Abstract
Infective endocarditis in drug users commonly targets the tricuspid valves, whereas pulmonic valve endocarditis is a rare occurrence. Staphylococcus aureus rather than Streptococcus species is the culprit organism. Streptococcal pneumonia is also not commonly seen as an etiological agent for endocarditis. Here we present a case of a 57-year-old man with a history of HIV infection on antiretroviral therapy who was admitted for sepsis and found to have pneumococcal bacteremia with vegetation on a pulmonic valve. He had been vaccinated with both pneumococcal conjugate vaccine 13 and pneumococcal polysaccharide vaccine 23 at the time of his HIV diagnosis. Pulmonic valve endocarditis is unusual in the setting of pneumococcal bacteremia in HIV patients previously vaccinated for pneumococcal disease.
Keywords: Endocarditis, HIV, pneumococcal, pulmonic valve, vaccination
Infective endocarditis is common in intravenous drug users, with an incidence of 2% to 5% per year and an overall mortality rate of 5% to 10%.1 HIV infection further raises the risk for developing endocarditis. Overall, the most common organism is Staphylococcus aureus, with most cases (60%–70%) involving the tricuspid valve; pulmonic valve infection is rare (<1%).1 Streptococcus pneumoniae is an unusual organism to cause infective endocarditis. Pulmonic valve endocarditis secondary to this organism has been reported in only 70 cases between 1979 and 2013.2 We present a case of a patient with HIV who was admitted with sepsis and found to have infective endocarditis with S. pneumoniae.
CASE PRESENTATION
A 57-year-old man with HIV well controlled on antiretroviral therapy presented with generalized pain in multiple joints including the left wrist, right knee, and back for 3 days. His past medical history included intravenous drug use, gout, treated hepatitis C, and septic arthritis from pneumococcal bacteremia that led to the diagnosis of HIV 3 years earlier. He had no prior history of sinus pathology. He denied licking or sharing needles before intravenous drug administration. On presentation, he was febrile at 38.8°C, tachycardic (105 beats/min), and normotensive, with an arterial oxygen saturation >90% on room air. On exam, he was alert and oriented to his name and place only. Lungs were clear on auscultation, and no heart murmur was appreciated. His right knee was warm and tender to touch.
Complete blood count revealed a normal white blood cell count at 6.5 × 109/L with a normal differential, hemoglobin of 14.3 g/dL, and platelets of 196 × 109/L. His CD4 was 791 cells/mm3, and HIV viral load was undetectable. Erythrocyte sedimentation rate (40 mm/h) and C-reactive protein (1.1 mg/dL) were mildly elevated. His liver function tests revealed elevated aspartate aminotransferase at 118 U/L, alanine aminotransferase of 206 U/L, albumin of 2.6 g/dL, total bilirubin of 0.9 mg/dL, and a normal international normalized ratio. A basic metabolic panel, urinalysis, lactate, and computed tomography angiogram of the chest and abdomen/pelvis were unremarkable. His urine drug screen was positive for cocaine, amphetamines, and cannabis. He had an elevation of troponin at 0.4 ng/mL, which had a flat trend and peaked at 0.530. An electrocardiogram showed T wave inversions in lateral leads without any changes in reciprocal leads.
He was admitted for sepsis of an unclear source. Given his right knee pain, he underwent right knee arthrocentesis, which was not convincing of septic arthritis with 3543 total nucleated cell count/mm3, comprising 94% neutrophils with a fluid glucose of 102 mg/dL. Synovial fluid cultures were negative. Lumbar puncture was unrevealing for infection. The patient was started on broad-spectrum antibiotics.
