Abstract
Background
Adolescents aged 10–19 years living with human immunodeficiency virus (HIV) (ALHIV), both perinatally infected adolescents (APHIV) and behaviorally infected adolescents (ABHIV), are a growing population with distinct care needs. We characterized the epidemiology of HIV in adolescents included in Population-based HIV Impact Assessments (2015–2017) in Zimbabwe, Malawi, Zambia, Eswatini, and Lesotho.
Methods
Adolescents were tested for HIV using national rapid testing algorithms. Viral load (VL) suppression (VLS) was defined as VL <1000 copies/mL, and undetectable VL (UVL) as VL <50 copies/mL. Recent infection (within 6 months) was measured using a limiting antigen avidity assay, excluding adolescents with VLS or with detectable antiretrovirals (ARVs) in blood. To determine the most likely mode of infection, we used a risk algorithm incorporating recency, maternal HIV and vital status, history of sexual activity, and age at diagnosis.
Results
HIV prevalence ranged from 1.6% in Zambia to 4.8% in Eswatini. Of 707 ALHIV, 60.9% (95% confidence interval, 55.3%–66.6%) had HIV previously diagnosed, and 47.1% (41.9%–52.3%) had VLS. Our algorithm estimated that 72.6% of ALHIV (485 of 707) were APHIV, with HIV diagnosed previously in 69.5% of APHIV and 39.4% of ABHIV, and with 65.3% of APHIV and 33.5% of ABHIV receiving ARV treatment. Only 67.2% of APHIV and 60.5% of ABHIV receiving ARVs had UVL.
Conclusions
These findings suggest that two-thirds of ALHIV were perinatally infected, with many unaware of their status. The low prevalence of VLS and UVL in those receiving treatment raises concerns around treatment effectiveness. Expansion of opportunities for HIV diagnoses and the optimization of treatment are imperative.
Keywords: HIV, southern Africa, adolescents, perinatal HIV infection, national survey data
Based on national surveys, fewer than half of adolescents living with human immunodeficiency virus (HIV) in 5 countries in southern Africa were virally suppressed, and 68.7% classified as perinatally infected had undiagnosed HIV or HIV diagnosed at age ≥10 years.
With the scale-up in pediatric antiretroviral (ARV) treatment (ART) in sub-Saharan Africa, the number of children perinatally infected with human immunodeficiency virus (HIV) who reach adolescence has increased. However, adolescents aged 10–19 years living with HIV (ALHIV) have increasing mortality rates, owing to inadequate case finding and high rates of treatment attrition [1–4]. Accurate estimates of ALHIV are critical for health service planning, particularly in southern and eastern Africa, where 60% of ALHIV reside [2, 5, 6]. Improved efficacy of ART for prevention of vertical transmission and pediatric care has changed some of the modelling parameters, suggesting that further evidence is needed for refining estimates [3, 7].
Classifying ALHIV as adolescents with behavioral infection (ABHIV) or with perinatal HIV infection (APHIV) is important for accurately describing the epidemiology in ALHIV and for appropriate targeting of services; ALHIV have different clinical and psychosocial needs, depending on the mode of transmission. Untreated APHIV are likely to have multiple comorbid conditions, including chronic lung disease and stunting [8–12]. Furthermore, while mortality rates are high among HIV-positive infants [3], studies have revealed large numbers of APHIV who are identified only as a result of acute illness [9].
The Population-based HIV Impact Assessments (PHIAs) were national surveys designed to measure HIV-related indicators. These household-based surveys provide an opportunity to evaluate HIV outcomes in adolescents and to identify APHIV who have survived into adolescence without treatment and those lost to care. We aimed to estimate the number of ALHIV in Malawi, Eswatini, Zimbabwe, Zambia, and Lesotho, some of the countries with the highest HIV burdens, describe their virologic and immune outcomes, and assess probable modes of HIV acquisition.
METHODS
Survey Design and Participants
PHIAs in Malawi, Eswatini, Zimbabwe, Zambia, and Lesotho were conducted in each country between 2015 and 2017. All PHIAs used a 2-stage sampling design to select a nationally representative sample and were powered to achieve precision with a relative standard error of 30% around a national estimate of HIV incidence in adults (aged 15–49 years). Further details are available in Supplementary Appendix 1.
Consenting heads of households completed a household roster of members and questionnaire. Biological parents were linked to their children (aged 0–17 years) through the roster, and absent or deceased parents were recorded. Adolescents aged 18–19 years were linked to their cohabitating parents via the household roster, but parental vital status was not collected [13]. Each household member provided consent to participate in an interview and to HIV testing. A guardian or parent provided permission for interviewers to approach children aged 10–17 years who provided assent. The PHIA protocol was approved by ethics committees in each country and the institutional review boards at Columbia University and the US Centers for Disease Control and Prevention.
Procedures
Staff administered questionnaires using Google Nexus 9 tablets. Older adolescent participants (aged 15–19 years) answered an adult questionnaire, including questions on sexual behavior and clinical history. Young adolescent participants (aged 10–14 years) answered the adolescent questionnaire, which covered sexual history, everywhere but Malawi. Parents or legal guardians provided information on young adolescents’ clinical history.
Rapid HIV testing was conducted according to each country’s national algorithm. Depending on the country’s guidelines on age of consent for HIV testing, the results were either returned directly to the adolescent, or to their parent/guardian [14]. CD4 cell counts were measured using the PIMA CD4 Analyzer (Abbott Laboratories). HIV-seropositive results were verified using the Geenius HIV-1/2 supplemental assay (Bio-Rad Laboratories). HIV-1 RNA was measured in plasma, or in dried blood spot (DBS) samples if plasma was unavailable, with real-time polymerase chain reaction using the CobasTaqMan (Roche Diagnostics) or the Abbott M2000 (Abbott Laboratories) platform. Viral load (VL) suppression (VLS) was defined as VL <1000 copies/mL, per the World Health Organization definition [15], p 480. We classified VL <50 copies/mL as undetectable VL (UVL) for plasma samples, and we excluded DBS samples owing to the lower limit of detection of 400 copies/mL [16].
Qualitative screening for the 3 most commonly used ARVs with long half-lives—efavirenz, nevirapine, and lopinavir—was conducted on DBS specimens, using high-performance liquid chromatography coupled with tandem mass spectrometry [17]. Atazanavir was tested for in Malawi and Zambia. ARV detection was performed at the University of Cape Town, South Africa. Recent infection was defined using the HIV-1 Limiting Antigen–Avidity EIA (Sedia Biosciences), in which specimens with a median normalized optical density ≤ 1.5, a VL ≥1000 copies/mL, and no detectable ARVs were classified as recent infection (within the past 6 months) [18].
