Abstract
Background
Limitations in the sensitivity and accessibility of diagnostic tools for childhood tuberculosis contribute to the substantial gap between estimated cases and cases notified to national tuberculosis programs. Thus, tools to make accurate and rapid clinical diagnoses are necessary to initiate antituberculosis treatment in more children.
Methods
We analyzed data from a prospective cohort of children <13 years old being routinely evaluated for pulmonary tuberculosis in Cape Town, South Africa, from March 2012 to November 2017. We developed a regression model to describe the contributions of baseline clinical evaluation to the diagnosis of tuberculosis using standardized, retrospective case definitions. We included baseline chest radiographic and Xpert MTB/RIF assay results to the model to develop an algorithm with ≥90% sensitivity in predicting tuberculosis.
Results
Data from 478 children being evaluated for pulmonary tuberculosis were analyzed (median age, 16.2 months; interquartile range, 9.8–30.9 months); 242 (50.6%) were retrospectively classified with tuberculosis, bacteriologically confirmed in 104 (43.0%). The area under the receiver operating characteristic curve for the final model was 0.87. Clinical evidence identified 71.4% of all tuberculosis cases in this cohort, and inclusion of baseline chest radiographic results increased the proportion to 89.3%. The algorithm was 90.1% sensitive and 52.1% specific, and maintained a sensitivity of >90% among children <2 years old or with low weight for age.
Conclusions
Clinical evidence alone was sufficient to make most clinical antituberculosis treatment decisions. The use of evidence-based algorithms may improve decentralized, rapid treatment initiation, reducing the global burden of childhood mortality.
Keywords: Diagnostic algorithm, diagnostic system, pediatric, clinical evidence, Diagnosis, clinical decision-making
Analysis of data from children evaluated for pulmonary tuberculosis in a high-burden setting suggests that clinical evidence is sufficient to treat many children. Diagnostic testing/imaging may be reserved for children not meeting criteria for treatment based on clinical evidence alone.
Each year, 1.2 million children are estimated to develop tuberculosis, and about one-quarter of those children die [1]. This places tuberculosis in the top 10 causes of death among children <5 years old worldwide. Globally, >96% of deaths in children with tuberculosis occur among those not receiving treatment [2].
Childhood tuberculosis is generally paucibacillary, limiting the sensitivity of bacteriological tests, including rapid molecular diagnostic tests, such as the Xpert MTB/RIF assay (Xpert) [3]. Findings on chest radiography (CR) are similarly less sensitive among children [4]. In addition to diagnostic limitations, accessing these tests may be challenging—especially in low-income and middle-income countries that bear the greatest burden of tuberculosis [5]. These limitations in sensitivity and accessibility contribute to the substantial gap between the estimated 1.2 million annual incident cases of childhood tuberculosis and the approximate 500 000 annual cases notified to the World Health Organization (WHO) [1].
Decentralized diagnosis and treatment for childhood tuberculosis may reduce the risk of untreated tuberculosis and improve treatment outcomes by shortening the delay to treatment initiation [6–10]. To that end, the WHO and the International Union against Tuberculosis and Lung Disease suggest treating children for whom there is sufficient clinical evidence of tuberculosis, even in the absence of further diagnostic investigation [11, 12]; however, it is not clear what clinical evidence is sufficient to start treatment. Practical, data-driven treatment-decision algorithms could help support more effective and uniform treatment decision making at peripheral health facilities [13].
A recent study among children living with human immunodeficiency virus (HIV) demonstrated that antituberculosis treatment decisions may be made using clinical evidence alone [14]. We present a complementary study, in which we analyze data from HIV-uninfected children from a well-characterized prospective cohort of young children routinely evaluated for pulmonary tuberculosis in Cape Town, South Africa. We aimed to investigate the relative contributions of baseline clinical characteristics, baseline CR findings, and baseline Xpert findings to the diagnosis of childhood pulmonary tuberculosis in a high-tuberculosis burden setting. We used this evidence to develop a practical algorithm to assist in making sensitive and rapid decisions to initiate antituberculosis treatment.
METHODS
Participants
Children <13 years old routinely evaluated for pulmonary tuberculosis were prospectively identified for participation in a diagnostic study [15–17]. Children were recruited from inpatient wards and emergency rooms at Tygerberg Hospital and Karl Bremer Hospital, referral hospitals in Cape Town, South Africa, from March 2012 to November 2017. Eligibility criteria reflected the WHO and national criteria for the evaluation of childhood tuberculosis and were any of the following: cough for ≥2 weeks, unexplained fever for ≥1 week, poor growth or weight loss over the preceding 3 months, or cough for <1 week with known tuberculosis exposure in the previous 12 months, positive tuberculin skin test (TST) results, or CR appearance suggestive of tuberculosis, as evaluated by study physicians. Children were not eligible if they had received antituberculosis treatment for >1 day or had extrapulmonary tuberculosis without also being evaluated for pulmonary tuberculosis.
Procedures and Definitions
At the time of enrollment each participant underwent a standardized clinical examination performed by study physicians; TST; bacteriological testing for Mycobacterium tuberculosis using acid-fast bacilli smear microscopy, Xpert, and mycobacteria growth indicator tube (MGIT) liquid culture from a minimum of 2 respiratory specimens (1 specimen of either gastric aspirate for children <5 years old or spontaneously produced sputum for older children able to expectorate, and 1 specimen of induced sputum); and anteroposterior and lateral CR. CR studies were read by 2 independent pulmonology and/or pediatric tuberculosis experts blinded to the clinical history using a standardized evaluation tool.
