Abstract
Background:
Our previous work has linked childhood violence exposure in Black youth to functional changes in the hippocampus, a brain region sensitive to stress. However, different contexts of violence exposure (e.g., community, home, school) may have differential effects on circuitry. We investigated the unique effect of community violence in predicting resting-state functional connectivity (rsFC) in the hippocampus.
Methods:
Fifty-two (26F) violence-exposed Black youth ages 8–15 performed resting-state functional neuroimaging scans while looking at a fixation cross for seven minutes with eyes open. Seed-based analyses were conducted to examine the association between total violence exposure and rsFC of the hippocampus to the whole brain. Follow-up hierarchical regression analysis were performed to specifically investigate community violence.
Results:
Violence exposure was associated with higher hippocampus rsFC with a core node of the Default Mode Network (i.e., posterior cingulate cortex) and lower hippocampal rsFC with a core node of the Salience Network (i.e., insula). Community violence uniquely associated with lower hippocampus-insula rsFC, after controlling for home and school violence, sex and age. Age-related decreases in hippocampus-insula rsFC were also present in youth with lower violence exposure, but not in youth with higher violence exposure.
Conclusions:
This is one of the first studies to investigate the unique impact of community violence, above home and school violence, on threat circuitry. Our data suggest functional alterations in the hippocampus in violence-exposed youth, and that violence in the community may be a more salient form of threat exposure compared to other forms of violence experienced by youth.
Keywords: Childhood, PTSD, Trauma, fMRI, Violence, Development
Introduction
Exposure to childhood adversity (CA) is prevalent (~58.3% of youth in the United States) and may explain about 30% of psychiatric disorders (McLaughlin et al., 2012). Violence is an extremely common form of CA, with national survey data indicating that 20–50% of youth in the United States are exposed to violence in their home, community, or school (Finkelhor and Dziuba-Leatherman, 1994; Taylor et al., 2003; Finkelhor et al., 2009). Violence exposure is particularly common in urban settings (Schwab-Stone et al., 1995) and disproportionately affects Black American youth (~12.7% as compared to 6.2% of their White counterparts) (Crouch et al., 2019).
A recent approach to understanding the impact of CA on brain and behavioral development in youth differentiates between threat- (e.g., abuse, violence) and deprivation-related exposures (e.g., neglect, poverty) (McLaughlin et al., 2014; Mclaughlin et al., 2019). Violence exposure is considered to be a salient form of threat-related adversity, and our previous work has linked violence exposure to structural and functional neural changes in youth (van Rooij et al., 2020). Exposure to violence and CA has been shown to strongly impact the developing brain, particularly brain regions implicated in threat processing (Herringa et al., 2013). Functional magnetic resonance imaging (fMRI) studies have demonstrated alterations in emotional processing (Tottenham et al., 2010) in CA-exposed youth as compared to controls. Violence exposure has also been associated with structural and functional changes in the hippocampus, which is involved the context processing of fear memories (Ji and Maren, 2007; Liberzon and Abelson, 2016). Altered functional connectivity of the hippocampus has been reported in CA-exposed youth (Sheynin et al., 2020; van Rooij et al., 2020) and has been hypothesized to be involved in the context-specificity in fear extinction (Ji and Maren, 2007). Alterations in hippocampal connectivity may be associated with altered processing of safe contexts in adolescents exposed to adversity.
Few studies have investigated the impact of violence exposure on functional connectivity of the hippocampus in youth. We recently demonstrated that childhood violence exposure is associated with lower volume of the hippocampus, higher volume of the amygdala, and heightened functional connectivity between the amygdala and brainstem during an emotional Go/NoGo task (van Rooij et al., 2020). Exposure to a specific type of childhood violence, community violence, is particularly common in low-income urban neighborhoods and may present unique challenges for neural development (Cross et al., 2017). In a recent study, increased self-reported community violence was associated with structural brain changes, such as decreased hippocampus and amygdala volume 3–5 years post-interview (Saxbe et al 2018). Furthermore, greater community violence exposure was associated with increased resting-state functional connectivity (rsFC) between the right hippocampus and insula (Saxbe et al., 2018).
Present Study
The present study examined self-reported violence in the community, along with home and school violence, in order to assess the unique variance accounted for by this specific trauma context on Black youth recruited from an urban population with high rates of community violence exposure (Cross et al., 2017). Second, MRI scans were collected to measure rsFC and examine the impact of violence exposure on rsFC threat-relevant neural circuitry in a sample of Black youth. We focused on rsFC of the hippocampus, a core region involved in threat processing. Given that community violence exposure is extremely common in urban settings and may have unique impacts on the developing brain (Saxbe et al., 2018; van Rooij et al., 2020), we examined the unique impact of community violence on hippocampal rsFC and above violence experienced in the home or school. Based on a recent review of CA-exposed youth, we hypothesized that greater violence exposure would be associated with lower rsFC of the hippocampus with regions involved in threat circuitry (e.g., amygdala, vmPFC) (Cisler and Herringa, 2020), and that community violence exposure would be most strongly predictive of this association. Second, given evidence for age-related changes in activation and connectivity within threat-processing circuitry across childhood and adolescence (cf. (Gee et al., 2013; van Rooij et al., 2020; Herzberg et al., 2021), we tested age x violence exposure interactions in rsFC, and hypothesized that age-related differences would be moderated by violence.
