Abstract
Divergence of ecological niches in phylogenetically closely related species indicates the importance of ecology in speciation, especially for sympatric species are considered. Such ecological diversification provides an advantage of alleviating interspecies competition and promotes more efficient exploitation of environmental resources, thus being a basis for ecological speciation. We analyzed a group of closely related species from the subgenus Neritrema (genus Littorina, Caenogastropoda) from the gravel‐bouldery shores. In two distant sites at the Barents and Norwegian Sea, we examined the patterns of snail distribution during low tide (quantitative sampling stratified by intertidal level, presence of macrophytes, macrophyte species, and position on them), shell shape and its variability (geometric morphometrics), and metabolic characteristics (metabolomic profiling). The studied species diversified microbiotopes, which imply an important role of ecological specification in the recent evolution of this group. The only exception to this trend was the species pair L. arcana / L. saxatilis, which is specifically discussed. The ecological divergence was accompanied by differences in shell shape and metabolomic characteristics. Significant differences were found between L. obtusata versus L. fabalis and L. saxatilis / L. arcana versus L. compressa both in shell morphology and in metabolomes. L. saxatilis demonstrated a clear variability depending on intertidal level which corresponds to a shift in conditions within the occupied microhabitat. Interestingly, the differences between L. arcana (inhabiting the upper intertidal level) and L. compressa (inhabiting the lower one) were analogous to those between the upper and lower fractions of L. saxatilis. No significant level‐dependent changes were found between the upper and lower fractions of L. obtusata, most probably due to habitat amelioration by fucoid macroalgae. All these results are discussed in the contexts of the role of ecology in speciation, ecological niche dynamics and conservatism, and evolutionary history of the Neritrema species.
Keywords: cryptic species, ecological niche, ecological speciation, geometric morphometrics, habitat amelioration, Littorina, metabolomics, microhabitat distribution, morphological disparity, niche differentiation, shell shape
Five sympatric Littorina (Neritrema) species demonstrate clearly different ecological preferences within the intertidal zone (except L. arcana and L. saxatilis pair). The degree of between‐species niche differentiation correlates with metabolomic characteristics and shell shape differences.

1. INTRODUCTION
Ever since the Darwinian description of the Galapagos finches, sympatric populations of phylogenetically close species have attracted the attention of evolutionary biologists. Natural case studies of this kind provide an opportunity to trace the divergence of ancestral species and hypothesize about the drivers of speciation and the mechanisms of emergence of reproductive isolation between nascent species. Notable examples of such model systems are speciation within associations between the host–plant and the phytophagous insect (Futuyma & McCafferty, 1990; Nyman et al., 2006; Peccoud et al., 2010; Percy, 2003, etc.), divergence via host switching in parasites (Cox‐Singh, 2012; Duval & Ariey, 2012; Reed et al., 2007, etc.), and transition to endosymbiotic lifestyle in microorganisms (Moran & Wernegreen, 2000; Moya et al., 2008). In all these cases, a shift to an alternative niche accompanying species divergence allows the exploitation of different environmental resources even by young species coexisting within the same biocoenosis, that is, sympatric species in a broad sense.
Complexes of sibling species living in sympatry (and, by definition, occupying nonequal niches) are quite common. Their existence highlights the importance of ecology in evolution and the broad occurrence of the so‐called “ecological speciation.” There are two possible scenarios for the formation of such complexes. The first one involves allopatric speciation due to genetic and ecological drift followed by secondary contacts and reinforcement by premating reproductive isolation (Mayr, 1970). The second scenario suggests diversification of ecological niches in situ according to the concept of sympatric speciation (Nosil, 2012; Schluter, 2000). The latter scenario is driven by the ecological specialization of parts of an ancestral population to heterogeneous environments. Genetic processes occurring during sympatric speciation are diverse and cannot be reduced to a single evolutionary pathway (Richards et al., 2019).
Both allo‐ and sympatric ecological speciation models imply niche divergence as a reflection and/or cause of morphological and physiological differences in incipient species. If divergence and speciation events occur, these species expectedly inherit close ecological niches and become ecologically similar. For this reason, ecologically similar species tend to be phylogenetically related (Doenz et al., 2018; Harvey & Rembaut, 2000; McGee et al., 2020; Price, 1997). Numerous observations indicate that some nonrandom mechanisms are responsible for limiting ecological niche expansion during species proliferation (either adaptive or not) and phylogenetic niche conservatism1 (Baniaga et al., 2020; Losos, 2008; McGee et al., 2020; Pyron et al., 2015; Wiens & Graham, 2005). Although phylogenetic niche conservatism is a common phenomenon, “niche similarity” is not the same as “niche equivalence” (Warren et al., 2008). The conclusion about the existence of niche conservatism in a particular case depends on the scale of observations (spatial, temporal, environmental, and phylogenetic): Although sister species tend to be more ecologically similar to each other than predicted by random models, their similarity is often not greater than that of more distantly related taxa (Graham et al., 2004; Knouft et al., 2006; Losos et al., 2003; Pearman et al., 2008; Warren et al., 2008). Moreover, stabilizing forces promoting niche conservatism of sympatric species may be opposed by the forces favoring diversification and niche shifts during speciation (Losos, 2008; Losos et al., 2003; Pitteloud et al., 2017; Scriven et al., 2016). From the perspective of population genetics, ecological specialization to different parts of the environment would prevent species from hybridizing and reinforce later divergence (Ackerly, 2003). Such a specialization provides an ecological advantage, alleviating interspecies competition and promoting more efficient exploitation of environmental resources (Gause, 1934; Hardin, 1960; Schluter, 2000).
The present study focuses on the exploitation of different aspects of heterogeneous and dynamic environments within the marine intertidal zone by a set of closely related snail species. Gastropods of the Littorina (Neritrema) subgenus are a striking example of adaptive radiation within the intertidal zone. On the European North Atlantic shores, the subgenus Neritrema comprises two cryptic groups of closely related species—the “obtusata” group (L. obtusata [Linnaeus, 1758] and L. fabalis [Turton, 1825]) and “saxatilis” group (L. saxatilis [Olivi, 1792], L. arcana Hannaford‐Ellis, 1978 and L. compressa Jeffreys, 1865). Ecotypes of L. saxatilis and, to the lesser extent, L. obtusata and L. fabalis are the best studied Neritrema species, while L. arcana and L. compressa are the least studied because of the difficulties involved in their identification. These species have been examined in many aspects such as morphology and parasitology, phylogeny, genetics and reproductive barriers, adaptive mechanisms, and proteomics (Conde‐Padin et al., 2007; Granovitch et al., 2009; Johannesson, 2003; Maltseva et al., 2016; Maltseva et al., 2020; Panova et al., 2014; Rolán‐Alvarez et al., 2015; Sokolova & Pörtner, 2001a, 2001b, etc). Several studies concerning ecological preferences of species or ecotypes of particular species have been conducted (Granovitch et al., 2013; Rolán & Templado, 1987; Sacchi, 1966; Tatarenkov & Johannesson, 1998; Warmoes et al., 1992; Watson & Norton, 1987; Williams, 1995). However, these studies focus on the ecology of one particular Neritrema species (or more than one, but never all the five coexisting species) and usually ignore the others, not even mentioning their presence or absence, which might affect the microbiotopic distribution of the species under study. In this way, an important factor is left out of the analysis. While snails of the subgenus Neritrema have been used as a model in studies of recent and current speciation (Butlin et al., 2014; Johannesson, 2003, 2016; Johannesson et al., 2017; Morales et al., 2019; Ravinet et al., 2016; Rolán‐Alvarez, 2007; Rolán‐Alvarez et al., 2015), there is still no clear picture of their niche diversification within the complex of sympatric populations.
To fill this gap, we set out to describe the basic parameters of ecological niches of all the five sympatric Neritrema species using evidence from their populations in the northeastern Atlantic, where they coexist within the same intertidal zone. We implemented a complex approach with the following characteristics. (a) Two distant sites of the Northern Norway coast (the Barents Sea and the Norwegian Sea) with gravel/stony shores, typical of the northeastern parts of distribution of the studied species, were chosen as models. At these types of coasts, quantitative sampling is possible throughout the intertidal zone. (b) The original sampling method enabled a replicated evaluation of populations’ composition in diverse biotopes regarding tidal level, zonality of macroalgae distribution and open gravel/stony microsites. (c) For the first time, a “layered” sampling was performed to account for the position of the snails in the macroalgal canopy: on the surface, in the depth of the canopy, or on the gravel under it. (d) The species identification was based on the reproductive system morphology to ensure its accuracy. (e) The analysis of the population ecology was supplemented by the geometric morphometric evaluation of shell shape and by metabolomic profiling aimed at revealing the main distinctive physiological features. Such an approach provides an opportunity to reveal unique preferred microhabitats for each species and to match these data with either shell morphology or biochemical characteristics.
Neritrema species exhibit a species‐specific microdistribution pattern along the vertical shore gradient (Granovitch et al., 2013), but with a significant overlap: Both L. arcana and L. saxatilis are present in the fucoid‐free high‐shore zone, while four other species (L. compressa, L. fabalis, L. obtusata, and L. saxatilis) inhabit fucoid‐overgrown low shore. We hypothesized that these species should be ecologically segregated within the zones of the overlap. We also tested the possibility of shore‐level‐related shifts in the preferred microhabitat, as different levels provide different sets of microhabitats and coexisting species.
Periwinkles are well known for their adaptive plasticity at both morphological and physiological levels. For example, there are adaptive differences between ecotypes of L. fabalis and L. saxatilis (reviewed in Johannesson, 2003, 2016; Rolán‐Alvarez, 2007; Rolán‐Alvarez et al., 2015). M and H ecotypes of L. saxatilis from British rocky shores are differentiated in relation to wave action: Snails of high‐shore (H) ecotype, which are exposed to a strong wave impact, have a smooth fragile shell with a wide aperture, while snails of M (mid‐shore) ecotype have a narrower aperture and a thicker shell wall. Similar tendencies in the shell shape modification depending on the strength of wave action have been shown for exposed and sheltered ecotypes of L. fabalis. In various parts of its distribution such as Galicia and Sweden, L. saxatilis is known to form a certain ecotype (SU and E, respectively) under strong wave impact, characterized by a small fragile shell with a wide aperture to accommodate a large foot helping to avoid dislodgement (Le Pennec et al., 2017), and a certain ecotype (RB and C, respectively) in the presence of predatory crabs, characterized by a large armored shell with a narrow aperture to withstand crab attacks and excessive desiccation during exposure. Importantly, there is no obvious shore‐level‐related gradient in the strength of wave action on a flat intertidal zone, also, no records of a predatory crab presence at the Northern Norway coasts. Accordingly, we checked the snails under study for the presence of any shell shape changes correlating with the shore level and universal among the species. In particular, we looked for the shell shape changes comparable with those described for ecotypes, such as a globose shell with a wider aperture at the low shore versus a relatively slim elongate shell with a narrow aperture at the high shore, when the action of factors generally accepted as drivers of such morphological changes is absent or very weak.
Morphological specialization contributes much to the adaptation to the microenvironment (e.g., in gastropods, see Vermeij, 1973; Stanley, 1988; Walker & Grahame, 2011; Kistner & Dybdahl, 2013) and seems to be an important part of ecologically based divergence in different organisms (e.g., flies, Filchak et al., 2000; fishes, Doenz et al., 2018; Raffini et al., 2020; palms, Savolainen et al., 2006). Moreover, there are studies implying that the probability of coexistence of morphologically similar species is low (Vodă et al., 2015). Interestingly, no records of distinct ecotypes are available from the northeastern part of the Neritrema species distribution ranges, possibly owing to the absence of the predatory crabs at the intertidal zone and the dominance of flat gravel‐stony coastal types. Nevertheless, physiological specialization to contrasting shore levels has been described in L. saxatilis. Differences in water conservation abilities, thermal stress resistance, and metabolic rates during low tide have been revealed between L. saxatilis snails inhabiting high and low shores (Smith et al., 1995; Sokolova et al., 2000; Sokolova & Pörtner, 2001a, 2001b). Here, we used metabolomic profiling to reveal the species‐specific metabolic background of the snails inhabiting certain microhabitats. Special attention was paid to the compounds of anaerobic metabolism, which are expected to be more abundant in low‐shore snails due to their higher metabolic rate.
We believe that a comparative analysis of the complexes of potentially adaptive traits and ecological preferences, characteristic of closely related snail species, may shed light on their evolutionary history and the role of ecology in their origin. It may also elucidate some fundamental aspects of the mechanisms allowing cryptic species to coexist as well as the dynamics of environmental niches during their evolution.
2. MATERIALS AND METHODS
2.1. Study sites
Two distant sites at a similar type of the intertidal zone, gravel‐stony with abundant fucoid algae, were chosen for a detailed population analysis of five littorinid species (L. saxatilis, L. arcana, L. compressa, L. obtusata, and L. fabalis) at coasts of the Norwegian Sea (S1, Saltstraumen, 66°58′10.2″N 13°58′26.5″E, collection dates 29.06–5.07.2019; the average July sea temperature is 13.6℃,2 the average length of the day in July is 22 h2; the average rainfall in July is 86 mm2) and the Barents Sea (V1, Varangerfjord, 70°04′03.9″N 29°58′40.1″E, collection dates 09.07–12.07.2019; the average July sea temperature is 8 °C2, the average length of the day in July is 23.8 h2; the average rainfall in July is 62 mm2). While there are no reports on the Littorina species composition in the Norwegian Sea near S1, the set of Littorina species in the Barents region has been previously characterized (see, e.g., Galaktionov & Bustnes, 1999; Muraeva et al., 2016; Repkin et al., 2020). Both sites have a flat, moderately exposed gravel‐boulder intertidal zone, about 100 m wide from the lowest to the highest water edge (the map and photographs are in Appendix S1). The middle and the lower part of the intertidal zone at both sites are heavily overgrown by brown macroalgae with a similar species composition. The canopy‐forming macroalgae are key ecosystem engineers within the marine coastal zone, providing habitats for many invertebrates including Littorina species. Although the mass of the canopy and the distribution pattern of intertidal fucoids are affected in a complex manner by a number of both abiotic (e.g., wave action) and biotic (e.g., presence of herbivores) factors (e.g., Jonsson et al., 2006; Lappalainen et al., 2019), only the distribution pattern of macroalgae, as the most proximate factor, was used as a proxy to determine the intertidal zonation, which was similar in two sites studied. We followed the generalized zonation scheme of Stephenson and Stephenson (1949, 1972). This scheme does not link the zones to exact tidal heights but determines the relative positions of the major community types across moderately exposed rocky shores (Raffaelli & Hawkins, 1996). The upper part included stones of the littoral fringe and open gravel with sparse clumps of Fucus vesiculosus, which do not form a continuous belt or a massive canopy. The lower part was completely covered by macroalgae, with occasional patches of open gravel. The species composition of macroalgae changed seawards in the following manner: F. vesiculosus only, Ascophyllum nodosum interspersed by F. vesiculosus, and Fucus serratus interspersed by F. vesiculosus.
