Abstract
Background
The growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis modulates critical metabolic pathways; however, little is known regarding effects of augmenting pulsatile GH secretion on immune function in humans. This study used proteomics and gene set enrichment analysis to assess effects of a GH releasing hormone (GHRH) analog, tesamorelin, on circulating immune markers and liver tissue in people with human immunodeficiency virus (HIV) (PWH) and nonalcoholic fatty liver disease (NAFLD).
Methods
92 biomarkers associated with immunity, chemotaxis, and metabolism were measured in plasma samples from 61 PWH with NAFLD who participated in a double-blind, randomized trial of tesamorelin versus placebo for 12 months. Gene set enrichment analysis was performed on serial liver biopsies targeted to immune pathways.
Results
Tesamorelin, compared to placebo, decreased interconnected proteins related to cytotoxic T-cell and monocyte activation. Circulating concentrations of 13 proteins were significantly decreased, and no proteins increased, by tesamorelin. These included 4 chemokines (CCL3, CCL4, CCL13 [MCP4], IL8 [CXCL8]), 2 cytokines (IL-10 and CSF-1), and 4 T-cell associated molecules (CD8A, CRTAM, GZMA, ADGRG1), as well as ARG1, Gal-9, and HGF. Network analysis indicated close interaction among the gene pathways responsible for these proteins, with imputational analyses suggesting down-regulation of a closely related cluster of immune pathways. Targeted transcriptomics using liver tissue confirmed a significant end-organ signal of down-regulated immune activation pathways.
Conclusions
Long-term treatment with a GHRH analog reduced markers of T-cell and monocyte/macrophage activity, suggesting that augmentation of the GH axis may ameliorate immune activation in an HIV population with metabolic dysregulation, systemic and end organ inflammation.
Clinical Trials Registration. NCT02196831.
Keywords: HIV-infection, nonalcoholic fatty liver disease, growth hormone, growth hormone releasing hormone, immune activation
Treatment with a growth hormone releasing hormone analog in people with human immunodeficiency virus and nonalcoholic fatty liver disease decreased circulating proteins associated with immune activation pathways. Gene set enrichment analysis of RNA-seq in liver confirmed down-regulation of end-organ immune activation pathways.
People living with human immunodeficiency virus (HIV) (PWH) demonstrate increased immune activation in association with metabolic comorbidities including nonalcoholic fatty liver disease (NAFLD) [1]. Tesamorelin, a growth hormone releasing hormone (GHRH) agonist, was recently shown to improve liver fat and clinical indices of inflammation and to prevent progression of liver fibrosis in PWH with NAFLD [2]. A key unanswered question for PWH, as well as for other populations with inflammation and ectopic adipose tissue, is whether there are unique immunological effects of augmenting pulsatile GH release that may reduce systemic immune activation and ameliorate clinical disease.
The GH/insulin-like growth factor-1 (IGF-1) axis participates in immune regulation and inflammatory response. GH receptors are present on T-cells, B-cells, natural killer (NK) cells, monocytes, and neutrophils, and many immune cells synthesize GH that may act in an autocrine or paracrine fashion [3, 4]. GH signals through the Janus kinase family-signal transducers and activators of transcription (JAK-STAT) pathway common to many immune and inflammatory pathways [5]. IGF-1 receptors are present on monocytes, NK cells, T-cells, and B-cells [6]. Both GH and IGF-1 are known to increase thymic mass and function [4, 7, 8]. Mice with knockout of GH releasing hormone (GHRH-/-), causing severe GH and IGF-1 deficiency, have splenic atrophy, relative B-cell lymphopenia, and deficient response to Streptococcus pneumoniae vaccine as well as S. pneumoniae infection [9, 10].
The role of GH/IGF-1 in immune regulation in humans is complex. Adults with pituitary GH deficiency have increased markers of systemic inflammation, including increased C-reactive protein (CRP), tumor necrosis factor alpha (TNFα), and interleukin-6 (IL6), and these levels decrease with GH treatment [11, 12]. Further, studies of both children and adults have demonstrated strong inverse associations between GH secretory capacity and systemic markers of inflammation, including CRP [13–17]. Clinical studies of treatment with GHRH agonist have suggested benefit to reduce certain markers of systemic inflammation, [18], but more detailed studies using proteomic analyses and liver tissue have not been performed.
The purpose of the current study was to comprehensively assess the immunological effects of augmenting pulsatile GH secretion among a population with HIV and NAFLD, leveraging data from a 12-month randomized trial of tesamorelin. Using proteomic analyses of plasma along with RNA-seq of liver tissue to confirm end-organ changes in immune pathways, we demonstrate that, compared to placebo, long-term treatment with tesamorelin down-regulates pathways of cytotoxic T-cell and monocyte activation. Further, we demonstrate a strong parallel signal of tesamorelin to reduce immune activation in the liver, using a targeted transcriptomic approach.
METHODS
This analysis utilizes a 12-month, double-blind, randomized, placebo-controlled trial of tesamorelin in men and women with HIV-infection and NAFLD [2]. Sixty-one individuals (31 randomized to tesamorelin and 30 to placebo) participated, and 47 individuals (21 tesamorelin and 26 placebo) completed [2]. The study was performed at the NIH Clinical Center and the Massachusetts General Hospital (MGH).
Eligibility criteria included age 18–70 years; HIV-infection; liver fat fraction of ≥5% on proton magnetic resonance spectroscopy (MRS); no excessive alcohol use; no cirrhosis, hepatitis B, active hepatitis C, or other known liver disease; HbA1c ≤ 7%; no use of insulin or thiazolidinediones; stable use of antiretroviral regimen; CD4+ count ≥ 100 cells/mm3; and HIV viral load ≤ 400 copies/mL [2]. All participants provided written informed consent, and the study was approved by the institutional review boards at the MGH and NIH.
Intervention and Study Procedures
Participants were randomized in a 1:1 ratio to self-administer tesamorelin 2 mg subcutaneously daily versus identical placebo for 12 months. Study procedures were previously described in detail [2]. The screening visit included fasting labs and MRS for quantification of hepatic fat fraction (HFF); the baseline and 12-month visits included additional fasting blood sampling and ultrasound-guided percutaneous liver biopsy. HFF was assessed using 1H-MRS performed in the morning, fasting [2]. Histopathological analysis of liver biopsies was conducted by a single, blinded pathologist (D.E.K.), who also scored samples based on NAFLD activity score (NAS) [19].
