Abstract
Purpose
Emerging evidence has indicated that oxidative stress (OS) contributes to periodontitis. Periodontal ligament cells (PDLCs) are important for the regeneration of periodontal tissue. Quercetin, which is extracted from fruits and vegetables, has strong antioxidant capabilities. However, whether and how quercetin affects oxidative damage in PDLCs during periodontitis remains unknown. The aim of this study was to assess the effects of quercetin on oxidative damage in PDLCs and alveolar bone loss in periodontitis and underlying mechanisms.
Materials and Methods
The tissue block culture method was used to extract human PDLCs (hPDLCs). First, a cell counting kit 8 (CCK-8) assay was used to identify the optimal concentrations of hydrogen peroxide (H2O2) and quercetin. Subsequently, a 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) probe, RT-qPCR, Western blotting and other methods were used to explore the effects of quercetin on OS in hPDLCs and the underlying mechanism. Finally, quercetin was administered to mice with periodontitis through gavage, and the effect of quercetin on the level of OS and alveolar bone resorption in these mice was observed by immunofluorescence, microcomputed tomography (micro-CT), hematoxylin and eosin staining (H&E) staining and so on.
Results
Quercetin at 5 μM strongly activated NF-E2–related factor 2 (NRF2) signaling, alleviated oxidative damage and enhanced the antioxidant capacity of hPDLCs. In addition, quercetin reduced cellular senescence and protected the osteogenic ability of hPDLCs. Finally, quercetin activated NRF2 signaling in the periodontal ligaments, reduced the OS level of mice with periodontitis, and slowed the absorption of alveolar bone in vivo.
Conclusion
Quercetin can increase the antioxidant capacity of PDLCs and reduce OS damage by activating the NRF2 signaling pathway, which alleviates alveolar bone loss in periodontitis.
Keywords: periodontitis, periodontal ligament cells, oxidative stress, cellular senescence and quercetin
Introduction
Periodontitis is defined as a chronic inflammation of periodontal tissue, which affects approximately 10% of the global population.1 This condition is also the main cause of tooth loss in adults. The decrease in the quality and volume of the alveolar bones due to inflammation has a serious impact on oral prosthodontics and implantation. In recent years, increasing evidence has shown that oxidative stress (OS) plays an important role in the pathogenesis of various types of chronic inflammation, including periodontitis.2
OS is a state of imbalance between reactive oxygen species (ROS) levels and the endogenous antioxidant capacity. During the development of periodontitis, neutrophils are overactivated and release ROS, such as H2O2 and superoxide, in response to bacteria.3 Excessive ROS will cause damage to cells and tissues, such as increased lipid peroxidation metabolites and DNA and protein damage. Studies have shown that patients with periodontitis have elevated levels of OS in gingival crevicular fluid, saliva, and serum.4–6 As a signaling factor, ROS activate the NF-κB signaling pathway to promote the expression of inflammatory factors, and participate in the differentiation and activation of osteoclasts through the RANKL signaling pathway, ultimately leading to alveolar bone resorption and periodontal tissue injury.7,8 Previous studies on ROS and inflammation have mainly focused on the activation of osteoclasts. Currently, an increasing number of studies have shown that changes in the OS state of periodontal ligamental cells (PDLCs) during periodontitis play an important role in maintaining the stability of periodontal tissues.
PDLCs are the main cells in the periodontal ligament and are important for periodontal tissue reconstruction and repair.9 Studies have found that PDLCs show decreased proliferation, increased apoptosis and autophagy, impaired osteogenesis, and even cellular senescence under OS.10−12 Antioxidant therapies can effectively relieve the oxidative damage of PDLCs and restore their osteogenic and regenerative abilities.13,14 Therefore, it is important to maintain the balance between oxidation and antioxidation in periodontal tissues, especially in PDLCs.