2.2. Microhabitat distribution analysis
The samples were collected at the upper and the lower part of the intertidal zone, the parts of collection being separated by approximately 20 m. At each site, all the samples were collected during the same low tide. There were four types of substrates in the lower part (open gravel, F. vesiculosus, A. nodosum, and F. serratus) and three types of substrates in the upper part (open gravel, stone, and F. vesiculosus) (Appendix S1). The samples were collected from haphazardly placed quadrats (0.04 m2, 0.20 m × 0.20 m) in five replicates per substrate type (with two exceptions at the lower level on A. nodosum, where six samples in Saltstraumen and four in the Varangerfjord were taken). Sampling on fucoids was carried out in layers: the snails were collected separately from the surface of the canopy, from the “depth” of the fucoid canopy, and from the substrate below the canopy. At the upper intertidal level at Saltstraumen, the snails from the depth and the surface of F. vesiculosus were collected together and assigned to the depth layer, as there was no clear distinction between these microhabitats. Snails from the open gravel and the fucoid surface were collected immediately from quadrats; then, all the fucoids were cut out and placed into plastic bags together with periwinkles hidden inside for subsequent sorting; finally, the snails were collected from the gravel beneath the canopy (numbers of samples collected for the analysis of microhabitat distribution can be found in Appendix S2).
All the snails were transported to the laboratory for measurements and photodocumentation, followed by dissection for a check for trematode infection, which is known to affect Littorina shell shape (Sergievsky & Granovitch, 1999) and for species identification. Snails from the “depth” samples were washed off the algae, which were then weighed. All individuals were dissected under MBI‐10 and Leica EZ4HD stereomicroscopes. Species identification was performed after removing the shell and opening the mantle based on species‐specific characteristics of the reproductive system. Defining features in males included the penis basement and filament form, as well as shape, number, and pattern of penial glands; in females, the decisive characteristics were the morphology of bursa copulatrix and the proportions of the auxiliary gland of the pallial complex (for more details, see previous descriptions in: Granovitch et al., 2008, 2013; Maltseva et al., 2016, 2020; Reid, 1996).
2.3. Geometric morphometric data
Photographic pictures were taken with MBI‐10 binocular microscope coupled with MFU photo adapter and Canon EOS 1200D camera. The camera settings remained unaltered throughout the study. The snails were photographed in a standard position, with the ventral surface of the shell facing up and the aperture in the same plane as the objective, being attached with plasticine for stability. For geometric morphometric analysis, the images were digitized using Tps‐software package (Rohlf, 2015; The Stony Brook Morphometrics); tpsUtil v.1.74 program was used to generate TPS files from two‐dimensional images. The coordinate locations of landmarks and semilandmarks were marked on the images in tpsDig2 v.2.3. The shell shape of the mollusks was described by 11 landmarks and 56 semilandmarks3 forming 6 curves (Figure 1). LM1 is in the inner part of the aperture where the parietal wall meets the ultimate whorl; LM2 is at the end of the suture; LM3‐6 are on the right border of the profile of the shell at the end of the suture; LM7 is the apex of the shell; LM8‐10 are on the left border of the profile of the shell at the end of the suture; LM11 is where the outer contour of the columellar lip meets the border of the profile of the shell. In L. obtusata, LM6‐8 were located at equal intervals between LM5 and LM9. In total, the analysis was based on 126 individuals of L. saxatilis (50–141 mm), 32 individuals of L. arcana (81–130 mm), 47 individuals of L. compressa (61–113 mm), 128 individuals of L. obtusata (55–167 mm), and 41 individuals of L. fabalis (50–147 mm); more details can be found in Appendix S3.
FIGURE 1.

The scheme showing the position of landmarks (I–XI) and semilandmarks (12–69) on periwinkle shells used in this study
2.4. Metabolomic data
Metabolomic data were obtained using nontargeted GC‐MS (gas chromatography mass spectrometry)‐based profiling of the trimethylsilyl derivatives. Ninety‐eight adult littorinid snails (28 L. saxatilis, 14 L. arcana, 14 L. compressa, 24 L. obtusata, and 13 L. fabalis) were collected from the two study sites, an approximately equal number from each site. All the snails were uninfected with parasites and had a well‐developed reproductive system. L. saxatilis and L. obtusata were collected from both intertidal levels where they occur, 5–7 snails from each level. At each site, the snails were collected during the same low tide, at a similar time moment after air exposure. After collection, the shell and the operculum were removed and the molluskan bodies were individually fixed whole in tubes containing 1 ml of 100% methanol (Merck), frozen at −20℃, and transported to the laboratory, where they were kept at −80℃ until use. Frozen tissues were homogenized in Mixer Mill MM 400 (Retsch) for 20 min at a frequency of 30 Hz with two stainless steel beads (Qiagen). Tissue debris was sedimented by centrifugation at 11 500 g for 15 min; supernatants were collected, vacuum‐dried (CentriVap, Labconco) at 4℃, and frozen at −80℃ until use. Tissue debris was also dried and weighed on Sartorius CPA‐324S analytical balance; weights were used to normalize chromatograms. Before gas chromatography (GC), vacuum‐dried samples (supernatants) were dissolved in 40 µl pyridine (Merck) containing 1 mg/ml of tricosane (nC23) (Sigma‐Aldrich), used as an internal standard for GC‐quantitation, by incubation 10–15 min in ultrasonic bath Elma Elmasonic S100H. Derivatization of analytes by silylation was performed to reduce polarity of compounds (Halket & Zaikin, 2003). For silylation, N,O‐bis(trimethylsilyl)‐trifluoroacetamide with 1% trimethylsilyl chloride (Sigma‐Aldrich) was added to each sample before the analysis. The samples were loaded to glass vials with Teflon septa (Agilent Technologies). The GC‐MS analysis was carried out on a gas chromatograph with a time‐of‐flight mass spectrometer Pegasus 4D GCxGC‐TOF MS (Leco); the carrier gas was helium, and the column was Zorbax DB5 (length 30 m, inner diameter 0.25 mm, film thickness 0.25 µm). The initial temperature was 70℃, the final temperature was 320℃, and the gradient was 6℃ per minute. The injector temperature was 250℃. The scanning frequency was ten spectra per second in a range of masses 50–800 Da. Metabolites were identified using MS‐library NIST10 (National Institute of Standards and Technology) based on match factor and retention indices and using the chromatograms of available standard single amino acids, monosaccharides, fatty acids, and their mixtures. Quantitation of metabolites was performed by the peak total ion current (TIC) on the chromatogram.
2.5. Statistical analysis
2.5.1. Analysis of microhabitat distribution
Microhabitat distribution of the periwinkles was analyzed using partial Distance‐based Redundancy Analysis (dbRDA; Legendre & Anderson, 1999; McArdle & Anderson, 2001), based on Bray–Curtis dissimilarities (Bray & Curtis, 1957) on square root‐transformed densities (to reduce the effect of more abundant species on ordination; Legendre & Legendre, 2012), with vegan package (Oksanen et al., 2019) of R (R Core Team, 2020). First, the effect of the microhabitat on the distribution of periwinkles was tested separately in the upper and the lower parts of the intertidal zone; the abundances of the snails sampled from different layers of fucoids were pooled for this analysis. The partial dbRDA model included microhabitat as a predictor (gravel, stone, and Fucus vesiculosus in the upper shore; gravel, F. vesiculosus, F. serratus, and Ascophyllum nodosum in the lower shore), and two conditioning variables (site and fucoid weight). Next, the effects of the fucoid species and the position of the snails within the canopy were tested on the macroalgae‐associated samples from the lower part of the intertidal zone. The partial dbRDA model included two predictors: microhabitat (F. vesiculosus, F. serratus, and Ascophyllum nodosum) and layer in the fucoid‐related microhabitat (surface or depth of the fucoid mass and gravel underneath it), and two conditioning variables (site and fucoid weight). To account for nonindependence of layers within a quadrat, it was used as a blocking factor to restrict permutations in tests (which is the way random factors are treated in permutational tests after dbRDA; Oksanen et al., 2019). Finally, the samples taken on F. vesiculosus, the only fucoid species present in both the upper and the lower intertidal levels, were used to test the effect of the intertidal level and layer within the fucoid canopy. The model included two predictors: shore level (upper and lower) and layer in the fucoid‐related microhabitat (the same levels as in the previous analysis), and two conditioning variables, site and fucoid weight; permutations were restricted within quadrats. Significance tests were carried out using 9,999 permutations.
2.5.2. Geometric morphometric analysis
Digital images of the shells of the five species of periwinkles were obtained during the survey of microhabitat distribution. Only the photographs of healthy adult snails (no trematode infection, well‐developed reproductive system) were used for geometric morphometric analysis. Statistical analysis was performed in R using geomorph and RRPP packages (Adams & Otárola‐Castillo, 2013; Collyer & Adams, 2018). Landmark coordinates were aligned using the Generalized Procrustes Analysis (Gower, 1975) to remove nonshape variation (effects of position, orientation, and scale). Size (centroid size) and shape (Procrustes coordinates) of each snail were estimated during this procedure. Centroid size was calculated as the square root of the sum of squared distances of landmarks from their centroid.
Measurement error resulting from digitalization of landmarks and semilandmarks was evaluated on a random subsample of 30 snails, which were digitized twice. A Procrustes linear model with an individual as a categorical factor was fitted; mean squares in the resulting ANOVA table were used to calculate the repeatability index as described in Arnqvist and Martensson (1998). A high repeatability index (R = 96.5%) indicated a minimal measurements error relative to among‐individual variation.
To visualize shape variation, Procrustes coordinates were subjected to Principal Component Analysis (PCA). Patterns of shape variation were statistically evaluated using nonparametric multivariate analysis of variance (perMANOVA; Anderson, 2001) with randomized residual permutation procedure (RRPP; Collyer & Adams, 2018) on the matrix of Procrustes coordinates. The model for interspecific comparisons of the shape of all five littorinid species included the following predictors: species/subpopulation (a categorical predictor with seven levels defined by species and subpopulation: the upper or the lower shore for L. saxatilis and L. obtusata), a random effect of collection site, log‐transformed centroid size (to account for possible allometric effects; Bookstein, 1991), and all two‐ and three‐way interactions of these factors. In addition, separate models with the same set of predictors were fitted for shape comparisons within “saxatilis” and “obtusata” species groups. Predicted shell shapes for species/subpopulations were obtained from these models at the mean centroid size of the respective group to correct for allometric changes. Shell shape predictions were visualized using thin‐plate spline transformation grids. Morphological disparity was quantified for different species and their subpopulations from the upper and the lower intertidal levels. It was estimated as the Procrustes variance using residuals of the model (with the function pairwise() in the package geomorph). 95% bias‐corrected and accelerated (BCa) confidence intervals for morphological disparities were estimated using a nonparametric bootstrap (DiCiccio & Efron, 1996) with 999 iterations using the package boot (Canty & Ripley, 2020; Davison & Hinkley, 1997). Post hoc comparisons of mean shapes, allometric vectors, and morphological disparities among species/subpopulations were done using the above‐mentioned models for “saxatilis” and “obtusata” species groups. The analyses were performed after accounting for the site‐specific allometric differences, that is, the null model, which was used to extract residuals for RRPP included log‐centroid size, collection site, and their interaction. The tests were performed using 999 permutations. p‐values in post hoc tests were corrected for multiple testing using Holm–Bonferroni procedure (Holm, 1979). Sample sizes in these analyses were unbalanced. Less accurately estimated means (Cardini & Elton, 2007) and larger variation in small samples (Tversky & Kahneman, 1971) may lead to an overestimation of the differences in small groups from the larger ones (Cardini et al., 2015). Thus, the differences between L. arcana, whose sample size was the smallest (altogether 32 snails: 8 in Saltstraumen and 24 in Varangerfjord), and the other species should be treated with caution.
2.5.3. Metabolomic analysis
Raw metabolomic data (detection intensities) were log‐transformed, normalized by sample weight (NormalizeMets package; De Livera & Olshansky, 2018), and quantile normalized (limma package; Ritchie et al., 2015). The metabolomes were visualized using nonmetric multidimensional scaling (nMDS) based on Euclidean distances among the samples (vegan package; Oksanen et al., 2019). Interspecies differences among metabolomes were tested using perMANOVA with 9,999 permutations in vegan package, after testing for the assumption of homogeneity of within‐group dispersions, which was met. To note, the sample sizes were unbalanced in this analysis, but perMANOVA is robust to sample imbalance in the absence of heterogeneity of within‐group dispersions (Anderson & Walsh, 2013). The sets of metabolites that best distinguished the species were obtained using principal least squares discriminant analysis (PLS‐DA; Barker & Rayens, 2003) using mixOmics package (Rohart et al., 2017). The concentration of several anaerobic metabolites such as succinate, lactate, and malate was compared in L. saxatilis and L. obtusata snails from different intertidal levels using the moderated t test (Smyth, 2004) in the limma package (Ritchie et al., 2015). The same comparison was performed for L. arcana and L. compressa. The moderated t test is more powerful than an ordinary t test, especially for small samples, because it moderates the sample variances for each metabolite using the information on the distribution of sample variances across all metabolites with the help of an empirical Bayes method.