Proteomics Analysis
Proteomics was conducted from fasting plasma (EDTA) samples by Olink (Watertown, MA), using high-multiplex immunoassays to investigate curated panels of biomarkers. This analysis presents results from the Immuno-Oncology panel of protein biomarkers selected for involvement in tumor immunity, chemotaxis, vascular and tissue remodeling, apoptosis, metabolism, and autophagy. Data are reported in Normalized Protein Expression units, in Log2 scale, with higher values indicating greater concentrations of protein. Information regarding the panel, including assay performance characteristics for each protein, as well as the assay technique is available at https://www.olink.com/resources-support/document-download-center/.
Hepatic Gene Set Enrichment Analysis
To investigate whether the changes seen in plasma were reflected in gene expression in an available tissue, the liver, targeted Gene Set Enrichment Analysis (GSEA) was performed using the Blood Transcriptional Modules (BTM) established by Li and colleagues [20] as signatures of immune system response to vaccination. A tissue core obtained at liver biopsy was placed in RNAlater (Qiagen) and frozen (-80°C) for RNA sequencing performed by the Broad Institute (Cambridge, MA), using standard methodologies [21]. GSEA was performed using the desktop module from the Broad (www.broadinstitute.org/gsea/) using the-BTM [20]. Gene sets with a false discovery rate (FDR-q) < 0.05 were considered enriched. We have previously reported on tesamorelin effects in this study on different gene sets focusing on metabolism [21], without comprehensively assessing immunological pathways in the liver. Mean leading edge gene expression levels were used for correlation analyses with plasma proteins.
Statistical Analysis
Changes in circulating proteins in tesamorelin versus placebo were assessed with random intercept mixed effects modeling for continuous repeat measures utilizing all available data, employing restricted maximum likelihood to assess the effect estimate for the time × randomization interaction. Two data points, a baseline alanine aminotransferase value and a baseline C-reactive protein value, both of which were more than 5 standard deviations above the sample mean, were excluded as outliers [2]. Baseline between-group comparisons were performed using Student’s t-test. We determined between-group statistical difference in changes in protein expression levels over time using the T-statistic. We used a Benjamini-Hochberg method-based FDR corrected q value of < 0.1 to indicate significance consistent with the exploratory nature of the work and with the approach used in similar proteomic analyses [22–24]. All protein changes with q values highlighted by this approach (i.e., q < 0.1) also achieved nominal significance (unadjusted P-value < .05). Within group changes over 12 months were also assessed using paired t-testing. Correlations were performed using Pearson correlation. All statistical analyses were two-sided and were performed using SAS 9.4 or JMP 15 (both SAS Institute, Cary, NC).
RESULTS
Baseline clinical characteristics have been previously reported [2] and are shown in Supplementary Table 1. The cohort was 79% male with an average age of 53 ± 7 years. Tesamorelin and placebo groups were similar at baseline with regard to demographic characteristics, measures of HIV immunologic control, and measures of NAFLD. All subjects had HIV viral load < 400 copies/mL, and 90% had undetectable viral load (<20 copies/mL) at study entry. HIV viral load at 12 months was also <400 copies/mL for all subjects. As previously reported, tesamorelin significantly reduced HFF (absolute effect size -4.1% [95% CI -7.6, -0.7]; relative effect size -37% [-67, -7]), visceral adipose tissue (VAT) area (effect size -35cm2 [-66, -4]), and C-reactive protein (effect size -4.7mg/L [-9.2, -0.2]) [2]. As shown in Supplementary Table 1, approximately one-third of the cohort had histological NASH at baseline. NAS score was reduced most over time among those with highest baseline NAS score in the tesamorelin group (r = -0.48, P = .04), though the overall change in NAS score between groups did not reach statistical significance over 12 months [2].
Changes in Plasma Proteins
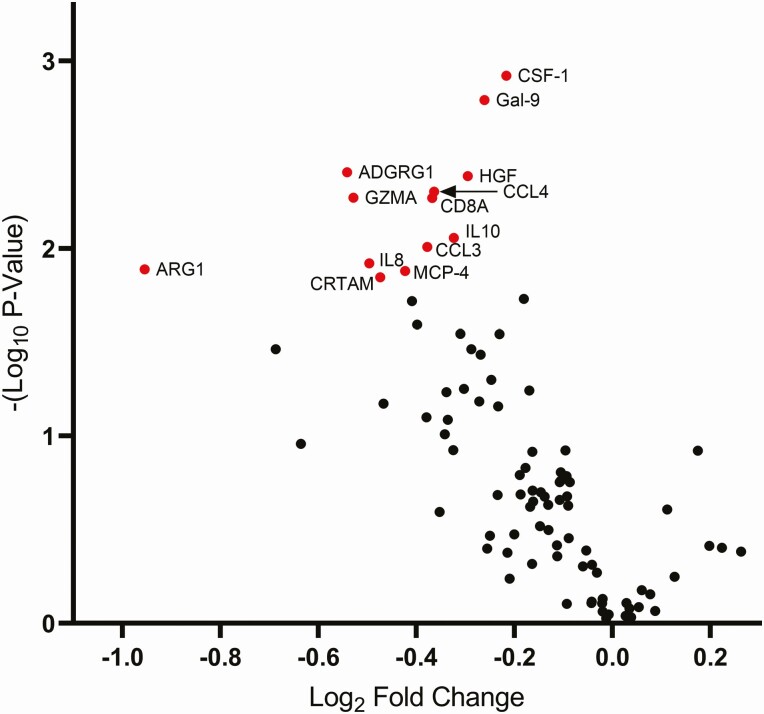
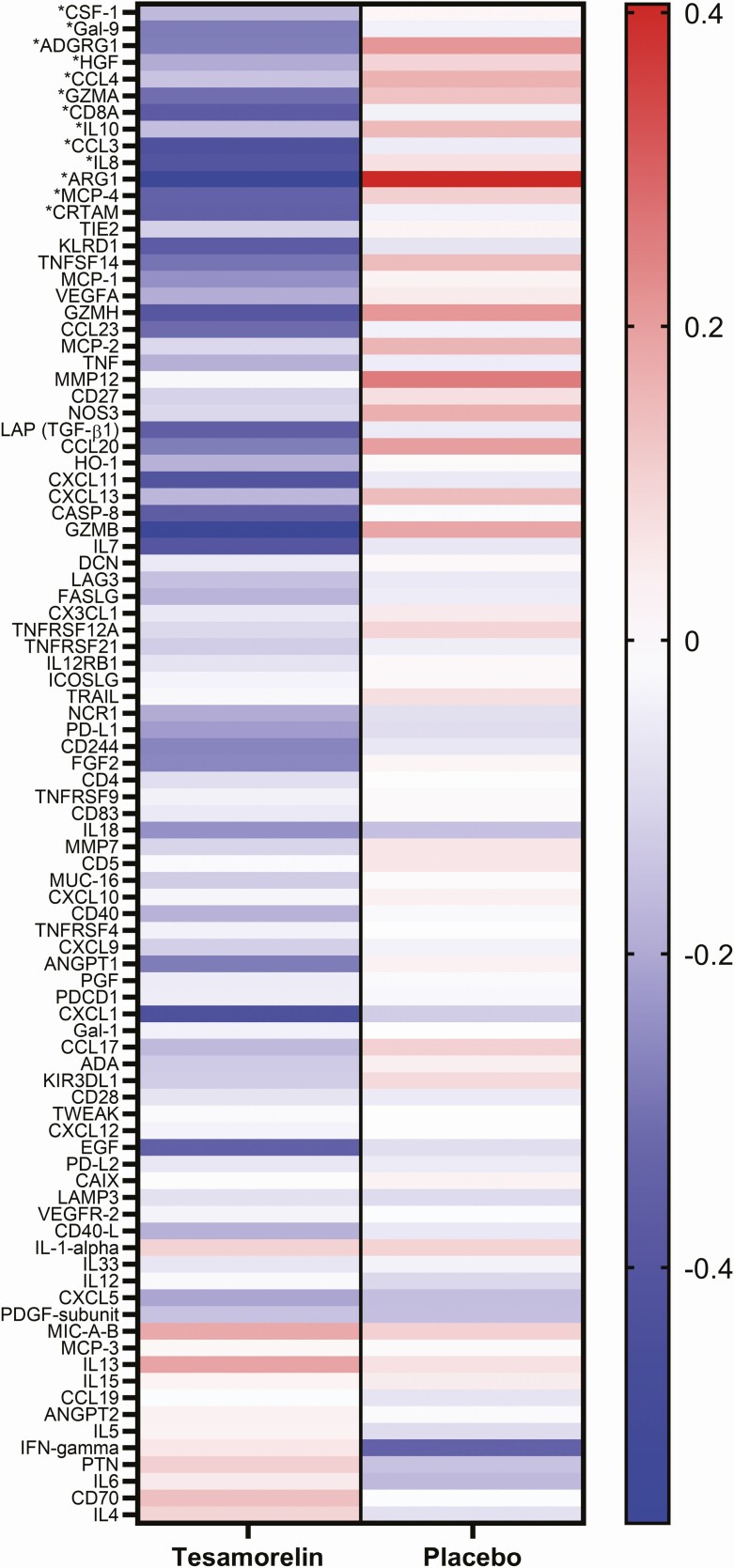
