient populations. Noteworthy, the A5274 protocol was amended in February 2012 to include a requirement for urine sample collection and storage from each participant for testing using a new diagnostic assay, Alere’s Determine TB-LAM, at study entry. A sample was collected at the next study visit for the participants (11%) who enrolled before the amendment, and those participants were excluded from this analysis. Due to different approval timelines for the ethics boards for the study sites, there were differences in the number of participants who had urine LAM testing by site.
Study Population
The study population was HIV-positive adults who were ART naive, aged ≥18 years with pre-ART CD4 counts <50 cells/µL and no evidence of active TB. Potential participants were screened for TB prior to study entry using a symptom screen, physical examination, and locally available diagnostic tools. The TB symptom screen included cough ≥2 weeks, any current fever >38°C, hemoptysis, night sweats within the past 2 weeks, unintentional weight loss >10% in the past 30 days, or enlarged axillary or cervical lymph nodes. Locally available diagnostics included sputum staining for acid-fast bacilli, chest radiograph, and Xpert MTB/RIF implemented at screening in 5 sites only.
Study Procedures and Data Collection
Study procedures for the primary study A5274 have been published elsewhere [7]. Urine samples were stored for retrospective batch testing. The tests were positive if 2 readers agreed. A positive urine LAM test for this protocol was grade 1 or higher. Development of symptomatic TB disease was defined as having clinical symptoms of pulmonary or extrapulmonary tuberculosis with or without demonstrable Mycobacterium tuberculosis from any specimens [8].
Statistical Analysis
The A5274 primary endpoint was survival (death or unknown vital status) 24 weeks postrandomization. However, since there were very few participants with unknown vital status, we have concentrated on deaths only for this analysis. The proportion of participants who died and the incidence of confirmed or probable TB was compared between the arms using χ 2 test (or Fisher exact test, where appropriate). The Kaplan-Meier method was used to estimate mortality and TB incidence rates by week 24, and the rates were compared by the z test. Time to confirmed or probable TB was compared by the log-rank test. For the time-to-event analyses, modified intent-to-treat analyses were conducted by excluding participants who were retrospectively identified as TB positive at the time of study entry through urine LAM testing, and conducted among all participants with LAM testing performed at baseline including 3 participants with inconclusive results. All analyses were conducted in SAS version 9.4 software.
RESULTS
Baseline Characteristics of Urine LAM–Tested Participants
A total of 850 participants (424 in the empiric arm and 426 in the IPT arm) were enrolled to the A5274 study. Baseline characteristics were similar across arms (shown elsewhere) [9]. Of the 850 enrolled participants, 566 (67%) had urine samples collected at baseline that were tested for LAM antigen retrospectively (283 in each arm). LAM testing ranged from 20% in South Africa to 100% in Peru and Brazil. Of those who had urine LAM testing, the median age was 36 (interquartile range [IQR], 30–42) years, 311 (55%) were male, 491 (87%) were black, and the median viral load was 5.3 (IQR, 4.9–5.7) log10 copies/mL (Table 1). The median baseline CD4 count was higher among those who did not have urine LAM testing compared to those who had urine LAM testing (21 [IQR, 11–35] cells/μL vs 17 [IQR, 8–31] cells/µL; P = .002). The rest of the baseline characteristics were similar between the 2 populations (Table 1).
Table 1.