3. RESULTS
3.1. Ecological mapping
Littorina (Neritrema) species composition differed in the upper and lower intertidal zones. L. fabalis was registered only in the lower part, while L. arcana was registered only in the upper part. The upper and lower parts of the intertidal zone also differed as to the dominant species of fucoid macroalgae and their projective cover. Only F. vesiculosus was present in the upper part, with a patchy distribution and a relatively low projective cover (0%–25% in any quadrat within the upper shore area). The lower part was almost completely covered by three species of fucoids (F. vesiculosus, F. serratus, and A. nodosum), and patches of open sediment were rare and scattered.
3.1.1. The upper intertidal zone
Four species of periwinkles (L. arcana, L. compressa, L. obtusata, and L. saxatilis) inhabited three microhabitats (open stones, open gravel, and F. vesiculosus clumps) in the upper intertidal. The effect of microhabitat on species composition was significant after controlling for between‐site differences (permutational test after partial dbRDA, p < .001; Appendix S2) and canonical axes constrained by this factor together explained 44.2% of the total variation. L. arcana and L. saxatilis mainly occupied open microniches (gravel and stones), L. arcana tended to be more common on gravel, and L. saxatilis, on stones (Figure 2). L. compressa and L. obtusata showed an “attraction” to F. vesiculosus.
FIGURE 2.

Distribution of the Littorina species across microhabitats in the upper littoral zone. The effect of microhabitat on littorine species composition was analyzed after controlling for between‐site differences using partial distance‐based redundancy analysis (dbRDA). Bray–Curtis dissimilarities were calculated from the square roots of species abundances in samples. Microhabitats: open stones (M:stone), open gravel (M:gravel), clumps of Fucus vesiculosus (M:F.ves); Littorina species: arc—L. arcana, comp—L. compressa, obt—L. obtusata, sax—L. saxatilis. The relative position of the microhabitat centroids (blue) and species (red) reflects the predominant spatial distribution of the snails in the intertidal zone
3.1.2. The lower intertidal zone
Harbored a different set of the four Littorina species (L. compressa, L. fabalis, L. obtusata, and L. saxatilis) in four principal microhabitats (F. vesiculosus, F. serratus, A. nodosum, and open gravel) than the upper intertidal zone. We performed a two‐step partial dbRDA analysis of the littorine distribution after controlling for between‐site variation: across all microhabitats when snails from different layers of the fucoid canopy were pooled (Figure 3a) and across a subset of fucoid‐related microhabitats accounting for their layered structure (surface, depth of the fucoid canopy, and gravel under it; Figure 3b). The canonical axes in the resulting models explained 20.1% and 22.3% of total variation, respectively.
FIGURE 3.

Distribution of the Littorina species across microhabitats in the lower intertidal zone. The partial dbRDA ordinations explore differences in Littorina species composition (a) across microhabitats and (b) stratification in the fucoid‐related microhabitats after controlling for between‐site differences. The analyses were based on Bray–Curtis dissimilarities calculated from the square roots of species abundances in samples. Microhabitats: open gravel (M:gravel), F. serratus (M:F.ser), A. nodosum (M:A.nod), F. vesiculosus (M:F.ves); layer in the fucoid‐related microhabitat: surface of the fucoid mass (L:s), depth of the fucoid mass (L:d), gravel under the fucoid mass (L:g), Littorina species: comp—L. compressa, fab—L. fabalis, obt—L. obtusata, sax—L. saxatilis. The relative position of the microhabitat centroids (blue) and species (red) reflects the predominant spatial distribution of the snails in the intertidal zone
Microhabitat type significantly affected the composition of Littorina species at the lower intertidal level (p < .001 in both analyses; Appendix S2). Two species, L. obtusata and L. fabalis, were strongly associated with fucoid macroalgae: the former with A. nodosum (slightly stronger) and F. vesiculosus, and the latter with F. serratus. Conversely, L. saxatilis was most commonly registered in open gravel microhabitats or, to a lesser extent, associated with F. vesiculosus (Figure 3a). The layer of fucoid‐associated habitats also significantly affected Littorina species distribution (p ≤ .05, Appendix S2). L. obtusata was usually buried in the depth of the fucoid mass, while L. fabalis was equally abundant in the depth and on the surface (Figure 3b). L. saxatilis, when associated with F. vesiculosus, kept on the gravel under the fucoid canopy. The fourth species, L. compressa, was predominantly associated with the surface of F. vesiculosus and was also found, albeit very rarely, in open gravel microhabitats.
3.1.3. Association with F. vesiculosus in different levels
Fucus vesiculosus is the only fucoid macroalga present both in the lower and upper parts of the intertidal zone. All the five Littorina species were associated with it to some degree. In the F. vesiculosus samples, the variation of littorine species composition across shore levels was not significant after controlling for between‐site variation; in contrast, the effect of the layer within F. vesiculosus canopy was significant (partial dbRDA, p = .23 and p < .001, respectively; Figure 4, Appendix S2). Taken together, these factors explained 25.5% of the total variation.
FIGURE 4.

Distribution of the Littorina species in association with Fucus vesiculosus. The dbRDA ordination explores variation in the distribution of littorines within F. vesiculosus clumps in the upper and lower parts of the intertidal zone after accounting for between‐site differences. The analysis was based on the Bray–Curtis dissimilarities on square roots of species abundances in samples. Shore level: upper (Z:upper) or lower (Z:lower) and stratification by layers of the fucoid‐related microhabitats: surface of fucoid mass (L:s), depth of the fucoid mass (L:d), gravel under fucoid mass (L:g); Littorina species: arc—L. arcana, comp—L. compressa, fab—L. fabalis, obt—L. obtusata, sax—L. saxatilis. The relative position of the microhabitat centroids (blue) and species (red) reflects the predominant spatial distribution of the snails in the intertidal zone
L. arcana, when associated with F. vesiculosus at the upper level, was present only on the gravel below the canopy, while L. compressa occurred at both shore levels, either inside algal clumps or on the gravel beneath them. L. fabalis was present in the lower part only and was associated with both the surface and the depth of F. vesiculosus canopy. L. obtusata and L. saxatilis displayed association with this fucoid macroalgae species predominantly in the upper part of the littoral zone. The former species was detected usually within the depth of the fucoid canopy and the latter on the gravel beneath it.
3.2. Shell morphology
It is often regarded as an adaptive trait in snails (Kistner & Dybdahl, 2013; Stanley, 1988; Vermeij, 1973; Walker & Grahame, 2011). As Littorina species studied do differ as to the occupied microhabitats, we evaluated possible interspecific shell shape differences and the level of intraspecies variability. When we visualized the shell shape variation using PCA, the first principal component explained almost a half of the total variability, indicating prominent differences between the “saxatilis” and “obtusata” species groups in the relative shell width, general globosity, and spire height (Figure 5). The Procrustes linear model confirmed the significance of “species/subpopulation” (upper or lower intertidal levels), “collection site”, and interaction of these factors. There was no effect of sex on shell shape, except Saltstraumen population of L. compressa (Appendix S3). Due to the significant allometric effect and its interactions, the correction for the site‐specific allometric differences was included in all the subsequent analyses (more details are in Appendix S3).
FIGURE 5.

Shell shape variation in five Littorina species. Principal component analysis ordination of shell shapes grouped by species and subpopulation (upper or lower intertidal level). Thin‐plate spline transformation grids represent shell shape changes between the mean shape and minimum and maximum values of PC1 and PC2. For clarity, the magnitude of shell shape changes on the positive end of PC1 was reduced 0.6 times
3.2.1. In the “saxatilis” species group
Shell shape depended strongly on site (Procrustes linear model, p = .001, standardized effect size Z = 6.4; Appendix S3) and its interaction with species/subpopulation (p = .001, Z = 5.6), which indicates site‐specific differences among species/subpopulations. Allometric relationships did not significantly differ between species/subpopulations (p = .241, Z = 0.7) and were site‐specific (p = .001, Z = 2.6). The species‐specific allometries did not differ between sites (p = .119, Z = 1.1).
Average shell shapes of L. compressa significantly differed from those of L. arcana and L. saxatilis from both shore levels after site‐specific allometric differences had been removed (Figure 6, Table 1). Its predicted shell shape was slimmer and had a more rounded aperture than that of the other species of the “saxatilis” group (Figure 6). The strongest shape differences in average shapes were found between L. compressa and L. saxatilis from the upper and the lower intertidal levels (p = .001 in both cases, Z = 9.8 and Z = 6.3, correspondingly; Table 1). The differences between L. arcana and L. saxatilis were weak and not significant (Figure 6, Table 1), those between L. arcana and upper subpopulations of L. saxatilis being the weakest (p = .175, Z = 0.9). Comparison of allometric angles yielded a similar pattern of differences (Table 2; Appendix S3). Based on the model predictions, L. saxatilis at the lower level, similarly to L. obtusata, tends to possess a more widely open aperture and a generally more globose shell (Figure 6, Table 1).
FIGURE 6.
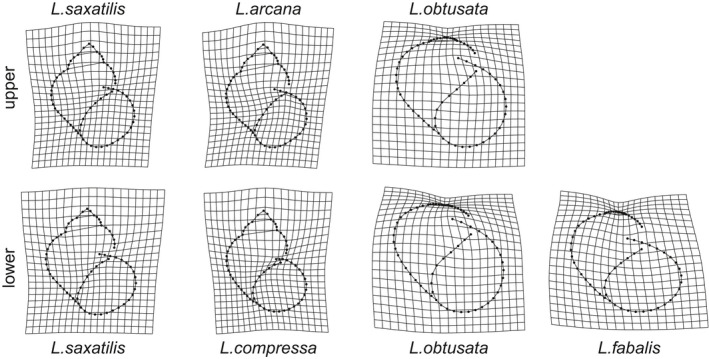
Predicted mean shapes of five Littorina species. Predictions for “saxatilis” and “obtusata” groups were made from separate Procrustes linear models, both of which included species/subpopulation, site, log‐centroid size, and their interactions. Shell shapes averaged across sites were predicted at the mean centroid size of the corresponding species group to correct for allometric changes. Thin‐plate spline transformation grids reflect modification of shell shape relative to the overall mean shape. For clarity, shell shape changes were magnified 1.5 times
TABLE 1.
Post hoc comparisons of mean shell shape between the Littorina (Neritrema) species
| Contrast | d | UCL (95%) | Z | p | p adj. |
|---|---|---|---|---|---|
| L. arcana : L. compressa | 0.047 | 0.032 | 4.6 | .001 | .006 |
| L. arcana : L. saxatilis_high | 0.032 | 0.036 | 0.9 | .175 | .175 |
| L. arcana : L. saxatilis_low | 0.042 | 0.042 | 1.8 | .052 | .123 |
| L. compressa : L. saxatilis_high | 0.053 | 0.021 | 9.8 | .001 | .006 |
| L. compressa : L. saxatilis_low | 0.046 | 0.025 | 6.3 | .001 | .006 |
| L. saxatilis_high : L. saxatilis_low | 0.024 | 0.022 | 2.0 | .041 | .123 |
| L. fabalis–L. obtusata_high | 0.067 | 0.037 | 6.4 | .001 | .003 |
| L. fabalis–L. obtusata_low | 0.083 | 0.048 | 6.6 | .001 | .003 |
| L. obtusata_high–L. obtusata_low | 0.041 | 0.031 | 3.4 | .005 | .005 |
Analyses within the “saxatilis” and “obtusata” groups were performed independently. Regional allometric differences had been removed. Significantly differing contrasts are written in bold.
Abbreviations: d, pairwise distances between means; p adj., adjusted p‐values; p, p‐values; UCL (95%), upper confidence limit; Z, standardized effect size.
TABLE 2.
Post hoc comparisons of the direction of allometric shape change in the Littorina (Neritrema) species
| Contrast | r | Angle | UCL (95%) | Z | p | p adj. |
|---|---|---|---|---|---|---|
| L. arcana : L. compressa | −.332 | 109.40 | 105.22 | 1.9 | .035 | .175 |
| L. arcana : L. saxatilis_high | .736 | 42.59 | 68.95 | −0.04 | .463 | 1.0 |
| L. arcana : L. saxatilis_low | −.061 | 93.49 | 127.35 | −0.5 | .679 | 1.0 |
| L. compressa : L. saxatilis_high | −.382 | 112.45 | 87.96 | 3.4 | .002 | .012 |
| L. compressa : L. saxatilis_low | .117 | 83.30 | 84.04 | 1.8 | .055 | .220 |
| L. saxatilis_high : L. saxatilis_low | .424 | 64.90 | 89.02 | −0.7 | .749 | 1.0 |
| L. fabalis–L. obtusata_high | .825 | 34.43 | 42.74 | 0.6 | .261 | .783 |
| L. fabalis–L. obtusata_low | .909 | 24.60 | 39.88 | −0.7 | .737 | .930 |
| L. obtusata_high–L. obtusata_low | .947 | 18.81 | 28.62 | <0.1 | .465 | .930 |
Analyses within the “saxatilis” and “obtusata” groups were performed independently. Regional allometric differences had been removed. Significantly differing contrasts are written in bold.
Abbreviations: angle, angles between vectors; deg, the correlation between the vectors is the cosine of the angle between the vectors (the smaller the angle, the larger the correlation); p adj., adjusted p‐values; p, p‐values; r, pairwise correlations between vectors of shape change; UCL (95%), upper confidence limit; Z, standardized effect size.