Proteomics analysis of the 92-protein immuno-oncology set revealed significant changes in 13 proteins (Table 1, Figure 1). These proteins include 4 chemokines (C-C motif chemokine ligand 3 [CCL3], also known as macrophage inflammatory protein 1-alpha; C-C motif chemokine ligand 4 [CCL4], also known as macrophage inflammatory protein 1-beta; C-C motif chemokine ligand 13 [CCL13], also known as monocyte chemoattractant protein 4; C-X-C motif chemokine ligand 8 [CXCL8], also known as interleukin-8); 2 cytokines (interleukin-10 [IL-10]; colony stimulating factor 1 [CSF-1], also known as macrophage colony stimulating factor); and 4 T-cell associated molecules (CD8a, cytotoxic and regulatory T-cell molecule [CRTAM], granzyme A [GZMA], and adhesion G protein-coupled receptor G1 (ADGRG1, also known as G protein-coupled receptor 56 [GPR56]). Additional down-regulated proteins were arginase 1 (ARG1), galectin 9 (Gal-9, also known as lectin, galactoside-binding, soluble 9 [LGALS9]), and hepatocyte growth factor (HGF). Changes for all proteins in the Immuno-Oncology panel, along with effect size and FDR q-values for the treatment effect of tesamorelin versus placebo, are shown in Supplementary Table 2. Intensity of within group changes in the tesamorelin and placebo groups are displayed in Figure 2 in a heatmap indicating the strength and contrasting directionality of the within-group changes over 12 months.
Table 1.
Plasma Proteins Significantly Down-Regulated by Tesamorelin Relative to Placebo
| Protein | Estimate (Log2 fold change) | T-value | Unadjusted P-value | FDR q-value | Classification and Function in Immune Response |
|---|---|---|---|---|---|
| C-C motif chemokine 3 (CCL3) | -0.38 | -2.71 | .01 | 0.099 | Chemokine; secreted by immune cells including CD8+ T-cells, binds to C-C motif chemokine receptors 1, 4, and 5 to induce immune response [37, 41]. |
| C-C motif chemokine 4 (CCL4) | -0.36 | -2.97 | .005 | 0.07 | Chemokine; secreted most highly by CD8+ T-cells, binds to C-C motif chemokine receptor 5 to induce immune response [37, 41]. |
| C-C motif chemokine 13 (MCP-4) | -0.42 | -2.59 | .01 | 0.0999 | Chemokine; secreted by monocytes to attract additional monocytes, lymphocytes, basophils, and eosinophils [37, 41]. |
| Interleukin-8 (IL8, CXCL8) | -0.50 | -2.63 | .01 | 0.0999 | Chemokine; secreted most highly by neutrophils to attract additional neutrophils, basophils, and T-cells. Involved in neutrophil activation [37, 41]. |
| Interleukin-10 (IL10) | -0.32 | -2.75 | .009 | 0.099 | Cytokine; secreted primarily by monocytes and, to a lesser degree, T-cells. Has anti-inflammatory effect to limit damage caused by inflammation [37, 41]. |
| Macrophage colony-stimulating factor 1 (CSF-1) | -0.22 | -3.48 | .001 | 0.07 | Cytokine, and growth factor; secreted by multiple cell types and plays fundamental role in proliferation and differentiation of monocytes and macrophages [37, 41]. |
| T-cell surface glycoprotein CD8 alpha chain (CD8A) | -0.37 | -2.94 | .005 | 0.07 | T-cell associated; membrane glycoprotein on cytotoxic T-cells [37, 41]. |
| Cytotoxic and regulatory T-cell molecule (CRTAM) | -0.47 | -2.56 | .01 | 0.0999 | T-cell associated; transmembrane receptor expressed on NK cells and CD8+ T-cells upon activation [42] |
| Granzyme A (GZMA) | -0.53 | -2.94 | .005 | 0.07 | T-cell associated; protease in the cytosolic granules of CD8+ T-cells and NK cells [37, 41]. |
| Adhesion G-protein coupled receptor G1 (ADGRG1, GPR56) | -0.54 | -3.05 | .004 | 0.07 | T-cell associated, and G protein-coupled receptor; expressed by cytotoxic NK and T-cells with significant upregulation during peak immune response to stimulus [43]. |
| Arginase-1 (ARG1) | -0.95 | -2.60 | .01 | 0.0999 | Enzyme; expressed by neutrophils, monocytes, and macrophages in response to inflammatory stimuli and is thought to exert anti-inflammatory effect [37, 41, 44]. |
| Galectin-9 (Gal-9, LGALS9) | -0.26 | -3.37 | .002 | 0.07 | Lectin; produced by myeloid, lymphoid, and endothelial cells and fibroblasts in response to inflammatory stimuli; plays an important immunoregulatory role with largely anti-inflammatory actions [45–47]. |
| Hepatocyte growth factor (HGF) | -0.30 | -3.04 | .004 | 0.07 | Growth factor; stimulates proliferation of hepatocytes, also widely expressed outside of liver, upregulated in response to inflammatory stimuli; exerts anti-inflammatory function [37, 41, 48], |
Abbreviations: FDR, false discovery rate; NK, natural killer.
