Abstract
Background
Chagas disease is an infectious disease caused by the parasite Trypanosoma cruzi and is endemic from Latin American countries. The goal of our study was to identify novel genetic loci associated with chronic Chagas cardiomyopathy development in Chagas disease patients from different Latin American populations.
Methods
We performed a cross-sectional, nested case-control study including 3 sample collections from Colombia, Argentina, and Bolivia. Samples were genotyped to conduct a genome-wide association study (GWAS). These results were meta-analyzed with summary statistic data from Brazil, gathering a total of 3413 Chagas disease patients. To identify the functional impact of the associated variant and its proxies, we performed an in silico analysis of this region.
Results
The meta-analysis revealed a novel genome-wide statistically significant association with chronic Chagas cardiomyopathy development in rs2458298 (OR = 0.90, 95%CI = 0.87–0.94, P-value = 3.27 × 10-08), nearby the SAC3D1 gene. In addition, further in silico analyses displayed functional relationships between the associated variant and the SNX15, BAFT2, and FERMT3 genes, related to cardiovascular traits.
Conclusions
Our findings support the role of the host genetic factors in the susceptibility to the development of the chronic cardiac form of this neglected disease.
Keywords: Chagas disease, cardiomyopathy, genetic association, GWAS, Latin American, meta-analysis, Trypanosoma cruzi
A genome-wide association study of 3413 samples from different Latin American countries identifies a significant genetic loci associated with the differential development of chronic Chagas cardiomyopathy.
Chagas disease is an infectious disease caused by the parasitic protozoan Trypanosoma cruzi [1] that, according to the World Health Organization (WHO), affects about 6–7 million people and its endemic areas are spread in 21 Latin American countries (www.who.int/health-topics/chagas-disease#tab=tab_1). Chagas disease is considered as an emerging infection as cases have been reported out of the endemic areas of transmission, mainly in the United States and Europe, being a consequence of migration and globalization processes [1]. The disease comprises an acute phase, which occurs after the entry of the parasite into the host, and a chronic phase, which can occur even decades after the first contact with the infectious agent; however, approximately 30% of patients evolve to this chronic phase [1]. The hypothesis of the implication of host genetic component in the differential susceptibility to Chagas disease arises from the high exposure to T. cruzi and the differential susceptibility to the infection in endemic areas, in addition to the remarkable variation in the disease progression during the chronic phase [1, 2]. In spite of the intensive investigation of the genetic basis of Chagas disease using the candidate gene strategy, our understanding of it remains elusive. These studies have analyzed specific genetic polymorphisms mainly located in genes involved in the immune response, such as chemokines, cytokines, and the major histocompatibility complex; however there are recognized important limitations to identify solid genetic associations [3, 4]. Some of these limitations include reduced sample sizes and the assessment of a limited number of variants, usually based on plausible biological pathways involved in the trait or disease, precluding replication [4]. In this sense, genome-wide association studies (GWAS) have contributed to the assessment of thousands of genetic variants associated with the susceptibility to complex traits, taking a step forward in the understanding of its genetic basis [5]. Regarding Chagas disease, only one GWAS has been performed in Brazilian population in chronic Chagas cardiomyopathy showing suggestive associations [6].
In order to better elucidate the genetic basis of Chagas disease and chronic Chagas cardiomyopathy, herein we perform a large GWAS and a meta-analysis of Latin American populations that, in combination with complementary in -silico functional evidence, would provide further insights into the pathogenesis of this neglected disease.
MATERIALS AND METHODS
Study Populations, Sample Collection and Ethical Considerations
Samples from 3 different Latin American countries: Colombia, Bolivia, and Argentina were included in this study and meta-analyzed with data from the previous GWAS in a Brazilian population, comprising a total of 3699 genomic DNA recruited samples. The description of sample origin is available elsewhere [7]. Chagas disease cases were defined by their serological status using recombinant antigen and a commercial indirect hemagglutination test for ELISA assays. Participants were classified as seropositive and seronegative for T. cruzi antigens and comparisons were performed among them in order to assess susceptibility to the infection in the Colombian and Argentinian cohort. The Bolivian and Brazilian cohorts were composed only of seropositive individuals. In addition, all the seropositive patients recruited in this study were subjected to electrocardiograms, echocardiograms, and chest radiography to identify cardiac involvement. For the assessment of the chronic Chagas cardiomyopathy susceptibility, those patients with cardiac abnormalities were compared to asymptomatic individuals. Data included from the Brazilian cohort were described elsewhere [6]. Sample size and demographic information are summarized in Table 1.