Classification of Mode of Infection
The most likely mode of HIV acquisition was classified using a risk algorithm whereby participants were classified as ABHIV using the following sequence: (1) if they tested as recently infected; (2) if not, if their biological mother tested HIV negative during the survey; and (3) if neither or unknown, if they reported sexual activity before HIV diagnosis. Remaining participants were further classified as APHIV based on 2 criteria: if they reported their HIV diagnosis or initiation into care before the age of 15 years (criterion 1) and if older adolescents (aged 15–19 years) had a biological mother who tested HIV positive during the survey or who was reported as deceased by the head of household, or, if maternal data were missing, if the participant reported never having been sexually active (criterion 2).
Statistical Analyses
We included adolescents with HIV test results. Design weights were calculated based on sampling design, including probabilities of household and individual selection that were adjusted for nonresponse at the household, individual, and biomarker levels. The final adjustment calibrated the nonresponse-adjusted individual and biomarker weights to make the sum of each set of weights conform to the national population total by 5-year age groups and sex distribution of the most recent national population projections. This calibration was used to estimate the total number of ALHIV in each country. The Joint United Nations Programme on HIV/AIDS (UNAIDS) provided Spectrum estimates of ALHIV population size and mode of acquisition for the survey years, which were generated using models based on program data on HIV prevalence in women of childbearing age, pregnant women receiving prophylaxis, and ART coverage in children [19]. Supplementary Appendix 1 provides information on a sensitivity analysis done to compare our results with those of another algorithm estimating mode of acquisition [20].
Analyses were conducted with Stata software, version 15.1, using weighted data, with jackknife replicate weights for variance estimation. All presented proportions are weighted, whereas numerators and denominators are unweighted. Comparisons between weighted pooled averages were performed using Student t tests. ALHIV were classified as having a previous diagnosis if they or their guardian reported their HIV-positive status or if they tested positive for ARVs; they were also classified as receiving ART based on self-report or guardian report of current ART, or if they had detectable ARVs in the blood [21].
RESULTS
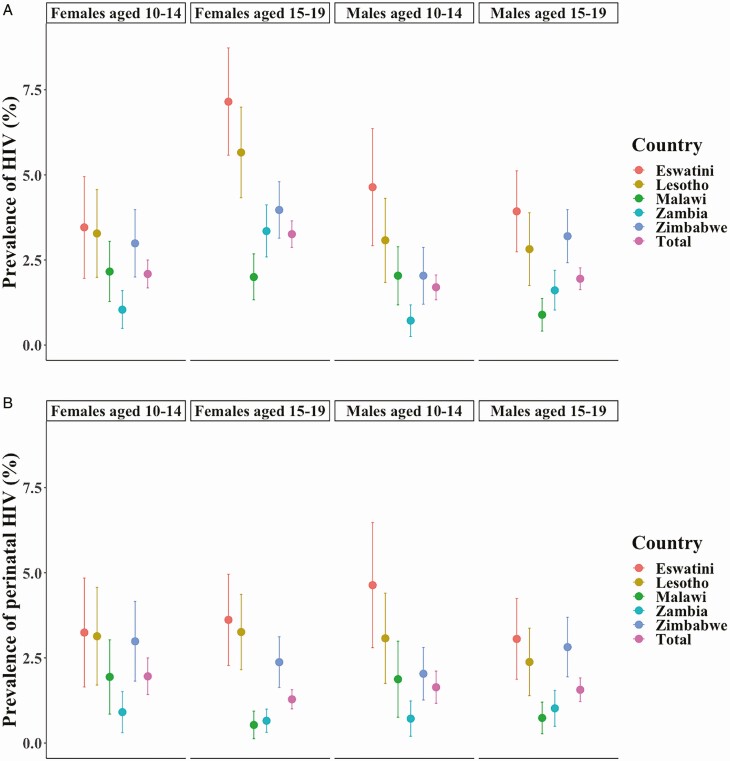
Overall, 109 970 adults and children were enrolled, including 24 805 adolescents. Unweighted response rates among adolescents ranged from 69.3% in Malawi to 87.7% in Eswatini (Table 1). The lowest adolescent HIV prevalence was in Zambia (1.6%; 95% confidence interval [CI], 1.3%–2.0%) and the highest in Eswatini (4.8%; 3.9%–5.7%). Prevalence was higher in older adolescents in every country except Malawi (Figure 1A). One-third of adolescents (31.3%) reported sexual activity, with no difference by sex. Of adolescents with data on parental vital status, 9.0% (2026 of 18 364) had deceased mothers, 16.5% (3773 of 17 920) had deceased fathers, and 4.2% (1029 of 17 884) were orphans, with both parents deceased. HIV prevalence was significantly higher in orphans (9.5%) than in those with ≥1 living parent (1.8%; P < .001). The data predicted 262 000 (95% CI, 233 000–291 000) ALHIV across the 5 countries, which is within UNAIDS plausibility bounds (PBs), with the highest number in Zimbabwe (Table 2).
Table 1.