Figure 1.
Between group changes in immuno-oncology proteins with tesamorelin treatment. Volcano plot showing the Log2 Fold Change (x-axis) and -(Log10 P-value) (y-axis) for each protein in the immuno-oncology panel. Proteins shown in red and labeled had False Discovery Rate < 0.1. Abbreviations: ADGRG1, adhesion G-protein coupled receptor G1; ARG1, Arginase-1; CCL3, C-C motif chemokine 3; CCL4, C-C motif chemokine 4; CD8A, T-cell surface glycoprotein CD8 alpha chain; CRTAM, cytotoxic and regulatory T-cell molecule; CSF-1, Macrophage colony-stimulating factor 1; Gal-9, Galectin-9; GZMA, Granzyme A; HGF, Hepatocyte growth factor; IL8, Interleukin-8; IL10, Interleukin-10; MCP-4, C-C motif chemokine 13.
Figure 2.
Heatmap comparing within-group changes in immuno-oncology proteins. Heatmap indicating the strength and directionality of the within-group log2 fold changes over 12 months in the tesamorelin (left) and placebo (right) treatment groups. Proteins are ordered from top to bottom according to t-statistic for the between-group comparison, with the lowest t-statistic at the top. Blue indicates decrease and red increase. *Denotes proteins with statistically significant changes over time (FDR-q < 0.1) between tesamorelin and placebo groups. Abbreviations: ADGRG1, adhesion G-protein coupled receptor G1; ARG1, Arginase-1; CCL3, C-C motif chemokine 3; CCL4, C-C motif chemokine 4; CD8A, T-cell surface glycoprotein CD8 alpha chain; CRTAM, cytotoxic and regulatory T-cell molecule; CSF-1, Macrophage colony-stimulating factor 1; FDR, false discovery rate; Gal-9, Galectin-9; GZMA, Granzyme A; HGF, Hepatocyte growth factor; IL8, Interleukin-8; IL10, Interleukin-10; MCP-4, C-C motif chemokine 13.
Association Between Changes in Plasma Proteins and Changes in Alanine Aminotransferase, VAT, and IGF-I
Associations between the reductions in plasma proteins and clinical changes in serum alanine aminotransferase (ALT), VAT area and IGF-1 among the cohort are shown in Table 2. Reductions in many of the plasma proteins were strongly associated with reductions in serum ALT, a marker of liver injury as well as changes in IGF-1, a marker of GH activity. Reductions in a limited number of proteins, CRTAM, ARG1, and Gal-9, were significantly associated with reductions in VAT.
Table 2.
Reductions in Plasma Proteins Are Associated with Reductions in ALT and VAT and Increases in IGF-1
| Change in ALT | Change in VAT | Change in IGF-1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| r | β* | P | r | β* | P | r | β* | P | |
| Δ CCL3 | 0.58 | 0.22 | <.0001 | 0.31 | 0.02 | .05 | -0.30 | -0.02 | .05 |
| Δ CCL4 | 0.31 | 0.10 | .046 | 0.25 | 0.02 | .11 | -0.36 | -0.02 | .02 |
| Δ MCP-4 | 0.40 | 0.18 | .008 | 0.06 | 0.005 | .71 | -0.21 | -0.01 | .17 |
| Δ CXCL8 (IL8) | 0.39 | 0.20 | .01 | 0.00 | 0.0001 | .99 | -0.24 | -0.02 | .12 |
| Δ IL10 | 0.42 | 0.14 | .005 | -0.02 | -0.001 | .92 | -0.41 | -0.02 | .006 |
| Δ CSF-1 | 0.56 | 0.09 | <.0001 | 0.10 | 0.003 | .53 | -0.38 | -0.01 | .01 |
| Δ CD8A | 0.50 | 0.17 | .0007 | 0.19 | 0.01 | .23 | -0.31 | -0.02 | .04 |
| Δ CRTAM | 0.26 | 0.12 | .09 | 0.39 | 0.04 | .01 | -0.46 | -0.03 | .002 |
| Δ GZMA | -0.01 | -0.004 | .96 | 0.16 | 0.02 | .31 | -0.09 | -0.007 | .56 |
| Δ ADGRG1 | 0.57 | 0.26 | <.0001 | 0.18 | 0.02 | .24 | -0.42 | -0.03 | .005 |
| Δ ARG1 | 0.21 | 0.22 | .17 | 0.52 | 0.11 | .0004 | -0.14 | -0.02 | .35 |
| Δ Gal-9 | 0.53 | 0.11 | .0003 | 0.39 | 0.02 | .01 | -0.30 | -0.01 | .05 |
| Δ HGF | 0.51 | 0.14 | .0005 | 0.26 | 0.01 | .09 | -0.25 | -0.01 | .10 |
r- and P-values for Pearson correlation of changes over 12 months, and β-estimate (n = 43 individuals for whom baseline and 12-month data were available).