The angles between allometric relationships of L. compressa also differed significantly from those of L. arcana and L. saxatilis from the upper shore level after site‐specific allometric differences had been removed (Table 2). The difference between L. compressa and L. saxatilis from the upper intertidal level was the strongest (p = .002, Z = 3.4; Table 2), while the difference between L. compressa and the lower shore L. saxatilis was weaker and not significant (p = .055, Z = 1.8; Table 2). L. compressa had the highest morphological disparity among the species of the “saxatilis” group, which significantly differed from L. arcana and L. saxatilis from both shore levels (Appendix S3).
3.2.2. In the “obtusata” species group
The shell shape was significantly affected by the collection site (Procrustes linear model, p = .001, Z = 7.1; Appendix S3) and by the interaction with species/subpopulation factor (p = .001, Z = 4.9), which was similar to the situation observed in the “saxatilis” group of species. Site‐specific allometric shape changes were significant (p = .001, Z = 4.9); while species allometries did not differ significantly (p = .303, Z = 0.5), the species‐specific allometric relationships significantly differed between sites (p = .002, Z = 2.9).
Average shell shapes of L. fabalis significantly differed from L. obtusata of both shore levels (p = .001 in both cases) after site‐specific allometric differences had been removed (Table 1, Figure 6). These shape differences were much stronger than the differences between L. obtusata from the upper and the lower intertidal levels (Z = 6.4 and Z = 6.6, respectively, versus Z = 3.4, p = .005). The shell of L. fabalis has a more expanded outer lip and a more rounded aperture (Figure 6). Angles between allometric relationships of L. fabalis did not significantly differ from those of L. obtusata from both shore levels after site‐specific allometric differences had been removed (Table 2; Appendix S3). L. fabalis had a significantly higher morphological disparity than L. obtusata from the upper and lower shore levels (p = .001 in the both cases, Z = 5.3 and Z = 6.4, correspondingly; Appendix S3). Importantly, there were no significant differences between the upper and lower fractions of L. obtusata populations (Z = −0.5, p = .638; Tables 1 and 2).
Littorina obtusata individuals with strongly deformed shells, with a pressed spire and a right‐angle fin on the body whorl or, vice versa, with a very high spire, were registered in the Varangerfjord population. Their frequency was approximately the same at the upper and lower levels: 7.2% and 6.3%, respectively. Both these forms of monstrosity were described by Reid (1996). No such forms were found in the Saltstraumen population of L. obtusata and in both populations of L. fabalis.
3.3. Physiological variation among species and subpopulations
We applied a metabolomic approach to juxtapose physiology and niche differentiation of the Littorina species. We analyzed “instantaneous snapshots of metabolism” as all the samples from each site were collected in parallel during the same low tide. The metabolomes varied significantly across species/subpopulations (p = .0001, perMANOVA) and sampling sites (p = .0001), the pattern of variation differed at different sites (interaction was significant, p = .0002) (Figure 7, Appendix S5).
FIGURE 7.

nMDS ordination of whole metabolomes based on dry weight and quantile normalized quantitative data and selected component comparison. (a) ordination of whole metabolomes by species, 95% confidence intervals of centroids are visualized. (b) ordination of whole L. saxatilis and L. obtusata metabolomes regarding collection site and intertidal level, 95% confidence intervals of centroids are visualized
3.3.1. Interspecific variation
Significant differences were detected between the species of the “obtusata” group, L. fabalis and L. obtusata, at both intertidal levels (Figure 7a). Interspecies differences were also registered within the “saxatilis” group (Figure 7a). There was no overlap in the distribution of metabolomes between L. arcana and L. compressa (p = .002, post hoc perMANOVA), while the metabolome of L. saxatilis overlapped both with that of L. arcana and, to a much lesser extent, with that of L. compressa. The fraction of L. saxatilis overlapping with L. arcana was mainly represented by high‐shore inhabitants (see Appendix S5 and below). As a result, these two species were metabolically indistinguishable at the upper intertidal level. At the same time, the differences between L. compressa and L. saxatilis from both levels as well as between L. arcana and lower shore L. saxatilis were significant (p = .002, post hoc perMANOVA). Species‐distinguishing metabolites, according to PLS‐DA, were mainly represented by saturated and unsaturated fatty acids, cholesterol, adenosine, tryptophan, and pantothenic acid (the full list is given in Appendices S4 and S6).
3.3.2. Shore‐level effects
Two species, L. saxatilis and L. obtusata, from, respectively, the “saxatilis” and the “obtusata” cryptic group were collected from two intertidal levels. The shore level did not significantly affect the metabolome of L. obtusata but did affect that of L. saxatilis (Figure 8b). The differences in L. saxatilis metabolomes at different shore levels were more prominent in Saltstraumen than in Varangerfjord.
FIGURE 8.

Comparison of abundances of anaerobic metabolites (in detection intensity units) at different collection sites and intertidal levels. Means and 95% confidence intervals are shown. p‐values were obtained from the moderated t test (Smyth, 2004)
Since air exposure during low tide is known to onset the anaerobic metabolic pathways, the concentrations of anaerobic metabolic compounds were specifically analyzed. These compounds did not differ significantly in L. obtusata from different intertidal levels (Figure 8, Appendix S6). At the same time, in L. saxatilis, lactate and malate were significantly more abundant in lower shore snails (p = .015, moderated t test; Figure 8), with the increase in the succinate, though insignificant, showing the same tendency. Finally, succinate was significantly more abundant in L. compressa than in L. arcana (p = .043, moderated t test; Figure 8), with malate and lactate showing the same tendency. Amino acids involved in anaerobic metabolism, aspartate and alanine, demonstrated no clear tendency in any of the species studied (Appendix S6).
4. DISCUSSION
In this study, we evaluated the shell morphology and the metabolomes in a group of closely related sympatric Littorina (Neritrema) species in the context of occupied microhabitats. All the five Neritrema species studied at the intertidal zone of the Barents and the Norwegian Sea coasts clearly demonstrated niche conservatism (the whole family Littorinidae includes more than 200 exclusively inter‐ and supratidal species; Bouchet et al., 2005). Nevertheless, some niche specialization and differentiation between species are expected, as phylogenetically related species usually occupy similar but not equivalent niches (Losos, 2008; Pitteloud et al., 2017; Scriven et al., 2016; Wiens & Graham, 2005). The prevalence of niche divergence over niche conservatism is stronger for sympatric species due to such mechanisms as interspecies competition alleviation and efficient exploitation of environmental resources (Losos et al., 2003; Pitteloud et al., 2017; Schluter, 2000; Silvertown et al., 2001). Here, we present a detailed study of microhabitat distribution of five coexisting littorinid species, demonstrating their niche nonequivalence to provide possible adaptive interpretations of observed differences in shell morphology and physiology. Based on our results, we cannot conclude whether the occupied microhabitats belong to their optimal range. We can just pinpoint the fact of their ecological segregation, owing to either differential adaptive scope, or typical biotic associations, or competition, or a combination of these factors. Nevertheless, inhabiting a certain type of microhabitat requires morphological and physiological adaptations, which are a precondition of ecology‐driven speciation. Thus, along with microdistribution patterns, we analyzed morphological and physiological differences between the Neritrema species and inferred their possible adaptive relevance.
4.1. Ecology
Littorina obtusata seems to be the most ecologically distinct species. It preferentially occupies the depth of the fucoid canopy: A. nodosum in the lower part of the littoral zone and F. vesicolosus in the upper part. L. fabalis is associated with another fucoid, F. serratus, occupying mainly surface of the fucoid canopy. This difference in localization between the two “obtusata” species concords with the results of an earlier study, which showed that L. obtusata was negatively phototactic and tended to retreat into the algal mass while L. fabalis actively crawled on the algal surface during air exposure (Guiterman, 1970).
Two species from the “saxatilis” group, L. saxatilis and L. arcana, inhabit stony and gravel substrates, mainly in the upper part of the intertidal zone. The former spreads lower down the littoral on the gravel under F. vesiculosus. L. compressa seems to be an “ecological stranger” within the “saxatilis” group. It preferentially dwells in the lower part of the intertidal zone in association with F. vesiculosus, on the surface and beneath the fucoid mass. Unexpectedly, individuals of L. arcana were occasionally registered in a similar microhabitat. These results demonstrate a clear partitioning of the environments by five closely related periwinkle species.
Species of the “obtusata” group favor different “host” alga species, and it was probably this “host” switch that promoted the divergence of L. fabalis and L. obtusata. Moreover, L. obtusata is not only epiphytic but also phytophagous, feeding on the thalli of Fucus and Ascophyllum and displaying resistance to their toxic polyphenols, while L. fabalis prefers to graze on bacterial films on the surface of the thalli (Norton et al., 1990; Norton & Manley, 1990; Reid, 1996). In the “saxatilis” group, L. saxatilis, besides grazing solid substrates, is known to feed on seedlings of the green alga Enteromorpha but avoids feeding on brown algae (Lotze & Worm, 2000), which corresponds to our observation of its preferred occurrence under the fucoid canopy in the lower level. Thus, although the distribution of L. obtusata partially overlaps with that of both L. fabalis and L. saxatilis during low tide, these three species are ecologically separated: L. obtusata and L. fabalis inhabit clumps of various macroalgae, while L. saxatilis occupies gravel under the macroalgal canopy.
Littorina compressa is the only species of the “saxatilis” group tightly associated with fucoid macroalgae. Being a micrograzer, L. compressa keeps to F. vesiculosus, probably scraping the bacterial film from its surface, as L. fabalis does on the surface of F. serratus. Similarly to L. saxatilis, L. compressa spatially overlaps with L. obtusata but their niches are different due to the specificity of feeding. On the whole, when the microenvironment is taken into account, there is no obvious phylogenetic signal (correspondence between phylogenetic relatedness and ecological niche similarity; Blomberg & Garland, 2002) in ecological preferences of the studied species, as L. compressa, being a species of the “saxatilis” group, demonstrates a pattern more typical of “obtusata” species. This observation is in line with the rapid rate of ecological divergence between sympatric species and the absence of a clear correlation between the ecological similarity of sympatric species and their phylogenetic proximity (Losos et al., 2003; Warren et al., 2008).
It should be emphasized that the habitat preferences of the periwinkles described in this paper are not universal. For example, L. obtusata appears to prefer F. vesiculosus to A. nodosum as “host” species and feeding substrate in Scotland (Watson & Norton, 1987) but favors A. nodosum in populations of Wales and Norway (Williams, 1995). L. fabalis ecotypes in the Iberian Peninsula are associated with either Fucus vesiculosus, or Zostera spp. or Mastocarpus (as Gigartina) stellatus, depending on the wave exposure of a coast (Reid, 1996; Rolán & Templado, 1987). In Sweden, L. fabalis is found throughout the eulittoral zone on the open stones or in the canopy of F. serratus, F. vesiculosus, F. spiralis, and A. nodosum, not being confined to the lower littoral part (Tatarenkov & Johannesson, 1998). At the same time, in Wales and Scotland, the distribution pattern of L. fabalis is very similar to the one described here (Reimchen, 1981; Watson & Norton, 1987). Even when only moderately exposed coasts are compared, ecological preferences of L. fabalis (and other species, as expected) vary in Galicia, Sweden, and Norway. To be more precise, the ecological preferences of each species form a unique pattern in every particular coast type. Nevertheless, the analysis of a variety of the distribution patterns could be used for understanding these preferences.
Another important point to keep in mind is a temporal variation of the environmental regime in the littoral zone: Intertidal organisms are regularly emerged and submerged during the tidal cycle. Besides typical emerged and submerged states in all littoral inhabitants, there is a specific regime of their change and specific mechanisms regulating the physiological transition between these two states. Ecological niche characteristics include parameters of emerged and submerged conditions and transitions between them, requiring adequate adaptations. Furthermore, there are fluctuations in temperature, humidity, salinity, etc., even if only submerged or emerged state analyzed due to day–night and seasonal variations. Irregular variability also occurs. In our study, only one dimension of this complex system was analyzed—“the instantaneous ecology and physiology of emerged state.” This description, though incomplete, is undoubtedly informative as it allows one to outline at least a subset of possible stressors and adaptations to withstand them.
4.2. Shell morphology
We found that shell morphology was highly variable in three of the studied species: L. obtusata, L. fabalis, and L. saxatilis. In the latter two species, shell shape polymorphism has been found to be associated with shore conditions such as wave exposure, temperature stress, and crab predation, and contrasting ecotypes have been identified (Reid, 1996). In L. obtusata, a significant variability in shell shape has been noted (Reid, 1996) but no ecotypes have been recognized. To note, two geographical morphs, the “northern” turbinate palliata and the “southern” globose retusa, have been described in L. obtusata (Reid, 1996). L. fabalis, though similar in shell shape to L. obtusata and forming similar geographical morphs, the “northern” turbinate limata and the “southern” globose typica, is more patulous and has a thicker columellar lip (Reid, 1996). A hypothetical explanation is that these differences reflect a stronger ability of L. fabalis to attach to a substrate and thus to occupy more exposed habitats (Tatarenkov & Johannesson, 1998; Williams, 1990, 1992, 1995). In line with this hypothesis, a geometric morphometric analysis showed significant shell shape differences between L. obtusata and all three L. fabalis ecotypes in the Iberian Peninsula (Carvalho, 2014). Our morphometric analysis confirmed that in Norway the shell of L. fabalis differs from that of L. obtusata, having a more expanded aperture. This probably means that L. fabalis has a more massive foot, which is consistent with the above hypothesis.