Characteristics of Adolescents Aged 10–19 Years Living with Human Immunodeficiency Virus (HIV) in the Southern African Population-Based HIV Impact Assessments, 2015–2017a
| Characteristic | Zimbabwe 2015–2016 | Malawi 2015–2016 | Zambia 2016 | Eswatini 2016–2017 | Lesotho 2016–2017 | Total |
|---|---|---|---|---|---|---|
| Among all adolescents | ||||||
| Response rate, no./total (unweighted %) | 6310/8023 (78.6) | 5240/7561 (69.3) | 6477/8871 (73.0) | 3213/3664 (87.7) | 3565/4260 (83.7) | 24 805/32 379 (76.6) |
| Age, median age (IQR), y | 14 (12–17) | 14 (12–17) | 14 (12–17) | 15 (12–17) | 14 (12–17) | 14 (12–17) |
| History of sexual activity, % (no./total)b | 19.1 (1307/5409) | 52.6 (1606/3118) | 30.2 (1949/5452) | 17.1 (549/2742) | 32.0 (1024/2952) | 31.3 (6435/19 673) |
| HIV prevalence, % (no./total) | ||||||
| Total | 3.0 (208/6310) | 1.8 (95/5240) | 1.6 (112/6477) | 4.8 (157/3213) | 3.7 (135/3565) | 2.2 (707/24 805) |
| Female sex | 3.4 (120/3247) | 2.1 (59/2689) | 2.1 (78/3403) | 5.3 (92/1609) | 4.5 (87/1884) | 2.6 (436/12 832) |
| Male sex | 2.7 (88/3063) | 1.5 (36/2551) | 1.1 (34/3074) | 4.3 (65/1604) | 2.9 (48/1681) | 1.9 (271/11 973) |
| Death of parents (at age 10–17 y), % (no./total)c | ||||||
| Mother | 13.3 (693/4753) | 6.0 (239/3868) | 7.6 (359/4762) | 10.6 (290/2407) | 16.2 (445/2574) | 9.0 (2026/18 364) |
| Father | 22.1 (1129/4550) | 11.7 (524/3836) | 14.9 (759/4734) | 21.1 (546/2359) | 33.0 (815/2441) | 16.5 (3773/17 920) |
| Both parents | 7.2 (371/4532) | 2.0 (98/3836) | 3.5 (186/4728) | 4.5 (128/2351) | 9.5 (246/2437) | 4.2 (1029/17 884) |
| Among HIV-positive adolescents, no./total (%) | ||||||
| Age group | ||||||
| 10–14 y | 61/208 (41.6) | 42/95 (63.0) | 20/112 (28.7) | 45/157 (41.9) | 45/135 (43.6) | 213/707 (44.6) |
| 15–19 y | 147/208 (58.4) | 53/95 (37.0) | 92/112 (71.3) | 112/157 (58.1) | 90/135 (56.4) | 494/707 (55.4) |
| Sex | ||||||
| Female | 120/208 (55.3) | 59/95 (58.7) | 78/112 (65.3) | 92/157 (55.3) | 87/135 (59.9) | 436/707 (58.9) |
| Male | 88/208 (44.7) | 36/95 (41.3) | 34/112 (34.7) | 65/157 (44.7) | 48/135 (40.1) | 271/707 (41.1) |
| HIV previously diagnosed, % (95% CI)d | ||||||
| Total | 71.4 (64.3–78.6) | 57.9 (43.5–72.2) | 40.0 (29.2–50.8) | 77.8 (70.8–84.8) | 76.7 (68.6–84.9) | 60.9 (55.3–66.6) |
| Age 10–14 y | 77.9 (65.5–90.4) | 64.9 (44.3–85.5) | 43.0 (19.3–66.7) | 84.0 (72.5–95.4) | 79.6 (66.3–92.8) | 67.9 (57.6–78.3) |
| Age 15–19 y | 66.9 (58.5–75.4) | 47.1 (32.2–62.0) | 38.8 (26.7–50.9) | 73.6 (64.6–82.5) | 74.6 (63.9–85.3) | 55.6 (49.6–61.6) |
| Age at HIV diagnosis, % (no./total)e | ||||||
| 0–9 y | 48.4 (44/108) | 59.5 (19/40) | 42.4 (12/31) | 39.9 (34/101) | 40.4 (27/75) | 49.6 (136/355) |
| 10–14 y | 42.0 (48/108) | 34.4 (18/40) | 34.1 (10/31) | 40.0 (41/101) | 40.2 (30/75) | 38.6 (147/355) |
| 15–19 y | 9.6 (16/108) | 6.1 (3/40) | 23.6 (9/31) | 20.1 (26/101) | 19.4 (18/75) | 11.9 (72/355) |
| Proportion on ART, % (95% CI)f | ||||||
| Total | 66.7 (59.2–74.2) | 51.8 (37.0–66.5) | 37.3 (26.2–48.4) | 73.1 (65.3–80.9) | 72.8 (64.2–81.4) | 56.3 (50.6–62.1) |
| Age 10–14 y | 76.1 (63.3–88.9) | 58.2 (36.9–79.5) | 43.0 (19.3–66.7) | 84.0 (72.5–95.4) | 77.0 (63.3–90.7) | 64.6 (54.1–75.2) |
| Age 15–19 y | 60.2 (51.2–69.2) | 41.8 (26.4–57.2) | 35.0 (22.8–47.1) | 65.5 (56.3–74.8) | 69.6 (58.6–80.6) | 50.1 (43.9–56.2) |
| Detectable ARVs, % (95% CI) | ||||||
| Total | 61.1 (53.4–68.7) | 46.4 (32.2–60.6) | 33.6 (23.2–44.0) | 66.6 (59.0–74.3) | 66.7 (58.2–75.1) | 51.2 (45.5–56.8) |
| Age 10–14 y | 68.4 (55.1–81.7) | 50.9 (30.3–71.5) | 38.5 (16.3–60.7) | 78.5 (66.2–90.9) | 71.6 (57.7–85.5) | 57.7 (47.4–68.0) |
| Age 15–19 y | 55.8 (46.4–65.2) | 38.8 (24.0–53.6) | 31.6 (19.5–43.8) | 58.1 (48.7–67.4) | 62.8 (51.5–74.0) | 45.9 (39.8–52.0) |
| Detected ARVs, % (no./total) | ||||||
| None | 38.9 (78/204) | 53.6 (52/94) | 66.4 (73/112) | 33.4 (56/157) | 33.3 (45/134) | 48.9 (304/701) |
| Nevirapine | 12.6 (23/204) | 26.1 (51/94) | 11.3 (11/112) | 32.1 (44/157) | 21.0 (27/134) | 17.4 (130/701) |
| Efavirenz | 46.9 (100/204) | 20.3 (17/94) | 21.8 (27/112) | 30.4 (50/157) | 41.5 (57/134) | 32.6 (251/701) |
| Lopinavir | 1.6 (3/204) | 0 | 0.5 (1/112) | 4.1 (7/157) | 4.2 (5/134) | 1.2 (16/701) |
| Detectable ARVs based on reported ART status, % (no./total) | ||||||
| Unaware of HIV status | 27.9 (26/86) | 11.2 (6/45) | 9.7 (7/73) | 25.1 (11/47) | 24.7 (10/40) | 17.2 (60/290) |
| Not on ART | 24.9 (26/95) | 9.9 (6/49) | 10.9 (8/77) | 23.5 (12/56) | 24.3 (11/45) | 16.3 (63/322) |
| On ART | 90.2 (98/106) | 87.7 (34/40) | 87.0 (29/33) | 92.2 (87/96) | 87.7 (66/76) | 89.0 (314/351) |
| Virologic suppression, % (95% CI) | ||||||
| VLS | ||||||
| Total | 52.3 (45.1–59.4) | 45.5 (32.4–58.6) | 36.2 (25.5–46.9) | 54.3 (45.0–63.6) | 57.6 (48.1–67.1) | 47.1 (41.9–52.3) |
| Age 10–14 y | 54.0 (40.3–67.7) | 49.2 (29.2–69.2) | 50.0 (23.5–76.5) | 60.5 (42.8–78.2) | 67.1 (51.7–82.5) | 52.6 (42.9–62.2) |
| Age 15–19 y | 51.0 (42.2–59.9) | 39.3 (24.0–54.7) | 30.7 (18.5–42.8) | 49.8 (40.7–58.9) | 50.1 (38.0–62.2) | 42.6 (36.9–48.3) |
| VL <50 copies/mL | ||||||
| Total | 43.0 (35.9–50.2) | 31.9 (20.2–43.6) | 28.0 (18.0–37.9) | 46.2 (36.1–56.4) | 48.3 (38.6–58.1) | 36.9 (31.9–42.0) |