Baseline Characteristics for Participants by Urine Lipoarabinomannan Testing
| Characteristic | LAM Not Tested (n = 284) | LAM Tested (n = 566) | Total (N = 850) | P Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 139 (49) | 311 (55) | 450 (53) | .098a |
| Female | 145 (51) | 255 (45) | 400 (47) | |
| Race | ||||
| Black | 277 (98) | 491 (87) | 768 (90) | <.001a |
| Other | 7 (2) | 75 (13) | 82 (10) | |
| Age | ||||
| No. | 284 | 566 | 850 | .533b |
| Mean (SD) | 36.8 (8.2) | 36.4 (8.9) | 36.5 (8.7) | |
| Min, Max | 22, 65 | 18, 70 | 18, 70 | |
| Median (IQR) | 36 (31–41) | 36 (30–42) | 36 (30–42) | |
| <20 | 0 (0) | 5 (1) | 5 (1) | |
| 20–29 | 57 (20) | 125 (22) | 182 (21) | |
| 30–39 | 138 (49) | 250 (44) | 388 (46) | |
| 40–49 | 65 (23) | 144 (25) | 209 (25) | |
| 50–59 | 21 (7) | 34 (6) | 55 (6) | |
| 60–69 | 3 (1) | 7 (1) | 10 (1) | |
| ≥70 | 0 (0) | 1 (0) | 1 (0) | |
| CD4 count at baseline, cells/μL | ||||
| No. | 284 | 563 | 847 | .002b |
| Mean (SD) | 25.6 (20.6) | 21.6 (17.7) | 22.9 (18.8) | |
| Min, Max | 0, 154 | 0, 145 | 0, 154 | |
| Median (IQR) | 21 (11–35) | 17 (8–31) | … | |
| <25 | 162 (57) | 361 (64) | 523 (62) | |
| 25–49 | 100 (35) | 165 (29) | 265 (31) | |
| 50–100 | 18 (6) | 36 (6) | 54 (6) | |
| 101–200 | 4 (1) | 1 (0) | 5 (1) | |
| Invalid result/result not obtained | 0 (0) | 3 (1) | 3 (0) | |
| HIV-1 RNA at baseline, log10 copies/mL | ||||
| No. | 281 | 563 | 844 | .594b |
| Mean (SD) | 5.3 (0.7) | 5.3 (0.7) | 5.3 (0.7) | |
| Min, Max | 1.7, 7.0 | 1.7, 7.0 | 1.7, 7.0 | |
| Median (IQR) | 5.3 (4.9–5.7) | 5.3 (4.9–5.7) | 5.3 (4.9–5.7) | |
| <1000 | 2 (1) | 7 (1) | 9 (1) | |
| 1000–9999 | 5 (2) | 7 (1) | 12 (1) | |
| 10 000–99 999 | 80 (28) | 150 (27) | 230 (27) | |
| 100 000–499 999 | 121 (43) | 256 (45) | 377 (44) | |
| ≥500 000 | 73 (26) | 143 (25) | 216 (25) | |
| Result not obtained | 3 (1) | 3 (1) | 6 (1) | |
| Country | ||||
| South Africa | 140 (49) | 36 (6) | 176 (21) | <.001a |
| India | 6 (2) | 23 (4) | 29 (3) | |
| Zambia | 6 (2) | 30 (5) | 36 (4) | |
| Malawi | 12 (4) | 181 (32) | 193 (23) | |
| Kenya | 87 (31) | 65 (11) | 152 (18) | |
| Haiti | 27 (10) | 82 (14) | 109 (13) | |
| Peru | 0 (0) | 39 (7) | 39 (5) | |
| Zimbabwe | 1 (0) | 51 (9) | 52 (6) | |
| Brazil | 0 (0) | 15 (3) | 15 (2) | |
| Uganda | 5 (2) | 44 (8) | 49 (6) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: HIV-1, human immunodeficiency virus type 1; IQR, interquartile range; LAM, lipoarabinomannan; SD, standard deviation.
aχ 2 test.
bWilcoxon test.
cFisher exact test.
Clinical Outcomes of Participants Who Had a Positive Urine LAM Test
Of the 566 who had urine LAM testing, 28 (5%) were positive (21 [7%] and 7 [2%] in the empiric and IPT arms, respectively) (Table 2). Of the 28 participants with a positive test, most (23 [82%]) participants did not develop TB. Only 5 (18%) participants developed TB and they were all in the empiric arm, and 1 participant in each arm died from other TB-unrelated causes (Table 3). The 3 participants who had inconclusive urine LAM test results were excluded from Table 3, but they were included in the subsequent survival analysis.
Table 2.
Urine Lipoarabinomannan Results by Study Arm
| Strategy Arm | ||||||
|---|---|---|---|---|---|---|
| Urine LAM Result | Empiric (n =283) | IPT (n = 283) | Total (N = 566) | |||
| Negative | 260 | (92) | 275 | (97) | 535 | (94) |
| Positive | 21 | (7) | 7 | (2) | 28 | (5) |
| Inconclusive | 2 | (1) | 1 | (1) | 3 | (1) |
Data are presented as No (%).
Abbreviations: IPT, isoniazid preventive therapy; LAM, lipoarabinomannan.
Table 3.
