Abstract
Familial cold autoinflammatory syndrome (FCAS) is a cryopyrin-associated periodic syndrome that presents with episodic fever, skin rash, and joint pain after exposure to cold temperatures. Although the diagnosis is often singular, there are several instances of concurrent underlying autoimmune pathologies with either rheumatoid arthritis (RA) or amyloidosis. Because symptoms of the two entities overlap, it can be difficult to address a potential dual diagnosis of FCAS and an autoimmune disorder. We found seven previously reported cases of FCAS and amyloidosis and five cases of FCAS and RA and present another case of an FCAS-RA dual diagnosis.
Keywords: Autoimmune disorder, familial cold autoinflammatory syndrome, FCAS
Autoinflammatory syndromes present as recurrent, febrile episodes accompanied by various cutaneous, mucosal, serosal, and osteoarticular manifestations. Familial cold autoinflammatory syndrome (FCAS) is a cryopyrin-associated periodic syndrome caused by an overproduction of cytokines due to defects in inflammasomes1; it typically presents in infancy with episodic fever, skin rash, and joint pain after exposure to cold temperatures. FCAS is rare, with only 1 to 2 cases per million in the USA, and is caused by an autosomal dominant mutation in the NLRP3 gene at 1q44, leading to an overproduction of interleukin-1β by inflammasomes.2–4 Here, we discuss both FCAS and its even rarer associations with autoimmune disorders, including rheumatoid arthritis (RA) and amyloidosis.
CASE PRESENTATION
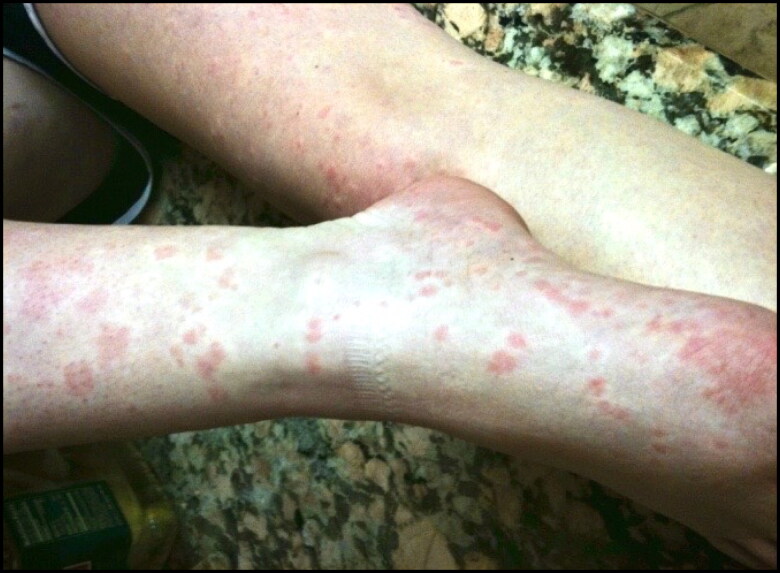
Four family members—a 49-year-old white man and his 18-year-old daughter, 58-year-old female first cousin, and 28-year-old male second cousin—presented with episodic nonscaly, erythematous, painful, and burning papules and plaques prominent on the extremities and buttocks, lasting 3 to 4 hours after exposure to cold (Figure 1). The rash was always associated with fever, chills, and profuse sweating and resolved 24 hours after onset. All four patients underwent extensive laboratory workup with pertinent lab findings including normal cell blood count, normal C3/C4 and immunoglobulin levels, and negative antinuclear antibodies and monoclonal proteins. All were found to be heterozygous for the L355P mutation in the NLRP3 gene. Based on history, symptoms, and genetic testing, the diagnosis of FCAS was confirmed for all four family members.
Figure 1.
Erythematous papules and plaques on the lower extremities.