Table 1.
Demographical Characteristics and Classification of Data Collections
| Seropositive | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Colombia | Bolivia | Argentina | Brazila | Seronegative | |||||
| CCC | ASY | CCC | ASY | CCC | ASY | CCC | ASY | Colombia | |
| Subjects, n | 577 | 442 | 97 | 518 | 100 | 62 | 207 | 306 | 1104 |
| Sex (% females) | 280 (48 %) | 282 (64%) | 57 (59%) | 364 (70%) | 65 (65%) | 40 (65%) | 81 (39%) | 154 (53%) | 672 (61%) |
| Age (Mean ± SD) | 67.4 ± 14.3 | 57.2 ± 15.6 | 50.7 ± 9.4 | 46.4 ± 9.5 | 58.2 ± 9.4 | 47.5 ± 14.0 | - | - | 49.5 ± 16.7 |
The sample sizes refer to data passing the genotyping quality controls.
Abbreviations: CCC, chronic Chagas cardiomyopathy; ASY, asymptomatic.
a Data set from Deng et al, 2013
The study was approved by the Ethics Committees from the Industrial University of Santander and Cardiovascular Foundation, Colombia (Act No. 15/2005), the Vall D’Hebron University Hospital, Barcelona, Spain (PR (AMI) 297/2016), and the National Hospital of Clinics, National University of Cordoba, Argentina (CIEIS HNC 118/2012 and 2/16/2017). The Ethics Committees for the Brazilian cohort was described elsewhere [6]. Protocols used in the study followed the principles of the Declaration of Helsinki, and all individuals included in the study signed written informed consents.
Genotyping, Quality Control and Imputation
Genomic DNA from blood samples from the Colombia, Bolivian, and Argentinian cohorts were isolated using the QIAamp MidiDNA Kit following manufacturer’s recommendations. The samples were genotyped using the Global Screening Array Platform (Illumina Inc., San Diego, CA, USA). Quality controls (QCs) of genotyped data and consistency of sex assignment were performed using Plink v.1.9 [8]. Individuals and variants with missing genotype rate <5% were excluded. Single nucleotide polymorphisms (SNPs) with different call rates between cases and controls (P-value < .05), or deviating from Hardy-Weinberg equilibrium (HWE) with a P-value ≤ 1 × 10-06 in the control group were removed.
A principal component analysis (PCA) was performed using Plink1.9 to compare our data with the data from “The 1000 Genomes Project” (1KGP). The genomic inflation factor (λ) was calculated to control the type I error rate with the package “gap” for R version 3.6.1.
After QCs, genotyped data were imputed with the Michigan Imputation Server using the admixed American population from 1KGP phase 3 as reference panel [9]. After imputation, variants were filtered out by their minor allele frequency (MAF < 1%) and the imputation quality metric Rsq < 0.3.
Statistical Analysis
For each case-control collection, association testing for imputed allele dosages was performed using a mixed model with EMMAX [10] implemented in EPACTS (https://genome.sph.umich.edu/wiki/EPACTS). Given the common environmental exposure to triatomine vectors of individuals living together in endemic areas, related individuals were maintained in the analysis. In this sense, genotyped data were used to calculate the pairwise identity by descent (IBD) proportion (PI_HAT), duplicated individuals were removed (PI_HAT > 0.8) and related individuals were considered with a PI_HAT ≥ 0.4. EMMAX calculates the kinship matrix to include it as a covariate in the statistical analysis, as well as sex and age. The summary-level statistics of each cohort, including the Brazilian cohort, were meta-analyzed using METASOFT [11] under a fixed and random-effect models based on variants’ heterogeneity (Cochran’s Q test P-value). As most of imputed variants (>95%) did not show heterogeneity (Cochran’s Q test P-value > .05), those analyzed under a random-effect model were omitted from further analysis. Conservative suggestive associations were established at a P-value < 1 × 10-06 and genome-wide significance at P-value < 5 × 10-08 as usually employed for GWAS. The SNPnexus software (https://www.snp-nexus.org/v4/) was used to annotate the suggestive and significant signals, and LocusZoom (http://locuszoom.org/) for the regional association plots.