Experimental Procedures
Participants
Sixty-eight Black youth were recruited through the Grady Trauma Project in Atlanta, GA, to participate in the brain imaging study. Following exclusions for quality assurance (detailed below), the final sample consisted of 52 participants (27 female), ages 8–15 (M = 10.86, SD = 1.70 years). Structural MRI and task-based fMRI data from an overlapping sample have been previously reported (van Rooij et al., 2020). This is the first report of rsFC data collected in this sample. Study oversight was conducted by the Emory University Institutional Review Board and the Research Oversight Committee at Grady Memory Hospital. The age range (8–15 years) was selected to span pre-and post-pubertal ages, to examine developmental changes emerging across adolescence. Exclusion criteria consisted of the following: cognitive disability, head injury with loss of consciousness, epilepsy, neurological disorder, autism spectrum disorder, or brain tumor, metal in the body, or hearing or vision impairment unable to be corrected by glasses. Verbal or written informed assent was obtained by youth < age 11 or ages 11 and above, respectively. Written informed consent was obtained from a parent or legal guardian for all participants.
Violence Exposure
Structured interviews were conducted by trained research staff to assess youth self-reported exposure to violence using the Violence Exposure Scale for Children-Revised (VEX-R)(Fox and Leavitt, 1995), which has been validated in the Grady Trauma Project population (Cross et al., 2017). This 25-item questionnaire presents sex-matched images of children experiencing or witnessing violence (e.g., seeing someone getting arrested), after which participants endorse whether a similar event has happened to them during their lifetime (yes/no). A total VEX-R score was calculated, which represented the number of different types of violence that the child endorsed experiencing and/or witnessing. Possible VEX-R scores ranged from 0 to 25. After each item, the experimenters asked whether the event happened in a school, community, and/or home context. One female participant (12 years) was a potential outlier (Z > |3|) for total VEX-R scores, which was related to a high incidence of school violence. We imputed the mean to reduce the impact on this potential outlier on the results and all reported results remained consistent with or without this mean substitution.
Clinical Assessments
The Behavioral Assessment for Children – Revised, Second Edition (BASC-2) was used to measure youth depression and anxiety symptoms (Reynolds and Kamphaus, 2004). We utilized age- and sex-normed T-scores on the child self-report scale. Child self-reported PTSD symptoms were measured using the UCLA Child PTSD Reaction Index, UCLA-RI (Steinberg et al., 2004). A summative symptom score of intrusive thoughts, avoidance, hyperarousal, and negative affect was calculated to determine if participants met for PTSD diagnosis on DSM-5 criteria.
Imaging Data Acquisition
Scans were acquired using two separate 3.0-T Siemens Trio (whole-body) MR scanners equipped with a 32-channel head coil at the Facility for Education and Research in Neuroscience (FERN; n = 47) and the Center for Systems Imaging Core (n = 5) at Emory University. Effects of scanner were considered by performing a secondary analysis only on the participants scanned at FERN. Results were consistent with the findings with the total N = 52 (see Supplemental Material). Resting state fMRI data were acquired while youth directed their attention to a fixation cross for seven minutes with eyes open. Image parameters included 180 acquired T2-weighted images with the following parameters: acquisition order = interleaved descending, repetition time [TR] = 2,330 ms, echo time [TE] = 30 ms, and voxel size 3.0 × 3.0 × 2.5 mm. A T1-weighted image was collected during the same scan for structural/functional co-registration, using the following parameters: 176 slices, repetition time [TR] = 2,250 ms, echo time [TE] = 4.18 ms, and voxel size 1 × 1 × 1 mm.
Imaging Data Processing and Motion Correction
Preprocessing was performed in CONN toolbox v19. After discarding the first four volumes of fMRI data, the following preprocessing steps were applied: functional realignment, unwarping, slice-timing correction, outlier identification (see below), direct segmentation, normalization to the Montreal Neurological Institute (MNI) template, and functional smoothing using an 8 mm gaussian kernel. Multiple procedures were implemented to mitigate the impact of head motion on the data. First, prior to the scan, participants were acclimated to the scan environment using a mock scanner. Second, during outlier identification, high movement frames (i.e., > 0.9 mm framewise displacement) and/or frames that exceeded a 5 mm global z-signal threshold were flagged for subsequent nuisance regression. Third, following preprocessing, participants with excess remaining motion (> 0.3 mm; n = 10) or with < 5 minutes of data (i.e., 129 frames; n = 6) were excluded. Average head motion, estimated by framewise displacement (FD), in the final sample was 0.1609 mm. Fourth, we performed three alternate processing pipelines that used various motion corrected strategies (i.e., global signal regression, despiking, more conservative motion thresholds) to test whether our main results were robust to denoising procedures and processing parameters. See ‘Alternate Processing Pipelines’ in the Supplemental Material, for more information.