Contrasting ecotypes of L. saxatilis in environmental gradients of rocky shores have been extensively studied (e.g., Carvajal‐Rodriguez et al., 2005; Faria et al., 2019; Galindo et al., 2010; Johannesson et al., 1997; Ravinet et al., 2016; Rolán‐Alvarez, 2007, etc.). They are usually represented by either a globose shell with a wide aperture, which is better suited for withstanding wave impact, or a higher, often thickened and armored shell with a narrower aperture, which allows the snail to endure higher temperature/desiccation stress and/or to resist the attacks of crabs (Johannesson, 2003; Mill & Grahame, 1995; Rolán‐Alvarez et al., 2015). In this study, we found similar tendencies in the predicted mean shapes of L. saxatilis and L. obtusata, though they were weak for the former and weaker still for the latter species (Figure 7). Our study sites were not steep rocky but flat gravel‐stony, with a moderate wave action and no expected gradient in this action along shore height; additionally, there are no predatory crabs in the area. This is probably the reason behind the weaker differences between the high‐shore and the low‐shore subpopulations found in our study. Nevertheless, the factor underlying the presence of these differences is still of high interest. In an experimental work of Kemp and Bertness (1984) on L. littorea, similar shell shape changes were not adaptive but were caused by differences in metabolic and growth rates. Snails living on poor diet developed elongate shell with narrow aperture, while the mollusks on a rich diet had thin globose shell with expanded aperture (Kemp & Bertness, 1984). It seems self‐consistent that periwinkles of high‐shore have less ample feeding regime due to a longer exposure during the low tide. Thus, shore level‐related difference in shell morphology observed in the studied Neritrema species may be partially nonadaptive but neutral and caused by different allometric patterns.
In our study, L. arcana most closely resembled the higher fraction of L. saxatilis both in shell shape and microhabitat preferences, as well as in metabolome characteristics and the proteome (Maltseva et al., 2016, 2020). This contradicts earlier studies of this species pair based on linear morphometric analysis, which has shown not only significant differences between sympatric L. arcana and L. saxatilis in British populations but also the nonequivalence of L. saxatilis depending on the presence of L. arcana (Grahame & Mill, 1989). L. arcana has a more open aperture than L. saxatilis and has been reported to possess a larger foot (Grahame & Mill, 1986). It has been hypothesized that these features explain the ability of L. arcana to occupy more exposed sites and substrates than L. saxatilis in Britain (Dytham et al., 1990). The discrepancy between our results and earlier studies might be due to the differences between the coast types: strongly exposed in Britain versus moderately exposed in Norway. However, it is more likely that it results from specific features of the British Littorina populations, which have a long history of isolation from the mainland ones (Doellman et al., 2011; Maltseva et al., 2020; Panova et al., 2011). Indeed, other morphometric studies of British periwinkles revealed shell shape differences in L. saxatilis and L. arcana, comparable with the differences between either of them and L. compressa (Caley et al., 1995; Conde‐Padin et al., 2007).
According to our results, L. compressa significantly differed from L. arcana and L. saxatilis in the shell shape, while the latter two species were barely distinguishable in this respect, especially if only the upper fraction of L. saxatilis was taken into account. The shell of L. compressa is relatively high and slim, with a small round aperture (Figure 7) but it is difficult to find any adaptive background in this. These differences correspond to our long‐term field experience, which suggests that L. compressa may be recognized based on shell morphology, and not only the shape but also the texture of the shell (our observations, unpublished), and to the results of the metabolomic analysis.
4.3. Metabolome similarity
Instantaneous metabolic “snapshots” were obtained to describe the physiological status of the periwinkles under study and to trace a correlation with their ecological preferences. In general, the metabolomes of these phylogenetically close littorinid species were, expectedly, rather similar (Figure 7). Nonetheless, there were significant interspecies differences, while the effect of the intertidal level was less certain. We have found no metabolomic (and no shell shape) differences between the upper and lower fractions of L. obtusata populations, but found robust differences between L. obtusata and L. fabalis. The latter two species occupy ecologically different microhabitats (in terms of intertidal level, preferred species of fucoid macroalgae, and position in/on “host” fucoid during low tide), which may explain the observed differences in metabolomes (here) and proteomes (Maltseva et al., 2020). L. obtusata switches a preferred “host” macroalgae from A. nodosum on the lower/mid‐shore to F. vesiculosus on the upper shore, where the former fucoid is absent. Robert McMahon suggested the existence of two adaptive strategies in intertidal gastropods (McMahon, 1990). Inhabitants of the eulittoral zone (the low‐ to mid‐shore zone) struggle to retain metabolic activities even when air‐exposed during low tide, while the dwellers of the littoral fringe (the high‐shore zone) slow down metabolic processes and isolate themselves by closing shells with opercula. We suggest that the upper and lower subpopulations of L. obtusata do not differ significantly in metabolomic features due to habitat amelioration by fucoid macroalgae: The microclimate within a canopy of the “host” fucoid remains relatively stable during the tidal cycle, even though the fucoids are different at different levels. Thus, L. obtusata physiologically represents eulittoral fauna even in the upper part of the shore in association with F. vesiculosus.
Unlike L. obtusata, L. saxatilis showed significant variation of the metabolome depending on the intertidal level. This result is consistent with many previous studies on the vertical subdivision of L. saxatilis populations (not considering contrasting ecotypes) in neutral genetic markers (Sokolov et al., 2003), general proteomic features (Maltseva et al., 2016), water conservation abilities, behavior, and metabolic enzymes stability and productiveness (Smith et al., 1995; Sokolova et al., 2000; Sokolova & Pörtner, 2001a, 2001b). In particular, regarding the last point, it was shown that the allele of a less productive aspartate aminotransferase (AAT) enzyme is more common among the upper shore L. saxatilis snails (Panova & Johannesson, 2004). AAT catalyzes transamination reaction during the transition to anaerobic metabolism, which leads to the accumulation of end products of diverse pathways of pyruvate transformation (with tissue‐dependent intensity): succinate, malate, and lactate (De Zwaan, 1983; Livingstone & De Zwaan, 1983; Sokolova et al., 2000). Metabolic depression in the upper shore L. saxatilis fraction during low tide was confirmed in the White and North Sea populations (Sokolova et al., 2000; Sokolova & Pörtner, 2001a, 2001b). Accordingly, in our study, the average concentrations of succinate, malate, and lactate were slightly higher in the metabolomes of the lower level L. saxatilis snails (Figure 8, for more details, see Appendix S6). Notably, there was no such clear tendency in the case of L. obtusata (Figure 8). This confirms the existence of two different adaptive strategies in L. saxatilis snails from the upper and the lower shore levels (littoral fringe and eulittoral following McMahon, 1990) in contrast to L. obtusata.
The differences between the other two species of the “saxatilis” group, L. arcana (the species of the littoral fringe) and L. compressa (the species of the eulittoral zone), tended to be similar to those between the upper and the lower fractions of L. saxatilis (Figure 8). This concerns both a set of metabolites differentiating the upper and the lower shore L. saxatilis and anaerobic metabolism compounds (Appendix S6). Such metabolome properties are well consistent with the ecological preferences of these species. Both L. arcana and L. saxatilis inhabit stony and gravel biotopes on the upper shore, which are prone to acute desiccation, temperature variation, and other stressors. At the same time, both L. compressa and L. saxatilis are associated with F. vesiculosus in the low shore, where environmental conditions are milder. Interestingly, the metabolome of L. compressa still differed from that of the lower L. saxatilis (Appendix S6), probably reflecting the difference in their microhabitats: gravel under the fucoid canopy (L. saxatilis) versus the surface of the fucoid mass (L. compressa).
Our results, supplemented by the published data, provide a backbone to the reconstruction of the niche divergence and the evolution of species‐specific features in the complex of phylogenetically close snail species. The estimated divergence time between “saxatilis” and “obtusata” groups is ~3.25–2.83 Mya (Reid et al., 1996, 2012). The event was probably related to the specialization of flat periwinkles (“obtusata” group) to association with and feeding on brown macroalga (fucoids) against rough periwinkle L. islandica, a presumable ancestor of the three species of the “saxatilis” group, grazing bacterial biofilms from solid substrates (Reid, 1996). The feeding specialization was accompanied by a modification of the radula, which is clearly different in grazers on macroalgae (“obtusata”) compared with micrograzers of the “saxatilis” group (Reid, 1996). Differences in shell morphology between the species groups also became apparent: more globose shells in “obtusata” versus coniform shells with pronounced concaved suture in “saxatilis.”
Diversification within groups seems to be due to inhabiting different microhabitats, which can be partially characterized through their typical position during low tide. L. obtusata and L. fabalis developed an association with different fucoid macroalgae: A. nodosum and F. vesiculosus versus F. serratus, respectively. The “host” algae, in turn, determined their distribution along the vertical intertidal gradient (L. obtusata occupies a higher zone than L. fabalis) as well as the complexes of morphological and physiological adaptations. The differences in the “host” alga species, the position on it, the preferred feeding substrate, and the distribution pattern (differential regimes of air exposure, wave action, etc.) are reflected in clear differences in the metabolomes and the shell morphology.
Among the “saxatilis” species, L. saxatilis represents a typical generalist, being able to spread from the upper to the lower intertidal levels, inhabit both sheltered and exposed shores, and enter estuarine areas. Owing to this, it has the widest geographical range in the subgenus (Reid, 1996). This species is associated with gravel, open at the upper intertidal level and covered by fucoid macroalgae at the lower one. This microhabitat shift is linked to different regimes of exposure to air and extreme temperatures, desiccation, and other stressors. These differences underlie shore level‐dependent variation in shell shape and metabolomic characteristics, illustrating the ability of this species to form partially isolated specialized subpopulations. In contrast, L. obtusata populations occupy a fairly stable microhabitat within the fucoid canopy, which weakens level‐dependent effects. L. compressa is tightly associated with F. vesiculosus, and this association seems to limit the distribution of this species to the uppermost part of the intertidal zone and to determine its metabolomic and conchological peculiarity. L. arcana closely resembles L. saxatilis at the upper intertidal level in shell shape, metabolome, and ecological preferences. Some fraction of L. arcana descends into the lower part of the intertidal zone in association with F. vesiculosus. This fraction was not analyzed in this study, but is expected to differ from the upper part, similarly to the upper and the lower shore L. saxatilis based on our previous results (Maltseva et al., 2016). Moreover, some laboratory and field observations suggest the possibility of a restricted gene flow between L. arcana and L. saxatilis, the existence of presumable hybrids, and interspecies copulations in wild populations (Granovitch et al., 2013; Maltseva et al., 2021; Mikhailova et al., 2009; Warwick et al., 1990). Thus, the divergence between these two species is of particular interest since the ecological specification can hardly be its driving force. Some postmating prezygotic reproductive isolation mechanisms, for example, those associated with polymorphism of gamete interaction proteins, could be expected to function in this pair of species and to contribute to their nonecological and nongeographical speciation (Lobov et al., 2018; Maltseva et al., 2021).
5. CONCLUSIONS
The Neritrema species are a vivid example of niche nonequivalence between sympatric close relatives, although in general they retain niche conservatism, remaining within the intertidal zone. We detected a clear differentiation of potentially adaptive traits related to shell morphology and metabolite composition between species with clearly delineated microhabitats. On the other hand, there was no such differentiation of the metabolomes and the shell morphology between the species inhabiting the same biotope in the high‐shore (L. saxatilis and L. arcana). These findings accentuate the role of adaptation in the divergence of the Northern Atlantic Neritrema species and provide a rationale for considering them as a living example of recent ecological speciation, with the exception of L. arcana/L. saxatilis pair. The driving force of divergence in the latter species pair can become a hot spot of future research.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Arina L. Maltseva: Conceptualization (lead); Data curation (lead); Methodology (lead); Supervision (equal); Validation (equal); Writing‐original draft (lead). Marina A. Varfolomeeva: Data curation (equal); Methodology (lead); Software (lead); Validation (equal); Visualization (lead); Writing‐original draft (equal). Roman V. Ayanka: Data curation (supporting); Methodology (equal); Software (supporting); Visualization (supporting); Writing‐original draft (supporting). Elizaveta R. Gafarova: Methodology (equal); Writing‐original draft (equal). Egor A. Repkin: Methodology (equal); Writing‐original draft (equal). Polina A. Pavlova: Methodology (equal); Writing‐original draft (equal). Alexei L. Shavarda: Methodology (lead); Writing‐original draft (supporting). Natalia A. Mikhailova: Conceptualization (equal); Methodology (equal); Supervision (supporting); Writing‐review & editing (supporting). Andrei I. Granovitch: Conceptualization (lead); Data curation (equal); Funding acquisition (lead); Methodology (equal); Project administration (lead); Supervision (equal); Validation (equal); Writing‐review & editing (lead).
OPEN RESEARCH BADGES
This article has earned an Open Data Badge for making publicly available the digitally‐shareable data necessary to reproduce the reported results. The data is available at [https://doi.pangaea.de/10.1594/PANGAEA.923735].
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
ACKNOWLEDGMENTS
The project was supported by the Russian Scientific Foundation grant 19‐14‐00321, PI Andrei Granovitch. Metabolomic analysis was performed with the use of the equipment of the Resource Center of St. Petersburg State University “Development of Molecular and Cell Technologies.” Adaptation of the GC‐MS protocol for the TMS‐analysis and in‐house database were made during the BIN research project AAAA‐A18‐118032390136‐5 “Assessment of changes in the correlation structure of metabolic networks in the process of growth and development of fungi and plants from the viewpoint of systemic biology.” We thank Prof. Michael Collyer for the kind help with the bootstrap procedure used for the computation of confidence limits of morphological disparity. We are grateful to Natalia Lentsman for the thorough review and proofreading of the manuscript.
Maltseva, A. L., Varfolomeeva, M. A., Ayanka, R. V., Gafarova, E. R., Repkin, E. A., Pavlova, P. A., Shavarda, A. L., Mikhailova, N. A., & Granovitch, A. I. (2021). Linking ecology, morphology, and metabolism: Niche differentiation in sympatric populations of closely related species of the genus Littorina (Neritrema). Ecology and Evolution, 11, 11134–11154. 10.1002/ece3.7901
ENDNOTES
Phylogenetic niche conservatism is a phenomenon of closely related species being more similar ecologically than would be expected by simple Brownian motion (Wiens & Graham, 2005; Losos, 2008).