Some children underwent additional sampling for other respiratory specimens for M. tuberculosis confirmation, including nasopharyngeal aspirate and stool, as part of investigational substudies. At 2 months, all study participants were evaluated irrespective of tuberculosis diagnosis at baseline. All children with an ongoing suspicion for tuberculosis, regardless of the decision to treat for tuberculosis, had respiratory samples taken during follow-up at 1, 2, and/or 6 months or as clinically needed for smear microscopy, MGIT culture, and Xpert. Data were dual entered into standard case report forms. Managing clinical teams made the decision to treat.
Study participants were retrospectively classified by the study team as having confirmed, unconfirmed, or unlikely tuberculosis, using standardized clinical case definitions developed for the evaluation of diagnostics for childhood pulmonary tuberculosis (Supplementary Table 1) [18]. These definitions considered clinical history from baseline evaluations, immunological evidence of M. tuberculosis infection, consistency of CR appearance with tuberculosis as evaluated by experts blinded to the clinical history, confirmation of M. tuberculosis by Xpert or MGIT culture of respiratory specimens collected at baseline or in follow-up, and follow-up evaluation to assess for resolution or persistence of symptoms. All available information was used to inform classification of tuberculosis using these definitions. Given the epidemiological difference in the risk of tuberculosis and severe forms of disease [19], we defined 2 risk groups in our population: higher-risk children <2 years of age or with a weight-for-age z score below −2, and lower-risk children ≥2 years of age and with a weight-for-age z score of at least −2.
Statistical Analysis
We used logistic regression to develop a model to predict confirmed and unconfirmed tuberculosis restricted to data from the baseline evaluation of children with complete predictor information. We identified candidate predictors from the baseline clinical evaluation (initial clinical history and physical examination) used in previous scoring systems to diagnose childhood pulmonary tuberculosis, as well as from a nested case-control analysis of our data, in which we defined cases as having any bacteriological confirmation of M. tuberculosis over the study period and controls as those retrospectively classified as unlikely tuberculosis with the additional requirement that they completed the study without ever receiving antituberculosis treatment.
We carried out backward variable selection from the full model containing only predictors from the baseline clinical evaluation to develop the first model (clinical model). We used an inclusion P value cutoff informed by variable degree of freedom as per Akaike information criterion in model selection [20]. We added results from the baseline CR and Xpert performed on all respiratory specimens collected at baseline only to obtain the second model (investigational model). Though MGIT culture is more sensitive for M. tuberculosis than Xpert, we include Xpert in our models, given improved accessibility in many settings and shorter time to result.
All predictors were binary variables to reflect their presence or absence in the child, except cough duration, which we categorized as no cough or cough lasting <1, 1–2, 2–3, or >3 weeks. A list of all relevant candidate predictors and their definitions as relevant to this study are provided in the Supplementary Material. Analysis was performed using R software, version 4.0.1.
Given that a positive Xpert result was sufficient to classify a child as having tuberculosis by the reference standard, coefficient and standard error estimates for the investigational model were obtained by means of Firth’s logistic regression analysis, using function brglm in R package brglm. We examined separation by plotting the receiver operating characteristic (ROC) curve for each model and assessing the area under the ROC curve (AUC), using the R package pROC. We used the function roc.test to compare whether the models had statistically significant AUCs, using DeLong’s test for correlated ROC curves. We used leave-one-out cross-validation using the function cv.glm in the R package boot to assess out-of-sample predictive performance.
Treatment-Decision Algorithm Development
We scaled the coefficient estimates for the parameters in each model, such that a score of >100 constituted a sensitivity of ≥90% to diagnose pulmonary tuberculosis, consistent with the WHO target product profile of a community-based triage test to identify tuberculosis (scaling methods described in the Supplementary Material) [21]. To develop a treatment-decision algorithm, we examined how study participants met criteria for diagnosis disaggregated by contribution from baseline clinical evidence, baseline CR findings consistent with tuberculosis, and baseline Xpert findings with respiratory specimens.
Ethical Considerations
Data collection and analysis was approved by the Stellenbosch University Health Research Ethics Committee (reference no. N11/09/282). Written informed consent for study participation was obtained from parents or legal caregivers, and written assent was obtained from children ≥7 years old. This analysis was approved via expedited review by the Yale Institutional Review Board (reference no. 2000028046) and did not require specific consent because it was a secondary analysis of previously collected data.