Quercetin is a typical flavonoid compound found in fruits and vegetables, such as apples, potatoes, tomatoes and onions.15 This molecule has been widely researched due to its strong antioxidant, anticancer and anti-inflammatory properties.16 Studies have found that quercetin can protect nerve cells from oxidative damage, thereby alleviating diseases such as Alzheimer’s disease and Parkinson’s disease.17,18 Moreover, previous reports have found that quercetin can inhibit the release of inflammatory cytokines in gingival fibroblasts in vitro19 and alleviate alveolar bone resorption.20 However, few studies have examined the direct relationship between the antioxidant effect of quercetin in PDLCs and its effects on periodontitis.
Therefore, we established an OS-induced PDLC model through H2O2 exposure to explore the effect of quercetin on oxidative damage in PDLCs and the potential mechanism. And then ligature-induced periodontitis model of mice was established to explore the therapeutic effect of quercetin on periodontitis.
Materials and Methods
Cell Culture
hPDLCs were obtained from patients aged 18–25 years old with orthodontic extraction of premolars or third molars. The acquisition criteria were healthy periodontal tissue, no smoking, no diabetes and other chronic diseases or other infections. The study was reviewed by the Medical Ethics Committee of the Stomatological Hospital of Tongji University, patients were provided informed consent in accordance with the relevant ethical requirements (no.[2020]-R-003).
hPDLCs were prepared according to previously published protocols.22 In brief, the extracted teeth were placed immediately (within 2 h) in phosphate-buffered saline (PBS) (HyClone, South Logan, UT, USA) with 2% penicillin/streptomycin, and the periodontal ligament tissue in 1/3 of the root was scraped with a scalpel and cut into small pieces of 1 mm3 in a 25 mL cell culture flask. The flask was turned upside down with 5 mL of α-MEM (HyClone) medium containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C in a 5% CO2 humidified incubator for 4 h, and then the flask was turned over and cultured. The cell culture medium was changed every 3 days. hPDLCs were passaged when they reached approximately 90% confluence. Cells at 3rd-5th passage (P3-P5) were used for further treatments.
Cell Counting Kit 8 (CCK-8) Assays
A CCK-8 assay (Beyotime, Shanghai, China) was used to choose the optimal concentrations of quercetin (Sigma, USA) and H2O2 (Sigma). hPDLCs were seeded in 96-well culture plates at a density of 2000 cells per well and incubated for 24 h before the indicated experiments were performed. hPDLCs were treated with different concentrations of H2O2 (0 μM, 50 μM, 100 μM, 200 μM, 300 μM, 500 μM and 1000 μM) for 2 h/24 h or quercetin (0 μM, 5 μM, 10 μM, 20 μM, 30 μM, 50 μM and 100 μM) for 24 h. Then, the culture medium was exchanged for 100 µL of medium containing 10 µL of CCK‐8 solution and incubated for 2 h at 37°C. The absorbance was measured at 450 nm. The OD values are expressed as an average of the three wells for each group. The percentage of treated cells relative to control cells was calculated and defined as cell viability. The experiment was performed three times.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted using RNAiso Plus reagent (TaKaRa, Japan) according to the manufacturer’s protocol. cDNA was synthesized using the PrimeScript™ RT reagent kit with gDNA Eraser (Takara). RT-qPCR was performed using a Hieff® qPCR SYBR Green Master Mix (Yeasen, China). Fold changes in mRNA were determined based on the expression of GAPDH. The primer information for related mRNAs is shown in Table 1.
Table 1.