| Age 10–14 y | 43.6 (31.0–56.1) | 32.3 (16.2–48.2) | 31.6 (9.2–54.5) | 56.4 (38.6–74.1) | 58.1 (41.8–74.4) | 38.8 (30.0–47.7) |
| Age 15–19 y | 42.7 (34.3–51.0) | 31.3 (16.7–45.8) | 26.5 (15.5–37.6) | 38.8 (29.5–48.1) | 40.9 (29.5–52.4) | 35.4 (29.8–41.0) |
| VLS in those reporting ART use | ||||||
| Total | 78.0 (69.7–86.4) | 63.8 (43.4–84.3) | 76.2 (60.0–92.4) | 73.8 (63.6–84.1) | 75.4 (67.0–83.8) | 73.8 (67.0–80.7) |
| Age 10–14 y | 70.9 (56.3–85.5) | 61.0 (32.3–89.6) | 71.6 (32.4–100) | 75.3 (58.1–92.5) | 81.9 (68.4–95.3) | 68.7 (56.6–80.8) |
| Age 15–19 y | 84.2 (76.1–92.3) | 70.0 (49.9–90.1) | 78.4 (63.4–93.5) | 72.5 (62.0–83.0) | 70.1 (58.9–81.3) | 78.9 (72.8–84.9) |
| VLS in those with detectable ARVs | ||||||
| Total | 79.7 (72.3–87.1) | 73.9 (52.8–95.0) | 84.5 (69.2–99.8) | 78.6 (68.9–88.3) | 80.0 (71.9–88.2) | 79.0 (72.3–85.6) |
| Age 10–14 y | 75.1 (62.8–87.4) | 69.2 (39.6–98.8) | 80.0 (39.6–100) | 77.1 (61.3–92.8) | 84.7 (71.9–97.5) | 74.4 (62.7–86.1) |
| Age 15–19 y | 83.7 (75.0–92.4) | 84.4 (67.7–100) | 86.7 (74.3–99.1) | 80.1 (70.1–90.1) | 75.9 (65.1–86.6) | 83.6 (77.8–89.4) |
| VL <50 copies/mL in those with detectable ARVs | ||||||
| Total | 67.4 (58.4–76.3) | 56.7 (35.8–77.6) | 76.0 (58.6–93.4) | 68.3 (56.2–80.5) | 67.5 (57.6–77.5) | 66.1 (58.7–73.5) |
| Age 10–14 y | 62.1 (47.6–76.7) | 48.7 (21.2–76.2) | 77.7 (32.7–100) | 71.8 (54.3–89.2) | 73.1 (56.3–90.0) | 60.4 (48.0–72.8) |
| Age 15–19 y | 72.0 (61.4–82.5) | 74.1 (53.7–94.4) | 75.3 (59.3–91.2) | 64.5 (51.0–78.8) | 62.7 (49.8–75.6) | 71.8 (64.6–79.0) |
Abbreviations: ART, antiretroviral treatment; ARV, antiretroviral; CI, confidence interval; HIV, human immunodeficiency virus; IQR, interquartile range; VL, viral load; VLS, VL suppression <1000 copies/mL.
aAll numerators and denominators in this table are crude, whereas percentages are weighted unless otherwise indicated.
bMalawi did not include an interview for adolescents aged 10–14 year; therefore, no data on sexual activity were included for this group.
cParental vital status collected only in participants aged 10–17 years.
dAdolescents with previously diagnosed HIV include those who reported prior diagnosis of HIV or who had detectable ARVs.
eAge at HIV diagnosis in those with previously diagnosed HIV. This was not collected for young adolescents in Malawi, or for those who did not report prior diagnosis.
f“On ART” includes those who reported taking ART or those who had detectable ARVs.
Figure 1.
A, Human immunodeficiency virus (HIV) prevalence by age, sex, and country in adolescents aged 10–19 years in the southern African Population-based HIV Impact Assessments (PHIAs), 2015–2017. Data are weighted estimates and include jackknife estimates of variance; bars represent 95% confidence intervals (CIs). B, Prevalence of perinatally acquired HIV by age, sex, and country in adolescents aged 10–19 years in the southern African PHIAs, 2015–2017. Data are weighted estimates and include jackknife estimates of variance. Perinatal classification was based on criterion 2 of the mode of transmission algorithm. Colored bars indicate 95% CIs.
Table 2.
Population Projections of Adolescents Aged 10–19 Years Living with Human Immunodeficiency Virus (HIV) Based on Southern African Population-Based HIV Impact Assessment Data and Joint United Nations Programme on AIDS/HIV Spectrum Models, 2015–2017
| Estimated Population | Zimbabwe 2015–2016 | Malawi 2015–2016 | Zambia 2016 | Eswatini 2016–2017 | Lesotho 2016–2017 | Total |
|---|---|---|---|---|---|---|
| Spectrum estimate of ALHIV, no. (PB)a | 78 000 (55 000–100 000) | 63 000 (44 000–81 000) | 67 000 (47 000–89 000) | 11 000 (7600–14 000) | 14 000 (9300–21 000) | 233 000 (163 000–305 000) |
| PHIA estimate of total ALHIV, no. (95% CI) | 101 000 (84 000–118 000) | 71 000 (51 000–92 000) | 61 000 (49 000–73 000) | 12 000 (10 000–14 000) | 16 000 (12 000–19 000) | 262 000 (233 000–291 000) |
| PHIA estimate of ALHIV with undiagnosed HIV, no. (95% CI)b | 28 000 (20 000–37 000) | 28 000 (16 000–41 000) | 37 000 (26 000–47 000) | 3000 (2000–4000) | 4000 (2000–5000) | 100 000 (81 000–118 000) |
| Estimates of ALHIV classified as APHIV, no. (% of all ALHIV) |
||||||
| Spectrum estimatea (%) | ||||||
| Female | 29 000 (64) | 23 000 (59) | 21 000 (49) | 3000 (43) | 4000 (44) | 80 000 (56) |
| Male | 29 000 (89) | 23 000 (94) | 22 000 (80) | 4000 (99) | 4000 (84) | 82 000 (88) |
| PHIA estimate: criterion 1c (%) | ||||||
| Female | 35 000 (60) | 23 000 (55) | 11 000 (27) | 3000 (49) | 5000 (53) | 77 000 (49) |
| Male | 26 000 (60) | 23 000 (78) | 10 000 (49) | 4000 (74) | 5000 (73) | 68 000 (64) |
| PHIA estimate: criterion 2c (%) | ||||||
| Female | 45 000 (77) | 26 000 (62) | 15 000 (37) | 4000 (64) | 7000 (72) | 97 000 (62) |
| Male | 40 000 (93) | 26 000 (89) | 16 000 (71) | 5000 (90) | 6000 (93) | 92 000 (87) |
Abbreviations: ALHIV, adolescents living with human immunodeficiency virus (HIV); APHIV, perinatally infected ALHIV; CI, confidence interval; PB, plausibility bound; PHIA, Population-based HIV Impact Assessment; UNAIDS; Joint United Nations Programme on HIV/AIDS.