Tuberculosis Diagnoses and Survival Status Within 24 Weeks by Study Arm and Urine Lipoarabinomannan Status
| Empiric (n = 281a) | IPT (n = 282a) | Total (N = 563a) | ||||
|---|---|---|---|---|---|---|
| Status | LAM Positive (n = 21) | LAM Negative (n = 260) | LAM Positive (n = 7) | LAM Negative (n = 275) | LAM Positive (n = 28) | LAM Negative (n = 535) |
| TB | 5 (24) | 10 (4) | 0 (0) | 5 (2) | 5 (18) | 15 (3) |
| No TB | 16 (76) | 250 (96) | 7 (100) | 270 (98) | 23 (82) | 520 (97) |
| Death | 1 (5) | 13 (5) | 1 (14) | 13 (5) | 2 (7) | 26 (5) |
| Alive | 20 (95) | 247 (95) | 6 (86) | 262 (95) | 26 (93) | 509 (95) |
Data are presented as No (%).
Abbreviations: IPT, isoniazid preventive therapy; LAM, lipoarabinomannan; TB, tuberculosis.
aDenotes number of participants with conclusive LAM test result.
Clinical Outcomes Excluding Participants Who Had a Positive Urine LAM Test
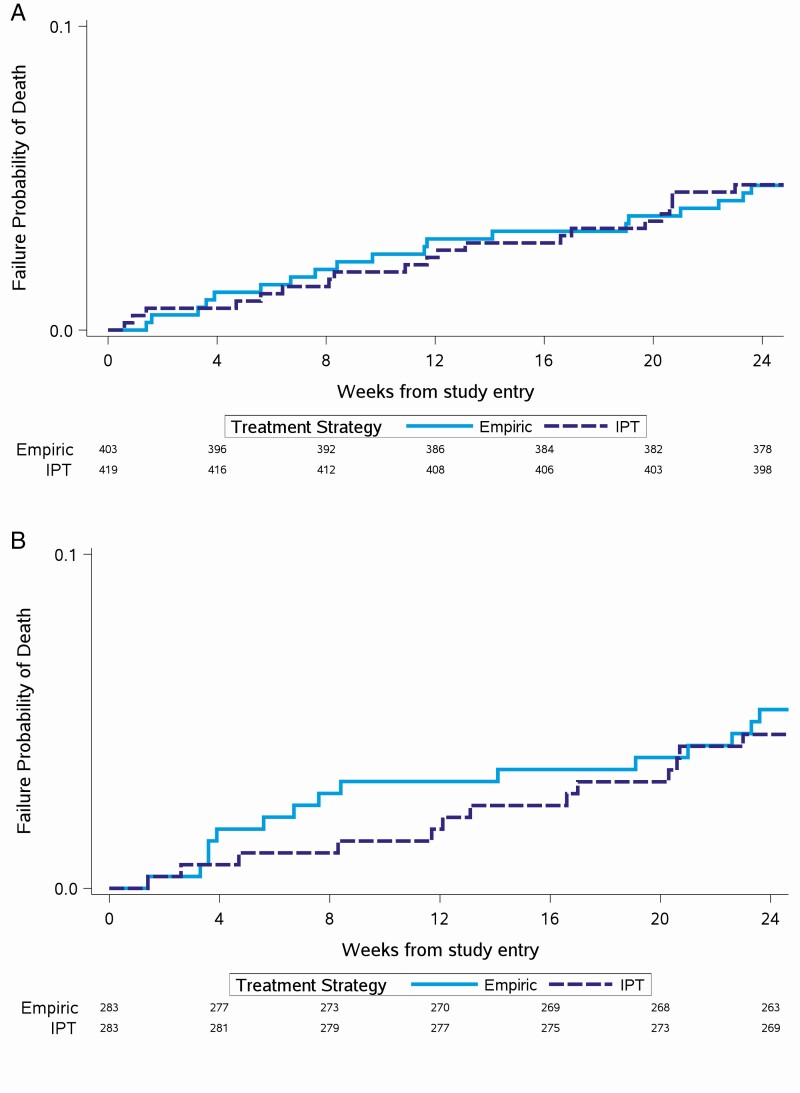
Overall, at week 24, there were 20 deaths in the empiric arm and 22 deaths in the IPT arm, resulting in a similar mortality rate of 4.8% (95% confidence interval [CI]: 3.1%–7.3%) for the empiric arm and 5.2% (95% CI: 3.4%–7.8%) for the IPT arm and resulting in an absolute risk difference of 0.4% (95% CI: −2.5% to 3.3%) (P = .78). After excluding the 28 LAM-positive participants from the analysis, there were 19 deaths in the empiric arm and 21 deaths in the IPT arm. Similarly, the mortality rate across arms was similar at 4.8% (95% CI: 3.1%–7.4%) for the empiric arm and 5% (95% CI: 3.3%–7.6%) for the IPT arm, resulting in an absolute risk difference of 0.3% (95% CI: −2.7% to 3.2%) (P = .86). There was no difference in the time to death across arms with all participants [9], participants after excluding LAM positives, and participants with LAM testing conducted (Figure 1A and 1B).