Functional Annotation of Associated Variants
An insilico approach was used to investigate the potential biological consequences of the associated variant. The LDlink tool [12] was used to calculate proxies in high linkage disequilibrium (LD) with the top signal (r2 > 0.8) and different databases were queried. The Open Targets Genetic was used to assess their functional implication (https://genetics.opentargets.org/), and complemented with PheWeb version 1.1.17 (http://pheweb.sph.umich.edu/SAIGE-UKB/) to assess these variants in previous Phenome-Wide Association Studies (PheWAS). We used HaploReg v4.1 [13] to evaluate regulatory genomic regions and Capture HiC Plotter [14] to assess long-distance physical interactions. RegulomeDB (https://regulomedb.org/regulome-search/) and GTEx (https://gtexportal.org/home/) were used to evaluate expression-quantitative trait loci (eQTLs) in relevant tissues.
RESULTS
A total of 3413 postQCs samples from 4 independent Latin American cohorts from Colombia, Bolivia, Argentina, and Brazil were included in this study. The Supplementary Table 1 summarizes the pre and post QC information within each dataset. After QCs and imputation, 7 846 902 SNPs for the Colombian and 8 408 292 SNPs for the Argentinian cohorts were analyzed correcting by age, sex, and kinship in the case of susceptibility to the infection by T. cruzi analysis, while in the case of chronic Chagas cardiomyopathy development, a total of 8 218 190 SNPs were tested in the meta-analysis including the Colombia, Bolivian, Argentinian, and Brazilian populations.
Population Structure
Given the ethnic admixture of Latin American populations, the first 2 principal components were used to evaluate the genetic heterogeneity existing in the samples included in this study and in comparison with the admixed American subpopulation from 1KGP (Supplementary Figure 1). The results of these analyses showed that the heterogeneity present in our samples is consistent with the observed in the admixed American subpopulation of 1KGP. Despite this, the Argentinian and Bolivian sample collections seemed to be more homogenous, while a considerable genetic variability with the Brazilian cohort was detected, reinforcing the high degree of genetic admixture of this population. In the association analyses within each cohort, the effect of population structure was taken into account by using a mixed-model of association [10].
Differential Susceptibility to Trypanosoma cruzi Infection
Individuals from the Colombian and Argentinian cohorts were classified and compared according to their serological status in seropositive and seronegative for T. cruzi infection. No statistically relevant results were identified in the association test in the Argentinian population, mainly due to the reduced sample size of this cohort. However, in the case of the Colombian cohort, we detected 7 suggestive associations (P-value < 1 × 10-06) contained in 4 different loci due to the LD (Table 2, Supplementary Figure 2). The strongest association is an intronic variant of the EBF2 gene (rs147475322; P-value = 2.15 × 10-07, Odds Ratio [OR] = 1.20, 95% Confidence Interval [95%CI] 0.76–1.90). The other 3 SNPs are located in an intronic region of CD247 gene (rs554994388) and map downstream and upstream of SIK1 (rs229347) and IL18 (rs4937075) genes, respectively (Table 2). Remarkably, a different SNP within the IL18 gene was previously associated in a candidate gene assessment [7]; however, this variant was nominally associated in our study and did not reach the suggestive level of significance.
Table 2.
Suggestivea Signals Associated with Susceptibility to Trypanosoma cruzi Infection in the Colombian Cohort
| Chr | Locus | Bp | SNP | SNP status | Rsq | Effect allele | Allele Freq | P -value | OR (95% CI) | Function |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CD247 | 167431884 | rs554994388 | Imputed | 0.50 | G | 0.03 | 5.35E-07 | 1.33 (1.19–1.49) | Intronic |
| 8 | EBF2 | 25979367 | rs147475322 | Imputed | 0.86 | A | 0.03 | 2.15E-07 | 1.20 (0.76–1.90) | Intronic |
| 11 | IL18 | 112127040 | rs4937075 | Imputed | 0.77 | C | 0.77 | 8.34E-07 | 1.02 (0.86–1.21) | Intergenic |
| 21 | SIK1 | 43428190 | rs229347 | Imputed | 0.88 | A | 0.60 | 8.93E-07 | 1.07 (1.04–1.10) | Intergenic |
aSuggestive associations were considered with a P-value ≤ 1E-06
Associated loci were determined in order to the LD of contained variants and the most significant SNP of each loci were reported in the table.
The r-squared metric (Rsq) indicates the correlation between input genotypes and imputed dosages.
Abbreviations: Allele Freq, allele frequency; bp, base pair; chr, chromosome; CI, confidence interval; OR, odds ratio; SNP info, SNP information.