Functional Connectivity Analysis
Following preprocessing, signals from white matter, cerebrospinal fluid, and motion parameters were regressed out of the data and a band-pass filter was applied (between 0.008 – 0.09 Hz). (Nieto-Castanon, 2020). Left and right hippocampus were selected as two seed regions based on our recent work in an overlapping sample demonstrating an association between higher levels of violence exposure and increased hippocampal activation during an emotional response inhibition task (van Rooij et al., 2020). The hippocampus seed was defined anatomically using the Harvard-Oxford atlas (Caviness et al., 1996; Makris et al., 1999, 2004, 2005). RsFC of the seed with the rest of the brain (i.e., seed to voxel analysis) was computed using bivariate correlation in CONN toolbox v19. Resulting connectivity maps were Fisher-z-transformed.
Statistical Analyses
Second-level analyses examined the impact of violence exposure on rsFC of the seed region. Linear regression analyses were performed in CONN toolbox, with total VEX-R scores entered as the regressor of interest, mean motion as the nuisance regressor, and rsFC as the outcome measure. Significant clusters were defined using a threshold of p < 0.05, cluster-level false discovery rate (FDR) corrected at the whole brain level. For clusters showing significant main effects of violence exposure, rsFC values were extracted from each participant using the Fisher-transformed bivariate correlation coefficients for secondary analyses in IBM SPSS v.26. RsFC values were converted to Pearson correlation coefficients to examine raw connectivity values and interpret directionality of findings. Secondary analyses were used to test whether the overall main effects of violence exposure (total VEX-R) were driven by community violence or other contexts (i.e., home, school) using multiple hierarchical regression analysis in SPSS. Sex and age were entered in the first step, home and school violence in the second step, and community violence in the third step. In order to test for interactions of age and violence, we entered categorical age and violence exposure as between-subject factors into an ANOVA with rsFC as the dependent variable. We computed categorical age groups by stratifying by age (<10 and >=10), based on our prior findings indicating a developmental shift around age 10 (Gee et al., 2013; van Rooij et al., 2020) and categorical violence exposure groups by a median split variable. We then conducted a post-hoc linear regression, entering age as a continuous variable and categorical violence exposure groups with a median split variable. All results were considered significant at p < .05 (two-tailed).
Results
Violence Exposure
All participants reported exposure to at least one violent event. On average, participants endorsed having 9 violence exposures out of a possible 25 (see Table 1). Violence exposure was positively associated with age (r = .354, p = .01). There were no sex differences in total violence score or among violence contexts (ps > .05), and violence exposure was not associated with anxiety or depressive symptoms (p’s > .05). PTSD symptoms were significantly associated with total violence exposure (r = .370, p = .007), however, only two participants met for PTSD diagnosis (UCLA-RI>35).
Table 1.
Participant demographics.
| All | Age < 10 years | Age >=10 years | Group Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 52 | N = 20 | N = 32 | ||||||||
| N | M | SD | N | M | SD | N | M | SD | p | |
| Sex (females) | 27 | 10 | 17 | |||||||
| Age (years) | 10.86 | 1.7 | 9.28 | 0.55 | 11.85 | 1.40 | .001*** | |||
| Violence Exposure | ||||||||||
| Total Violence Exposure | 9.24 | 4.57 | 7.85 | 5.30 | 10.11 | 3.88 | .152 | |||
| Home Violence | 3.65 | 2.07 | 2.15 | 2.35 | 2.91 | 2.40 | .273 | |||
| Community Violence | 3.53 | 2.75 | 2.85 | 2.46 | 4.28 | 3.38 | .264 | |||
| School Violence | 2.45 | 2.73 | 3.15 | 3.03 | 3.96 | 2.52 | .298 | |||
Note.
p < .05.
p < .01.
p < .001.
Impact of overall violence exposure on rsFC
There was a significant main effect of violence exposure on hippocampal rsFC at the whole brain corrected threshold (see Table 2). Greater violence exposure was associated with lower rsFC between the left hippocampus and right insula (Fig 1A) and higher rsFC between the left hippocampus and posterior cingulate cortex (PCC) (see Fig. 1B). There were no significant regions associated with right hippocampus.
Table 2.
Effects of violence exposure on rsFC of left and right hippocampus in whole-brain analysis.
| Seed | Target cluster (maxima) | Directionality | x | y | z | Cluster size (number of voxels) | p-FDR |
|---|---|---|---|---|---|---|---|
| Left Hippocampus | Right Insula | Negative | 32 | 26 | 2 | 272 | .005** |
| PCC | Positive | −10 | −50 | 28 | 166 | .030* | |
| Right Hippocampus | No significant clusters | ||||||
Note. N = 52. rsFC = Resting State Functional Connectivity. PFC = Prefrontal Cortex, PCC = Posterior Cingulate Cortex.
p < .05.
p < .01.
Fig. 1.