Climatic data from the Weather Atlas https://www.weather‐atlas.com.
Landmarks are the anatomical loci that can be reliably defined on the structures studied (Bookstein, 1991). The same set of landmarks should be recognised on all specimens. Semilandmarks are used to describe the shape of curves where it is impossible to identify discrete anatomical loci repeatedly. Semilandmarks are series of points located at equal intervals along the curves (Bookstein, 1997).
DATA AVAILABILITY STATEMENT
Microhabitat distribution (counts in quantitative samples), shell shape (TPS files with landmark positions and metadata), and metabolome compositions (normalized detection intensities) will be available at Pangaea.de (https://doi.org/10.1594/PANGAEA.923735).
REFERENCES
- Ackerly, D. D. (2003). Community assembly, niche conservatism, and adaptive evolution in changing environments. International Journal of Plant Sciences, 164(S3), S165–S184. 10.1086/368401 [DOI] [Google Scholar]
- Adams, D. C., & Otárola‐Castillo, E. (2013). geomorph: An R package for the collection and analysis of geometric morphometric shape data. Methods in Ecology and Evolution, 4(4), 393–399. [Google Scholar]
- Anderson, M. J. (2001). A new method for non‐parametric multivariate analysis of variance. Austral Ecology, 26(1), 32–46. 10.1111/j.1442-9993.2001.01070.pp.x [DOI] [Google Scholar]
- Anderson, M. J., & Walsh, D. C. (2013). PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecological Monographs, 83(4), 557–574. 10.1890/12-2010.1 [DOI] [Google Scholar]
- Arnqvist, G., & Martensson, T. (1998). Measurement error in geometric morphometrics: Empirical strategies to assess and reduce its impact on measures of shape. Acta Zoologica Academiae Scientiarum Hungaricae, 44(1–2), 73–96. [Google Scholar]
- Baniaga, A. E., Marx, H. E., Arrigo, N., & Barker, M. S. (2020). Polyploid plants have faster rates of multivariate niche differentiation than their diploid relatives. Ecology Letters, 23(1), 68–78. 10.1111/ele.13402 [DOI] [PubMed] [Google Scholar]
- Barker, M., & Rayens, W. (2003). Partial least squares for discrimination. Journal of Chemometrics: A Journal of the Chemometrics Society, 17(3), 166–173. 10.1002/cem.785 [DOI] [Google Scholar]
- Blomberg, S. P., & Garland, T.Jr (2002). Tempo and mode in evolution: Phylogenetic inertia, adaptation and comparative methods. Journal of Evolutionary Biology, 15(6), 899–910. 10.1046/j.1420-9101.2002.00472.x [DOI] [Google Scholar]
- Bookstein, F. L. (1991). Morphometric tools for landmark data: Geometry and biology. Cambridge University Press. [Google Scholar]
- Bookstein, F. L. (1997). Landmark methods for forms without landmarks: Morphometrics of group differences in outline shape. Medical Image Analysis, 1, 225–243. 10.1016/S1361-8415(97)85012-8 [DOI] [PubMed] [Google Scholar]
- Bouchet, P., Fryda, J., Hausdorf, B., Ponder, W. F., Valdes, A., Waren, A., & Rocroi, J. P. (2005). Working classification of the Gastropoda. Malacologia, 47(1–2), 239–283. [Google Scholar]
- Bray, J. R., & Curtis, J. T. (1957). An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs, 27(4), 326–349. 10.2307/1942268 [DOI] [Google Scholar]
- Butlin, R. K., Saura, M., Charrier, G., Jackson, B., André, C., Caballero, A., Coyne, J. A., Galindo, J., Grahame, J. W., Hollander, J., Kemppainen, P., Martínez‐Fernández, M., Panova, M., Quesada, H., Johannesson, K., & Rolán‐Alvarez, E. (2014). Parallel evolution of local adaptation and reproductive isolation in the face of gene flow. Evolution, 68(4), 935–949. 10.1111/evo.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caley, K., Grahame, J., & Mill, P. J. (1995). A geographically based study of shell shape in small rough periwinkles. Hydrobiologia, 309, 181–193. 10.1007/BF00014486 [DOI] [Google Scholar]
- Canty, A., & Ripley, B. (2020). boot: Bootstrap R (S‐Plus) functions. R package version 1.3‐25. [Google Scholar]
- Cardini, A., & Elton, S. (2007). Sample size and sampling error in geometric morphometric studies of size and shape. Zoomorphology, 126(2), 121–134. 10.1007/s00435-007-0036-2 [DOI] [Google Scholar]
- Cardini, A., Seetah, K., & Barker, G. (2015). How many specimens do I need? Sampling error in geometric morphometrics: Testing the sensitivity of means and variances in simple randomized selection experiments. Zoomorphology, 134(2), 149–163. 10.1007/s00435-015-0253-z [DOI] [Google Scholar]
- Carvajal‐Rodriguez, A., Conde‐Padin, P., & Rolán‐Alvarez, E. (2005). Decomposing shell form into size and shape by geometric morphometric methods in two sympatric ecotypes of Littorina saxatilis . Journal of Molluscan Studies, 71(4), 313–318. 10.1093/mollus/eyi037 [DOI] [Google Scholar]
- Carvalho, J. G. M. (2014). Study on the diversification of flat periwinkles (Littorina fabalis and L. obtusata): Insights from genetics and geometric morphometrics. Dissertação Mestrado em Biologia Evolutiva e do Desenvolvimento. Universidade de Lisboa, Portugal. [Google Scholar]
- Collyer, M. L., & Adams, D. C. (2018). RRPP: An r package for fitting linear models to high‐dimensional data using residual randomization. Methods in Ecology and Evolution, 9(7), 1772–1779. [Google Scholar]
- Conde‐Padin, P., Grahame, J. W., & Rolán‐Alvarez, E. (2007). Detecting shape differences in species of the Littorina saxatilis complex by morphometric analysis. Journal of Molluscan Studies, 73(2), 147–154. 10.1093/mollus/eym009 [DOI] [Google Scholar]
- Cox‐Singh, J. (2012). Zoonotic malaria: Plasmodium knowlesi, an emerging pathogen. Current Opinion in Infectious Diseases, 25(5), 530–536. 10.1097/QCO.0b013e3283558780 [DOI] [PubMed] [Google Scholar]
- Crockett, E. L. (1998). Cholesterol function in plasma membranes from ectotherms: Membrane‐specific roles in adaptation to temperature. American Zoologist, 38(2), 291–304. 10.1093/icb/38.2.291 [DOI] [Google Scholar]
- Davison, A. C., & Hinkley, D. V. (1997). Bootstrap methods and their application (vol 1). Cambridge University Press. [Google Scholar]
- De Livera, A. M., & Olshansky, G. (2018). NormalizeMets: Analysis of metabolomics data. R Package Version 0.25. https://CRAN.R‐project.org/package=NormalizeMets [Google Scholar]
- De Zwaan, A. (1983). Carbohydrate catabolism in bivalves. In Hochachka P. W. (Ed.), The Mollusca: Metabolic biochemistry and molecular biomechanics (pp. 137–175). Academic Press. 10.1016/B978-0-12-751401-7.50011-9 [DOI] [Google Scholar]
- DiCiccio, T. J., & Efron, B. (1996). Bootstrap confidence intervals. Statistical Science, 11, 189–212. [Google Scholar]
- Doellman, M. M., Trussell, G. C., Grahame, J. W., & Vollmer, S. V. (2011). Phylogeographic analysis reveals a deep lineage split within North Atlantic Littorina saxatilis . Proceedings of the Royal Society B: Biological Sciences, 278(1722), 3175–3183. 10.1098/rspb.2011.0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenz, C. J., Bittner, D., Vonlanthen, P., Wagner, C. E., & Seehausen, O. (2018). Rapid buildup of sympatric species diversity in Alpine whitefish. Ecology and Evolution, 8(18), 9398–9412. 10.1002/ece3.4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval, L., & Ariey, F. (2012). Ape Plasmodium parasites as a source of human outbreaks. Clinical Microbiology and Infection, 18(6), 528–532. 10.1111/j.1469-0691.2012.03825.x [DOI] [PubMed] [Google Scholar]
- Dytham, C., Grahame, J., & Mill, P. J. (1990). Distribution, abundance and shell morphology of Littorina saxatilis (Olivi) and Littorina arcana Hannaford Ellis at Robin Hood’s Bay, North Yorkshire. Hydrobiologia, 193, 233–240. 10.1007/BF00028080 [DOI] [Google Scholar]
- Faria, R., Chaube, P., Morales, H. E., Larsson, T., Lemmon, A. R., Lemmon, E. M., & Westram, A. M. (2019). Multiple chromosomal rearrangements in a hybrid zone between Littorina saxatilis ecotypes. Molecular Ecology, 28(6), 1375–1393. 10.1111/mec.14972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filchak, K. E., Roethele, J. B., & Feder, J. L. (2000). Natural selection and sympatric divergence in the apple maggot Rhagoletis pomonella . Nature, 407(6805), 739–742. [DOI] [PubMed] [Google Scholar]
- Futuyma, D. J., & McCafferty, S. S. (1990). Phylogeny and the evolution of host plant associations in the leaf beetle genus Ophraella (Coleoptera, Chrysomelidae). Evolution, 44(8), 1885–1913. 10.1111/j.1558-5646.1990.tb04298.x [DOI] [PubMed] [Google Scholar]
- Galaktionov, K. V., & Bustnes, J. O. (1999). Distribution patterns of marine bird digenean larvae in periwinkles along the southern coast of the Barents Sea. Diseases of Aquatic Organisms, 37(3), 221–230. 10.3354/dao037221 [DOI] [PubMed] [Google Scholar]
- Galindo, J., Grahame, J. W., & Butlin, R. K. (2010). An EST‐based genome scan using 454 sequencing in the marine snail Littorina saxatilis . Journal of Evolutionary Biology, 23(9), 2004–2016. 10.1111/j.1420-9101.2010.02071.x [DOI] [PubMed] [Google Scholar]
- Gause, G. F. (1934). The struggle for existence (pp. 163). Williams and Wilkins. [Google Scholar]
- Gower, J. C. (1975). Generalized Procrustes analysis. Psychometrika, 40, 33–51. [Google Scholar]
- Graham, C. H., Ron, S. R., Santos, J. C., Schneider, C. J., & Moritz, C. (2004). Integrating phylogenetics and environmental niche models to explore speciation mechanisms in dendrobatid frogs. Evolution, 58(8), 1781–1793. 10.1554/03-274 [DOI] [PubMed] [Google Scholar]
- Grahame, J., & Mill, P. J. (1986). Relative size of the foot of two species of Littorina on a rocky shore in Wales. Journal of Zoology, 208(2), 229–236. 10.1111/j.1469-7998.1986.tb01510.x [DOI] [Google Scholar]
- Grahame, J., & Mill, P. J. (1989). Shell shape variation in Littorina saxatilis and L. arcana: A case of character displacement? Journal of the Marine Biological Association of the United Kingdom, 69(4), 837–855. 10.1017/S0025315400032203 [DOI] [Google Scholar]
- Granovitch, A. I., Loskutova, Z. I., Gracheva, Y. A., & Mikhailova, N. A. (2008). Morphometric comparison of the copulatory organ in mollusks of «saxatilis» species complex (Coenogastropoda, Littorinidae): Problems of identification of species and species status. Zoologicheskiy Zhurnal, 87(12), 1425–1436. (In Russian). [Google Scholar]
- Granovitch, A. I., Maximovich, A. N., Avanesyan, A. V., Starunova, Z. I., & Mikhailova, N. A. (2013). Micro‐spatial distribution of two sibling periwinkle species across the intertidal indicates hybridization. Genetica, 141(7–9), 293–301. 10.1007/s10709-013-9728-3 [DOI] [PubMed] [Google Scholar]
- Granovitch, A. I., Yagunova, E. B., Maximovich, A. N., & Sokolova, I. M. (2009). Elevated female fecundity as a possible compensatory mechanism in response to trematode infestation in populations of Littorina saxatilis (Olivi). International Journal for Parasitology, 39(9), 1011–1019. 10.1016/j.ijpara.2009.02.014 [DOI] [PubMed] [Google Scholar]
- Guerzoni, M. E., Lanciotti, R., & Cocconcelli, P. S. (2001). Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus . Microbiology, 147(8), 2255–2264. 10.1099/00221287-147-8-2255 [DOI] [PubMed] [Google Scholar]
- Guiterman, J. D. (1970). The population biology of Littorina obtusata (L.) (Gastropoda: Prosobranchiata). Unpublished Ph.D. thesis. University College of Wales, Bangor, UK. [Google Scholar]
- Halket, J. M., & Zaikin, V. G. (2003). Derivatization in mass spectrometry — 1. Silylation. European Journal of Mass Spectrometry, 9(1), 1–21. 10.1255/ejms.527 [DOI] [PubMed] [Google Scholar]
- Hannaford Ellis, C. J. (1979). Morphology of the oviparous rough winkle . Littorina arcana Hannaford Ellis, 1978, with notes on the taxonomy of the L. saxatilis species‐complex (Prosobranchia: Littorinidae). Journal of Conchology, 30(1), 43–56. [Google Scholar]
- Hardin, G. (1960). The competitive exclusion principle. Science, 131(3409), 1292–1297. [DOI] [PubMed] [Google Scholar]
- Harvey, P. H., & Rambaut, A. (2000). Comparative analyses for adaptive radiations. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 355(1403), 1599–1605. 10.1098/rstb.2000.0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6(2), 65–70. [Google Scholar]
- Houlihan, D. F., Innes, A. J., & Dey, D. G. (1982). The influence of mantle cavity fluid on the aerial oxygen consumption of some intertidal gastropods. Journal of Experimental Marine Biology and Ecology, 49, 57–68. 10.1016/0022-0981(81)90147-7 [DOI] [Google Scholar]
- Johannesson, K. (2003). Evolution in Littorina: Ecology matters. Journal of Sea Research, 49(2), 107–117. 10.1016/S1385-1101(02)00218-6 [DOI] [Google Scholar]