RESULTS
Population
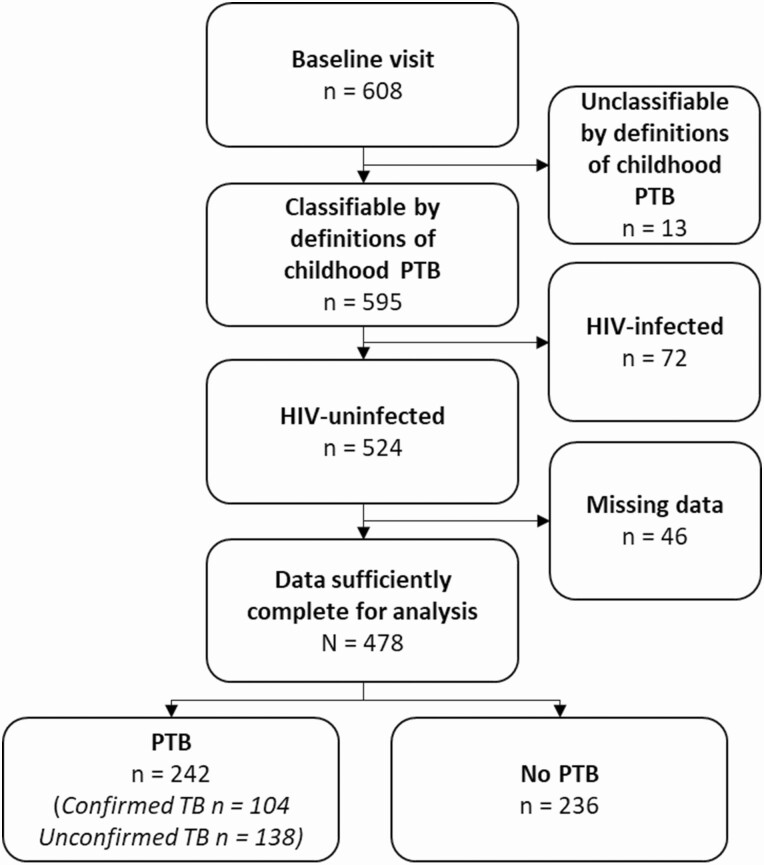
Data were available for 608 children who completed evaluation for the prospective study, of whom 478 HIV-uninfected participants had sufficiently complete data for this analysis (Figure 1). A total of 242 children (50.6%) were retrospectively classified as having confirmed or unconfirmed pulmonary tuberculosis using the clinical case definitions, with bacteriological confirmation in 104 of them (43.0%). See Supplementary Table 2 for differences between populations included versus excluded from this analysis because of missing variables.
Figure 1.
Flow diagram demonstrating participant eligibility for this analysis [17]. Pulmonary tuberculosis was confirmed or unconfirmed using the retrospective, standardized clinical case definitions). Abbreviations: HIV, human immunodeficiency virus; PTB, pulmonary tuberculosis; TB, tuberculosis.
Table 1 displays the demographics and candidate predictors for children with sufficiently complete data for this analysis. Of 478 children, 223 (46.7%) were female; their median age (interquartile range) was 16.2 (9.8–30.9) months, and their median weight-for age z score was −1.58 (−2.7 to −0.7). We classified 378 children (79.1%) as at higher-risk for tuberculosis and severe disease. Details concerning these higher- and lower-risk subpopulations are provided in the Supplementary Tables 3 and 4.
Table 1.
Demographics and Candidate Predictors From Clinical Evaluation and Diagnostic Imaging/Testing of Human Immunodeficiency Virus–Uninfected Participantsa
| Children, No. (%)b | ||
|---|---|---|
| Variable | Tuberculosis (n = 242) | Not Tuberculosis (n = 236) |
| Demographics | ||
| Sex | ||
| Male | 127 (52) | 128 (54) |
| Female | 115 (48) | 108 (46) |
| Age, median (IQR), mo | 18.09 (10.14–32.1) | 15.28 (9.36–27.52) |
| Age group, y | ||
| 0–1 | 15 (64) | 161 (68) |
| 2–4 | 60 (25) | 62 (26) |
| ≥5 | 27 (11) | 13 (6) |
| Weight-for-age z score , median (IQR) | −1.71 (−3.01 to −0.66) | −1.46 (−2.47 to −0.69) |
| Weight-for-age z score below −2 | 105 (43) | 92 (39) |
| Clinical history at baseline | ||
| Cough duration, wk | ||
| No cough | 46 (19) | 55 (23) |
| <1 | 74 (31) | 97 (41) |
| 1–2 | 43 (18) | 32 (14) |
| 2–3 | 23 (1) | 16 (7) |
| >3 | 56 (23) | 36 (15) |
| Fever | 147 (61) | 105 (44) |
| Failure to thrive/weight loss | 111 (46) | 87 (37) |
| Poor appetite | 137 (57) | 122 (52) |
| Lethargy | 104 (43) | 74 (31) |
| History of tuberculosis contact | 128 (53) | 55 (23) |
| Clinical examination at baseline | ||
| Lymphadenopathy | 151 (62) | 145 (61) |
| Stridor | 6 (2) | 3 (1) |
| Wheeze | 55 (23) | 58 (25) |
| Hepatomegaly | 42 (17) | 19 (8) |
| Splenomegaly | 19 (8) | 6 (3) |
| Diagnostic testing/imaging at baseline | ||
| CR findings consistent with pulmonary tuberculosis at baseline | 131 (54) | 22 (9) |
| Xpert-confirmed Mycobacterium tuberculosis on respiratory specimens at baseline | 62 (26) | 0 (0) |
| Retrospective clinical case definitions | ||
| Confirmed tuberculosis | 104 (43) | 0 (0) |
| Unconfirmed tuberculosis | 138 (57) | 0 (0) |
| Unlikely tuberculosis | 0 (0) | 236 (0) |
Abbreviations: CR, chest radiography; IQR, interquartile range; Xpert, Xpert MTB/RIF assay.
aParticipants with sufficiently complete data for this analysis.
bData represent no. (%) of participants, unless otherwise identified as median (IQR).