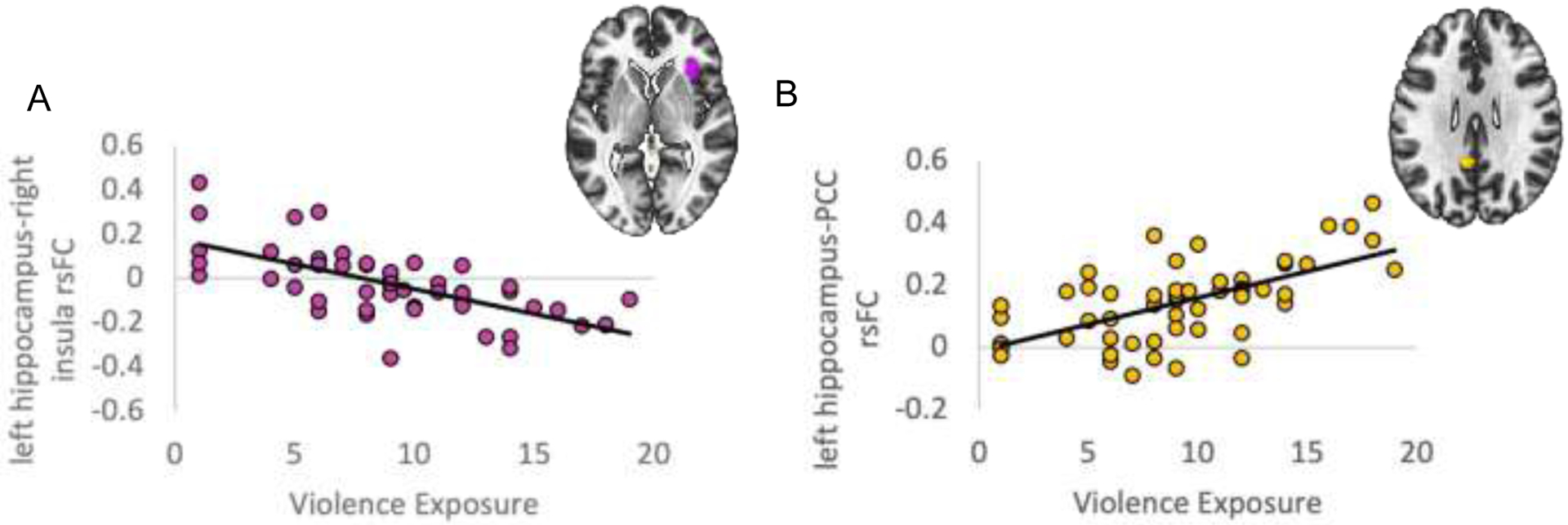
Impact of violence exposure on rsFC with left hippocampus seed. Greater violence exposure (total VEX-R) scores are associated with (A) lower left hippocampus-right insula rsFC, (B) higher left hippocampus-PCC rsFC. All results are significant at whole-brain p-FDR < 0.05. Connectivity values are Pearson correlations. PCC = Posterior Cingulate Cortex.
Unique impact of community violence exposure on rsFC
After controlling for home and school violence, hierarchical regression analyses showed that community violence alone accounted for 10% of the variance in left hippocampus-right insula rsFC (see Table 3A) and 10% of the variance in left hippocampus-right PCC rsFC (see Table 3B).
Table 3.
Hierarchical Regression Model Predicting rsFC by Sex, Age, Home Violence, School Violence, and Community Violence for (A) Left Hippocampus – Right Insula and (B) Left Hippocampus – PCC.
| A | Predicting Left Hippocampus - Right Insula rsFC | β | t | R | R 2 | ΔR 2 | ΔF | p |
|---|---|---|---|---|---|---|---|---|
| Step 1 | .422 | .178 | .178 | 5.317 | .008** | |||
| Sex | −.142 | −1.088 | ||||||
| Age | −.412 | −3.167** | ||||||
| Step 2 | .549 | .301 | .123 | 4.12 | .002* | |||
| Sex | −.111 | −.864 | ||||||
| Age | −.335 | −2.619* | ||||||
| Home Violence | −.275 | −2.076* | ||||||
| School Violence | −.151 | −1.072 | ||||||
| Step 3 | .634 | .402 | .101 | 7.75 | < .001*** | |||
| Sex | −.055 | −.453 | ||||||
| Age | −.225 | −1.786 | ||||||
| Home Violence | −.179 | −1.390 | ||||||
| School Violence | −.150 | −1.136 | ||||||
| Community Violence | −.356 | −2.785** | ||||||
| B | Predicting Left Hippocampus - PCC rsFC | β | t | R | R2 | ΔR2 | ΔF | p |
| Step 1 | .193 | .037 | .037 | .946 | 0.395 | |||
| Sex | .178 | 1.263 | ||||||
| Age | .094 | .667 | ||||||
| Step 2 | .600 | .359 | .322 | 11.8 | <.001*** | |||
| Sex | .111 | .901 | ||||||
| Age | −.043 | −.347 | ||||||
| Home Violence | .399 | 3.148** | ||||||
| School Violence | .307 | 2.278* | ||||||
| Step 3 | .680 | .462 | .103 | 8.775 | < .001*** | |||
| Sex | .054 | .472 | ||||||
| Age | −.153 | −1.285 | ||||||
| Home Violence | .302 | 2.478* | ||||||
| School Violence | .305 | 2.447* | ||||||
| Community Violence | .359 | 2.962** |
Note. N = 52. rsFC = Resting State Functional Connectivity, PCC = Posterior Cingulate Cortex.
p < .05.
p < .01.
p < .001.