- Johannesson, K. (2016). What can be learnt from a snail? Evolutionary Applications, 9(1), 153–165. 10.1111/eva.12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesson, K., Butlin, R. K., Panova, M., & Westram, A. M. (2017). Mechanisms of adaptive divergence and speciation in Littorina saxatilis: Integrating knowledge from ecology and genetics with new data emerging from genomic studies. In Oleksiak M., & Rajora O. (Eds.), Population genomics: Marine organisms (pp. 277–301). Springer. 10.1007/13836_2017_6 [DOI] [Google Scholar]
- Johannesson, K., Rolán‐Alvarez, E., & Erlandsson, J. (1997). Growth rate differences between upper and lower shore ecotypes of the marine snail Littorina saxatilis (Olivi) (Gastropoda). Biological Journal of the Linnean Society, 61(2), 267–279. 10.1111/j.1095-8312.1997.tb01790.x [DOI] [Google Scholar]
- Jonsson, P. R., Granhag, L., Moschella, P. S., Åberg, P., Hawkins, S. J., & Thompson, R. C. (2006). Interactions between wave action and grazing control the distribution of intertidal macroalgae. Ecology, 87(5), 1169–1178. [DOI] [PubMed] [Google Scholar]
- Joseph, J. D. (1989). Distribution and composition of lipids in marine invertebrates. In Ackman R. G. (Ed.), Marine biogenic lipids fats and oils (pp. 49–143). CRC Press. [Google Scholar]
- Kemp, P., & Bertness, M. D. (1984). Snail shape and growth rates: Evidence for plastic shell allometry in Littorina littorea . Proceedings of the National Academy of Sciences, 81(3), 811–813. 10.1073/pnas.81.3.811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistner, E. J., & Dybdahl, M. F. (2013). Adaptive responses and invasion: The role of plasticity and evolution in snail shell morphology. Ecology and Evolution, 3(2), 424–436. 10.1002/ece3.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knouft, J. H., Losos, J. B., Glor, R. E., & Kolbe, J. J. (2006). Phylogenetic analysis of the evolution of the niche in lizards of the Anolis sagrei group. Ecology, 87(sp7), S29–S38. [DOI] [PubMed] [Google Scholar]
- Lappalainen, J., Virtanen, E. A., Kallio, K., Junttila, S., & Viitasalo, M. (2019). Substrate limitation of a habitat‐forming genus Fucus under different water clarity scenarios in the northern Baltic Sea. Estuarine, Coastal and Shelf Science, 218, 31–38. 10.1016/j.ecss.2018.11.010 [DOI] [Google Scholar]
- Le Pennec, G., Butlin, R. K., Jonsson, P. R., Larsson, A. I., Lindborg, J., Bergström, E., Westram, A. M., & Johannesson, K. (2017). Adaptation to dislodgement risk on wave‐swept rocky shores in the snail Littorina saxatilis . PLoS One, 12(10), e0186901. 10.1371/journal.pone.0186901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre, P., & Anderson, M. J. (1999). Distance‐based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecological Monographs, 69(1), 1–24. [Google Scholar]
- Legendre, P., & Legendre, L. (2012). Numerical ecology. Elsevier. [Google Scholar]
- Livingstone, D. R., & De Zwaan, A. (1983). Carbohydrate metabolism of gastropods. In Hochachka P. W. (Ed.), The Mollusca: Metabolic biochemistry and molecular biomechanics (pp. 177–242). Academic Press. 10.1016/b978-0-12-751401-7.50012-0 [DOI] [Google Scholar]
- Lobov, A. A., Maltseva, A. L., Starunov, V. V., Babkina, I. Y., Ivanov, V. A., Mikhailova, N. A., & Granovitch, A. I. (2018). LOSP: A putative marker of parasperm lineage in male reproductive system of the prosobranch mollusk Littorina obtusata . Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 330(4), 193–201. 10.1002/jez.b.22803 [DOI] [PubMed] [Google Scholar]
- Losos, J. B. (2008). Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecology Letters, 11(10), 995–1003. 10.1111/j.1461-0248.2008.01229.x [DOI] [PubMed] [Google Scholar]
- Losos, J. B., Leal, M., Glor, R. E., de Queiroz, K., Hertz, P. E., Rodriguez Schettino, L., Chamizo Lara, A., Jackman, R. R., & Larson, A. (2003). Niche lability in the evolution of a Caribbean lizard community. Nature, 424, 542–545. 10.1038/nature01814 [DOI] [PubMed] [Google Scholar]
- Lotze, H. K., & Worm, B. (2000). Variable and complementary effects of herbivores on different life stages of bloom‐forming macroalgae. Marine Ecology Progress Series, 200, 167–175. 10.3354/meps200167 [DOI] [Google Scholar]
- Maltseva, A. L., Varfolomeeva, M. A., Lobov, A. A., Mikhailova, N. A., Renaud, P. E., Grishankov, A. V., Volovik, K. Y., & Granovitch, A. I. (2016). Measuring physiological similarity of closely related littorinid species: A proteomic insight. Marine Ecology Progress Series, 552, 177–193. 10.3354/meps11770 [DOI] [Google Scholar]
- Maltseva, A. L., Varfolomeeva, M. A., Lobov, A. A., Tikanova, P., Panova, M., Mikhailova, N. A., & Granovitch, A. I. (2020). Proteomic similarity of the Littorinid snails in the evolutionary context. PeerJ, 8, e8546. 10.7717/peerj.8546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltseva, A. L., Varfolomeeva, M. A., Lobov, A. A., Tikanova, P. O., Repkin, E. A., Babkina, I. Y., Panova, M., Mikhailova, N. A., & Granovitch, A. I. (2021). Premating barriers in young sympatric snail species. Scientific Reports, 11(1), 1–16. 10.1038/s41598-021-84407-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr, E. (1970). Populations, species, and evolution: An abridgment of animal species and evolution. Belknap Press of Harvard University Press. [Google Scholar]
- McArdle, B. H., & Anderson, M. J. (2001). Fitting multivariate models to community data: A comment on distance‐based redundancy analysis. Ecology, 82(1), 290–297. [Google Scholar]
- McGee, M. D., Borstein, S. R., Meier, J. I., Marques, D. A., Mwaiko, S., Taabu, A., & Seehausen, O. (2020). The ecological and genomic basis of explosive adaptive radiation. Nature, 586(7827), 75–79. [DOI] [PubMed] [Google Scholar]
- McMahon, R. F. (1988). Respiratory response to periodic emergence in intertidal molluscs. American Zoologist, 28(1), 97–114. 10.1093/icb/28.1.97 [DOI] [Google Scholar]
- McMahon, R. F. (1990). Thermal tolerance, evaporative water loss, air‐water oxygen consumption and zonation of intertidal prosobranchs: A new synthesis. In Johannesson K., Raffaelli D. G., & Hannaford Ellis C. J. (Eds.), Progress in Littorinid and Muricid Biology. Developments in hydrobiology (vol. 56, pp. 241–260). Springer. 10.1007/978-94-009-0563-4_20 [DOI] [Google Scholar]
- McMahon, R. F., Russell‐Hunter, W. D., & Aldridge, D. W. (1995). Lack of metabolic temperature compensation in the intertidal gastropods, Littorina saxatilis (Olivi) and L. obtusata (L.). In Mill P. J. & McQuaid C. D. (Eds.), Advances in Littorinid biology (pp. 89–100). Springer. [Google Scholar]
- Mill, P. J., & Grahame, J. (1995). Shape variation in the rough periwinkle Littorina saxatilis on the west and south coasts of Britain. Hydrobiologia, 309, 61–71. 10.1007/BF00014473 [DOI] [Google Scholar]
- Morales, H. E., Faria, R., Johannesson, K., Larsson, T., Panova, M., Westram, A. M., & Butlin, R. K. (2019). Genomic architecture of parallel ecological divergence: Beyond a single environmental contrast. Science . Advances, 5(12), eaav9963. 10.1126/sciadv.aav9963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, N. A., & Wernegreen, J. J. (2000). Lifestyle evolution in symbiotic bacteria: Insights from genomics. Trends in Ecology & Evolution, 15(8), 321–326. 10.1016/s0169-5347(00)01902-9 [DOI] [PubMed] [Google Scholar]
- Moya, A., Pereto, J., Gil, R., & Latorre, A. (2008). Learning how to live together: Genomic insights into prokaryote‐animal symbioses. Nature Reviews Genetics, 9(3), 218–229. 10.1038/nrg2319 [DOI] [PubMed] [Google Scholar]
- Muraeva, O. A., Maltseva, A. L., Mikhailova, N. A., & Granovitch, A. I. (2016). Mechanisms of adaption to salinity stress in marine gastropods Littorina saxatilis: A proteomic analysis. Cell and Tissue Biology, 10, 160–169. 10.1134/S1990519X16020085 [DOI] [PubMed] [Google Scholar]
- Mikhailova, N. A., Gracheva, Y. A., Backeljau, T., & Granovitch, A. I. (2009). A potential species‐specific molecular marker suggests interspecific hybridization between sibling species Littorina arcana and L. saxatilis (Mollusca, Caenogastropoda) in natural populations. Genetica, 137(3), 333. [DOI] [PubMed] [Google Scholar]
- Nilsson, G. E., & Lutz, P. L. (1992). Adenosine release in the anoxic turtle brain: A possible mechanism for anoxic survival. Journal of Experimental Biology, 162(1), 345–351. [Google Scholar]
- Norton, T. A., Hawkins, S. J., Manley, N. L., Williams, G. A., & Watson, D. C. (1990). Scraping a living: A review of littorinid grazing. In Johannesson K., Raffaelli D. G., & Hannaford Ellis C. J. (Eds.), Progress in Littorinid and muricid biology. Developments in hydrobiology (vol. 56, pp. 117–138). Springer. 10.1007/978-94-009-0563-4_10 [DOI] [Google Scholar]
- Norton, T. A., & Manley, N. L. (1990). The characteristics of algae in relation to their vulnerability to grazing snails. In Hughes R. N. (Ed.), Behavioural Mechanisms of Food Selection (pp. 461–478). Springer. 10.1007/978-3-642-75118-9_23 [DOI] [Google Scholar]
- Nosil, P. (2012). Ecological speciation. Oxford University Press. [Google Scholar]
- Nyman, T., Farrell, B. D., Zinovjev, A. G., & Vikberg, V. (2006). Larval habits, host‐plant associations, and speciation in nematine sawflies (Hymenoptera: Tenthredinidae). Evolution, 60(8), 1622–1637. 10.1554/05-674.1 [DOI] [PubMed] [Google Scholar]
- Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P. R., O'Hara, R. B., Simpson, G. L., Solymos, P., Stevens, M. H. H., Szoecs, E., & Wagner, H. (2019). vegan: Community Ecology Package. R package version 2.5‐6. https://CRAN.R‐project.org/package=vegan [Google Scholar]
- Panova, M., Blakeslee, A. M. H., Miller, A. W., Mäkinen, T., Ruiz, G. M., Johannesson, K., & Andre, C. (2011). Glacial history of the North Atlantic marine snail, Littorina saxatilis, inferred from distribution of mitochondrial DNA lineages. PLoS One, 6(3), e17511. 10.1371/journal.pone.0017511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panova, M., & Johannesson, K. (2004). Microscale variation in Aat (aspartate aminotransferase) is supported by activity differences between upper and lower shore allozymes of Littorina saxatilis . Marine Biology, 144, 1157–1164. 10.1007/s00227-003-1274-6 [DOI] [Google Scholar]
- Panova, M., Johansson, T., Canbäck, B., Bentzer, J., Rosenblad, M. A., Johannesson, K., Tunlid, A., & Andre, C. (2014). Species and gene divergence in Littorina snails detected by array comparative genomic hybridization. BMC Genomics, 15(687), 1–21. 10.1186/1471-2164-15-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearman, P. B., Guisan, A., Broennimann, O., & Randin, C. F. (2008). Niche dynamics in space and time. Trends in Ecology & Evolution, 23(3), 149–158. 10.1016/j.tree.2007.11.005 [DOI] [PubMed] [Google Scholar]
- Peccoud, J., Simon, J.‐C., von Dohlen, C., Coeur d’acier, A., Plantegenest, M., Vanlerberghe‐Masutti, F., & Jousselin, E. (2010). Evolutionary history of aphid‐plant associations and their role in aphid diversification. Comptes Rendus Biologies, 333(6–7), 474–487. 10.1016/j.crvi.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Pek, M., & Lutz, P. L. (1997). Role for adenosine in channel arrest in the anoxic turtle brain. Journal of Experimental Biology, 200(13), 1913–1917. 10.1242/jeb.200.13.1913 [DOI] [PubMed] [Google Scholar]
- Percy, D. M. (2003). Radiation, diversity, and host‐plant interactions among island and continental legume‐feeding psyllids. Evolution, 57(11), 2540–2556. 10.1111/j.0014-3820.2003.tb01498.x [DOI] [PubMed] [Google Scholar]
- Pitteloud, C., Arrigo, N., Suchan, T., Mastretta‐Yanes, A., Vila, R., Dincă, V., & Fumagalli, L. (2017). Climatic niche evolution is faster in sympatric than allopatric lineages of the butterfly genus Pyrgus . Proceedings of the Royal Society B: Biological Sciences, 284(1852), 20170208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, T. (1997). Correlated evolution and independent contrasts. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 352(1352), 519–529. 10.1098/rstb.1997.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyron, R. A., Costa, G. C., Patten, M. A., & Burbrink, F. T. (2015). Phylogenetic niche conservatism and the evolutionary basis of ecological speciation. Biological Reviews, 90(4), 1248–1262. 10.1111/brv.12154 [DOI] [PubMed] [Google Scholar]
- R Core Team . (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R‐project.org/ [Google Scholar]