aUNAIDS Spectrum estimates are from year of survey completion: 2016 in Zimbabwe, Malawi, and Zambia, and 2017 in Eswatini and Lesotho, and were generated in October 2018.
bEstimates of undiagnosed HIV were derived from the proportion of ALHIV who did not report a known HIV-positive status and who had no detectable antiretrovirals in the blood.
cCriterion 1 includes participants who did not test as recently infected with an HIV-1 limiting antigen avidity assay, did not have an HIV-negative mother or report sex before their HIV diagnosis, and who reported their HIV diagnosis or initiation into care before the age of 15 years. Criterion 2 builds on criterion 1 by also considering as APHIV those 15–19-year-olds with a biological mother who tested HIV positive during the survey or who was reported as deceased by the head of household, or, if maternal data were missing, if the participant reported never having been sexually active.
Most ALHIV had HIV previously diagnosed (60.9%; 95% CI, 55.3%–66.6%) (Table 1). Zambia had the lowest proportion of prior diagnoses (40.0%; 95% CI, 29.2%–50.8%), and Eswatini the highest (77.8%; 70.8%–84.8%), translating into a total of 100 000 ALHIV (81 000–118 000) with undiagnosed HIV across the 5 countries. Of those with diagnosed HIV, 91.6% reported receiving ART or had detectable ARV levels. The median reported age at ART initiation was 9 years (interquartile range, 6–13 years; n = 332); 19.5% had initiated ART before 5 years of age, 32.2% between 5 and 9 years, and 48.3% at ≥10 years. Half of ALHIV had detectable ARV levels (51.2%), a proportion was significantly higher among young adolescents than among older ones (57.7% vs 45.9%, respectively; P < .001). The most common ARV detected in young adolescents was nevirapine (29.9%), whereas more older adolescents were receiving efavirenz (37.1%); 1.2% were receiving a protease inhibitor–based regimen. Almost 1 in 5 ALHIV who were reported as having undiagnosed HIV (17.2%; 60 of 290) (Table 1) had detectable ARVs, while of those who reported being untreated, 16.3% (63 of 322) had detectable ARVs.
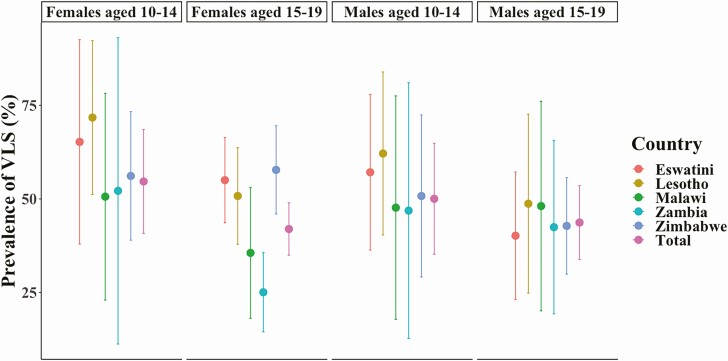
Fewer than half of ALHIV had VLS (47.1%; 95% CI, 41.9%–52.3%), with young adolescents slightly more likely to have VLS (52.6%; 42.9%–62.2%) (Figure 2) than older adolescents (42.6%; 36.9%–48.3%; P = .10). The proportion of ALHIV with VLS was lowest in Zambia (36.2%; 95% CI, 25.5%–46.9%) and highest in Lesotho (57.6%; 48.1%–67.1%). Only one-third of ALHIV (36.9%; 95% CI, 31.9%–42.0%) had a UVL. Overall, 73.8% (95% CI, 67.0%–80.7%) of those reporting ART use had VLS (young adolescents, 68.7% [56.6%–80.8%]; older adolescents, 78.9% [72.8%–84.9%]). Only two-thirds with detectable ARV levels had a UVL (66.1%; 95% CI, 58.7%–73.5%); this was lowest in Malawi, where only 56.7% (35.8%–77.6%) of those with detectable ARV levels overall, and fewer than half of young adolescents (48.7%; 21.2%–76.2%) had a UVL, although sample sizes were small for these populations.
Figure 2.
Prevalence of viral load suppression (VLS; viral load <1000 copies/mL) by age, sex, and country in adolescents living with human immunodeficiency virus (HIV) aged 10–19 years in the southern African Population-based HIV Impact Assessments, 2015–2017. All proportions are weighted using jackknife replicate weights for estimates of variance. Colored bars represent 95% confidence intervals.
Estimates of the Burden of Perinatal Infection
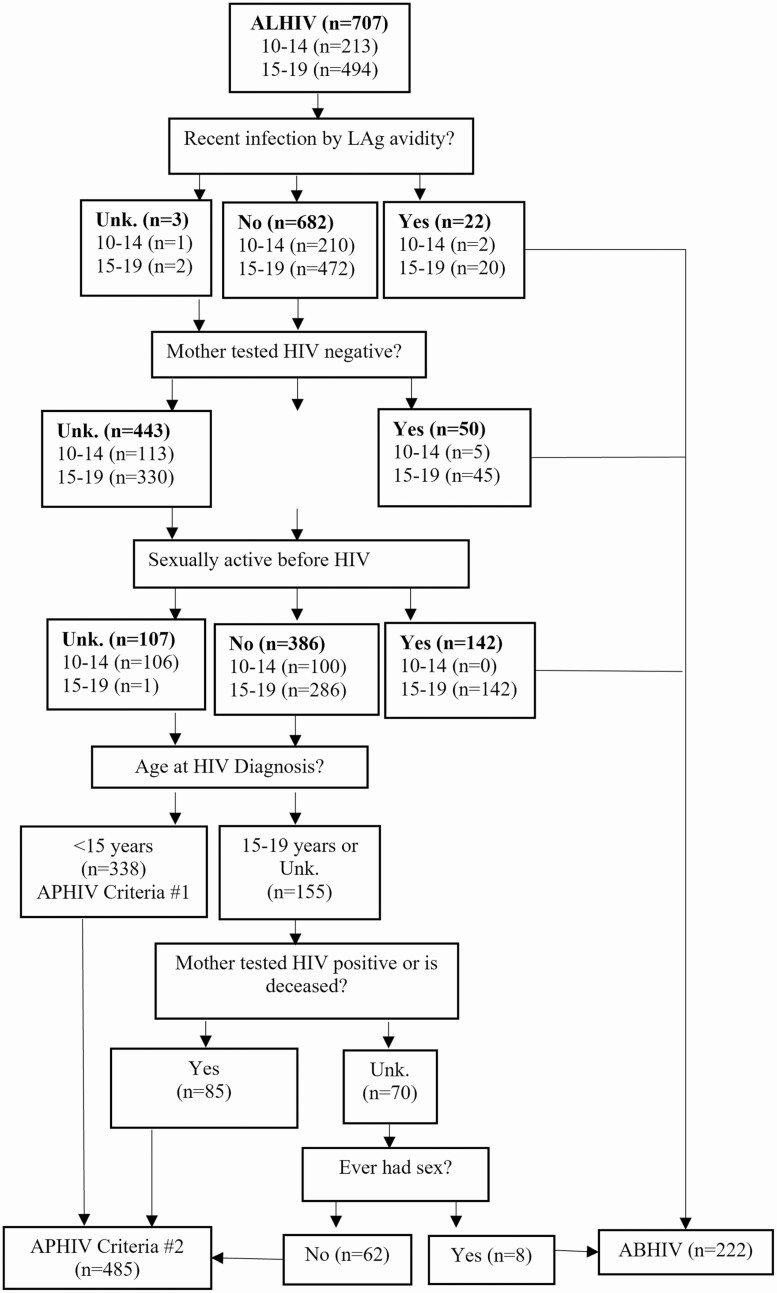
Of 707 ALHIV, 35.9% (n = 254) had a reliable maternal HIV result, and sexual history was collected in 84.0% (n = 594). Overall, 22 (3.1%) were classified as having recent infection (Figure 3). Of the remaining ALHIV, 50 had mothers with an HIV-negative result, and 142 reported sexual activity before receiving an HIV diagnosis, all of whom were aged 15–19 years. This amounted to 26.7% (n = 214) of ALHIV categorized as ABHIV, including 7 young adolescents. Of the 493 remaining ALHIV, 338 received an HIV diagnosis before the age of 15 years (APHIV criterion 1), which gives a weighted proportion of APHIV of 55.4%.