Figure 1.
A, Time to death, with urine lipoarabinomannan (LAM)–positive participants excluded. B, Time to death among participants with LAM testing done. Abbreviation: IPT, isoniazid preventive therapy.
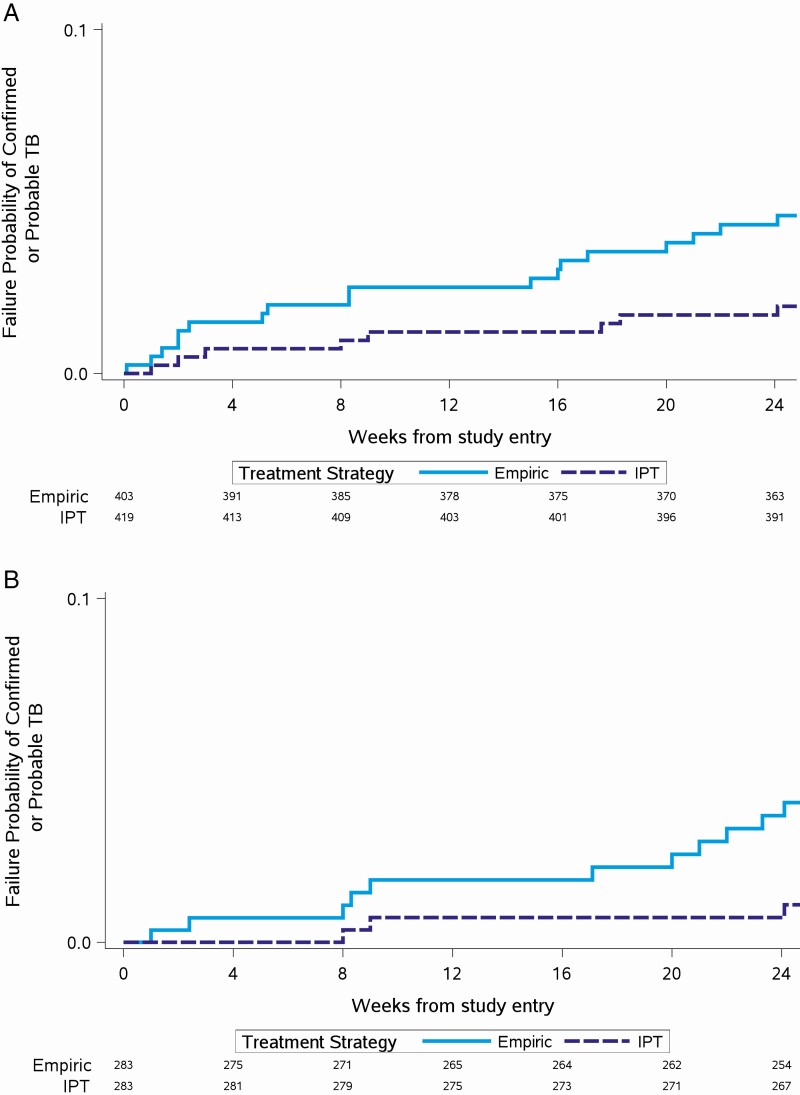
Overall, the incidence of confirmed or probable TB was higher in the empiric arm compared to the IPT arm (21 [5%] in the empiric arm vs 8 [2%] in the IPT arm; P = .01). The time to confirmed or probable TB was faster in the empiric arm compared to the IPT arm (P = .01) [9]. After excluding LAM-positive participants, the incidence of confirmed or probable TB remained higher (18 [4%] in the empiric arm vs 8 [2%] in the IPT arm; P = .045) and the time to TB remained faster in the empiric arm (P = .04). Among participants with LAM testing conducted, the incidence of confirmed or probable TB remained higher (11 [4%] in the empiric arm vs 3 [1%] in the IPT arm; P = .054) and the time to TB remained faster in the empiric arm (P = .03) (Figure 2A and 2B).