Differential Susceptibility to the Development of Chronic Chagas Cardiomyopathy
In order to identify novel genetic variants associated with differential chronic Chagas cardiomyopathy development, seropositive samples were stratified according to their cardiological status, and patients with cardiac abnormalities were compared to asymptomatic individuals in the association test.
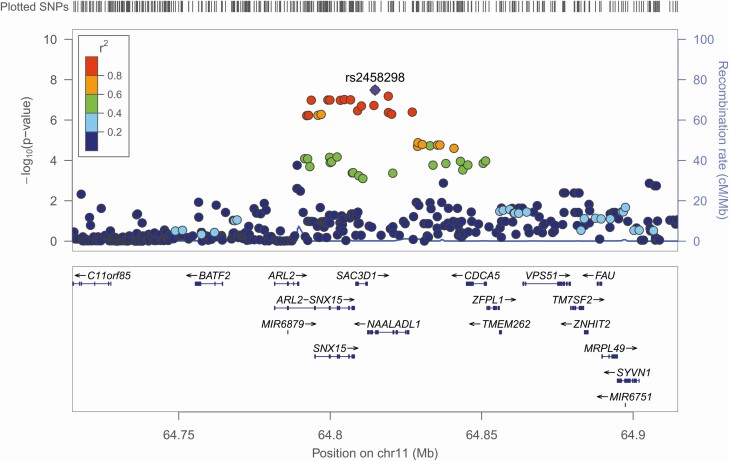
After QCs, genotype imputation, and filtering, a case-control association analysis from GWAS data was performed in the Colombian, Bolivian and Argentinian samples comprising a total of 2309 individuals (981 chronic cardiac patients and 1328 asymptomatic individuals). After correcting by age, sex, and kinship, deviations in genomic inflation factors were not observed (Supplementary Table 1, Supplementary Figure 3). Next, summary statistics from every cohort, including the Brazilian data, were meta-analyzed by an inverse variance-weighted effect size and those shared by at least 2 data sets were considered. Additionally, we did not observe inflation in the meta-analysis (λ = 1.03). As result, one statistically significant associated signal was identified at GWAS-level (P-value < 5 × 10-08) and 22 suggestive associations (P-value < 1 × 10-06) in 3 different autosomal loci (Table 3; Figure 1; Supplementary Table 2). The strongest association (rs2458298; P-value = 3.27 × 10-08, OR = 0.90, 95% CI 0.87–0.94) is located in chromosome 11 in an intronic region of the NAALADL1 gene. This signal is followed by several proxy variants in high or moderate LD (r2 > 0.4) located nearby NAALADL1, SAC3D1, and SNX15 genes (Figure 2). Regarding the rest of the suggestive signals, they are located in intergenic regions close to CDH8 and KLF4 genes (Table 3).
Table 3.
Genome-Wide Significant Association and Suggestivea Signals Associated with Chronic Chagas Cardiomyopathy Development in the Meta-Analysis
| Chr | Locus | Bp | SNP | SNP status | Rsq | Effect allele | Allele Freq | P-value Q | P -value | OR (95% CI) | Function |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | CDH8 | 18718887 | rs10472156 | Imputed | 0.81 | A | 0.73 | .72 | 8.89E-07 | 1.10 (1.06–1.14) | Intergenic |
| 9 | KLF4 | 107479876 | rs10759240 | Genotyped | - | C | 0.54 | .98 | 4.68E-07 | 0.92 (0.89–0.95) | Intergenic |
| 11 | SAC3D1 | 65047341 | rs2458298 | Imputed | 0.95 | G | 0.26 | .98 | 3.27E-08 | 0.90 (0.87–0.94) | Intronic |
aSuggestive associations were considered with a P-value ≤ 1E-06
Associated loci were determined in order to the LD of contained variants and the most significant SNP of each loci were reported in the table.
In boldface the genome-wide significant signal.
The r-squared metric (Rsq) indicates the correlation between input genotypes and imputed dosages.
Abbreviations: Allele Freq, allele frequency; bp, base pair; chr, chromosome; CI, confidence interval; OR, odds ratio; P-value Q Cochran’s Q test; rsq, r-square; SNP info, SNP information.
Figure 1.
Manhattan plot of the association test in chronic Chagas cardiomyopathy meta-analysis results. Y and X axes refer the –log10 transformed P-values and positions in chromosomes, respectively. The red horizontal line refers the genome-wide association threshold, stablished at P < 5 × 10-08, and the blue line refers the suggestive threshold (P < 1 × 10-06).
Figure 2.