Age by violence exposure interaction on rsFC between the left hippocampus and right insula
Left hippocampus-right insula rsFC was also predicted by age in Step 1 of the model (see Table 3A). We followed up on this main effect of age with an ANOVA to explore age by violence exposure interactions on rsFC. There was a significant main effect of age on rsFC, F(1,51) = 5.24, p = .027, which was driven by an age-related decrease in rsFC between the left hippocampus and right insula. There was also a main effect of higher vs lower violence exposure, F(1,51) = 19.28, p < .001, such that higher violence exposure was associated with lower rsFC. The age x violence interaction, F(1,51)=4.53, p = .038, was also significant. To follow-up the interaction, we conducted separate analyses to examine age-related changes in rsFC in youth exposed to lower and higher levels of violence. We found that the age-related decrease in rsFC was significant in the lower violence group (R2 = .263, p = .004; see Fig. 2), but not the higher violence group (R2 = .027, p > .05).
Fig. 2.
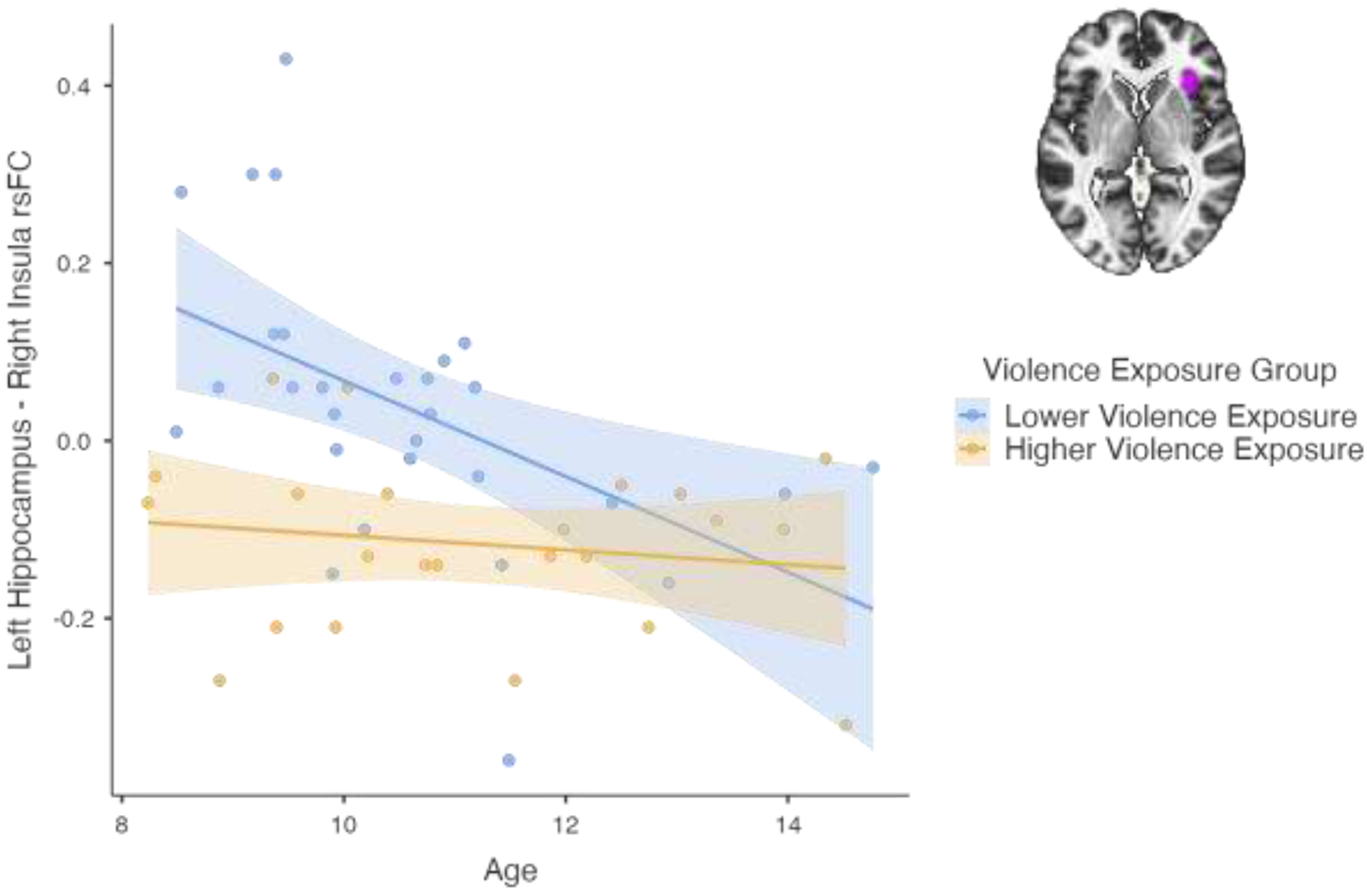
Effects of age and violence exposure on rsFC between left hippocampus and right insula. Blue depicts lower violence exposure group; orange depicts higher violence exposure group.
Discussion
We investigated the impact of violence exposure on rsFC of the hippocampus in a sample of urban youth. We used self-reported violence exposure to test the effect of this threat-related adversity on rsFC in the hippocampus, as well as the potential unique contribution of the context of community violence exposure. Our results indicated higher rsFC of the hippocampus to the PCC, but lower hippocampal connectivity with the insula in youth with greater violence exposure. Further, community violence exposure explained rsFC above and beyond other contexts of violence exposure, including home and school violence. We also observed age-related decreases in hippocampus-insula rsFC in the low violence (but not high violence) group. Together, these data suggest functional connectivity alterations between the hippocampus and insula in violence-exposed youth, and that community violence is associated with neural alterations above and beyond other forms of violence exposure.