Prediction Modeling
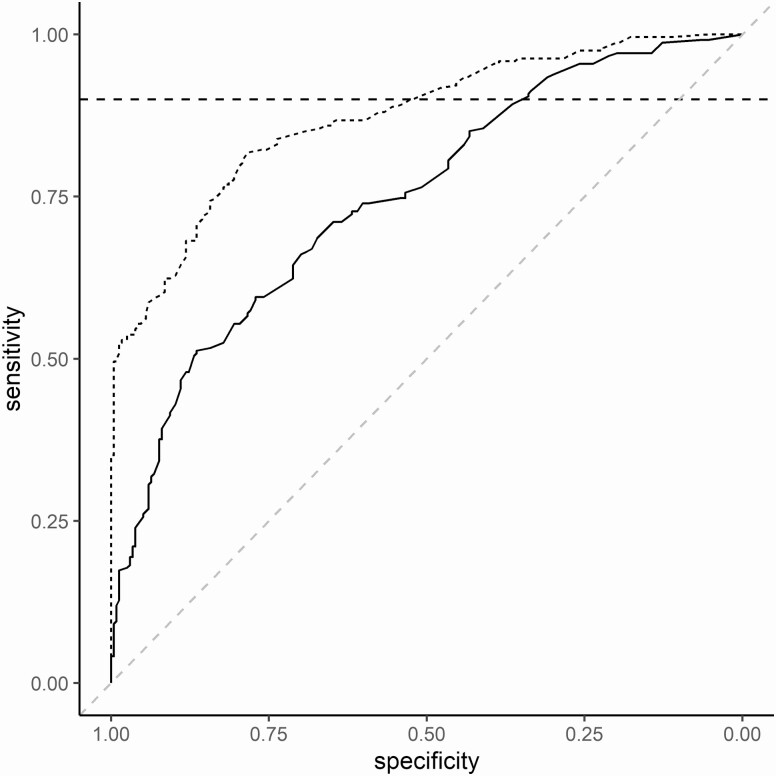
The predictors selected from baseline clinical evidence for inclusion in the final model were cough duration, fever, failure to thrive/weight loss, lethargy, history of tuberculosis exposure, and hepatomegaly. We added baseline CR and Xpert results to create the investigational model. Odds ratios, 95% confidence intervals, and P values for the predictors included in the clinical and investigational models along with AUC and leave-one-out cross-validation for each model are provided in Table 2, and the ROC curves for the models are presented in Figure 2. The clinical and investigational models had significantly different AUCs of 0.75 and 0.87, respectively (P < .001).
Table 2.
Prediction Models Using Baseline Clinical History and Physical Evaluation Findings With or Without Diagnostic Imaging and Microbiological Investigation
| Clinical Model: Clinical Evidence Only | Investigational Model: Clinical Evidence + CR + Xpert | |||||
|---|---|---|---|---|---|---|
| Predictor | OR | 95% CI (0.025–0.975) | P Value | OR | 95% CI (0.025-0.975) | P Value |
| Intercept | 0.22 | 0.12-0.37 | .00 | 0.10 | 0.04-0.18 | <.01 |
| Cough duration, wk | ||||||
| No cough | Reference | … | … | Reference | … | … |
| <1 | 0.68 | 0.39-1.19 | .18 | 0.62 | 0.31-1.18 | .15 |
| 1–2 | 1.51 | 0.78-2.97 | .22 | 1.29 | 0.59-2.85 | .52 |
| 2–3 | 2.29 | 1.01-5.29 | .05 | 1.35 | 0.48-3.76 | .56 |
| >3 | 2.27 | 1.20-4.35 | .01 | 2.48 | 1.19-5.49 | .02 |
| Fever present | ||||||
| No | Reference | … | … | Reference | … | … |
| Yes | 1.89 | 1.24-2.90 | .01 | 1.69 | 1.03-2.88 | .04 |
| Failure to thrive/weight loss | ||||||
| No | Reference | … | … | Reference | … | … |
| Yes | 1.66 | 1.10-2.54 | .02 | 1.80 | 1.10-3.04 | .02 |
| Lethargy | ||||||
| No | Reference | … | … | Reference | … | … |
| Yes | 1.40 | 0.90-2.18 | .14 | 1.68 | 0.98-2.97 | .06 |
| History of tuberculosis exposure | ||||||
| No | Reference | … | … | Reference | … | … |
| Yes | 5.13 | 3.33-8.05 | .01 | 6.99 | 4.20-13.00 | <.01 |
| Hepatomegaly | ||||||
| No | Reference | … | … | Reference | … | … |
| Yes | 2.62 | 1.38-5.13 | .01 | 1.18 | 0.52-2.71 | .69 |
| Baseline CR findings consistent with pulmonary tuberculosis | … | … | ||||
| No | … | … | … | Reference | … | … |
| Yes | … | … | … | 9.38 | 5.22-19.45 | <.01 |
| Baseline respiratory specimens positive for Mycobacteriun tuberculosis with Xpert | ||||||
| No | … | … | … | Reference | … | … |
| Yes | … | … | … | 90.41 | 10.69-Inf | <.01 |
| Leave-one-out cross-validation | 0.21 | … | … | 0.15 | … | … |
| Area under the ROC curve | 0.75 | … | … | 0.87 | … | … |
Abbreviations: CR, chest radiography; OR, odds ratio, ROC, receiver operating characteristic; Xpert, Xpert MTB/RIF assay.