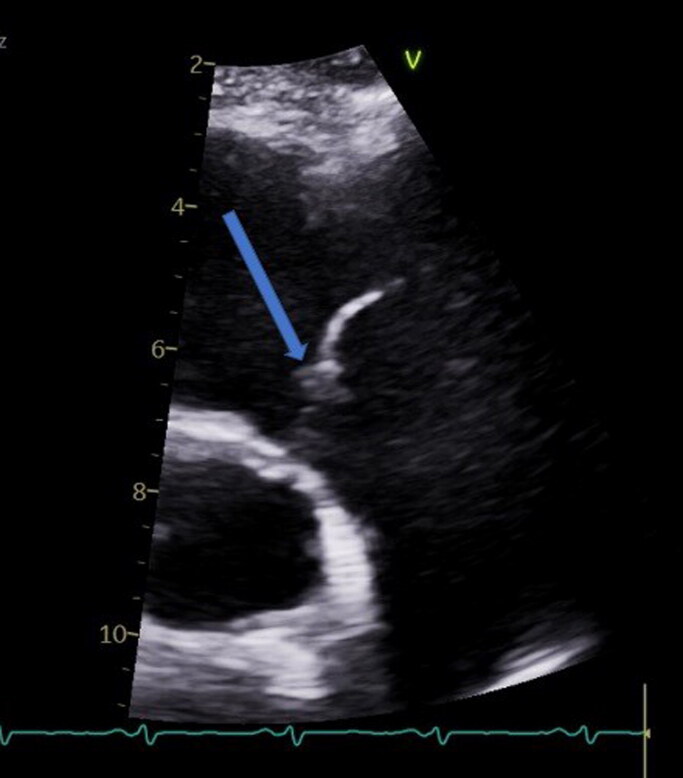
On day 2, his blood culture started growing S. pneumoniae. Magnetic resonance imaging of the whole spine did not show any bony involvement. An echocardiogram revealed an ejection fraction of 28% (50% 3 years ago) with pulmonic vegetation (Figure 1) and severe global hypokinesis. No vegetation was noted on other valves. He was switched to intravenous ceftriaxone per sensitivities with significant improvement in his clinical status and discharged on ceftriaxone for a total of 4 weeks. Interestingly, the patient had been immunized at the time of HIV diagnosis with both pneumococcal conjugate vaccine 13 (PCV13) and pneumococcal polysaccharide vaccine 23 (PPSV23), when his CD4 count was 496 cells/mm3 and viral load was 2420 RNA copies/mL.
Figure 1.
Echocardiogram showing pulmonic vegetation and an ejection fraction of 28%.
DISCUSSION
This case presents rare pulmonic valve endocarditis with pneumococcal bacteria in an intravenous drug user with S. pneumoniae despite vaccination with both PCV13 and PPSV23 and compliance with antiretroviral therapy. HIV-infected adults have a sevenfold higher incidence of developing invasive pneumococcal disease than HIV-uninfected adults.3 PPV23 and PCV13 cover 79.5% and 59.0% serotypes, respectively.3
In HIV patients, CD4 counts <200 cell/mL and viral loads >100,000 copies/µL lead to less immunogenicity for the pneumococcal vaccine.4,5 Our patient had a CD4 count of 496 cells/mL3 and viral load of 2420 RNA copies/mL at the time of vaccination. Higher antibody response to the pneumococcal vaccine has been noted in patients on antiretroviral therapy, with the response indirectly proportional to viral load.6 In our case, the likely explanation for developing an invasive disease is the lack of immune response to the vaccine due to compromised cellular immunity. In conclusion, we propose HIV patients should wait until they have undetectable viral load and CD4 count >200 cell/mL after initiation of antiretroviral therapy to receive a pneumococcal vaccination.
References
- 1.Miró JM, del Río A, Mestres CA.. Infective endocarditis in intravenous drug abusers and HIV-1 infected patients. Infect Dis Clin North Am. 2002;16(2):273–295. doi: 10.1016/S0891-5520(01)00008-3. [DOI] [PubMed] [Google Scholar]
- 2.Samaroo-Campbell J, Hashmi A, Thawani R, Moskovits M, Zadushlivy D, Kamholz SL.. Isolated pulmonic valve endocarditis. Am J Case Rep. 2019;20:151–153. doi: 10.12659/AJCR.913041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus JL, Baxter R, Leyden WA, et al. Invasive pneumococcal disease among HIV-infected and HIV-uninfected adults in a large integrated healthcare system. AIDS Patient Care STDS. 2016;30(10):463–470. doi: 10.1089/apc.2016.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nunes MC, Madhi SA.. Safety, immunogenicity and efficacy of pneumococcal conjugate vaccine in HIV-infected individuals. Hum Vaccin Immunother. 2012;8(2):161–173. doi: 10.4161/hv.18432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song JY, Cheong HJ, Noh JY, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in HIV-infected adults in the era of highly active antiretroviral therapy: analysis stratified by CD4 T-cell count. Hum Vaccin Immunother. 2020;16(1):169–175. doi: 10.1080/21645515.2019.1643677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordano Q, Falcó V, Almirante B, et al. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis. 2004;38(11):1623–1628. doi: 10.1086/420933. [DOI] [PubMed] [Google Scholar]