Figure 3.
Flowchart of determination of human immunodeficiency virus (HIV) acquisition risk among adolescents aged 10–19 years in the southern African Population-based HIV Impact Assessments (PHIAs), 2015–2017. Abbreviations: ABHIV, adolescents behaviorally infected with HIV; ALHIV, adolescents living with HIV; APHIV, adolescents perinatally infected with HIV; LAg, limiting antigen; Unk, unknown.
Using additional criteria in the remaining ALHIV whose HIV was not diagnosed before age 15 years, 54.8% (85 of 155) had a mother who tested positive or was deceased, and 88.6% (62 of 70) reported never having sex. This algorithm estimated a weighted proportion of 72.6% of ALHIV (485 of 707) being perinatally infected, or approximately 190 000 (95% PB, 164 000–216 000) adolescents, with previous diagnoses in 69.5% of APHIV and 39.4% of ABHIV. The proportion of infections classified as perinatal was significantly higher in boys than in girls (88.2% [235 of 268]) vs 61.9% [250 of 439], respectively; P < .001). The highest prevalence of perinatal infection was in young male adolescents in Eswatini (4.6%; 95% CI, 2.8%–6.5%), and the lowest in older female adolescents in Malawi (0.5%; .1%–.9%) (Figure 1B). The proportion of ALHIV who were APHIV was similar to cumulative Spectrum estimates (Table 2), although these proportions varied within each country. These results were very similar to estimates generated using the comparison algorithm (Supplementary Appendix, p 2, and Appendix Figure 1).
Treatment Outcomes by Estimated Mode of Transmission
Using criterion 2, 69.8% of APHIV (368 of 473) and 40.5% of ABHIV (95 of 222) had previously diagnosed HIV (P < .001); in 68.7% of APHIV (341 of 473), HIV was undiagnosed or diagnosed at age ≥10 years. These criteria estimated that 55 000 (95% PB, 40 000–71 000) APHIV had undiagnosed HIV. Overall, 65.3% (95% CI, 58.6%–72.0%) of APHIV and 33.5% (24.7%–42.2%) of ABHIV reported ART use (P < .001), and in those with previously diagnosed HIV, ART use was significantly higher in APHIV (93.6%; 95% CI, 89.8%–97.4%) than in ABHIV (82.7%; 72.1%–93.3%) (Table 3). The median age (interquartile range) at ART initiation was 9 (5–11) years for APHIV and 15 (11–17) years for ABHIV. There was no difference by mode of transmission in detection of ARV levels in those reporting ART use (P = .47). VLS was seen in 53.2% of APHIV (95% CI, 46.7%–59.7%) and 30.8% of ABHIV (22.8%–38.7%; P < .001). Of those classified as being on ART, 63.0% (95% CI, 55.2%–70.9%) of APHIV and 51.9% (34.8%–68.9%) of ABHIV had UVL, with slightly higher proportions in those with detectable ARV levels.
Table 3.
Virologic and Immune Outcomes by Previous Human Immunodeficiency Virus (HIV) Diagnosis and Estimated Mode of Transmission Among Adolescents Aged 10–19 Years in the Southern African Population-Based HIV Impact Assessments, 2015–2017a
| Adolescent Characteristic or Outcome | Perinatal HIV Infection | Behavioral HIV Infection | ||||
|---|---|---|---|---|---|---|
| Diagnoseda (n = 368) | Undiagnosed (n = 105) | Total (n = 473) | Diagnoseda (n = 95) | Undiagnosed (n = 127) | Total (n = 222) | |
| Adolescents on ART, % (95% CI)b,c | 93.6 (89.8–97.4) | 0 | 65.3 (58.6–72.0) | 82.7 (72.1–93.3) | 0 | 33.5 (24.7–42.2) |
| Age at ART initiation, median (IQR), yd | 9 (5–11) | NA | NA | 15 (11–17) | NA | NA |
| CD4 cell count, median (IQR), cells/µL | 636 (400–896) | 472 (333–638) | 586 (363–846) | 485 (341–651) | 487 (335–655) | 485 (337–655) |
| Adolescents with CD4 cell count <200/µL, % (95% CI)b | 6.5 (3.5–9.5) | 15.9 (6.8–25.0) | 9.5 (6.2–12.8) | 14.9 (5.4–24.3) | 9.5 (1.9–17.0) | 11.7 (6.1–17.3) |
| Adolescents with VLS, % (95% CI)b | 70.9 (63.6–78.2) | 14.9 (4.3–25.5) | 53.2 (46.7–59.7) | 58.6 (43.0–74.2) | 11.6 (3.7–19.5) | 30.8 (22.8–38.7) |
| Adolescents with VL <50 copies/mL, % (95% CI)b | ||||||
| Total | 59.4 (51.8–67.0) | 4.8 (0–10.2) | 41.9 (35.7–48.1) | 45.9 (31.4–60.5) | 8.8 (1.7–16.1) | 23.8 (16.3–31.4) |
| On ARTc | 63.0 (55.2–70.9) | NA | NA | 51.9 (34.8–68.9) | NA | NA |
| With detectable ARVs | 67.2 (59.3–75.1) | NA | NA | 60.5 (41.4–79.7) | NA | NA |
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; CI, confidence interval; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; VL, viral load; VLS, VL suppression (VL <1000 copies/ml).
aDiagnosed HIV is defined as reporting being aware of one’s HIV-positive status or having detectable ARVs in the blood.
bAll percentages are weighted. CIs were generated using jackknife replicates.
cOn ART is defined as reporting being on ART or having detectable ARVs in the blood.
dMedian age at ART initiation does not include those who did not report being on ART during the survey.