Figure 2.
Receiver operating characteristic curves of the clinical model (solid), including the baseline clinical evidence (cough duration, fever, failure to thrive/weight loss, lethargy, history of tuberculosis exposure, and hepatomegaly), and the investigational model (dashed), considering baseline clinical evidence, baseline chest radiographic findings, and Xpert MTB/RIF findings from respiratory specimens collected at baseline. Horizontal dashed line indicates a sensitivity of 90%.
Treatment-Decision Algorithm
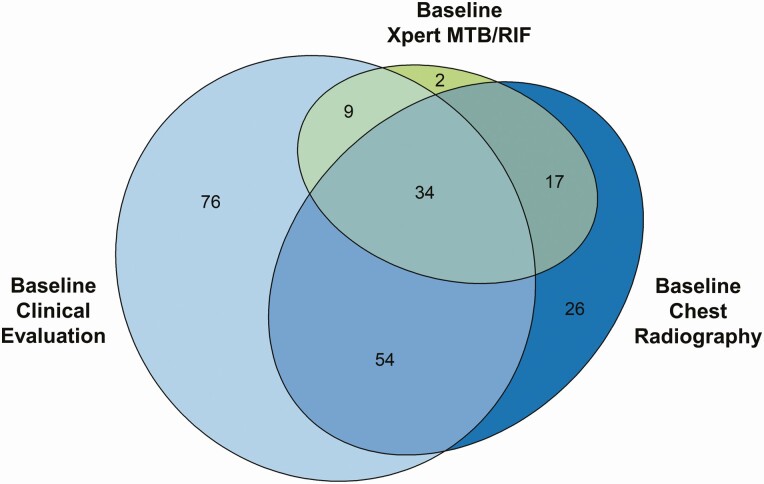
The probability threshold of the investigational model was set at 0.25 to classify tuberculosis with 90.1% sensitivity and 52.1% specificity. At this threshold, 173 (71.5%) of the 242 children with a diagnosis of tuberculosis could be identified using clinical evidence (Figure 3). Among those children not identifiable by clinical evidence, an additional 43 were identified with CR. Inclusion of CR results after clinical evidence increased the proportion of tuberculosis identified to 89.3%. Figure 4 shows the treatment-decision algorithm built from the investigational model. This algorithm failed to diagnose tuberculosis in 24 children (details in Supplementary Table 5). The sensitivity and specificity, compared with the retrospective reference standard, were 0.54 and 0.91, respectively, for baseline CR alone, and 0.26 and 1.0 for baseline Xpert alone.
Figure 3.
Venn diagram depicting how the 242 participants with tuberculosis in this cohort met criteria to be classified as having tuberculosis by the investigational model. Criteria were met by having sufficient evidence from baseline clinical evaluation, having baseline chest radiographic consistent with pulmonary tuberculosis, and/or having Mycobacterium tuberculosis confirmed by means of Xpert MTB/RIF assay of respiratory specimens collected at baseline. Note that 24 participants classified as having tuberculosis by the reference standard were missed by the investigational model.
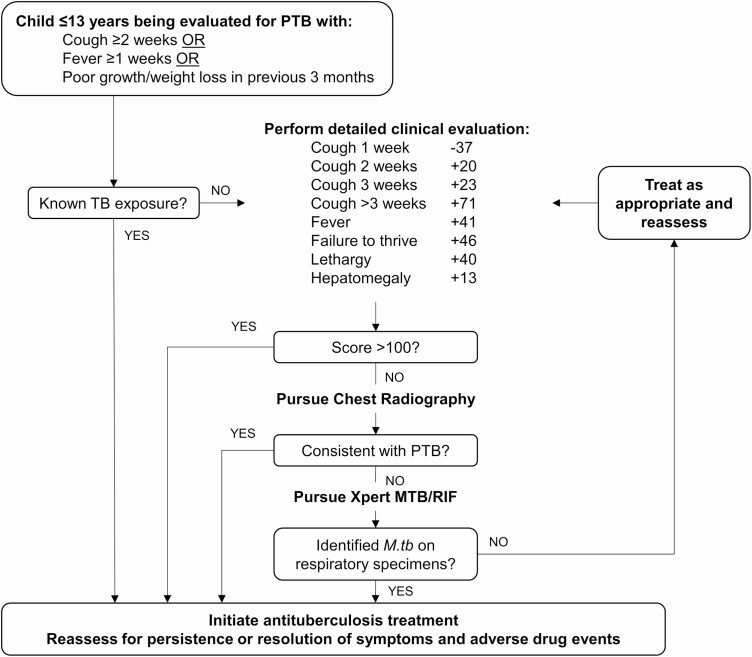
Figure 4.
Treatment-decision algorithm developed from the investigational model that includes baseline clinical evidence, baseline chest radiographic findings, and Xpert MTB/RIF assay findings from respiratory specimens collected at baseline. Abbreviations: M. tb, Mycobacterium tuberculosis; PTB, pulmonary tuberculosis; TB, tuberculosis.