Regional association plot for the genome-wide significant signal. The –log10 transformed P-values of associated variants are plotted by position. The genomic position indicated correspond the top variant associated in this region. The rest of the variants are colored in function of their degree of linkage disequilibrium (LD) with the associated signal. LD is based on pairwise r2 values from the American population from the 1000 Genomes Project. The blue line corresponds to the estimated recombination rates. Nearby genes to the associated and suggestive signals are shown in the bottom of the plot.
Functional Analysis
To explore functional features of the genomic association obtained from the meta-analysis in chronic Chagas cardiomyopathy, several databases were queried. Regarding the top associated variant rs2458298, although is located in an intronic region of NAALADL1, it is closer to the transcription start site (TSS) of SAC3D1, being this considered as its nearest gene. PheWAS information from Open Targets Genetics revealed a risk association of this SNP with cardiovascular traits such as hypertension (P-valuePheWas = 7.4 × 10-06) and high blood pressure (P-valuePheWas = 5.8 × 10-06) in the UK Biobank database, as well as with abdominal aortic aneurysm (P-valuePheWas = 1.9 × 10-03) in a subset of patients. Significant eQTLs (ie, loci that explain part of the variance in gene expression in specific tissues) were described identifying rs2458298 as correlated with SNX15 expression in heart atrial appendage (P-value = 9.9 × 10-10) and heart left ventricle (P-value = 1.3 × 10-11). The same SNP was also correlated with the expression of FERMT3 in a lymphoblastoid cell line. Using the Capture Hi-C Plotter platform, 42 interactions of this SNP with different gene regions across chromosome 11 were identified in the experiments done by Mifsud and colleagues [15] in a lymphoblastoid cell line (GM12878). One of those relevant promoter interactions is with the BATF2 gene, which has been previously implicated in T. cruzi infection [16]. All these results are summarized in Table 4.
Table 4.
In Silico Functional Analysis of the Associated Variant with Chronic Chagas Cardiomyopathy Development
| Chr | SNP | Nearest gene | eQTLs | C-HiC Genes | PheWAS |
|---|---|---|---|---|---|
| 11 | rs2458298 | SAC3D1 NAALADL1 | SNX15 a | BATF2 in GM12878b | Hypertension, high blood pressure and abdominal aortic aneurysm |
| FERMT3 b |
aeGENE in heart atrial appendage and heart left ventricle
blymphoblastoid cell line
Queried data bases were Open Targets Genetics, GTEx, RegulomeDB, Capture HiC Plotter, and PheWEB
Abreviations: Chr, chromosome; C-HiC, capture Hi-C; eQTL, expression quantitative trait loci; PheWAS, phenome-wide association study; SNP, single nucleotide polymorphism.
DISCUSSION
Several suggestive loci have been identified in association with differential susceptibility to T. cruzi infection in our study. Among these variants, highlight those located in intergenic regions near IL18 and CD247 given their implication in the immune response [17, 18]. IL18 encodes the interleukin-18, a proinflammatory cytokine that is involved in the innate and adaptive response, and is critical for T-cell differentiation into interferon-γ (IFN-γ) producing Th1-type T cells and for IFN-γ production by NK cells [18]. IFN-γ is a key player in the acute T. cruzi infection and a pathogen resistance gene, aiding the control of T. cruzi parasitism by stimulating surrounding cells to produce TNF-α and other inflammatory mediators leading to the generation of peroxynitrite [19]. Additionally, IFN-γ gene expression has been reported to increase in chronic cardiac patients in several studies conducted in Latin American populations, indicating an important role of this molecule in the pathogenesis of the chronic cardiac form through the induction of inflammatory damage [20]. Here, we confirm the association of this gene with the differential susceptibility to T. cruzi infection; although given the directed nature of candidate gene studies, different SNPs of the same locus were reported [7, 21].
Regarding CD247, this gene encodes a subunit of the T-cell receptor CD3 complex that plays an important role in antigen recognition [17]. In Chagas disease, previous works have confirmed lower expression of CD3 in isolated blood cells from individuals with high exposure to the parasite in Colombian populations [22]. Finally, genetic variants in the CD247 were also previously associated with autoimmunity [23–25] pointing to the existence of shared genes in autoimmune and infectious diseases as described elsewhere [26]. In this way, our results reinforce the potential implication of IL18 and CD247 during immune response against T. cruzi.