Primer Sequences Used for RT-qPCR Analysis
| Gene | Forward (5ʹ-3ʹ) | Reverse (5ʹ-3ʹ) |
|---|---|---|
| GADPH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
| NRF2 | TAGATGACCATGAGTCGCTTGC | GCCAAACTTGCTCCATGTCC |
| HO-1 | AAGACTGCGTTCCTGCTCAAC | AAAGCCCTACAGCAACTGTCG |
| NQO-1 | GAAGAGCACTGATCGTACTGGC | GGATACTGAAAGTTCGCAGGG |
| GPx3 | AGTCCACCGTGTATGCCTTCT | GAGACGCGACATTCTCAATGA |
| CAT | AGCGACCAGATGAAGCAGTG | TCCGCTCTCTGTCAAAGTGTG |
| p21 | CGATGGAACTTCGACTTTGTCA | GCACAAGGGTACAAGACAGTG |
| p53 | GAGGTTGGCTCTGACTGTACC | TCCGTCCCAGTAGATTACCAC |
| Alp | ACTGGTACTCAGACAACGAGAT | ACGTCAATGTCCCTGATGTTATG |
| Runx2 | TGGTTACTGTCATGGCGGGTA | TCTCAGATCGTTGAACCTTGCTA |
| Ocn | CACTCCTCGCCCTATTGGC | CCCTCCTGCTTGGACACAAAG |
Western Blotting Analysis
Harvested cells were washed with PBS 3 times and lysed in RIPA buffer (Beyotime) containing protease inhibitor cocktail (Beyotime) to extract total protein. For extraction of nuclear protein, a PARIS™ Kit (Invitrogen, USA) was used according to the manufacturer’s protocol, and protein was quantitatively analyzed using a bicinchoninic acid (BCA) kit (Beyotime). Western blotting was performed as previously reported.23 The following antibodies used in this study were purchased from Cell Signaling Technology (CST, Danvers, MA) and used at a dilution of 1:1000: p21 and GAPDH. The following antibodies used in this study were purchased from Abcam (China): NRF2 (1:1000) and Lamin B1 (1:10,000).
Immunocytochemistry
hPDLCs cultured in 24-well plates were fixed with 4% paraformaldehyde (PFA) (Sigma) for immunocytochemistry according to our previous study.24 The nuclei were stained with DAPI (1:1000, Sigma). The antibodies used in this experiment were as follows: Ki67 (1:250, Abcam), γH2AX (1:500, Abcam), Alexa Fluor® 488 donkey anti-rabbit IgG (H+L) (1:1000, Invitrogen) and Alexa Fluor® 488 goat anti-mouse IgG (H+L) (1:1000, Invitrogen). Digital images of ten randomly selected fields were acquired with a Nikon DS-Ri1 microscope.
Senescence-Associated β-Galactosidase Activity (SA-β-Gal) Assays
SA-β-gal staining was performed using an SA-β-gal staining kit (Beyotime). First, hPDLCs were fixed with 4% PFA for 15 min after 6 h of H2O2 treatment. The dyeing solution was added and incubated at 37°C for 24 h in dark. For determination of proportion of SA-β-gal-positive cells, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (1:1000, Sigma) staining was also performed. And digital images of ten randomly selected fields were acquired with a Nikon DS-Ri1 microscope.
Alkaline Phosphatase (ALP) Staining
ALP staining was determined using a BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime). hPDLCs were fixed with 4% PFA (Sigma) for 15 min after 7 days of osteogenic induction. Then, the cells were incubated in the dark (room temperature) with BCIP/NBT staining solution for 6 h. Digital images were taken under a film viewer by a Nikon Single Lens Reflex camera.
Detection of OS
OS was evaluated by detecting intracellular ROS generation, malondialdehyde (MDA) levels, and superoxide dismutase (SOD) activity.25 Intracellular ROS were detected using an ROS Assay Kit (Beyotime) in this study. After treatment, cells in 24-well plates were incubated with 0.1% DCFH-DA diluted in α-MEM at 37°C for 25 min. Green fluorescence was observed with a fluorescence microscope (Nikon, Japan). The excitation at 488 nm and emission at 525 nm were measured using a microplate reader (BioTek Synergy H1, USA) for quantitative analysis of ROS.
The methods for protein sample collection and quantification are described above. The SOD and MDA activities were detected using the Cu/Zn-SOD and Mn-SOD Assay Kit with WST-8 (Beyotime) and Lipid Peroxidation MDA Assay Kit (Beyotime) according to the manufacturer’s protocol. The OD value was measured by a microplate reader at wavelengths of 530 nm and 450 nm.
Blood samples were collected from the abdominal aorta of mice (n=5 each group). The samples were deposited at room temperature for 30 min and then centrifuged at 4 °C and 2500 rpm for 15 min. The supernatant was collected to detect the serum levels of MDA and SOD as described above.