Table 3 demonstrates the sensitivity, specificity, positive predictive value, and negative predictive value of the algorithm in the higher- and lower-risk subpopulations. The algorithm had a sensitivity and specificity of 91.8% and 51.6%, respectively, among higher-risk children, and 83.3% and 53.8% among lower-risk children. The algorithm built from the clinical model including only clinical evidence is shown in Supplementary Figure 1, with a sensitivity of 90.5% and specificity of 33.9% (Supplementary Figure 2). The sensitivity, specificity, positive predictive value, and negative predictive value of the algorithm in the higher- and lower-risk subpopulations are given in Supplementary Table 6).
Table 3.
Sensitivity, Specificity, and Positive and Negative Predictive Value of the Algorithm Developed From the Investigational Model, Including Baseline Clinical, Chest Radiographic, and Xpert MTB/RIF Assay Findings
| Risk for Tuberculosis and Severe Disease | Sensitivity, % | Specificity, % | PPV, % | NPV,% |
|---|---|---|---|---|
| High risk (age <2 y or weight-for-age z score below −2) | 91.8 | 51.6 | 66.7 | 85.6 |
| Low risk (age ≥2 y and weight-for-age z score of at least −2) | 83.3 | 53.8 | 62.5 | 77.8 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
DISCUSSION
Our analysis of a well-characterized, prospective cohort of young children evaluated for pulmonary tuberculosis demonstrates that a detailed clinical history and physical examination is sufficient to initiate treatment in most HIV-uninfected children. In our setting, CR and Xpert findings affected the decision to treat only a minority of children with symptoms suggestive of pulmonary tuberculosis. This suggests that diagnostic testing/imaging may be reserved for those children who do not meet criteria for treatment-initiation based on clinical evidence alone. We used these findings to construct a data-driven algorithm to promote sensitive and rapid antituberculosis treatment-initiation.
While the WHO does not define the target sensitivity and specificity of diagnostic tools for childhood tuberculosis, as compared with a composite reference standard, we fixed the sensitivity of our algorithm at 90% to be consistent with both the WHO-defined target for a community-based triage and the algorithm-building approach adopted by Marcy and colleagues [14, 21]. Our specificity fell short of the WHO-proposed target; however, given the severe consequences of failing to diagnose and treat a case of childhood tuberculosis, we elected to prioritize sensitivity over specificity.
Our results highlight the importance of a detailed clinical history and physical examination in making treatment initiation decisions for childhood tuberculosis. We identified clinical evidence suggestive of childhood pulmonary tuberculosis that is consistent with the literature [14, 22–25], and we quantitatively described their contribution to diagnosis. This analysis demonstrates that incorporating additional clinical characteristics may improve the specificity of treatment decisions without a substantial sacrifice in sensitivity among children identified by the WHO symptom screen. In addition, this approach allows health workers to identify those children with sufficient clinical evidence to begin antituberculosis treatment without the need for additional diagnostic imaging/testing. This supports rapid treatment initiation in settings where access to diagnostic imaging or testing is limited, as well as where negative results from available tests may not change management.
Our analysis suggests pursuing CR before Xpert among those children who do not meet criteria to receive antituberculosis treatment based on clinical evidence alone. This is reasonable, given the accessibility of CR in many settings and its utility in identifying other disease not related to tuberculosis. In addition, it does not require any invasive sampling procedures that may be needed to obtain samples from young children for microbiological confirmation. We note that the contribution to diagnosis that we present for CR in this analysis may be optimistic, given that high-quality images were obtained in a tertiary care setting with expert readings that may be unavailable in some high-burden, low-resource settings [26]. Prospective investigation into the use of standardized digital CR and enhanced reader training will be important to understand the use of CR in childhood tuberculosis diagnosis in settings with limited resources [27]. Furthermore, inclusion of specific findings on CR may increase the specificity of our algorithm [4].
Although we demonstrate that well-collected respiratory specimens for Xpert performed at baseline do not substantially improve our algorithm, we note that Xpert findings may provide important information on guiding treatment selection in settings where drug resistance is a concern. However, it is important to note that lack of access to microbiological testing and negative test results should not prevent children from accessing antituberculosis treatment when clinical criteria are met. Furthermore, while drug-resistant tuberculosis transmission is an important public health concern, the relative importance of microbiological tests in children should be informed by the local epidemiology of drug-resistant tuberculosis transmission [28]. Given limitations in the sensitivity of microbiological testing among children, obtaining a detailed exposure history that includes the drug susceptibility test profile of any potential source cases remains critical.
Good performance of this algorithm among younger or low weight-for-age children is encouraging, given a higher risk of severe tuberculosis in this group. The children missed by this algorithm were generally older, had a higher weight-for-age z score, and had a shorter cough duration. We believe that increased sensitivity of treatment decisions, rather than precise diagnosis, is likely to have a greater impact on child mortality rates, given the high proportion of young children who with undiagnosed tuberculosis. It may be necessary to accept some overtreatment with relatively safe antituberculosis therapy to reduce the preventable disease and death due to untreated tuberculosis [29, 30]. Diagnostic vigilance and careful follow-up are critically important for all children, regardless of the initial treatment-initiation decision, to consider competing diagnoses and monitor for adverse drug events.