Abbreviations: ADGRG1, adhesion G-protein coupled receptor G1; ALT, alanine aminotransferase; ARG1, arginase-1; CCL3, C-C motif chemokine 3; CCL4, C-C motif chemokine 4; CD8A, T-cell surface glycoprotein CD8 alpha chain; CRTAM, cytotoxic and regulatory T-cell molecule; CSF-1, macrophage colony-stimulating factor 1; CXCL8, interleukin-8; Gal-9, galectin-9; GZMA, granzyme A; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor 1; IL10, interleukin 10; MCP-4, C-C motif chemokine 13, also known as monocyte chemoattractant protein-4; VAT, visceral adipose tissue.
* β-estimate describes the Log2 fold change in plasma protein for each 10 U/L change in ALT, each 10 cm2 change in VAT, or each 10 ng/mL change in IGF-1.
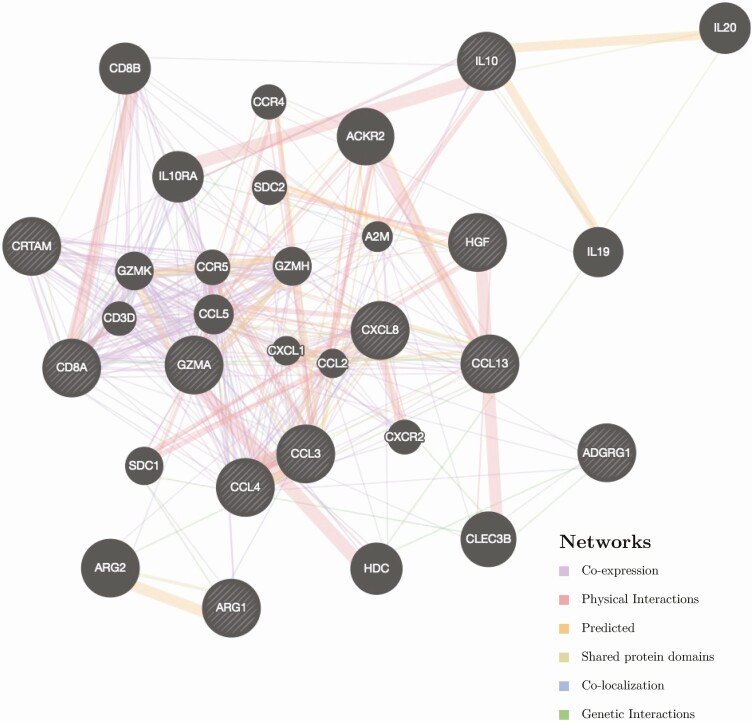
Network Analysis of Altered Proteins
Network analysis of the circulating proteins decreased by tesamorelin at an FDR-q < 0.1 revealed that many of the altered proteins were part of an interrelated network related to inflammation and immune function (Figure 3). Two additional proteins in the network were decreased by tesamorelin but did not achieve an FDR-q of <0.1: granzyme H, expressed in natural killer cells (GZMH, effect size -0.69 Log2 fold change, unadjusted P-value 0.03, FDR-q 0.16), and C-C motif chemokine ligand 2, a chemokine that regulates monocyte/macrophage infiltration (CCL2, also known as monocyte chemoattractant protein 1 [MCP-1], effect size -0.31 Log2 fold change, unadjusted P-value 0.03, FDR-q 0.14). The additional proteins in the network include chemokines (C-C motif chemokine ligand 5 [CCL5] and C-X-C motif chemokine ligand 1 [CXCL1]), chemokine receptors (C-C motif chemokine receptors 4 and 5 [CCR4 and CCR5]), cytokines (interleukin 19 and 20 [IL19 and IL20]), cytokine receptors (interleukin 10 receptor subunit A (IL10RA) and C-X-C motif chemokine receptor 2 [CXCR2]), T-cell associated proteins (CD8B, CD3D, and granzyme K [GZMK]), and monocyte associated proteins (syndecan 2 [SDC2]).
Figure 3.
Network analysis. Network analysis using GeneMANIA analytical software showing the potential relationships of proteins altered by tesamorelin with false discovery rate < 0.1 (denoted by light diagonal lines against black background), along with other proteins in the network (denoted by solid black fill). The line color connecting each of the proteins represents the different types of relationship between each respective protein, as shown in the legend in the bottom right of the figure. Line thickness represents relative strength of relationships. Abbreviations: ADGRG1, adhesion G-protein coupled receptor G1; ARG1, Arginase-1; CCL3, C-C motif chemokine 3; CCL4, C-C motif chemokine 4; CD8A, T-cell surface glycoprotein CD8 alpha chain; CRTAM, cytotoxic and regulatory T-cell molecule; CSF-1, Macrophage colony-stimulating factor 1; Gal-9, Galectin-9; GZMA, Granzyme A; HGF, Hepatocyte growth factor; IL8, Interleukin-8; IL10, Interleukin-10; MCP-4, C-C motif chemokine 13.
Changes in Hepatic Gene Expression
We utilized liver tissue to assess end-organ effects of tesamorelin, with the BTM gene sets chosen as reflective of immunologic and inflammatory changes [20]. GSEA using the BTM gene sets demonstrated 11 immunologic pathways that were significantly down-regulated by tesamorelin vs. placebo, as shown in Table 3, in which the Enrichment Score reflects the effect size of tesamorelin, in Log2 fold-change units, in each pathway [25]. These included pathways related to antigen presentation, complement activation, and inflammatory signaling. Relationships between changes in each plasma protein over 12 months and changes in gene set expression levels are shown in Supplementary Table 3. In these data, reductions in circulating plasma CCL3, MCP-4, CXCL8, CRTAM, ADGRG1, and HGF are associated with downregulation of hepatic expression of multiple BTM gene sets. Relationships between changes in gene set expression levels in the tesamorelin group and changes in serum ALT, HFF, and VAT over 12 months are shown in Supplementary Table 4. Changes in ALT and VAT are significantly associated with changes in some gene sets, whereas change in HFF is not associated with changes in the gene sets affected by tesamorelin.
Table 3.