Our 49-year-old patient was the only one who additionally presented with morning joint stiffness lasting approximately 1 hour, as well as joint pain and swelling involving bilateral metacarpophalangeal, proximal interphalangeal, and wrist joints unrelated to cold exposure. He was the only family member with a strongly positive anticyclic citrulline peptide antibody >250 units, rheumatoid factor of 20 IU/mL (upper limit of normal is 14 IU/mL), and both an elevated erythrocyte sedimentation rate and C-reactive protein. He was diagnosed with RA based on history and criteria of the American College of Rheumatology.5 X-rays of the bilateral hands showed no erosions, and administration of methotrexate, recurrent steroids, and nonsteroidal anti-inflammatory drugs failed to improve his symptoms. Unfortunately, this patient was lost to follow-up prior to treatment of his concurrent FCAS. All other family members dramatically improved with canakinumab 150 mg subcutaneously every 8 weeks.
DISCUSSION
FCAS, also known as familial cold urticaria or familial polymorphous cold eruption, is a rare autosomal dominant disorder and is frequently misdiagnosed as chronic urticaria.6 Most cases present with self-limited episodes of fever with synovial inflammation and cutaneous manifestations lasting <24 hours.5 Cutaneous findings involve urticarial wheals recurring in either symmetrically distributed crops mimicking urticarial vasculitis or in an annular configuration with a peripheral halo of vasoconstriction. Though the trunk is favored, lesions may involve the lower and upper extremities as well.6 The characteristic association with cold exposure and onset of symptoms is unique to FCAS, distinguishing it from others in the cryopyrin-associated periodic syndrome spectrum.
Thirty percent of FCAS patients present with musculoskeletal symptoms, a primary feature also seen in autoimmune disorders including RA and amyloidosis.6,7 Thus, it may be difficult to differentiate the two in patients with both FCAS and an underlying autoimmune pathology. FCAS existing concurrently with RA is exceedingly rare, and we presented the sixth case to date. Our literature search also revealed seven cases of FCAS-associated amyloidosis (Table 1).8
Table 1.
Previously reported patients with FCAS and other concurrent autoimmune disorders
| First author | Age (years) | Sex | Course of disease (years) | Related findings |
|---|---|---|---|---|
| Savic14 | 54 | M | 19 | NLRP3 mutation, anti-CCP antibody +, RF+ |
| Savic14 | 55 | F | 2 | MEFV mutation, anti-CCP antibody +, RF+ |
| Savic14 | 50 | M | 1 | NOD2 mutation, anti-CCP antibody +, RF+ |
| Savic14 | 42 | M | 2 | NOD2 mutation, anti-CCP antibody +, RF+ |
| Savic14 | 60 | F | 17 | No mutations, anti-CCP antibody –, RF + |
| Georgin-Lavialle8 | n/a | n/a | n/a | 7 cases of FCAS associated with amyloidosis |
CCP indicates cyclic citrullinated peptide; FCAS, familial cold autoinflammatory syndrome; RF, rheumatoid factor.
FCAS is caused by defects in the innate immune system, and autoimmune diseases, such as RA, affect acquired or adaptive immunity.9 Both autoinflammatory and autoimmune diseases show dysregulation of interleukin-1 signaling, which induces pro-inflammatory cytokine pathways, indicating that they may be part of a larger spectrum involving elements of both innate and adaptive immunopathy.7,9,10 It has also been reported that some RA patients have an NLPR3 polymorphism, which predisposes them to higher disease activity prior to treatment, but they do not demonstrate signs of FCAS.11
Though more commonly seen with Muckle-Wells, another cryopyrin-associated periodic syndrome, amyloidosis is known to be associated with approximately 2% of all FCAS cases.8 This systemic secondary complication is attributed to chronic inflammatory-induced aggregation of amyloid proteins that accumulate and deposit in major organs, particularly the kidneys and liver. These patients have an elevated C-reactive protein level and erythrocyte sedimentation rate as well as persistent joint and muscle pain, all of which are seen concurrently with FCAS. With deposition in the kidneys, urinalysis will demonstrate proteinuria and tissue biopsy will reveal amyloid fibrils, findings not seen in FCAS alone.
