Abstract
We describe a unique case of severe influenza A–induced rhabdomyolysis in a previously healthy 39-year-old man, who developed symptoms immediately following a 2-hour deep-tissue massage and improved with oseltamivir and intravenous fluids. This case describes a possible temporal association between tissue damage secondary to deep tissue massage and the subsequent exacerbation of rhabdomyolysis in the setting of an influenza A infection. Biological evidence of muscle cell viral invasion with massage supports the possibility of elevated influenza A pathogenicity.
Keywords: Creatine kinase, influenza virus, myoglobinuria, myotubes, rhabdomyolysis
Rhabdomyolysis is a potentially lethal sequela of influenza and is caused by the breakdown of muscle releasing toxic intracellular contents into the extracellular space. Here, we describe a possible temporal association between muscle tissue damage and the subsequent exacerbation of rhabdomyolysis secondary to influenza A infection.
CASE PRESENTATION
A previously healthy 39-year-old man with a noncontributive past medical history of traumatic brain injury and posttraumatic stress disorder presented to the emergency department with a 6-day history of generalized malaise, fatigue, myalgias, and cough. His symptoms began 1 hour following a 2-hour deep-tissue massage. This massage was a one-time event and was not done to palliate muscle soreness. He denied any medication use except for ibuprofen and acetaminophen/dextromethorphan in an unsuccessful attempt to palliate his symptoms. Of note, his children were reported to be sick with flu-like symptoms 2 weeks earlier. He initially went to an outside hospital 4 days after symptom onset, where he tested positive for influenza A but was not given oseltamivir. At home, symptoms progressed to somnolence, blurred vision, and extreme myalgias, prompting his visit to the emergency room. His illness was observed by his wife, who denied any trauma or seizure.
His temperature was 98.4°F; heart rate, 80 beats/min; blood pressure, 105/76 mm Hg; respiratory rate, 18 breaths/min; and oxygen saturation, 99% on room air. He had dry mucous membranes, bibasilar crackles, and a generalized ill appearance. Laboratory values on admission are shown in Table 1. A hepatitis panel, blood cultures, and urine drug screen were negative. Acetaminophen levels were below assay. A chest radiograph showed mild densities in the left lower lobe, and a nasopharyngeal swab was positive for influenza A.
Table 1.
Initial admission laboratory values
| Laboratory test | Value | Normal range |
|---|---|---|
| Blood urea nitrogen (mg/dL) | 53 | 7–22 |
| Creatinine (mg/dL) | 5.10 | 0.6–1.2 |
| Sodium (mEq/L) | 134 | 136–145 |
| Potassium (mEq/L) | 4.9 | 3.5–5.3 |
| Chloride (mEq/L) | 99 | 97–111 |
| Bicarbonate (mEq/L) | 17 | 22–30 |
| Calcium (mg/dL) | 5.9 | 8.6–10.5 |
| Ionized calcium (mg/dL) | 2.97 | 4.7–5.2 |
| Phosphorous (mEq/L) | 18 | 4–16 |
| Lactic acid (mmol/L) | 2.1 | 0.3–1.5 |
| Creatine kinase (U/L) | >426,700 | male 25–90 |
| Aspartate aminotransferase (U/L) | 2888 | 8–20 |
| Alanine aminotransferase (U/L) | 452 | 8–20 |
| Urinalysis (red blood cells per high-powered field) | 3-9 | 0–5 |
| Anion gap (mEq/L) | 18 | 6–12 |
The patient was treated with a 5-day course of oseltamivir as indicated for severely persistent influenza A infection even when symptomatic >48 hours, as well as ceftriaxone and doxycycline to cover for secondary pneumonia. He was also treated with calcium gluconate, sodium bicarbonate, a 2 L bolus of normal saline, and maintenance intravenous fluids at 500 mL/h infusion of normal saline. His initial urine output was 975 mL/day and increased by 2–3 L/day with 300 mL/h normal saline due to concern for acute kidney injury.
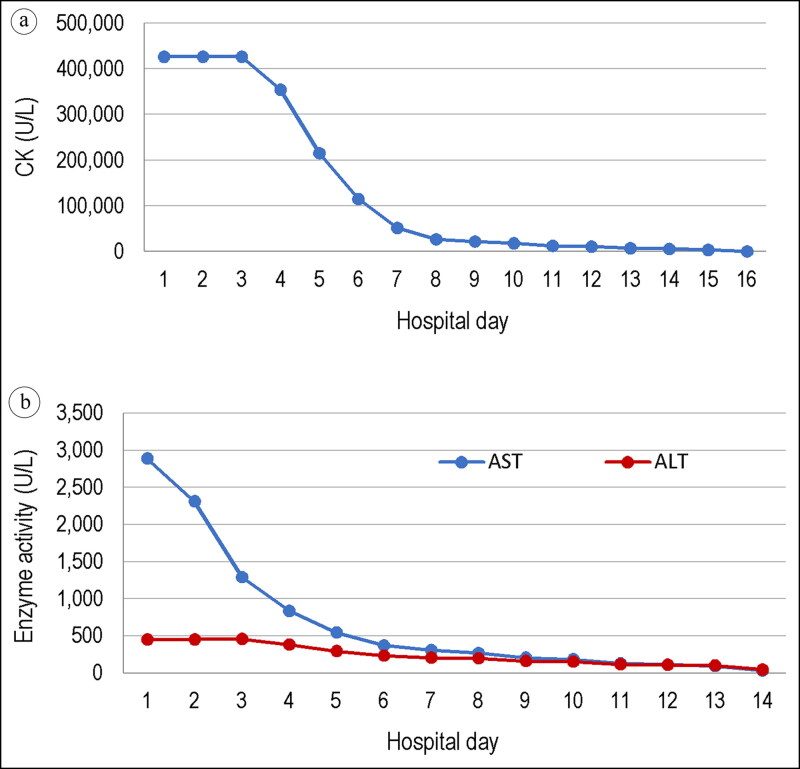
By hospital day 7, the patient had gained 50 lbs and was monitored for compartment syndrome. Furosemide was started, and fluids were decreased to 100 mL/h. By day 10, his creatinine peaked at 8.55 mg/dL (Figure 1a) and aspartate aminotransferase (AST)/alanine aminotransferase (ALT) decreased to 186 U/L and 154 U/L from 2888 U/L and 452 U/L, respectively (Figure 1b). Interestingly, he remained afebrile throughout his hospital course. All causes of rhabdomyolysis were investigated and excluded, including a history of trauma, heat exposure, prolonged immobilization, drugs, and connective tissue disease. Apart from the 2-hour deep tissue massage, he denied history of marathon-level muscle strain. On day 13, his creatinine kinase decreased below 10,000 U/L and he was discharged on day 16.