Remarkably, a statistically significant association at genomic level was identified near the SAC3D1 gene region (rs2458298) with the development of chronic Chagas cardiomyopathy. SAC3D1, also known as SHD1, has been identified as a transcriptional regulator of STAT5 [27], also associated with cardioprotection in humans [28]. Additionally, the STAT-5 signaling by IL-2, IL-7, and IL-15 receptors has been shown to be perturbed in peripheral and heart-infiltrating T cells in chronic Chagas cardiomyopathy [29].
An in silico functional assessment of the most associated variant and its proxies was performed in order to acknowledge the interactions and processes where they are involved. Interestingly, physical contact of chromatin regions in this genetic location revealed interaction with BATF2. This gene encodes a transcription factor (TF) that has been related with Chagas disease, regulating the IL-23-Th17 pathway and suggesting an immunoregulatory function during T. cruzi infection in a mouse model [16]. On the other hand, the top signal has been associated with several cardiovascular traits in PheWas data from UK Biobank. Additionally, this variant is an eQTL of SNX15 in heart atrial appendage and heart left ventricle. This gene encodes a member of the sorting nexin protein family, which has been related with cardiovascular diseases [30]. Additionally, the associated variant was also an eQTL for FERMT3 (Fermitin Family Member 3) in lymphoblastoid cell lines. The FERMT3 belongs to the Kindlins family and it encodes a protein that plays an important role in the regulation of thrombosis, as well as in maintaining the cytoskeleton of erythrocytes [31]. This gene was previously associated with serum triglyceride levels, considered as a risk factor for coronary heart disease [32]. Although the diagnosis of chronic Chagas cardiomyopathy involves the exclusion of other cardiomyopathies, the association with this gene may implicate common pathways among them.
Other suggestive signals are those located in chromosome 9 near to KLF4, which encodes the Kruppel-like factor 4, a TF related with cardiac mitochondrial homeostasis in a mouse model [33], as well as with a high expression of the inducible nitric oxide synthase and nitric oxide (NO) production in different cell types [34]. Taking into account that higher production of NO has been reported in chronic Chagas cardiomyopathy severity [35], it would be possible a relationship among KLF4 and chronic Chagas cardiomyopathy through the NO production.
This study has certain limitations. First, a cross-sectional assessment and the posterior seroconversion or development of cardiac events cannot be ruled out. In this case, the inclusion of cases in the control group will bias the results towards the null hypothesis of no association, weakening the risk of a false positive result. Second, the associated genes have been nominated as related with the disease through an functional assessment in publicly available non-endemic populations. Further functional analyses will guarantee the acceptance or rebuttal of these hypotheses. Finally, the small sample size in the overall study, but especially in the Chagas disease susceptibility assessment, bounds the statistical power for detecting lower frequency variants or with smaller effects. This is the reason why suggestive variants were highlighted, including their potential functional relevance.
The main strength of our study is that we reveal a consistent and reproducible association with chronic Chagas cardiomyopathy in 4 Latin American populations. Genetic studies in admixed populations are challenging given their differential ancestry proportions [36], which is further complicated when taking also into account the genetic diversity of the infectious agent [37]. This heterogeneity has been previously observed in a related parasitic disease such as Leishmania [38]. Further studies evaluating the human–parasite genetic interactions may improve the knowledge of disease pathogenesis.
Finally, the inclusion of underrepresented populations in genetic studies is a great opportunity to broaden the knowledge of complex diseases and deepen their molecular mechanisms [39], which is especially important in Chagas disease, due to its significant socio-economic burden in both endemic and nonendemic countries [40].
CONCLUSION
In summary, in this work we describe the results of a genome-wide meta-analysis of Chagas disease in Latin American populations, identifying a novel locus near the gene region of SAC3D1 in association with the development of chronic Chagas cardiomyopathy. Although larger studies are needed to validate our findings, this study provides important novel leads to understand the pathogenesis of this neglected disease.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Nonstandard Abbreviations: 1KGP, 1000 Genomes Project; CI, confidence interval, eQTL, expression-quantitative trail loci; GWAS, genome-wide association studies; HWE, Hardy-Weinberg equilibrium; IBD, identity by descent; LD, linkage disequilibrium; MAF, minor allele frequency; NO, nitric oxide; OR, Odds ratio; PCA, principal component analysis; PheWAS, Phenome-Wide Association Studies; QC, quality control; SNP, single nucleotide polymorphism; TSS, transcription start site; WHO, World Health Organization.