Childhood adversity has long-term effects on the brain, with different dimensions of adversity (threat vs deprivation) potentially leading to differential effects (Mclaughlin et al., 2019). A recent review found that even within threat-related adversity there are mixed findings as to whether adversity is associated with increased or decreased functional connectivity (Mclaughlin et al., 2019). Given this variability, more specific information about the type of adversity, as well as age-related differences, is of critical importance to better understanding the long-term consequences of CA.
Our results demonstrated an association between greater violence exposure and greater hippocampal functional connectivity with the PCC, a core node of the Default Mode Network (DMN), which is involved in the detection and integration of emotionally arousing stimuli (Taylor et al., 2009). Greater violence-associated functional coupling of the hippocampus and PCC may indicate a functional change in the DMN, an intrinsic connectivity network central to internally focused thought and self-reflection (Sripada et al., 2012; Ross and Cisler, 2020). Reduced coupling within DMN regions, which include the hippocampus, PCC and ventromedial prefrontal cortex (vmPFC) has been linked to posttraumatic stress disorder (PTSD) in adults (Lanius et al., 2010; McLaughlin et al., 2014; van Rooij et al., 2020). Most literature on the DMN has been conducted in adults with PTSD, and less is understood regarding adolescents with violence exposure in the absence of psychopathology. The youth in our study did not have high levels of PTSD symptoms, indicating an association between DMN functional connectivity and violence exposure in the absence of psychopathology. This suggests that violence exposure during childhood may manifest differently prior to adult-onset psychopathology, driving the development of more hypervigilant DMN neural pathways. Moreover, increased coupling between the DMN and Salience Network (SN) has also been linked to adult PTSD and has been theorized to indicate the dominance of threat-sensitive circuitry (Sripada et al., 2012). For instance, PCC-amygdala rsFC has been shown to predict PTSD symptoms in acutely traumatized adults (Keding and Herringa, 2015). While alterations in SN and DMN rsFC have been linked to adult psychopathology, these alterations may present differently in youth.
Our results also showed an association between greater violence exposure and lower hippocampal functional connectivity with a core node of the SN (i.e., insula), a network involved in the detection and integration of emotionally arousing stimuli (Taylor et al., 2009). The insula may filter sensory input (Raichle et al., 1996), and altered rsFC between the hippocampus and insula has been hypothesized to reflect the interaction between episodic future thinking and negative emotions in the context of risk-taking (Marusak et al., 2015). Violence-related heightened rsFC between the hippocampus and insula may indicate more coordinated activity between brain areas involved in threat processing and interoceptive emotion processing, respectively.
While we found opposite seed to cluster laterality effects (i.e., left hippocampus to right insula), our findings in adolescents may highlight (1) accelerated maturation of these pathways toward future development of internalizing symptoms such as anxiety and (2) unique biomarkers following community violence exposure in adolescents. Moreover, the central roles the insula and hippocampus structures play in the salience and default mode network, respectively, suggest that rsFC between these hubs may indicate functional changes on a network level. Greater SN rsFC has been linked to early adversity (Kalisch et al., 2005) and trauma exposure (Sheynin et al., 2020). The insula’s role in anticipatory anxiety (Taylor et al., 2003; Amodio and Frith, 2006) and emotional arousal (van Rooij et al., 2020) also suggest that functional changes in salience processing may predict the development of psychopathology, specifically in the context of internalizing disorders and PTSD. In conjunction with the evidence of decreased hippocampal volume associated with childhood adversity (Ross and Cisler, 2020), the developmental trajectories of this central hub of the SN may be best understood through a network-based approach (Burgess et al., 2001).
We found that community violence, above and beyond home and school violence, was associated with rsFC alterations between the hippocampus and PCC, as well as between the hippocampus and insula. Interestingly, a prior study of community violence exposure also reported altered rsFC between the right hippocampus and bilateral insula in urban youth (Saxbe et al., 2007). However, in that study, higher community violence exposure was associated with the opposite pattern (i.e., higher hippocampus-insula rsFC; Saxbe et al., 2007). Furthermore, they found significant results with two large bilateral clusters, while our study found a lateralized effect (i.e., right insula). This discrepancy may be due to the fact that they obtained scans 3–5 years after the violence assessment, therefore introducing the impact of development on the functional connectivity between these regions. Our study investigated neural circuitry patterns closer to the time of interview, in an attempt to control for these potential developmental effects.
Community violence in particular may be a salient exposure to youth due to the fact that it often occurs within an uncontrollable environmental context, therefore representing a significant form of threat. The hippocampus is a key node in context processing and may be particularly relevant in this environmental context (Holland and Bouton, 1999; Liberzon and Abelson, 2016). The ability to contextualize information is an adaptive function and is important in distinguishing between threat and safety, a process often altered in adult trauma-related psychopathology (Jovanovic et al., 2012; Norrholm and Jovanovic, 2018), that may be associated with hippocampal neurogenesis (Kheirbek et al., 2012). These findings in youth present potential functional alterations in context processing related to not only violence exposure but also community violence exposure in urban contexts.