Animals
Male C57BL/6 mice were purchased from Slaccas Laboratory Animal Corporation (Shanghai, China). All the mice were maintained in specific pathogen-free cages with a 12-hour light/dark cycle and moderate supplementation of water and food during the experiment. Mice aged 8 weeks were randomly assigned to three groups (n=12 each group): Control, periodontitis and periodontitis + quercetin (Q). Before the operation, the mice were anesthetized with an intraperitoneal injection of pentobarbital at a concentration of 60 mg/kg. Silk ligatures (6–0, Jinhuan, China) were tied around both second maxillary molars. During the experiment and routine feeding, animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH). The procedures performed on the mice were approved by the Institute of Animal Care and Use Committee of Tongji University (no. TJLAC-018-029).
Quercetin (Sigma) was given to the mice with periodontitis by oral gavage at a concentration of 50 mg/kg body weight on the first day after surgery, according to previous study.21 Quercetin was dissolved in 10% PEG 400 (90% ddH2O and 10% PEG 400) at the concentration of 5mg/mL.
Micro-CT Analysis
10 days after periodontitis, the maxillaries of the mice (n=6 each group) were isolated and directly scanned with high-resolution micro-CT (SkyScan1076, Bruker Micro-CT, USA). Image acquisition of the maxillaries was performed at an energy of 70 kV and a resolution of 14 μm. ImageJ software (NIH, Bethesda, MD, USA) was used to quantify the distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) of the maxillary second molars.
Histological Analysis
Maxillary specimens were fixed in 4% PFA (Sigma) for 48 h at 4°C and decalcified in 10% ethylenediaminetetraacetic acid (EDTA) (Sigma) for 4 weeks. Subsequently, the bone samples were embedded in paraffin wax and sectioned into 4-μm-thick slices. The loss of alveolar bone in the maxillary region was analyzed by H&E staining (Biotech Well, Shanghai, China).
For immunofluorescence staining, the bone sections were blocked with 10% goat serum (Maxim, China) and then incubated with a primary antibody at 4°C overnight and with a secondary antibody for 1 h at 37°C. Slides were then stained with DAPI (Sigma) and mounted with an anti-fade reagent (Invitrogen). The antibodies used in this experiment were as follows: NRF2 (1:100, Abcam) and Alexa Fluor® 488 donkey anti-rabbit IgG (H+L) (1:1000, Invitrogen). Images were acquired with a Nikon DS-Ri1 microscope.
Statistical Analysis
All analyses were conducted using SPSS 20.0 software (SPSS, Inc, Chicago, IL, USA). Data are expressed as the mean ± SD. Shapiro–Wilk test was used to verify whether the data in each group obeyed normal distribution. And for the data in animal studies, Q-Q plots were supplement for the normality analysis (Supplementary files). Levene test was used to verify whether the population shared the homogeneity of variance. If the above two conditions are met, the one-way ANOVA can be used (LSD-t test is used for pairwise comparison). Kruskal–Wallis test was used to compare the differences among groups in vivo studies for the data did not meet the normal distribution. Values of p<0.05 were considered statistically significant (*).