Human Genes: BATF2; CD247; FERMT3; IL18; KLF4; NAALADLI; SNX15; SPC3S1;
Notes
Acknowledgments. We thank all the patients who participated in this study and the Medical team from the different Latin American countries and Spain. This research is part of the doctoral degree awarded to D. C. M., within the Biomedicine program from the University of Granada entitled “Bases moleculares de la enfermedad de Chagas: Integrando Genómica, Transcriptómica y Epigenómica.”
Financial support. This research was supported by grants from Red Iberoamericana de medicina genómica en enfermedad de Chagas (RIMGECH–217RT0524)—CYTED and Ministerio de Ciencia y Tecnología de Córdoba (PID 2018) and Secretaría de Ciencia y Tecnología (Consolidar 2018–2021), Universidad Nacional de Córdoba, Argentina. M. A. H. was supported by Juan de la Cierva Incorporación fellowship (IJC2018-035131-I) from the Spanish Ministry of Science and Innovation. The SaMi-Trop cohort study is supported by the National Institutes of Health (P50 AI098461-02 and U19AI098461-06). A. L. P. R. is supported in part by CNPq (310679/2016-8 and 465518/2014-1) and by FAPEMIG (PPM-00428-17). E. S. was supported by the National Heart, Lung and Blood Institute Retrovirus Epidemiology Donor Study-II (REDS-II), International Component Contract (HHSN268200417175C). E. C. N. and C. C. were supported by an international program funded both by the Agence Nationale de Recherche (ANR, France) and the São Paulo State Research Funding Agency-(FAPESP, Brazil) agencies (Br-Fr-chagas). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. The authors report no conflict of interests. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Pérez-Molina JA, Molina I. Chagas disease. Lancet 2018; 391:82–94. [DOI] [PubMed] [Google Scholar]
- 2.Chapman SJ, Hill AV. Human genetic susceptibility to infectious disease. Nat Rev Genet 2012; 13:175–88. [DOI] [PubMed] [Google Scholar]
- 3.Cunha-Neto E, Chevillard C. Chagas disease cardiomyopathy: immunopathology and genetics. Mediators Inflamm 2014; 2014:683230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acosta-Herrera M, Strauss M, Casares-Marfil D, Martín J; Chagas Genetics CYTED Network . Genomic medicine in Chagas disease. Acta Trop 2019; 197:105062. [DOI] [PubMed] [Google Scholar]
- 5.Tam V, Patel N, Turcotte M, Bossé Y, Paré G, Meyre D. Benefits and limitations of genome-wide association studies. Nat Rev Genet 2019; 20:467–84. [DOI] [PubMed] [Google Scholar]
- 6.Deng X, Sabino EC, Cunha-Neto E, et al. ; REDSII Chagas Study Group from the NHLBI Retrovirus Epidemiology Donor Study-II Component International . Genome wide association study (GWAS) of Chagas cardiomyopathy in Trypanosoma cruzi seropositive subjects. PLoS One 2013; 8:e79629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strauss M, Acosta-Herrera M, Alcaraz A, et al. ; Chagas Genetics CYTED Network . Association of IL18 genetic polymorphisms with Chagas disease in Latin American populations. PLoS Negl Trop Dis 2019; 13:e0007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015; 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abecasis GR, Auton A, Brooks LD, et al. ; 1000 Genomes Project Consortium . An integrated map of genetic variation from 1092 human genomes. Nature 2012; 491:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang HM, Sul JH, Service SK, et al. Variance component model to account for sample structure in genome-wide association studies. Nat Genet 2010; 42:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han B, Eskin E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am J Hum Genet 2011; 88:586–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015; 31:3555–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 2012; 40:D930–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schofield EC, Carver T, Achuthan P, et al. CHiCP: a web-based tool for the integrative and interactive visualization of promoter capture Hi-C datasets. Bioinformatics 2016; 32:2511–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mifsud B, Tavares-Cadete F, Young AN, et al. Mapping long-range promoter contacts in human cells with high-resolution capture Hi-C. Nat Genet 2015; 47:598–606. [DOI] [PubMed] [Google Scholar]
- 16.Kitada S, Kayama H, Okuzaki D, et al. BATF2 inhibits immunopathological Th17 responses by suppressing Il23a expression during Trypanosoma cruzi infection. J Exp Med 2017; 214:1313–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alcover A, Alarcón B, Di Bartolo V. Cell biology of T cell receptor expression and regulation. Annu Rev Immunol 2018; 36:103–25. [DOI] [PubMed] [Google Scholar]