Immune-Related Changes in Hepatic Gene Transcription
| Gene Set | Enrichment Score | FDR q-value |
|---|---|---|
| Regulation of antigen presentation and immune response (M5.0) | -0.63 | 0.003 |
| Enriched in monocytes (II) (M11.0) | -0.56 | 0.003 |
| Myeloid cell enriched receptors and transporters (M4.3) | -0.72 | 0.005 |
| Enriched in antigen presentation (II) (M95.0) | -0.78 | 0.005 |
| TLR and inflammatory signaling (M16) | -0.65 | 0.01 |
| Complement activation (I) (M112.0) | -0.78 | 0.01 |
| Monocyte surface signature (S4) | -0.57 | 0.01 |
| MHC-TLR7-TLR8 cluster (M146) | -0.77 | 0.01 |
| T cell activation and signaling (M5.1) | -0.69 | 0.03 |
| Enriched in NK cells (I) (M7.2) | -0.58 | 0.03 |
| Resting dendritic cell surface signature (S10) | -0.54 | 0.03 |
GSEA using Blood Transcription Module (BTM) gene sets [20]. Notations in parentheses (e.g., “(M5.0)”) show the BTM ID for each gene set. More information for each set is available at https://github.com/shuzhao-li/BTM [20].
FDR-q-value was calculated based on all queried gene sets (e.g., all BTM sets); significant changes in only those sets related to inflammation or immune cell function are shown. A negative enrichment score indicates downregulation of the gene set in tesamorelin treated patients compared to placebo treated patients.
Abbreviations: FDR, false discovery rate; GSEA, Gene Set Enrichment Analysis; NK, natural killer.
DISCUSSION
Our data demonstrate that augmenting pulsatile endogenous GH secretion with tesamorelin significantly reduces circulating proteins associated with chemotaxis, inflammatory signaling, and activation of cytotoxic T-cells. Network analysis demonstrates that many of the proteins reduced by tesamorelin participate in a larger network of chemokine and cytokine signaling and T-cell activation. Further, GSEA, using established gene sets assessing immune transcription pathways on liver tissue confirmed an end-organ signal consistent with the changes seen in plasma, with reductions in multiple pathways associated with antigen presentation, complement activation, T-cell activation, and inflammatory signaling, as well as a clinically relevant signal strongly relating to reduction in ALT. Together, these data demonstrate a meaningful effect of long-term treatment with a GHRH agonist to reduce immune activation and systemic inflammation in individuals with HIV-infection and NAFLD.
The literature supports multiple possible mechanisms through which tesamorelin may reduce circulating inflammatory cytokines and chemokines. GH has been shown to induce suppressor of cytokine signaling 3 (SOCS3) in pro-B cells [26]. Further, promonocytic cells, monocytes, and macrophages that are pre-treated with GH secrete decreased amounts of TNFα in response to lipopolysaccharide challenge in association with a decrease in nuclear translocation of nuclear factor kappa-light-chain-enhancer of activated B cells [NF-κB] [27]. Literature also supports a direct effect of GH signaling to regulate macrophage activation in multiple tissues. In a macrophage-specific GH receptor-null (MacGHRKO) mouse, adipose tissue macrophage abundance was increased and skewed toward a pro-inflammatory (M1) phenotype, crown-like structures in adipose tissue were increased, and adipose tissue expression of pro-inflammatory cytokines (TNFα, IL1β, IL6, and NF-κB) was increased [28]. More recently, in a murine model of colitis, Soler Palacios and colleagues reported that GH treatment of murine macrophages significantly upregulated genes associated with the anti-inflammatory M2 phenotype and down-regulated expression of pro-inflammatory gene signature [29]. Recent models of macrophage activation suggest that CSF-1 is a central transcriptional regulator of macrophage activation [30]. Therefore, reductions in CSF-1 with tesamorelin may indicate anti-inflammatory effects, consistent with novel data showing a strong relationship of reduced CSF-1 to reduced ALT in the current study. Less direct evidence exists for an effect of GH or IGF-1 to decrease cytotoxic T-cells, although human studies of growth hormone deficiency [31, 32] and HIV-infection [8, 33–36] demonstrate that rhGH increases the CD4+-to-CD8+ ratio and/or reduces cytotoxic CD8+ T-cells. In a study administering supraphysiological doses of nonpulsatile rhGH to adults with HIV-infection, Napolitano and colleagues report significant increases in CD4+ T-cells with rhGH therapy, along with a significant reduction in T-cells with activation markers [36]. The authors note that, although the mechanism by which rhGH reduces activated T-cells is unclear, it may be due to combined effects of GH signaling through STAT5 and IGF-1 signaling to promote regulatory T-cells [36].
In contrast, we use a different approach, augmenting physiological pulsatile GH secretion. We previously reported neutral effects on CD4 and CD8, though significant effects to reduce CRP were observed using this approach [2]. Leveraging this study, we now demonstrate a more intense and consistent signal when performing deeper phenotyping assessing multiple novel markers, not previously investigated or routinely used to characterize clinical responses, paired with liver tissue analyses. In this regard, imputational network analysis suggested additional proteins that may be anticipated to change with tesamorelin and merit further investigation, such as interleukin 19, expressed by monocytes and known to activate of STAT3 [37], and C-C motif chemokine receptor 5 [CCR5], expressed by T-cells and macrophages and known to be a critical co-receptor for viruses including HIV [37]. Though our data show effects of GHRH agonist to down-regulate immune activation, further research will be required to determine the precise mechanisms by which these effects occur.
One consideration is that tesamorelin increases both circulating IGF-1 and pulsatile circulating GH [38], and literature suggests both may have physiologically relevant effects [4]. It is pertinent that animal studies show that continuous GH exposure results in 10%–20% of the maximal STAT5b signaling induced by a GH pulse [39], such that pulsatile GH may have a different magnitude of effect than apulsatile GH such as that achieved by rhGH administration. Moreover, GH receptor liver knockout animal models show profound increases in inflammatory pathways, which are not ameliorated by IGF-1, suggesting a direct effect of GH signaling on inflammation, at least with respect to the liver [40].
A second consideration in interpreting our data is that tesamorelin reduces visceral fat and hepatic triglyceride, and these changes may mediate effects of tesamorelin on circulating markers of immune activation and inflammation independent of GH or IGF-1 signaling. Individuals with HIV-infection have chronic systemic immune activation in the context of increased visceral adiposity and relative reductions in endogenous GH. These features also characterize adult obesity, such that our findings are likely relevant beyond the context of PWH with NAFLD.