Figure 1.
Trends in laboratory values over the hospital course for (a) creatine kinase, which decreased to <10,000 U/L by hospital day 16, and (b) aspartate aminotransferase (AST) and alanine aminotransferase (ALT), with an initial AST level of 2888 U/L on admission.
DISCUSSION
Rhabdomyolysis is commonly caused by drugs of abuse, alcohols, prolonged immobilization, infections, excessive muscle activity or muscle insult, compartment syndrome, and heat exposure.1 The most specific diagnostic laboratory value is an elevated creatinine kinase (>5000 U/L), indicative of significant muscle damage and myoglobinuria. This case highlights a first-ever reported theoretical association of deep-tissue massage exacerbating the pathophysiology of influenza-induced rhabdomyolysis.
The laboratory data revealed our patient’s serious illness. The blood urea nitrogen/creatinine ratio of <15 was significant for intrinsic renal pathogenesis and contributed to the borderline hyponatremia. Borderline hyperkalemia and acidosis supported the pathophysiological leaking of intracellular contents into the extracellular space in rhabdomyolysis. Additionally, hypocalcemia was noted from either deposition in damaged muscle cells or complexed with phosphate from hyperphosphatemia. Creatine kinase was elevated to such an extent that it was above the assay detection level of 426,700 U/L. Elevated AST supported rhabdomyolysis since AST is nonspecific to the liver and is present in muscle tissue. The elevated anion gap likely resulted from a lactic acidosis.
Influenza has very diverse phenotypic presentations, with cardiac, neurologic, renal, musculoskeletal, hepatic, hematologic, and endocrine involvement.2 Monitoring musculoskeletal and renal involvement is important when treating patients with severe acute influenza infection because rhabdomyolysis is a potentially lethal complication. The exact mechanism of influenza-induced rhabdomyolysis has not been determined, but it is hypothesized that direct muscle invasion by the virus results in direct muscle injury. Previous studies demonstrated that influenza directly infects skeletal muscle3 and displays tissue tropism by binding preferentially to α2-6 sialic acids.4 α2,3 and α2,6-linked sialic acid receptor expression exists on myotube surfaces and is necessary to facilitate viral replication.5 After one bout of deep-tissue massage, even in the absence of overt muscle injury, there is an associated increase in the activation of muscle stem cells, which aid in the recovery of the muscle.6 In response to activation, muscle stem cells differentiate to myocytes, which subsequently fuse to form myotubes.7 This biological evidence suggests that massage-induced increases in myotubes could grant viral particles increased access to 2,3 and α2,6-linked sialic acid receptors, thereby aiding infectivity and potential exacerbation of influenza-induced rhabdomyolysis. We hope future research will further test this hypothesis.
ACKNOWLEDGMENTS
The authors thank Maybelline Virginia Lezama, MD, for providing guidance throughout the writing process.
References
- 1.Vanholder R, Sever MS, Erek E, Lameire N.. Rhabdomyolysis. J Am Soc Nephrol. 2000;11(8):1553–1561. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 2.Sellers SA, Hagan RS, Hayden FG, FischerWA, II.. The hidden burden of influenza: a review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11(5):372–393. doi: 10.1111/irv.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis LE, Kornfeld M.. Experimental influenza B viral myositis. J Neurol Sci. 2001;187(1-2):61–67. doi: 10.1016/S0022-510X(01)00526-3. [DOI] [PubMed] [Google Scholar]
- 4.Walther T, Karamanska R, Chan RW, et al. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog. 2013;9(3):e1003223. doi: 10.1371/journal.ppat.1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desdouits M, Munier S, Prevost MC, et al. Productive infection of human skeletal muscle cells by pandemic and seasonal influenza A(H1N1) viruses. PLoS One. 2013;8(11):e79628. doi: 10.1371/journal.pone.0079628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt ER, Confides AL, Abshire SM, Dupont-Versteegden EE, Butterfield TA.. Massage increases satellite cell number independent of the age-associated alterations in sarcolemma permeability. Physiol Rep. 2019;7(17):e14200. doi: 10.14814/phy2.14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen JH, Chung JD, Lynch GS, Ryall JG.. The microenvironment is a critical regulator of muscle stem cell activation and proliferation. Front Cell Dev Biol. 2019;7:254. doi: 10.3389/fcell.2019.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]