Figure 2.
A, Time to confirmed or probable tuberculosis (TB), with urine lipoarabinomannan (LAM)–positive participants excluded. B, Time to confirmed or probable TB among participants with LAM testing done. Abbreviations: IPT, isoniazid preventive therapy; TB, tuberculosis.
DISCUSSION
In this study, the addition of urine LAM testing yielded 5% additional positive TB tests among outpatients with advanced HIV who had previously been systematically screened for TB using clinical diagnosis, microscopy, and Xpert MTB/RIF testing. Exclusion of LAM-positive participants did not alter the lack of effect of empiric TB treatment on survival or the risk of developing symptomatic TB disease in the empiric arm. About one-fifth of LAM-positive participants developed symptomatic TB disease, suggesting that the 4-drug anti-TB treatment in the empiric arm did not optimally prevent participants from developing symptomatic TB disease. This is one of the few studies that evaluated the role of urine LAM for diagnosis of TB among HIV-positive adults with advanced HIV disease who were systematically prescreened for TB.
In a recent Cochrane systematic review of 15 unique studies in the LMICs, pooled sensitivity and specificity of the Alere Determine TB LAM Ag assay among unselected participants not assessed for signs and symptoms of TB were 35% (95% CI: 22%–50%) and 95% (95% CI: 89%–96%), respectively. LAM sensitivity was higher among inpatients, severely ill patients, and high-TB-prevalence countries and increased with decreasing CD4 count [10]. Consequently, the WHO recently revised its policy recommendation in late 2019 to include urine LAM as an “add-on” test to assist in TB diagnosis in a broader patient setting, particularly more inclusive of outpatients [4]. The current consensus is that urine LAM testing has the potential to increase diagnostic yield among HIV-positive individuals and provide an alternative diagnostic method for TB in people who cannot produce sputum, especially in resource-limited settings where standard diagnostics for TB are scarce [10–12].
However, there are important differences in the diagnostic yield of urine LAM testing depending on setting. Some researchers have reported very low diagnostic yield of urine LAM testing. In Uganda, sensitivity and additional yield of urine LAM relative to sputum Xpert MTB/RIF was 7.9% and 1%, respectively. However, the study had a broad enrollment criteria of both inpatient and outpatient adults, HIV positive and HIV negative, undergoing sputum-based TB screening, which may have influenced their findings [11]. Among HIV-positive outpatient adults irrespective of TB symptoms initiating ART in Mozambique, urine LAM sensitivity was 3.5%. The early stage of HIV disease (67% WHO HIV stage 1) and higher CD4 count (median, 278 cells/µL) of the study participants could explain the low urine LAM sensitivity [13].
In contrast, other researchers have reported higher urine LAM diagnostic yield. In Kenya, in a cohort of outpatients who were either severely ill or with CD4 <200 cells/µL or with body mass index <17 kg/m2 and with symptoms of pulmonary TB, urine LAM sensitivity was 58% and the incremental yield to an algorithm based on clinical signs and smear microscopy was 12%. In another adult outpatient population with TB symptoms and CD4 count <200 cells/µL in Malawi, urine LAM sensitivity was 24.9% and urine LAM testing doubled the yield of TB cases identified relative to sputum Xpert MTB/RIF alone [12]. Both studies enrolled populations with TB symptoms for whom yield for TB diagnostics is bound to be high.
Our finding of a 5% diagnostic yield is arguably high, particularly in an outpatient population of patients who, despite having low CD4 counts of <50 cells/µL, were systematically prescreened for TB using standard TB diagnostic tools including Xpert MTB/RIF. Among participants screened out of A5274, a third had TB [7]. We hypothesize that the diagnostic yield could have been higher if these participants received urine LAM testing. In addition, we conducted retrospective testing on stored urine samples. Although no study has directly compared the diagnostic yield of LAM testing on fresh urine vs frozen urine, the Cochrane review showed that urine LAM sensitivity was higher on fresh nonstored urine [10]. The frozen urine samples and retrospective testing may have reduced the diagnostic yield of LAM testing in our study.