DISCUSSION
This is the first study to provide estimates of the number of undiagnosed ALHIV in several high-burden countries, including in adolescents with perinatal infection, based on robust national data. We found that almost 40% of ALHIV had not been previously diagnosed, most notably in Zambia, where 60% were undiagnosed. Our algorithm also suggests that 30% of those with perinatal infection were untreated but had survived into adolescence. Furthermore, in most ALHIV who were aware of their status, most HIV had had been diagnosed after 10 years of age. The protracted scale-up and expansion of early infant diagnosis services in many resource-limited settings during the time when current adolescents were infants meant many missed opportunities for early testing [22].
For those children who survived into adolescence without treatment, the lack of diagnosis could be attributed to limited availability of testing in pediatric care or adolescent-friendly testing services, as well as gaps in index testing of family members of HIV-positive adults [23, 24]. Stigma and discrimination by health facility staff often prevent young people from accessing testing [4, 25]. The high level of discordance between self-reported status and detectable ARVs, which was greater than in adults [21], likely reflects this stigma, and merits further exploration. Providers are also reluctant to offer testing to this age group, owing to legal issues around consent and disclosure to parents [4, 14]. Finally, adolescents overall have fewer encounters with health services than older adults [26].
Retention in care has also been shown to be problematic in adolescents [2, 7], and although >90% of ALHIV with previously diagnosed HIV reported use of ART, the low rates of VLS reflect high rates of inadequate adherence. Significant challenges remain in providing optimal pediatric HIV care, including the limited number of pediatric care providers in resource-limited settings, and complicated weight-based regimens [27]. The proportion of young adolescents with unsuppressed VL but detectable ARVs might also reflect acquired drug resistance, high levels of which have been shown to be circulating in these countries, although few studies have focused on ALHIV [28, 29]. As seen in our data, the predominant pediatric ART regimens continue to be nonnucleoside reverse-transcriptase inhibitor based [27, 30], which have low barriers to resistance, particularly nevirapine, which has higher rates of virologic failure than efavirenz [31]. Rapid transition to more optimal ART regimens, tailored adherence support, and timely management of elevated VL with a switch to second-line therapy could help improve VLS in this population.
Our data reveal that 55%–73% of ALHIV across the 5 countries were perinatally infected. Although our estimates of the burden of infection in adolescents fell within the Spectrum model PBs, there was significant variation for some countries. Spectrum models of pediatric survival are currently being revised to incorporate breastfeeding data to reflect the lower pediatric mortality rate associated with this mode of acquisition, owing to a lower viral set point in infants [32], which suggests that our estimates are relatively reliable. The significant variation across countries likely reflects historical prevention of mother-to-child transmission and pediatric treatment scale-up differences. The large proportion of APHIV supports the need for differentiated services for adolescents, including mental health screening, and more widely available diagnostics for comorbid conditions [10, 11]. Recognizing the long-term, potentially irreversible impacts of untreated perinatal infection, these adolescents will also need specialized interventions as they age into adulthood, and as they become sexually active and pregnant, if the low rates of VLS persist past adolescence [29]. ABHIV also have very low rates of awareness and VLS, with immunosuppression in 15% of those with HIV diagnosed, indicating that better adolescent-geared testing and care services are necessary as well.
The strengths of the current analysis are the nationally representative samples, the rigorous HIV testing methods for children and their parents, the use of the recency assay, and high participation rates. However, this analysis also has several limitations. We did not directly ask participants or their parents the most likely mode of HIV acquisition and are therefore dependent on several potentially inaccurately reported variables, including some variables with high rates of missing data. However, the testing for ARVs allowed us to correct for many instances of missing data on awareness and treatment.
We did not include a questionnaire for young adolescents in Malawi to ascertain sexual activity data, although we did not exclude any young adolescents as APHIV from the other countries based on this criterion. Adolescents in general, and girls in particular, might be underreporting sexual debut [8, 33]. The limiting antigen avidity assay has not been validated in children <15 years old, meaning that our results for adolescents 10–14 years old should be interpreted with caution. However, the similarities in results between our algorithm and the one used in Kenya [20], where the assay was not used, lends credence to our results. The low number of young adolescents with recent infection suggests that horizontal transmission is rare in children, but quantifying the rate of false recency in adolescents with documented perinatal acquisition is required for assay validation. Finally, World Health Organization treatment guidelines removed immune thresholds in 2015 [34]. Our data likely reflect the slow uptake of these changes, and we therefore expect considerable gains in treatment coverage since the surveys.
In conclusion, these data provide a comprehensive picture of the burden of HIV among adolescents in 5 high-prevalence countries, allowing targeted interventions to improve outcomes in a population with persistently high mortality rates [35–38]. The data demonstrate that many ALHIV have not yet had HIV diagnosed, including many with perinatal infection. To improve case identification, innovative strategies for adolescents should be further explored [12, 24]. Our findings also demonstrate that even among ALHIV with known status, there are critical gaps in VLS. Access to optimized ART, including prioritization of adolescents for transition to dolutegravir-based regimens [27], and adherence support are needed to ensure better treatment outcomes with both immediate and long-term benefits for individual health and epidemic control.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. The authors thank Population-based HIV Impact Assessment (PHIA) participants and survey staff, the Ministries of Health in Zimbabwe, Malawi, Zambia, Eswatini, and Lesotho, and colleagues from the PHIA survey group for their contributions to this study. They would also like to thank Jill Russell for her assistance as a medical editor on this manuscript.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Financial support. This work was supported by the President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention (grant U2GGH001226).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.UNICEF. HIV and AIDS in adolescents. Available at: https://data.unicef.org/topic/adolescents/hiv-aids/. Accessed 30 September 2019.