Although TSTs were used to establish the reference standard, we chose not to include it in our analysis because of the many participants with missing TST data (120 of 478) due to global tuberculin stockouts during the study. While immunological testing for M. tuberculosis infection may improve the specificity of the algorithms, limitations in sensitivity among young and malnourished children and lack of accessibility at peripheral health centers may discourage their inclusion in treatment-decision algorithms [31].
A source of potential bias in this analysis arises from the fact that the clinical evaluation, CR results, and Xpert results are included as predictors in the model and as components of the clinical reference standard. We believe that this may not be a major issue in the current study, given the high degree of microbiological confirmation. This is further supported by the similar operational characteristics of the algorithms in the nested case-control subpopulation, as compared with the development cohort (Supplementary Tables 7 and 8).
In addition, we must be careful not to overinterpret the generalizability of these algorithms, which were built from a cohort that was prescreened for tuberculosis and sourced from a tertiary care center. While the entry criteria for this development cohort reflects the WHO criteria for investigation for tuberculosis and a low value for cross-validation suggests generalizability and external validity, the positive predictive value of these algorithms may be lower where the baseline prevalence of tuberculosis is lower. Further evidence is required to determine the pretest probability of tuberculosis in children identified as having a positive WHO symptom screen across different settings, because this would have implications for the performance of this treatment-decision algorithm. Furthermore, randomized, interventional investigation is necessary to evaluate the morbidity and mortality impact of using data-driven, treatment-decision algorithms to guide antituberculosis treatment initiation in different settings.
This analysis outlines an approach to interpret clinical data to inform treatment initiation decisions for children being evaluated for pulmonary tuberculosis. It is important to recognize that this algorithm is context specific, and translation to other settings should be undertaken cautiously. Ideally, treatment-decision algorithms should be constructed locally to reflect the site-specific epidemiology, the quality and accessibility of diagnostic imaging and testing, and the relative consequence of overtreatment versus untreated child tuberculosis. Furthermore, these algorithms should be revised and recomputed as circumstance change—for example, as local capacity to incorporate additional tools changes or as improved diagnostic tools are discovered. Implementation of treatment-decision algorithms must include programmatic support and mentorship for the healthcare providers to use them effectively, as well as additional resources to support the families of the children initiated onto treatment [7].
We demonstrate that algorithms that incorporate evidence from a detailed clinical history and physical examination could play an important role in guiding sensitive treatment-initiation decisions for most children being evaluated for pulmonary tuberculosis. Data-driven treatment algorithms provide an important framework to consider the contribution of additional investigation, after detailed clinical evaluation. Algorithms that support rapid, decentralized antituberculosis treatment decision making are important tools to reduce the burden of disease and death associated with childhood tuberculosis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. K. S. G. and J. A. S. conceptualized and designed the study, and J. L. W. and T. C. provided input. E. W., M. M. v. d. Z., M. P., and A. C. H. collected the data from the original diagnostic study, K. S. G. and J. A. S. verified the data, and K. S. G., J. L. W., T. C., and J. A. S. analyzed and interpreted it. K. S. G. wrote the first draft of the manuscript, and all authors critically reviewed and approved the final manuscript.
Financial support. This work was supported by the US National Institutes of Health (medical scientist training program grant T32GM007205 to K. S. G.); the Fogarty International Center (Global Health Equity Scholars Program grant D43TW010540 to K. S. G.); the Infectious Diseases Society of America (mentorship grant to K. S. G.); the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) (clinical scientist fellowship to J. A. S., jointly funded under the MRC/DFID Concordat agreement [MR/R007942/1]); the European and Developing Countries Clinical Trials Partnership (grant 99726 TB-Lung FACT TMA 2015 CDF–1012 to M. M. v. d. Z.); the South African National Research Foundation, through a South African Research Chairs Initiative in Paediatric Tuberculosis (A. C. H.); and the Medical Research Council of South Africa (scholarship for doctoral studies to E. W. under the Medical Research Council Clinician Researcher Programme).