Results
Effects of Quercetin and H2O2 on Cell Viability
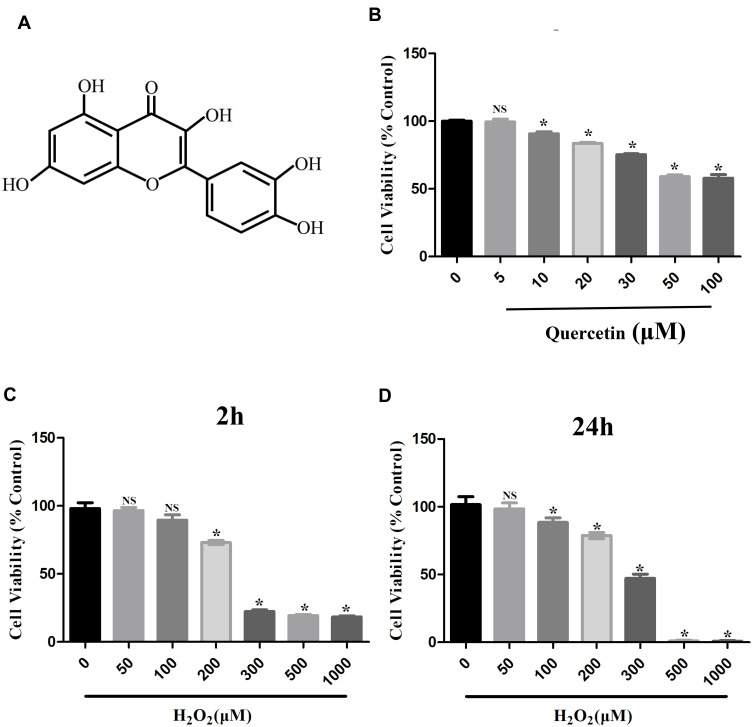
To detect the cytotoxicity of quercetin (Figure 1A), we cultured hPDLCs with different concentrations (0–100 μM) of quercetin for 24 h. As shown in Figure 1B, quercetin resulted in cytotoxicity at levels of more than 5 μM compared with the control (*p<0.05). To mimic the OS status in periodontitis, we treated hPDLCs with different concentrations of H2O2 (0–1000 μM) for 2 and 24 h. As shown in Figure 1C and D, a concentration of more than 200 μM had an obvious inhibitory effect on the cell activity of hPDLCs (*p<0.05). Based on the results achieved, we selected 5 μM quercetin and 200 μM H2O2 as target concentrations for the subsequent experiments.
Figure 1.
Effects of H2O2 and quercetin on cell viability of hPDLCs. (A) The chemical structural formula of quercetin. (B) Cell viability of hPDLCs treated with various concentrations of quercetin (0, 5, 10, 20, 30, 50 or 100 μM) for 24 h. (C) Cell viability of hPDLCs treated with various concentrations of H2O2 (0, 50, 100, 200, 300, 500 or 1000 μM) for 2 h. (D) Cell viability of hPDLCs treated with various concentrations of H2O2 (0, 50, 100, 200, 300, 500 or 1000 μM) for 24 h. All data are presented as the mean ± SD, *p < 0.05.
Quercetin Attenuated H2O2-Induced Oxidative Damage in hPDLCs by Activating the NRF2 Signaling Pathway
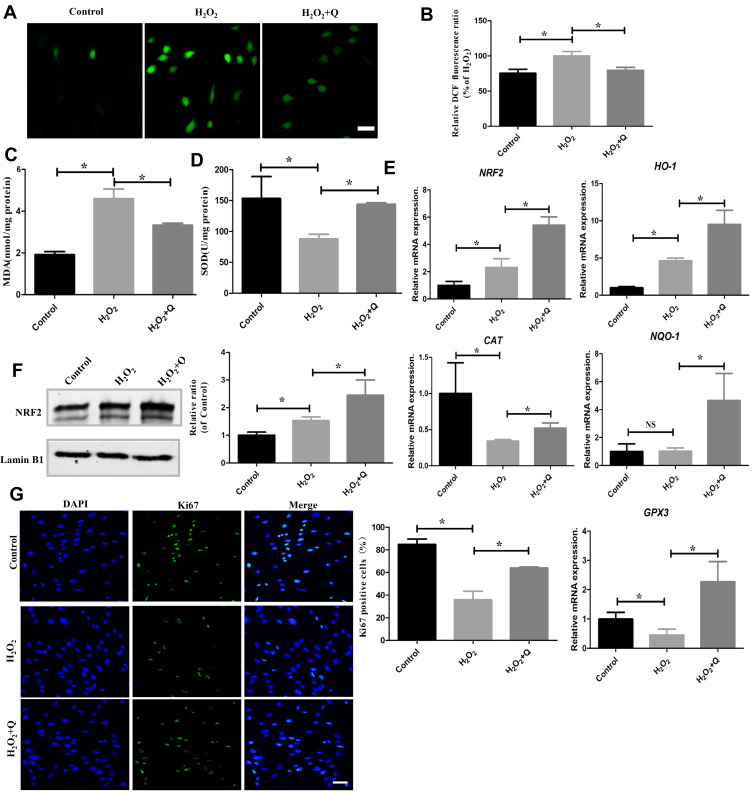
hPDLCs were pretreated with quercetin diluted in α-MEM supplemented with 10% FBS for 24 h before the addition of H2O2 to investigate its effects on cells under OS. The ROS level, MDA level and SOD activity were assessed to detect the effects of quercetin in vitro. The results showed that the levels of ROS and MDA were increased and that SOD activity was decreased in the H2O2-induced cells compared to the control cells (*p<0.05). Following the quercetin pretreatment, the levels of ROS and MDA were significantly reduced, and SOD activity was significantly increased (*p<0.05) (Figure 2A–D). The above results confirmed that quercetin attenuated H2O2-induced oxidative damage.