A third related consideration is that multiple tissues contribute to the circulating immune and inflammatory markers, and GH is known to affect multiple tissues, such as bone marrow, adipose tissue, and endothelial cells, that may have contributed to the changes seen in systemic circulation. With access to liver tissue to perform RNA-seq, we show a consistent pattern of down-regulation of inflammatory and immune activation pathways in the liver in association with circulating protein levels. Changes in hepatic expression of BTM gene sets were often associated with reductions in serum ALT, and some were associated with changes in VAT, whereas none were associated with changes in HFF. We hypothesize that the circulating markers likely reflect effects of tesamorelin to improve liver inflammation and gene expression, and may also relate to direct effects of augmented GH on circulating peripheral blood mononuclear cells. Further study will be important to elucidate the mechanisms and inter-dependencies of these changes.
In summary, we demonstrate that long-term treatment over 12 months with a GHRH analog among individuals with NAFLD reduces markers of T-cell and monocyte/macrophage activity in the circulation with a strong parallel effect on immune activation pathways in the liver. These data suggest that augmentation of the GH axis may reduce immune activation, systemic and end organ inflammation, and ectopic fat accumulation in an HIV population with metabolic dysregulation. Novel data from this study significantly expand our knowledge of the biological effects of a strategy to reduce ectopic fat, with strong immunomodulatory and anti-inflammatory effects in HIV. Further study is required to better define the effects of tesamorelin on specific immune cell populations as well as specific tissues.
Supplementary Material
Notes
Acknowledgments. The authors thank the participants of the study for their willingness to volunteer their time and effort and their dedication to research.
Financial support. This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (U01 AI115711). This research was also supported in part by the Intramural Research Program of the NIH, National Cancer Institute and the Nutrition Obesity Research Center at Harvard (P30 DK040561), and by NIH Grant Numbers R01 DK108370 (R. T. C.), R01 DK114144 (T. L. S. and K. E. C.), 1UL1TR002541-01 and 1UL1TR001102. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Theratechnologies provided study material under a material transfer agreement and had no role in the drafting of the manuscript.
Potential conflicts of interest. T. L. S. has received funding from Novo Nordisk for an investigator-initiated grant unrelated to the current project and has served as a consultant for Theratechnologies, Inc. L. T. F. has served as a consultant for Theratechnologies, Inc. J. M. B. has been awarded research funding from Boehringer Ingelheim to support bioinformatics analysis and software development in an unrelated project. K. C. has received grant funding from BMS and Boehringer Ingelheim as well as fees for consulting from Gilead, BMS and Novo Nordisk, unrelated to the current project. R. C. has received funding to the institution from Gilead, Merck, BMS, Janssen, Boehringer, Abbvie, and Roche unrelated to the current project. M. T. has nothing to disclose. D. E. K. is an employee of NIH. C. M. H. is an employee of NIH. S. K. G. has served as a consultant (personal) and currently serves as a consultant through an Institutional Consulting Agreement with Theratechnologies. The Mass General Hospital has a royalty and license agreement with Theratechnologies for Tesamorelin. S. K. G. is the inventor on a patent for GHRH or Analogues For Use Treatment of Hepatic Disease.
All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Maurice JB, Garvey L, Tsochatzis EA, et al. Monocyte-macrophage activation is associated with nonalcoholic fatty liver disease and liver fibrosis in HIV monoinfection independently of the gut microbiome and bacterial translocation. AIDS 2019; 33:805–14. [DOI] [PubMed] [Google Scholar]
- 2.Stanley TL, Fourman LT, Feldpausch MN, et al. Effects of tesamorelin on non-alcoholic fatty liver disease in HIV: a randomised, double-blind, multicentre trial. Lancet HIV 2019; 6:e821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab 2001; 86:4284–91. [DOI] [PubMed] [Google Scholar]
- 4.Hattori N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm IGF Res 2009; 19:187–97. [DOI] [PubMed] [Google Scholar]
- 5.Dehkhoda F, Lee CMM, Medina J, Brooks AJ. The growth hormone receptor: mechanism of receptor activation, cell signaling, and physiological aspects. Front Endocrinol (Lausanne) 2018; 9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodart G, Farhat K, Charlet-Renard C, Salvatori R, Geenen V, Martens H. The somatotrope growth hormone-releasing hormone/growth hormone/insulin-like growth factor-1 axis in immunoregulation and immunosenescence. Front Horm Res 2017; 48:147–59. [DOI] [PubMed] [Google Scholar]
- 7.Redelman D, Welniak LA, Taub D, Murphy WJ. Neuroendocrine hormones such as growth hormone and prolactin are integral members of the immunological cytokine network. Cell Immunol 2008; 252:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napolitano LA, Schmidt D, Gotway MB, et al. Growth hormone enhances thymic function in HIV-1-infected adults. J Clin Invest 2008; 118:1085–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodart G, Farhat K, Renard-Charlet C, et al. The severe deficiency of the somatotrope GH-releasing hormone/growth hormone/insulin-like growth factor 1 axis of Ghrh-/- mice is associated with an important splenic atrophy and relative B lymphopenia. Front Endocrinol (Lausanne) 2018; 9:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farhat K, Bodart G, Charlet-Renard C, et al. Growth hormone (GH) deficient mice with GHRH gene ablation are severely deficient in vaccine and immune responses against Streptococcus pneumoniae. Front Immunol 2018; 9:2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colao A, Di Somma C, Rota F, et al. Short-term effects of growth hormone (GH) treatment or deprivation on cardiovascular risk parameters and intima-media thickness at carotid arteries in patients with severe GH deficiency. J Clin Endocrinol Metab 2005; 90:2056–62. [DOI] [PubMed] [Google Scholar]
- 12.Serri O, St-Jacques P, Sartippour M, Renier G. Alterations of monocyte function in patients with growth hormone (GH) deficiency: effect of substitutive GH therapy. J Clin Endocrinol Metab 1999; 84:58–63. [DOI] [PubMed] [Google Scholar]