We found that age-related changes in rsFC between the left hippocampus and right insula were driven by youth with lower levels of violence exposure. Youth with higher violence exposure did not show age-related changes and demonstrated more negative rsFC at an earlier age than youth with lower violence. These findings are broadly consistent with reported patterns of accelerated maturation in youth experiencing high childhood adversity (Mclaughlin et al., 2019). For example, Gee and colleagues (2013) reported a similar lack of age-related changes in functional coupling between the amygdala and mPFC during an emotion face processing task in youth with maternal deprivation. Further, in that study, adversity-exposed youth demonstrated more negative amygdala-mPFC coupling at an earlier age than less exposed youth. Herzberg and colleagues (2021) also demonstrated accelerated maturation of threat circuitry (amygdala-vmPFC) in response to early life deprivation stress in a resting state fMRI paradigm. A similar pattern may be accounting for the absence of age-related effects on hippocampal-insula connectivity in youth with high levels of violence in this study. While our study did not primarily investigate deprivation as a form of adversity, we postulate that the context of threatening environments may also accelerate maturation of frontolimbic pathways.
This study had notable limitations. First, participants were recruited from a low-income urban setting, which may limit the generalizability of our findings. However, we focus on violence, and particularly community violence, as a form of threat-related CA that is common among urban settings and strongly linked to psychopathology. Second, although we found effects of age on rsFC, this study was cross-sectional in design. Future longitudinal studies will be better able to draw conclusions regarding age-related change on neural development associated with violence exposure. Third, we did not collect measures of deprivation-related CA in this sample, such as neglect or maternal deprivation. Therefore, future studies should examine the unique or interactive effects of threat- (e.g., violence) and deprivation-related CA.
Conclusion
In this neuroimaging study of Black youth in inner-city Atlanta, we found (a) an association between violence exposure and rsFC of threat circuitry regions, (b) unique impacts of community violence (as compared to home or school violence) on rsFC, and (c) age-related changes in hippocampal-insula circuitry linked to lower, but not higher, exposure to violence. Our data suggest that violence in the community has a greater impact on hippocampal functional connectivity, and it is possible that this increased connectivity may confer resilience in the face of adversity. Future studies should assess the link between differential violence exposure and whether specific types of threat are linked to functional and behavioral changes in youth. Due to the prevalence of altered threat circuitry functional connectivity in adults in response to stress and trauma, further investigating the developmental trajectories of these altered pathways can serve as an important indication to determine differential risk for later psychopathology and target at-risk youth for interventions.
Supplementary Material
Childhood violence exposure is associated with hippocampal circuitry
Hippocampus - insula functional connectivity is linked to childhood adversity
Community violence is a unique predictor of altered threat circuitry
Accelerated maturation of threat circuitry in youth with violence exposure
Acknowledgements:
Dr. Jovanovic reports funding from the National Institutes of Health (MH100122 and MH111682) and the Brain and Behavior Research Foundation (NARSAD). Dr. Marusak is supported by the National Institute of Mental Health award K01MH119241.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures and Conflicts of Interest:
The authors report no financial disclosures or conflicts of interest.
References
- Amodio DM, Frith CD (2006) Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 7:268–277 Available at: www.nature.com/reviews/neuro [Accessed January 31, 2021]. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD (2001) Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia 39:545–555 Available at: www.elsevier.com/locate/neuropsychologia [Accessed January 31, 2021]. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Meyer J, Makris N, Kennedy DN (1996) MRI-Based Topographic Parcellation of Human Neocortex: An Anatomically Specified Method with Estimate of Reliability. Available at: http://direct.mit.edu/jocn/article-pdf/8/6/566/1755442/jocn.1996.8.6.566.pdf [Accessed March 21, 2021]. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Herringa RJ (2020) Posttraumatic Stress Disorder and the Developing Adolescent Brain. Biological Psychiatry:1–8 Available at: 10.1016/j.biopsych.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D, Vance LA, Kim J, Ruchard AL, Fox N, Jovanovic T, Bradley B (2017) Trauma Exposure, PTSD, and Parenting in a Community Sample of Low-Income, Predominantly African American Mothers and Children. Available at: 10.1037/tra0000264 [Accessed February 28, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch E, Probst JC, Radcliff E, Bennett KJ, Mckinney SH (2019) Prevalence of adverse childhood experiences (ACEs) among US children. Child Abuse & Neglect 92:209–218 Available at: www.elsevier.com/locate/chiabuneg [Accessed February 26, 2021]. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Dziuba-Leatherman J (1994) Victimization of Children. American Psychologist 49:173–183. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Turner H, Ormrod R, Hamby SL (2009) Violence, abuse, and crime exposure in a national sample of children and youth. Pediatrics 124:1411–1423 Available at: https://pubmed.ncbi.nlm.nih.gov/19805459/ [Accessed December 12, 2020]. [DOI] [PubMed] [Google Scholar]