Several studies have reported benefits of urine LAM testing on clinical outcomes including mortality. Peter et al found that urine LAM–guided initiation of TB therapy reduced absolute risk of mortality by 4% and relative risk by 17% [14]. In the Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa trial, urine LAM testing significantly reduced the risk of mortality in 3 prespecified clinical subgroups: patients with CD4 counts <100 cells/µL, severely anemic patients, and those with clinically suspected TB [15]. Huerga et al reported that urine LAM grade was a marker for patients at higher risk of death [12] and a marker for severe disease and TB dissemination. Despite the growing evidence on mortality benefits of LAM testing, the uptake and implementation of LAM testing remains low in high-TB/HIV-burden countries—only 21% had implemented LAM testing by end 2019 [16]. In our study, regardless of the striking imbalance across arms in positive pre-ART urine LAM tests (21 cases in the empiric arm vs 7 cases in the IPT arm), exclusion of urine LAM-positive participants had no impact on the risk of developing symptomatic TB disease and mortality. Similar outcomes were seen in the Systematic Empirical vs. Test-guided Anti-TB Treatment Impact in Severely Immunosuppressed HIV-infected Adults Initiating ART With CD4 Cell Counts <100/mm3 [17] and TB Fast Track [18] studies.
Among the LAM-positive participants, 18% developed symptomatic TB disease—all in the empiric arm only (5 cases in the empiric arm and 0 cases in the IPT arm). This finding implies that the 4-drug anti-TB treatment in the empiric arm did not optimally prevent participants from developing symptomatic TB disease. Although self-reported drug adherence was similar between arms in A5274, there were more premature drug discontinuations in the empiric arm (47 empiric vs 18 IPT discontinuations) [7], which may have resulted in more symptomatic TB disease. In addition, despite the similar grade 3 or 4 adverse events between arms [7], the premature discontinuations may have been due to more grade 1 or 2 adverse events in the empiric arm compared to the IPT arm, which were not part of the A5274 primary analysis. Last, as the study was unblinded, diagnostic suspicion bias between the arms may have differed, resulting in more aggressive use of TB diagnostics or more liberal TB diagnosis in the empiric arm.
Our study enrolled HIV-positive outpatients who had screened negative for TB using standard TB diagnostic tools who started either empiric TB treatment or IPT plus ART. Despite the low CD4 count, in such a setting, TB cases are expected to be low, potentially limiting the generalizability of our results. In addition, the lack of an ART-only arm in the A5274 study may have masked the role of urine LAM testing in patients with subclinical disease who may have later developed active TB. Last, our results should be interpreted with caution as it was not a real randomized comparison, since we used a subset of the A5274 study participants and did not compare urine LAM results with microbiologically confirmed tests due to the lack of a perfect reference standard, particularly for patients with extrapulmonary TB and paucibacillary disease [19].
In our study, addition of LAM testing yielded more additional positive TB tests to clinical diagnosis, and microscopy and Xpert MTB/RIF testing among outpatients with advanced HIV who were systematically prescreened for TB; however, LAM testing was a poor marker for risk of development of symptomatic TB disease or mortality. Ultimately, our results support the current consensus that urine LAM testing has the potential to increase diagnostic yield among HIV-positive outpatients and provide an alternative diagnostic method for TB in people who cannot produce sputum, especially in resource-limited settings. Implementation and scale-up of existing LAM tests and development of next-generation assays such as the FujiLAM assay and the Foundation for Innovative New Diagnostics should be prioritized.
Notes
Acknowledgments. The authors acknowledge Jorge Alave, who was an investigator for the Reducing Early Mortality and Morbidity by Empiric TB Treatment (REMEMBER) study at the Asociacion Civil Impacta Salud y Educacion, Lima, Peru. The authors acknowledge Jing Bao for contributing toward statistical analysis for the REMEMBER study; the study teams and participants from all sites that were involved in the A5274 REMEMBER Study; and the Adult AIDS Clinical Trials Group and the Statistical and Data Management Center.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) (award numbers UM1AI068634, UM1AI068636, and UM1AI106701). M. M. M. has received support from the Fogarty Global Health Fellowship Program at NIH (award number D43 TW009340). A. G. has received support from the NIAID/NIH (grant numbers UM1AI069465 and RO1AI080417) and the Wyncote Foundation, Gilead Foundation, and Ujala Foundation. Y. C. M. has received support from the National Institute of Biomedical Imaging and Bioengineering/NIH (grant number U54EB007958); the Fogarty International Center/NIH (grant numbers D43TW009771, D43TW009340, and D43TW010132); and NIAID/NIH (grant number UM1AI068613).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.World Health Organization. Tuberculosis.2019. Available at: https://www.who.int/news-room/fact-sheets/detail/tuberculosis. Accessed 3 October 2019.