The original cohort study (principal investigator: E. W.) was also supported by funding from the Faculty of Medicine and Health Sciences at Stellenbosch University (early career grant and temporary research assistantship grant), the Harry Crossley Foundation, the South African National Research Foundation (Thuthuka programme funding for doctoral students), the South African Medical Research Council (Self-Initiated Research Programme), the Centers for Disease Control and Prevention Tuberculosis Trials Consortium (grant 15FED1511233), the Foundation for Innovative New Diagnostics, and the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT). Although this was not an IMPAACT network study, we acknowledge the network’s support of core Desmond Tutu TB Centre staff, infrastructure, and research activities. Overall support for the IMPAACT Network was provided by the National Institute of Allergy and Infectious Diseases, with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the National Institutes of Health (grants UM1AI068632 [IMPAACT Leadership and Operations Center], UM1AI068616 [IMPAACT Statistical and Data Management Center], and UM1AI106716 [IMPAACT Laboratory Center]), and by the National Institute of Child Health and Human Development (contract HHSN275201800001I).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.World Health Organization. Global tuberculosis report 2020. Geneva, Switzerland: World Health Organization, 2020. [Google Scholar]
- 2.Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health 2017; 5:e898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Detjen AK, DiNardo AR, Leyden J, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir Med 2015; 3:451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Villiers RV, Andronikou S, Van de Westhuizen S. Specificity and sensitivity of chest radiographs in the diagnosis of paediatric pulmonary tuberculosis and the value of additional high-kilovolt radiographs. Australas Radiol 2004; 48:148–53. [DOI] [PubMed] [Google Scholar]
- 5.MacPherson P, Khundi M, Nliwasa M, et al. Disparities in access to diagnosis and care in Blantyre, Malawi, identified through enhanced tuberculosis surveillance and spatial analysis. BMC Med 2019; 17:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyers N, Gie RP, Schaaf HS, et al. Delay in the diagnosis, notification and initiation of treatment and compliance in children with tuberculosis. Tuber Lung Dis 1994; 75:260–5. [DOI] [PubMed] [Google Scholar]
- 7.Zawedde-Muyanja S, Nakanwagi A, Dongo JP, et al. Decentralisation of child tuberculosis services increases case finding and uptake of preventive therapy in Uganda. Int J Tuberc Lung Dis 2018; 22:1314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacha JM, Ngo K, Clowes P, et al. Why being an expert—despite Xpert—remains crucial for children in high TB burden settings. BMC Infect Dis 2017; 17:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wobudeya E, Jaganath D, Sekadde MP, Nsangi B, Haq H, Cattamanchi A. Outcomes of empiric treatment for pediatric tuberculosis, Kampala, Uganda, 2010–2015. BMC Public Health 2019; 19:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naidoo P, Theron G, Rangaka MX, et al. The South African Tuberculosis Care Cascade: estimated losses and methodological challenges. J Infect Dis 2017; 216:702–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children.Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 12.Graham S.The Union’s desk guide for diagnosis and management of TB in children. 3rd ed. Paris, France: International Union Against Tuberculosis and Lung Disease, 2016. [Google Scholar]
- 13.Seddon JA, Whittaker E, Kampmann B, et al. The evolving research agenda for paediatric tuberculosis infection. Lancet Infect Dis 2019; 19:e322–9. [DOI] [PubMed] [Google Scholar]
- 14.Marcy O, Borand L, Ung V, et al. A treatment-decision score for HIV-infected children with suspected tuberculosis. Pediatrics 2019; 144:e20182065. [DOI] [PubMed] [Google Scholar]
- 15.Walters E, Demers AM, van der Zalm MM, et al. Stool culture for diagnosis of pulmonary tuberculosis in children. J Clin Microbiol 2017; 55:3355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walters E, van der Zalm MM, Palmer M, et al. Xpert MTB/RIF on stool is useful for the rapid diagnosis of tuberculosis in young children with severe pulmonary disease. Pediatr Infect Dis J 2017; 36:837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walters E, Scott L, Nabeta P, et al. Molecular detection of Mycobacterium tuberculosis from stools in young children by use of a novel centrifugation-free processing method. J Clin Microbiol 2018; 56:e00781-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis 2015; 61(suppl 3):S179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marais BJ, Gie RP, Schaaf HS, et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:278–85. [PubMed] [Google Scholar]
- 20.Sterberg E.Clinical prediction models: a practical approach to development, validation, and updating. New York, NY: Springer-Verlag New York, 2009. [Google Scholar]
- 21.World Health Organization. High-priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting.Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 22.Marais BJ, Gie RP, Hesseling AC, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics 2006; 118:e1350–9. [DOI] [PubMed] [Google Scholar]
- 23.Pearce EC, Woodward JF, Nyandiko WM, Vreeman RC, Ayaya SO. A systematic review of clinical diagnostic systems used in the diagnosis of tuberculosis in children. AIDS Res Treat 2012; 2012:401896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hesseling AC, Schaaf HS, Gie RP, Starke JR, Beyers N. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. Int J Tuberc Lung Dis 2002; 6:1038–45. [PubMed] [Google Scholar]
- 25.Marais BJ, Gie RP, Obihara CC, Hesseling AC, Schaaf HS, Beyers N. Well defined symptoms are of value in the diagnosis of childhood pulmonary tuberculosis. Arch Dis Child 2005; 90:1162–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seddon JA, Padayachee T, Du Plessis AM, et al. Teaching chest X-ray reading for child tuberculosis suspects. Int J Tuberc Lung Dis 2014; 18:763–9. [DOI] [PubMed] [Google Scholar]
- 27.ClinicalTrials.gov. TB-Speed Decentralisation Study. https://ClinicalTrials.gov/show/NCT04038632. Accessed 1 October 2020.
- 28.Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis 2016; 16:1193–201. [DOI] [PubMed] [Google Scholar]
- 29.Frydenberg AR, Graham SM. Toxicity of first-line drugs for treatment of tuberculosis in children: review. Trop Med Int Health 2009; 14:1329–37. [DOI] [PubMed] [Google Scholar]
- 30.Wobudeya E, Chabala C, Hesseling AC, et al. Shorter treatment for minimal tuberculosis in children: main findings from the SHINE trial. Int J Tuberc Lung Dis 2020; 24:s407-8. [Google Scholar]
- 31.Mandalakas AM, Detjen AK, Hesseling AC, Benedetti A, Menzies D. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis 2011; 15:1018–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.