- Fox Leavitt (1995) The violence exposure scale for children-VEX (preschool version). College Park, MD: Department of Human Development, University of Maryland. [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N (2013) A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience 33:4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ (2013) Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences 110:19119 LP – 19124 Available at: http://www.pnas.org/content/110/47/19119.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg MP, McKenzie KJ, Hodel AS, Hunt RH, Mueller BA, Gunnar MR, Thomas KM (2021) Accelerated maturation in functional connectivity following early life stress: Circuit specific or broadly distributed? Developmental Cognitive Neuroscience 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Bouton ME (1999) Hippocampus and context in classical conditioning. Cognitive Neuroscience 9:195–202 Available at: http://biomednet.com/elecref/0959438800900195 [Accessed March 21, 2021]. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S (2007) Hippocampal Involvement in Contextual Modulation of Fear Extinction. Hippocampus 17:749–758 Available at: www.interscience. [Accessed March 11, 2021]. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M (2012) Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Seymour B, O’doherty JP, Oakley DA, Allen P, Dolan RJ (2005) Anxiety Reduction through Detachment: Subjective, Physiological, and Neural Effects. [DOI] [PubMed] [Google Scholar]
- Keding TJ, Herringa RJ (2015) Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology 40:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R, Author NN (2012) Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders NIH Public Access Author Manuscript. Nat Neurosci 15:1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Théberge J, Neufeld RWJ, Williamson PC, Brimson M (2010) Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatrica Scandinavica 121:33–40 Available at: https://pubmed.ncbi.nlm.nih.gov/19426163/ [Accessed December 13, 2020]. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Abelson JL (2016) Perspective Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron 92:14–30 Available at: 10.1016/j.neuron.2016.09.039 [Accessed March 21, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, Caviness VS, Tsuang MT, Kennedy DN, Hyman SE, Rosen BR, Breiter HC (2004) Decreased Absolute Amygdala Volume in Cocaine Addicts. Pettit and Justice. [DOI] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS (1999) MRI-Based Topographic Parcellation of Human Cerebral White Matter and Nuclei II. Rationale and Applications with Systematics of Cerebral Connectivity. Available at: http://www.idealibrary.com. [DOI] [PubMed] [Google Scholar]
- Makris N, Schlerf JE, Hodge SM, Haselgrove C, Albaugh MD, Seidman LJ, Rauch SL, Harris G, Biederman J, Caviness VS, Kennedy DN, Schmahmann JD (2005) MRI-based surface-assisted parcellation of human cerebellar cortex: An anatomically specified method with estimate of reliability. NeuroImage 25:1146–1160. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Etkin A, Thomason ME (2015) Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. NeuroImage: Clinical 8:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC (2012) Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry 69:1151–1160 Available at: https://jamanetwork-com.proxy.lib.wayne.edu/journals/jamapsychiatry/fullarticle/1389368 [Accessed December 8, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Lambert HK (2014) Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience and Biobehavioral Reviews 47:578–591 Available at: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclaughlin KA, Weissman D, Bitrán D (2019) Childhood Adversity and Neural Development: A Systematic Review. Annual Review Developmental Psychology 1:277–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T (2018) Fear Processing, Psychophysiology, and PTSD. Harvard review of psychiatry 26:129–141. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Macleod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (1996) A default mode of brain function. Available at: www.pnas.org [Accessed January 31, 2021]. [DOI] [PMC free article] [PubMed]
- Reynolds CR, Kamphaus RW (2004) Behavior assessment system for children (2nd ed.). Bloomington, MN: Pearson Assessments. [Google Scholar]
- Ross MC, Cisler JM (2020) Altered large-scale functional brain organization in posttraumatic stress disorder: A comprehensive review of univariate and network-level neurocircuitry models of PTSD. NeuroImage: Clinical 27:102319 Available at: 10.1016/j.nicl.2020.102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe D, Khoddam H, Piero L del, Stoycos SA, Gimbel SI, Margolin G, Kaplan JT (2018) Community violence exposure in early adolescence: Longitudinal associations with hippocampal and amygdala volume and resting state connectivity. Developmental Science 21:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab-Stone ME, Ayers TS, Kasprow W, Voyce C, Barone C, Shriver T, Weissberg RP (1995) No Safe Haven: A Study of Violence Exposure in an Urban Community. Journal of the American Academy of Child and Adolescent Psychiatry 34:1343–1352 Available at: https://pubmed.ncbi.nlm.nih.gov/7592272/ [Accessed December 11, 2020]. [DOI] [PubMed] [Google Scholar]
- Sheynin J, Duval ER, Lokshina Y, Scott JC, Angstadt M, Kessler D, Zhang L, Gur RE, Gur RC, Liberzon I (2020) Altered resting-state functional connectivity in adolescents is associated with PTSD symptoms and trauma exposure. NeuroImage: Clinical 26 Available at: 10.1016/j.nicl.2020.102215 [Accessed February 27, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King APB, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I (2012) Neural Dysregulation in Posttraumatic Stress Disorder: Evidence for Disrupted Equilibrium between Salience and Default Mode Brain Networks. Psychosom Med 79:904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Decker KB, Pynoos RS, Address M (2004) The University of California at Los Angeles Post-traumatic Stress Disorder Reaction Index. [DOI] [PubMed]
- Taylor KS, Seminowicz DA, Davis KD (2009) Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping 30:2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I (2003) Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage 18:650–659. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti I-M, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ (2010) Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science 13:46–61 Available at: 10.1111/j.1467-7687.2009.00852.x [Accessed January 16, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Smith RD, Stenson AF, Ely TD, Yang X, Tottenham N, Stevens JS, Jovanovic T (2020) Increased activation of the fear neurocircuitry in children exposed to violence. Depression and Anxiety 37:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