- 13.Russell M, Bredella M, Tsai P, et al. Relative growth hormone deficiency and cortisol excess are associated with increased cardiovascular risk markers in obese adolescent girls. J Clin Endocrinol Metab 2009; 94:2864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misra M, Bredella MA, Tsai P, Mendes N, Miller KK, Klibanski A. Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am J Physiol Endocrinol Metab 2008; 295:E385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makimura H, Stanley T, Mun D, et al. Reduced growth hormone secretion is associated with increased carotid intima-media thickness in obesity. J Clin Endocrinol Metab 2009; 94:5131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Utz AL, Yamamoto A, Hemphill L, Miller KK. Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab 2008; 93:2507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bredella MA, Torriani M, Thomas BJ, et al. Peak growth hormone-releasing hormone-arginine-stimulated growth hormone is inversely associated with intramyocellular and intrahepatic lipid content in premenopausal women with obesity. J Clin Endocrinol Metab 2009; 94:3995–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makimura H, Feldpausch MN, Rope AM, et al. Metabolic effects of a growth hormone-releasing factor in obese subjects with reduced growth hormone secretion: a randomized controlled trial. J Clin Endocrinol Metab 2012; 97:4769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41:1313–21. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Rouphael N, Duraisingham S, et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol 2014; 15:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fourman LT, Billingsley JM, Agyapong G, et al. Effects of tesamorelin on hepatic transcriptomic signatures in HIV-associated NAFLD. JCI insight 2020; 5:e140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Statistical Society, Series B (Methodological) 1995; 57:289–300. [Google Scholar]
- 23.Wang X, Shojaie A, Zhang Y, et al. Exploratory plasma proteomic analysis in a randomized crossover trial of aspirin among healthy men and women. PLoS One 2017; 12:e0178444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Radabaugh T, Lu Z, et al. Exploration of early-life candidate biomarkers for childhood asthma using antibody arrays. Pediatr Allergy Immunol 2016; 27:696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005; 102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barclay JL, Anderson ST, Waters MJ, Curlewis JD. Regulation of suppressor of cytokine signaling 3 (SOC3) by growth hormone in pro-B cells. Mol Endocrinol 2007; 21:2503–15. [DOI] [PubMed] [Google Scholar]
- 27.Haeffner A, Thieblemont N, Déas O, et al. Inhibitory effect of growth hormone on TNF-alpha secretion and nuclear factor-kappaB translocation in lipopolysaccharide-stimulated human monocytes. J Immunol 1997; 158:1310–4. [PubMed] [Google Scholar]
- 28.Lu C, Kumar PA, Sun J, et al. Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity. J Biol Chem 2013; 288:15725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soler Palacios B, Nieto C, Fajardo P, et al. Growth hormone reprograms macrophages toward an anti-inflammatory and reparative profile in an MAFB-dependent manner. J Immunol 2020; 205:776–88. [DOI] [PubMed] [Google Scholar]
- 30.Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014; 40:274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caballero-Villarraso J, Aguado R, Canete MD, Roldan L, Canete R, Santamaria M. Hormone replacement therapy in children with growth hormone deficiency: impact on immune profile. Arch Physiol Biochem 2019:1–5. doi: 10.1080/13813455.2019.1628070 [DOI] [PubMed] [Google Scholar]
- 32.Lebl J, Sediva A, Snajderova M, Pruhova S, Rakosnikova V. Immune system in adults with childhood-onset growth hormone deficiency: effect of growth hormone therapy. Endocr Regul 2000; 34:169–73. [PubMed] [Google Scholar]
- 33.Herasimtschuk AA, Hansen BR, Langkilde A, Moyle GJ, Andersen O, Imami N. Low-dose growth hormone for 40 weeks induces HIV-1-specific T cell responses in patients on effective combination anti-retroviral therapy. Clin Exp Immunol 2013; 173:444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plana M, Garcia F, Darwich L, et al. ; Red de Investigación en Sida (RIS) . The reconstitution of the thymus in immunosuppressed individuals restores CD4-specific cellular and humoral immune responses. Immunology 2011; 133:318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen BR, Kolte L, Haugaard SB, et al. Improved thymic index, density and output in HIV-infected patients following low-dose growth hormone therapy: a placebo controlled study. AIDS 2009; 23:2123–31. [DOI] [PubMed] [Google Scholar]
- 36.Napolitano LA, Lo JC, Gotway MB, et al. Increased thymic mass and circulating naive CD4 T cells in HIV-1-infected adults treated with growth hormone. AIDS 2002; 16:1103–11. [DOI] [PubMed] [Google Scholar]
- 37.The Human Protein Atlas. Available at: http://www.proteinatlas.org. Accessed September 4, 2020.
- 38.Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK. Effects of a growth hormone-releasing hormone analog on endogenous GH pulsatility and insulin sensitivity in healthy men. J Clin Endocrinol Metab 2011; 96:150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gebert CA, Park SH, Waxman DJ. Down-regulation of liver JAK2-STAT5b signaling by the female plasma pattern of continuous growth hormone stimulation. Mol Endocrinol 1999; 13:213–27. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Cordoba-Chacon J, Kineman RD, et al. Growth hormone control of hepatic lipid metabolism. Diabetes 2016; 65:3598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science 2015; 347:1260419. [DOI] [PubMed] [Google Scholar]
- 42.Boles KS, Barchet W, Diacovo T, Cella M, Colonna M. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood 2005; 106:779–86. [DOI] [PubMed] [Google Scholar]
- 43.Peng YM, van de Garde MD, Cheng KF, et al. Specific expression of GPR56 by human cytotoxic lymphocytes. J Leukoc Biol 2011; 90:735–40. [DOI] [PubMed] [Google Scholar]
- 44.Thomas AC, Mattila JT. “Of mice and men”: arginine metabolism in macrophages. Front Immunol 2014; 5:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imaizumi T, Kumagai M, Sasaki N, et al. Interferon-gamma stimulates the expression of galectin-9 in cultured human endothelial cells. J Leukoc Biol 2002; 72:486–91. [PubMed] [Google Scholar]
- 46.Asakura H, Kashio Y, Nakamura K, et al. Selective eosinophil adhesion to fibroblast via IFN-gamma-induced galectin-9. J Immunol 2002; 169:5912–8. [DOI] [PubMed] [Google Scholar]
- 47.Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 2005; 6:1245–52. [DOI] [PubMed] [Google Scholar]
- 48.Molnarfi N, Benkhoucha M, Funakoshi H, Nakamura T, Lalive PH. Hepatocyte growth factor: A regulator of inflammation and autoimmunity. Autoimmun Rev 2015; 14:293–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.