Figure 2.
Quercetin alleviates OS by activating NRF2 signaling in vitro. (A) Representative images of DCFH-DA staining and (B) quantification through a microplate reader in the indicated groups. Scale bar: 50 μm. (C) MDA levels in different groups. (D) SOD levels in different groups. (E) mRNA expression levels of antioxidative genes (NRF2, HO-1, NQO-1, GPx3 and CAT). (F) Representative Western blotting images of NRF2 protein and quantification in hPDLCs. (G) Representative images and quantification of Ki67-positive cells stained with DAPI after different treatments (green: Ki67; blue: DAPI). Scale bar: 50 μm. All data are presented as the mean ± SD, *p < 0.05.
NRF2 is a key transcription factor that regulates antioxidant expression. To further verify the antioxidative capacity of quercetin, we detected the expression levels of NRF2 and NRF2-mediated downstream genes. The RT-qPCR results indicated that quercetin upregulated the mRNA expression of NRF2, heme oxygenase-1 (HO-1), NAD(P)H: quinone oxidoreductase 1 (NQO-1), glutathione peroxidase 3 (GPx3) and catalase (CAT) (*p<0.05) (Figure 2E). Moreover, the Western blotting results showed that the expression of intranuclear NRF2 protein was elevated after quercetin pretreatment (*p<0.05) (Figure 2F). Therefore, we concluded that quercetin attenuated oxidative damage by activating the NRF2 signaling pathway.
To further explore the effects of quercetin on cell viability, we detected the cell proliferation biomarker Ki67 through immunocytochemistry. And we found that hPDLCs in H2O2 treatment group showed a significant decrease in cell proliferation, while hPDLCs in the quercetin pretreatment group showed a higher proliferative capacity (*p<0.05) (Figure 2G).
Quercetin Protected Against Cellular Senescence and Preserved the Osteogenic Potential of hPDLCs
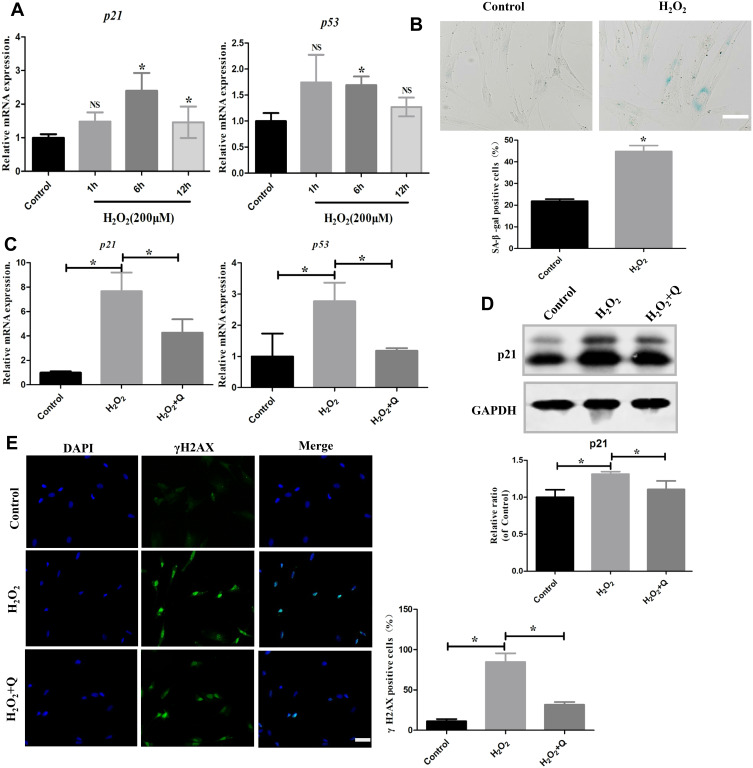