- Raffaelli, D., & Hawkins, S. J. (1996). Intertidal ecology. Chapman and Hall. [Google Scholar]
- Raffini, F., Schneider, R. F., Franchini, P., Kautt, A. F., & Meyer, A. (2020). Diving into divergence: Differentiation in swimming performances, physiology and gene expression between locally‐adapted sympatric cichlid fishes. Molecular Ecology, 29(7), 1219–1234. [DOI] [PubMed] [Google Scholar]
- Ravinet, M., Westram, A., Johannesson, K., Butlin, R., Andre, C., & Panova, M. (2016). Shared and nonshared genomic divergence in parallel ecotypes of Littorina saxatilis at a local scale. Molecular Ecology, 25(1), 287–305. 10.1111/mec.13332 [DOI] [PubMed] [Google Scholar]
- Reed, D. L., Light, J. E., Allen, J. M., & Kirchman, J. J. (2007). Pair of lice lost or parasites regained: The evolutionary history of anthropoid primate lice. BMC Biology, 5(1), 7. 10.1186/1741-7007-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, D. G. (1996). Systematics and evolution of Littorina. The Ray Society. [Google Scholar]
- Reid, D. G., Dyal, P., & Williams, S. T. (2012). A global molecular phylogeny of 147 periwinkle species (Gastropoda, Littorininae). Zoologica Scripta, 41(2), 125–136. 10.1111/j.1463-6409.2011.00505.x [DOI] [Google Scholar]
- Reid, D. G., Rumbak, E., & Thomas, R. H. (1996). DNA, morphology, and fossils: Phylogeny and evolutionary rates of the gastropod genus Littorina . Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 351, 877–895. 10.1098/rstb.1996.0082 [DOI] [PubMed] [Google Scholar]
- Reimchen, T. E. (1981). Microgeographical variation in Littorina mariae Sacchi & Rastelli and a taxonomic consideration. Journal of Conchology, 30, 341–350. [Google Scholar]
- Reipschlager, A. N. K. E., Nilsson, G. E., & Portner, H. O. (1997). A role for adenosine in metabolic depression in the marine invertebrate Sipunculus nudus . American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 272(1), R350–R356. 10.1152/ajpregu.1997.272.1.R350 [DOI] [PubMed] [Google Scholar]
- Renshaw, G. M., Kerrisk, C. B., & Nilsson, G. E. (2002). The role of adenosine in the anoxic survival of the epaulette shark, Hemiscyllium ocellatum . Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 131(2), 133–141. 10.1016/S1096-4959(01)00484-5 [DOI] [PubMed] [Google Scholar]
- Repkin, E. A., Maltseva, A. L., Varfolomeeva, M. A., Aianka, R. V., Mikhailova, N. A., & Granovitch, A. I. (2020). Genetic and morphological variation of metacercariae of Microphallus piriformes (Trematoda, Microphallidae): Effects of paraxeny and geographic location. International Journal for Parasitology: Parasites and Wildlife, 11, 235–245. 10.1016/j.ijppaw.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, E. J., Servedio, M. R., & Martin, C. H. (2019). Searching for sympatric speciation in the genomic era. BioEssays, 41(7), 1900047. 10.1002/bies.201900047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, M. E., Phipson, B., Wu, D. I., Hu, Y., Law, C. W., Shi, W., & Smyth, G. K. (2015). limma powers differential expression analyses for RNA‐sequencing and microarray studies. Nucleic Acids Research, 43(7), e47. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohart, F., Gautier, B., Singh, A., & Lê Cao, K. A. (2017). mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Computational Biology, 13(11), e1005752. 10.1371/journal.pcbi.1005752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlf, F. J. (2015). The tps series of software. Hystrix, 26(1), 9–12. [Google Scholar]
- Rolán, E., & Templado, J. (1987). Consideraciones sobre el complejo Littorina obtusata‐mariae (Mollusca, Gastropoda, Littorinidae) en el Noroeste de la peninsula Iberica. Thalassas, 5, 71–85. [Google Scholar]
- Rolán‐Alvarez, E. (2007). Sympatric speciation as a by‐product of ecological adaptation in the Galician Littorina saxatilis hybrid zone. Journal of Molluscan Studies, 73(1), 1–10. 10.1093/mollus/eyl023 [DOI] [Google Scholar]
- Rolán‐Alvarez, E., Austin, C. J., & Boulding, E. G. (2015). The contribution of the genus Littorina to the field of evolutionary ecology. In Hughes R. N., Hughes D. J., Smith I. P., & Dale A. C. (Eds.), Oceanography and marine biology: An annual review (vol 53, pp. 157–214). CRC Press. [Google Scholar]
- Sacchi, C. F. (1966). Littorina obtusata (L.) (Gastropoda, Prosobranchia): A problem of variability and its relation to ecology. Simposia Genetica Et Biologka Italica, 13, 521–572. [Google Scholar]
- Saito, H., & Aono, H. (2014). Characteristics of lipid and fatty acid of marine gastropod Turbo cornutus: High levels of arachidonic and n‐3 docosapentaenoic acid. Food Chemistry, 145, 135–144. 10.1016/j.foodchem.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Savolainen, V., Anstett, M. C., Lexer, C., Hutton, I., Clarkson, J. J., Norup, M. V., Powell, M. P., Springate, D., Salamin, N., & Baker, W. J. (2006). Sympatric speciation in palms on an oceanic island. Nature, 441(7090), 210–213. [DOI] [PubMed] [Google Scholar]
- Schluter, D. (2000). The ecology of adaptive radiation. Oxford University Press. [Google Scholar]
- Scriven, J. J., Whitehorn, P. R., Goulson, D., & Tinsley, M. C. (2016). Niche partitioning in a sympatric cryptic species complex. Ecology and Evolution, 6(5), 1328–1339. 10.1002/ece3.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergievsky, S. O., & Granovitch, A. I. (1999). Abnormal shell shape of the intertidal molluscs Littorina saxatilis and Littorina obtusata infected with trematodes. Parazitologiya, 33(1), 23–25. [Google Scholar]
- Silvertown, J., Dodd, M., & Gowing, D. (2001). Phylogeny and the niche structure of meadow plant communities. Journal of Ecology, 89(3), 428–435. 10.1046/j.1365-2745.2001.00553.x [DOI] [Google Scholar]
- Smith, D. C., Mill, P. J., & Grahame, J. (1995). Environmentally induced variation in uric acid concentration and xanthine dehydrogenase activity in Littorina saxatilis (Olivi) and L. arcana Hannaford Ellis. In Mill P. J., & McQuaid C. D. (Ed.), Advances in Littorinid biology. Developments in hydrobiology (vol. 111, pp. 111–116). Springer. 10.1007/978-94-011-0435-7_11 [DOI] [Google Scholar]
- Smyth, G. K. (2004). Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Statistical Applications in Genetics and Molecular Biology, 3(1), 1–25. 10.2202/1544-6115.1027 [DOI] [PubMed] [Google Scholar]
- Sokolov, E. P., Pörtner, H. O., Lucassen, M., & Sokolova, I. M. (2003). Microscale genetic differentiation along the vertical shore gradient in White Sea snails Littorina saxatilis (Olivi) assessed by microsatellite markers. Journal of Molluscan Studies, 69(4), 388–391. 10.1093/mollus/69.4.388 [DOI] [Google Scholar]
- Sokolova, I. M., Granovitch, A. I., Berger, V. J., & Johannesson, K. (2000). Intraspecific physiological variability of the gastropod Littorina saxatilis related to the vertical shore gradient in the White and North Seas. Marine Biology, 137(2), 297–308. 10.1007/s002270000343 [DOI] [Google Scholar]
- Sokolova, I. M., & Pörtner, H. O. (2001a). Physiological adaptations to high intertidal life involve improved water conservation abilities and metabolic rate depression in Littorina saxatilis . Marine Ecology Progress Series, 224, 171–186. 10.3354/meps224171 [DOI] [Google Scholar]
- Sokolova, I. M., & Pörtner, H. O. (2001b). Temperature effects on key metabolic enzymes in Littorina saxatilis and L. obtusata from different latitudes and shore levels. Marine Biology, 139(1), 113–126. 10.1007/s002270100557 [DOI] [Google Scholar]
- Soudant, P., Marty, Y., Moal, J., Masski, H., & Samain, J. F. (1998). Fatty acid composition of polar lipid classes during larval development of scallop Pecten maximus (L.). Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 121(3), 279–288. 10.1016/S1095-6433(98)10130-7 [DOI] [Google Scholar]
- Stanley, S. M. (1988). Adaptive morphology of the shell in bivalves and gastropods. The Mollusca, 11, 105–141. [Google Scholar]
- Stephenson, T. A., & Stephenson, A. (1949). The universal features of zonation between tide‐marks on rocky coasts. The Journal of Ecology, 38, 289–305. 10.2307/2256610 [DOI] [Google Scholar]
- Stephenson, T. A., & Stephenson, A. (1972). Life between tidemarks on rocky shores. W.H. Freeman. [Google Scholar]
- Tatarenkov, A., & Johannesson, K. (1998). Evidence of a reproductive barrier between two forms of the marine periwinkle Littorina fabalis (Gastropoda). Biological Journal of the Linnean Society, 63(3), 349–365. 10.1111/j.1095-8312.1998.tb01522.x [DOI] [Google Scholar]
- Tchernov, D., Gorbunov, M. Y., De Vargas, C., Yadav, S. N., Milligan, A. J., Häggblom, M., & Falkowski, P. G. (2004). Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proceedings of the National Academy of Sciences, 101(37), 13531–13535. 10.1073/pnas.0402907101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky, A., & Kahneman, D. (1971). Belief in the law of small numbers. Psychological Bulletin, 76(2), 105. 10.1037/h0031322 [DOI] [Google Scholar]
- Vermeij, G. J. (1973). Morphological patterns in high‐intertidal gastropods: Adaptive strategies and their limitations. Marine Biology, 20(4), 319–346. 10.1007/BF00354275 [DOI] [Google Scholar]
- Vodă, R., Dapporto, L., Dincă, V., & Vila, R. (2015). Why do cryptic species tend not to co‐occur? A case study on two cryptic pairs of butterflies. PLoS One, 10(2), e0117802. 10.1371/journal.pone.0117802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, T., & Grahame, J. (2011). Shell shape variation and fitness variables in the gastropod Littorina saxatilis . Marine Ecology Progress Series, 430, 103–112. 10.3354/meps09122 [DOI] [Google Scholar]
- Warmoes, T., Dumoulin, E., & Reid, D. G. (1992). The distribution of Littorina and Melarhaphe along the continental coast of the English Channel. In Grahame J., Mill P. J., & Reid D. G. (Eds.), Proceedings of the third international symposium on Littorinid biology (pp. 293–303). Malacological Society of London. [Google Scholar]
- Warren, D. L., Glor, R. E., & Turelli, M. (2008). Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution: International Journal of Organic Evolution, 62(11), 2868–2883. 10.1111/j.1558-5646.2008.00482.x [DOI] [PubMed] [Google Scholar]
- Warwick, T., Knight, A. J., & Ward, R. D. (1990). Hybridization in the Littorina saxatilis species complex (Prosobranchia: Mollusca). Hydrobiologia, 193(1), 109–116. 10.1007/BF00028070 [DOI] [Google Scholar]
- Watson, D. C., & Norton, T. A. (1987). The habitat and feeding preferences of Littorina obtusata (L.) and L. mariae Sacchi et Rastelli. Journal of Experimental Marine Biology and Ecology, 112(1), 61–72. 10.1016/S0022-0981(87)80015-1 [DOI] [Google Scholar]
- Wiens, J. J., & Graham, C. H. (2005). Niche conservatism: Integrating evolution, ecology, and conservation biology. Annual Review of Ecology, Evolution, and Systematics, 36, 519–539. 10.1146/annurev.ecolsys.36.102803.095431 [DOI] [Google Scholar]
- Williams, G. A. (1990). The comparative ecology of the flat periwinkles, Littorina obtusata (L.) and L. mariae Sacchi et Rastelli. Field Studies, 7, 469–482. [Google Scholar]
- Williams, G. A. (1992). The effect of predation on the life histories of Littorina obtusata and Littorina mariae . Journal of the Marine Biological Association of the United Kingdom, 72(2), 403–416. 10.1017/S0025315400037784 [DOI] [Google Scholar]
- Williams, G. A. (1995). Maintenance of zonation patterns in two species of flat periwinkle, Littorina obtusata and L. mariae . In Mill P. J., & McQuaid C. D. (Eds.), Advances in Littorinid biology. Developments in hydrobiology (vol. 111, 143–150). Springer. 10.1007/978-94-011-0435-7_15 [DOI] [Google Scholar]
- Zavras, E. T., & James, H. A. (1979). Carotenoids found in Littorina littorea and their relationship to parasitic infection by larva trematodes. Journal of Invertebrate Pathology, 34, 276–284. 10.1016/0022-2011(79)90074-0 [DOI] [Google Scholar]
- Zs.‐Nagy, I. (1977). Cytosomes (yellow pigment granules) of molluscs as cell organelles of anoxic energy production. International Review of Cytology, 49, 331–377. 10.1016/S0074-7696(08)61952-X [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Data Availability Statement
Microhabitat distribution (counts in quantitative samples), shell shape (TPS files with landmark positions and metadata), and metabolome compositions (normalized detection intensities) will be available at Pangaea.de (https://doi.org/10.1594/PANGAEA.923735).