The treatment of FCAS is targeted at interleukin-1 by drugs such as anakinra, rilonacept, and canakinumab.12 The role of anti-interleukin-1 drugs in RA alone has shown limited benefit compared to other medications12,13; however, Savic et al noted successful treatment of FCAS with concurrent RA utilizing interleukin-1 pathway blockade therapy.14
Ultimately, the clinician should consider a diagnosis of FCAS with an underlying autoimmune disease if the symptoms are sustained for >24 hours and persist beyond the initial flare. In addition to genetic testing, we recommend a workup including anticyclic citrulline peptide antibody, rheumatoid factor, erythrocyte sedimentation rate, C-reactive protein, serum and urine protein electrophoresis, and/or tissue biopsy.
References
- 1.Cush JJ.Autoinflammatory syndromes. Dermatol Clin. 2013;31:471–480. doi: 10.1016/j.det.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broderick L, De Nardo D, Franklin BS, Hoffman HM, Latz E.. The inflammasomes and autoinflammatory syndromes. Annu Rev Pathol Mech Dis. 2015;10:395–424. doi: 10.1146/annurev-pathol-012414-040431. [DOI] [PubMed] [Google Scholar]
- 3.Meng G, Zhang F, Fuss I, Kitani A, Strober W.. A mutation in the NLRP3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booshehri LM, Hoffman HM.. CAPS and NLRP3. J Clin Immunol. 2019;39:277–286. doi: 10.1007/s10875-019-00638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 6.Marzano AV, Damiani G, Genovese G, Gattorno M.. A dermatologic perspective on autoinflammatory diseases. Clin Exp Rheumatol. 2018;36(Suppl 110):32–38. [PubMed] [Google Scholar]
- 7.Houx L, Hachulla E, Kone-Paut I, et al. Musculoskeletal symptoms in patients with cryopyrin-associated periodic syndromes: a large database study. Arthritis Rheumatol. 2015;67:3027–3036. doi: 10.1002/art.39292. [DOI] [PubMed] [Google Scholar]
- 8.Georgin-Lavialle S, Stankovic Stojanovic K, Buob D, et al. French Amyloidosis CAPS study: AA amyloidosis complicating cryopyrin-associated periodic syndrome: a study on 14 cases and review of 53 cases from literature. Pediatr Rheumatol. 2015;13(Suppl. 1):P32. doi: 10.1186/1546-0096-13-S1-P32. [DOI] [Google Scholar]
- 9.Goldbach-Mansky R, Kastner DL.. Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory disease and implication for common illnesses. J Allergy Clin Immunol. 2009;124:1141–1151. doi: 10.1016/j.jaci.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman HM, Wanderer AA, Broide DH.. Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. J Allergy Clin Immunol. 2001;108:615–620. doi: 10.1067/mai.2001.118790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenko B, Praprotnik S, Tomšić M, Dolžan V.. NLRP3 and CARD8 polymorphisms influence higher disease activity in rheumatoid arthritis. J Med Biochem. 2016;35:319–323. doi: 10.1515/jomb-2016-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu JR, Leslie KS.. Cryopyrin-associated periodic syndrome: an update on diagnosis and treatment response. Curr Allergy Asthma Rep. 2011;11:12–20. doi: 10.1007/s11882-010-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh JA, Christensen R, Wells GA, et al. A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview. Can Med Assoc J. 2009;181:787–796. doi: 10.1503/cmaj.091391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savic S, Mistry A, Wilson AG, et al. Autoimmune-autoinflammatory rheumatoid arthritis overlaps: a rare but potentially important subgroup of diseases. RMD Open. 2017;3:e000550. doi: 10.1136/rmdopen-2017-000550. [DOI] [PMC free article] [PubMed] [Google Scholar]