- 2.Kranzer K, Bradley J, Musaazi J, et al. Loss to follow-up among children and adolescents growing up with HIV infection: age really matters. J Int AIDS Soc 2017; 20:21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F; Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children . Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004; 364:1236–43. [DOI] [PubMed] [Google Scholar]
- 4.Kranzer K, Meghji J, Bandason T, et al. Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: a mixed methods study. PLoS Med 2014; 11:e1001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joint United Nations Programme on HIV/AIDS. Improving UNAIDS’ paediatric and adolescent estimates. Available at: http://www.unaids.org/sites/default/files/media_asset/improving-unaids-paediatric-and-adolescent-estimates_en.pdf. Accessed 19 July 2019.
- 6.Slogrove AL, Schomaker M, Davies MA, et al. ; Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) Global Cohort Collaboration. The epidemiology of adolescents living with perinatally acquired HIV: A cross-region global cohort analysis. PLoS Med 2018; 15:e1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desmonde S, Tanser F, Vreeman R, et al. Access to antiretroviral therapy in HIV-infected children aged 0–19 years in the International Epidemiology Databases to Evaluate AIDS (IeDEA) Global Cohort Consortium, 2004–2015: a prospective cohort study. PLoS Med 2018; 15:e1002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrand RA, Munaiwa L, Matsekete J, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis 2010; 51:844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton JW, Garnett GP, Takavarasha FR, et al. Increasing adolescent HIV prevalence in eastern Zimbabwe—evidence of long-term survivors of mother-to-child transmission? PLoS One 2013; 8:e70447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrand RA, Desai SR, Hopkins C, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis 2012; 55:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHugh G, Rylance J, Mujuru H, et al. Chronic morbidity among older children and adolescents at diagnosis of HIV infection. J Acquir Immune Defic Syndr 2016; 73:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, Chapman J, Goldrath K, Ferrand RA. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis 2014; 14:627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Low A, Thin K, Davia S, et al. Correlates of HIV infection in adolescent girls and young women in Lesotho: results from a population-based survey. Lancet HIV 2019; 6:e613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinnon B, Vandermorris A. National age-of-consent laws and adolescent HIV testing in sub-Saharan Africa: a propensity-score matched study. Bull World Health Organ 2019; 97:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva, Switzerland: World Health Organization, 2016. [PubMed] [Google Scholar]
- 16.Zeh C, Ndiege K, Inzaule S, et al. Evaluation of the performance of Abbott m2000 and Roche COBAS Ampliprep/COBAS Taqman assays for HIV-1 viral load determination using dried blood spots and dried plasma spots in Kenya. PLoS One 2017; 12:e0179316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koal T, Burhenne H, Römling R, Svoboda M, Resch K, Kaever V. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2005; 19:2995–3001. [DOI] [PubMed] [Google Scholar]
- 18.Kim AA, Rehle T. Short communication: assessing estimates of HIV incidence with a recent infection testing algorithm that includes viral load testing and exposure to antiretroviral therapy. AIDS Res Hum Retroviruses 2018; 34:863–6. [DOI] [PubMed] [Google Scholar]
- 19.Mahy M, Penazzato M, Ciaranello A, et al. Improving estimates of children living with HIV from the Spectrum AIDS Impact Model. AIDS 2017; 31:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng’eno BN, Kellogg TA, Kim AA, et al. Modes of HIV transmission among adolescents and young adults aged 10-24 years in Kenya. Int J STD AIDS 2018; 29:800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Y, Milwid RM, Godin A, et al. Accuracy of self-reported HIV testing history and awareness of HIV-positive status among people living with HIV in four Sub-Saharan African countries. AIDS 2020; 35:503–10. [DOI] [PubMed] [Google Scholar]
- 22.Joint United Nations Programme on HIV/AIDS. Start free stay free AIDS free. 2017 progress report. Available at: https://www.unaids.org/sites/default/files/media_asset/JC2923_SFSFAF_2017progressreport_en.pdf. Accessed 1 September 2019.
- 23.Sam-Agudu NA, Folayan MO, Ezeanolue EE. Seeking wider access to HIV testing for adolescents in sub-Saharan Africa. Pediatr Res 2016; 79:838–45. [DOI] [PubMed] [Google Scholar]
- 24.Chikwari CD, Dringus S, Ferrand RA. Barriers to, and emerging strategies for, HIV testing among adolescents in sub-Saharan Africa. Curr Opin HIV AIDS 2018; 13:257–64. [DOI] [PubMed] [Google Scholar]
- 25.Strauss M, Rhodes B, George G. A qualitative analysis of the barriers and facilitators of HIV counselling and testing perceived by adolescents in South Africa. BMC Health Serv Res 2015; 15:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrand RA, Trigg C, Bandason T, et al. Perception of risk of vertically acquired HIV infection and acceptability of provider-initiated testing and counseling among adolescents in Zimbabwe. Am J Public Health 2011; 101:2325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penazzato M, Townsend CL, Rakhmanina N, et al. ; PADO4 participants . Prioritising the most needed paediatric antiretroviral formulations: the PADO4 list. Lancet HIV 2019; 6:e623–31. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. HIV drug resistance report 2019. Geneva, Switzerland: World Health Organization, 2019. [Google Scholar]
- 29.Tassiopoulos K, Moscicki AB, Mellins C, et al. ; Pediatric HIV/AIDS Cohort Study . Sexual risk behavior among youth with perinatal HIV infection in the United States: predictors and implications for intervention development. Clin Infect Dis 2013; 56:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. Antiretroviral medicines in low- and middle-income countries: forecasts of global and regional demand for 2014–2018. Available at: https://www.who.int/hiv/pub/amds/arv-forecast2014-2018/en/. Accessed 13 November 2019.
- 31.Lowenthal ED, Ellenberg JH, Machine E, et al. Association between efavirenz-based compared with nevirapine-based antiretroviral regimens and virological failure in HIV-infected children. JAMA 2013; 309:1803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becquet R, Marston M, Dabis F, et al. ; UNAIDS Child Survival Group . Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One 2012; 7:e28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowan FM, Pascoe SJ, Langhaug LF, et al. ; Regai Dzive Shiri trial team . The Regai Dzive Shiri project: results of a randomized trial of an HIV prevention intervention for youth. AIDS 2010; 24:2541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: World Health Organization, 2015. [PubMed] [Google Scholar]
- 35.Armstrong A, Nagata JM, Vicari M, et al. A global research agenda for adolescents living with HIV. J Acquir Immune Defic Syndr 2018; 78:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrand RA. Attrition from HIV care among adolescents and adults in a low-income setting. Public Health Action 2016; 6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boerma RS, Boender TS, Bussink AP, et al. Suboptimal viral suppression rates among HIV-infected children in low- and middle-income countries: a meta-analysis. Clin Infect Dis 2016; 63:1645–54. [DOI] [PubMed] [Google Scholar]
- 38.Enane LA, Vreeman RC, Foster C. Retention and adherence: global challenges for the long-term care of adolescents and young adults living with HIV. Curr Opin HIV AIDS 2018; 13:212–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.