- 2.World Health Organization. WHO End TB Strategy. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 3.Huerga H, Ferlazzo G, Bevilacqua P, et al. Incremental yield of including Determine-TB LAM assay in diagnostic algorithms for hospitalized and ambulatory HIV-positive patients in Kenya. PLoS One 2017; 12:e0170976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis of active tuberculosis in people living with HIV. Policy update 2019. Geneva, Switzerland: WHO, 2019.
- 5.Tenforde MW, Walker AS, Gibb DM, Manabe YC. Rapid antiretroviral therapy initiation in low- and middle-income countries: a resource-based approach. PLoS Med 2019; 16:e1002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta-Wright A, Fielding K, Wilson D, et al. Tuberculosis in hospitalized patients with HIV: clinical characteristics, mortality, and implications from the STAMP Trial. Clin Infect Dis 2019; 71:2618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosseinipour MC, Bisson GP, Miyahara S, et al. ; Adult AIDS Clinical Trials Group A5274 (REMEMBER) Study Team . Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): a multicountry open-label randomised controlled trial. Lancet 2016; 387:1198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Division of AIDS. Appendix 60 version 1.4, July 2012. Maryland: National Institute of Allergy and Infectious Diseases, National Institute of Health, 2012:1–121.
- 9.Hosseinipour MC, Bisson GP, Miyahara S, et al. Empiric tuberculosis therapy versus isoniazid in advanced HIV-infected adult outpatients initiating antiretroviral therapy: a multi-country randomized controlled trial. Lancet 2016; 387:1198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjerrum S, Schiller I, Dendukuri N, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev 2019; 10:CD011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andama A, Jaganath D, Crowder R, et al. Accuracy and incremental yield of urine Xpert MTB/RIF Ultra versus Determine TB-LAM for diagnosis of pulmonary tuberculosis. Diagn Microbiol Infect Dis 2020; 96:114892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huerga H, Rucker SCM, Bastard M, et al. Should urine-LAM tests be used in TB symptomatic HIV-positive patients when no CD4 count is available? A prospective observational cohort study from Malawi. J Acquir Immune Defic Syndr 2020; 83:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floridia M, Ciccacci F, Andreotti M, et al. Tuberculosis case finding with combined rapid point-of-care assays (Xpert MTB/RIF and Determine TB LAM) in HIV-positive individuals starting antiretroviral therapy in Mozambique. Clin Infect Dis 2017; 65:1878–83. [DOI] [PubMed] [Google Scholar]
- 14.Peter JG, Theron G, Muchinga TE, Govender U, Dheda K. The diagnostic accuracy of urine-based Xpert MTB/RIF in HIV-infected hospitalized patients who are smear-negative or sputum scarce. PLoS One 2012; 7:e39966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta-Wright A, Fielding KL, Oosterhout JJ, et al. Rapid urine-based screening for tuberculosis to reduce AIDS-related mortality in hospitalized patients in Africa (the STAMP trial): study protocol for a randomised controlled trial. BMC Infect Dis 2016; 16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singhroy DN, MacLean E, Kohli M, et al. Adoption and uptake of the lateral flow urine LAM test in countries with high tuberculosis and HIV/AIDS burden: current landscape and barriers. Gates Open Res 2020; 4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanc FX, Badje AD, Bonnet M, et al. ; STATIS ANRS 12290 Trial Team . Systematic or test-guided treatment for tuberculosis in HIV-infected adults. N Engl J Med 2020; 382:2397–410. [DOI] [PubMed] [Google Scholar]
- 18.Grant AD, Charalambous S, Tlali M, et al. Algorithm-guided empirical tuberculosis treatment for people with advanced HIV (TB Fast Track): an open-label, cluster-randomised trial. Lancet HIV 2019; 3018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulterys MA, Wagner B, Redard-Jacot M, et al. Point-of-care urine LAM tests for tuberculosis diagnosis: a status update. J Clin Med 2019; 9:111. [DOI] [PMC free article] [PubMed] [Google Scholar]