- 18.Kaplanski G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol Rev 2018; 281:138–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chevillard C, Nunes JPS, Frade AF, et al. Disease tolerance and pathogen resistance genes may underlie Trypanosoma cruzi persistence and differential progression to chagas disease cardiomyopathy. Front Immunol 2018; 9:2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunha-Neto E, Dzau VJ, Allen PD, et al. Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas’ disease cardiomyopathy. Am J Pathol 2005; 167:305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leon Rodriguez DA, Carmona FD, Echeverría LE, González CI, Martin J. IL18 gene variants influence the susceptibility to Chagas disease. PLoS Negl Trop Dis 2016; 10:e0004583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez-Olarte S, Bolaños NI, Cuéllar A, Puerta CJ, González JM. Diminished mitogen-induced T cell proliferation by Trypanosoma cruzi antigens associated with antigen-presenting cell modulation and CD3 signaling. Cell Immunol 2020; 348:103974. [DOI] [PubMed] [Google Scholar]
- 23.Radstake TR, Gorlova O, Rueda B, et al. ; Spanish Scleroderma Group . Genome-wide association study of systemic sclerosis identifies CD247 as a new susceptibility locus. Nat Genet 2010; 42:426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi T, Suzuki K. CD247 variants and single-nucleotide polymorphisms observed in systemic lupus erythematosus patients. Rheumatology (Oxford) 2013; 52:1551–5. [DOI] [PubMed] [Google Scholar]
- 25.Teruel M, McKinney C, Balsa A, et al. Association of CD247 polymorphisms with rheumatoid arthritis: a replication study and a meta-analysis. PLoS One 2013; 8:e68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matzaraki V, Kumar V, Wijmenga C, Zhernakova A. The MHC locus and genetic susceptibility to autoimmune and infectious diseases. Genome Biol 2017; 18:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima H, Tamura T, Ito M, et al. SHD1 is a novel cytokine-inducible, negative feedback regulator of STAT5-dependent transcription. Blood 2009; 113:1027–36. [DOI] [PubMed] [Google Scholar]
- 28.Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circ Res 2012; 110:111–5. [DOI] [PubMed] [Google Scholar]
- 29.Albareda MC, Perez-Mazliah D, Natale MA, et al. Perturbed T cell IL-7 receptor signaling in chronic Chagas disease. J Immunol 2015; 194:3883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Villar VAM, Rozyyev S, Jose PA, Zeng C. The emerging role of sorting nexins in cardiovascular diseases. Clin Sci (Lond) 2019; 133:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krüger M, Moser M, Ussar S, et al. SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell 2008; 134:353–64. [DOI] [PubMed] [Google Scholar]
- 32.Haas BE, Horvath S, Pietiläinen KH, et al. Adipose co-expression networks across Finns and Mexicans identify novel triglyceride-associated genes. BMC Med Genomics 2012; 5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao X, Zhang R, Lu Y, et al. Kruppel-like factor 4 is critical for transcriptional control of cardiac mitochondrial homeostasis. J Clin Invest 2015; 125:3461–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mo X, Chen J, Wang X, et al. Krüppel-like factor 4 regulates the expression of inducible nitric oxide synthase induced by TNF-α in human fibroblast-like synoviocyte MH7A cells. Mol Cell Biochem 2018; 438:77–84. [DOI] [PubMed] [Google Scholar]
- 35.Carvalho CM, Silverio JC, da Silva AA, et al. Inducible nitric oxide synthase in heart tissue and nitric oxide in serum of Trypanosoma cruzi-infected rhesus monkeys: association with heart injury. PLoS Negl Trop Dis 2012; 6:e1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conomos MP, Laurie CA, Stilp AM, et al. Genetic Diversity and Association Studies in US Hispanic/Latino Populations: applications in the Hispanic Community Health Study/Study of Latinos. Am J Hum Genet 2016; 98:165–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zingales B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop 2018; 184:38–52. [DOI] [PubMed] [Google Scholar]
- 38.Castellucci LC, Almeida L, Cherlin S, et al. A Genome-Wide Association Study Identifies SERPINB10, CRLF3, STX7, LAMP3, IFNG-AS1 and KRT80 as risk loci contributing to cutaneous leishmaniasis in Brazil. Clin Infect Dis 2021; 72:e515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genetics for all. Nat Genet 2019; 51: 579. [DOI] [PubMed] [Google Scholar]
- 40.Lidani KCF, Andrade FA, Bavia L, et al. Chagas disease: from discovery to a worldwide health problem. Front Public Health 2019; 7:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.