Since OS can induce cellular senescence, we detected cellular senescence markers in hPDLCs after treatment with H2O2 for 1 h, 6 h and 12 h (Figure 3A). We found that H2O2 treatment significantly increased the mRNA levels of p21 and p53 at 6 h after exposure (*p<0.05). Furthermore, SA-β-gal staining showed that senescent cells were significantly increased in the H2O2 treatment group (*p<0.05) (Figure 3B). However, following quercetin treatment, the mRNA levels of p21 and p53 were decreased (Figure 3C). The p21 protein expression was also decreased (*p<0.05) (Figure 3D). Moreover, we detected γH2AX, a marker of double-stranded DNA breaks. Immunocytochemistry results showed that quercetin significantly alleviated DNA damage in hPDLCs (*p<0.05) (Figure 3E). Overall, these results suggest that quercetin could alleviate cellular senescence induced by H2O2.
Figure 3.
Quercetin alleviates cellular senescence in vitro. (A) mRNA expression levels of senescence markers (p21 and p53) in hPDLCs treated with 200 μM H2O2 for 1 h, 6 h and 12 h. (B) Representative SA-β-gal staining images and quantification of cells in the different treatment groups. Scale bar: 50 μm. (C) mRNA expression levels of senescence markers (p21 and p53) in hPDLCs after quercetin treatment. (D) Representative Western blotting images of p21 protein and quantification in hPDLCs. (E) Representative images and quantification of γH2AX-positive cells stained with DAPI after different treatments (green: γH2AX; blue: DAPI). Scale bar: 50 μm. All data are presented as the mean ± SD, *p < 0.05.
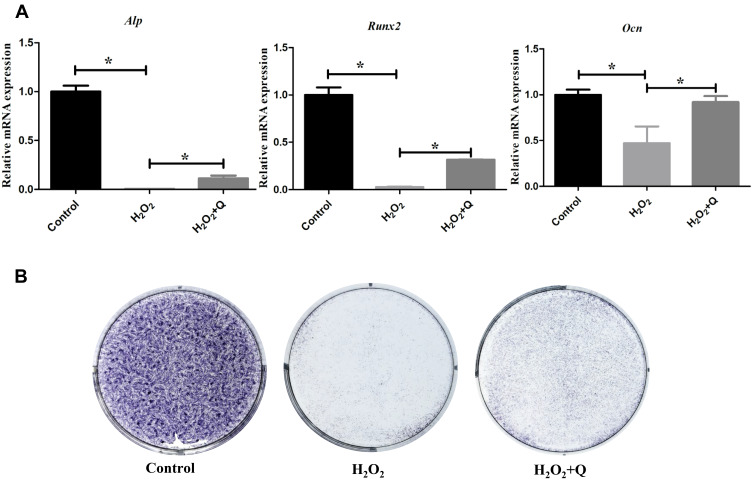
Then, we explored the ability of quercetin to protect the osteogenic potential of hPDLCs under OS. As shown in Figure 4A and B, quercetin elevated the mRNA expression of the osteogenic biomarkers Alp, Runx2 and Ocn and ALP activity (*p<0.05). Thus, quercetin has the potential to reverse the osteogenesis of hPDLCs.
Figure 4.
Quercetin protects the osteogenic potential of hPDLCs in vitro. (A) Expression of osteogenic genes (Alp, Runx2, and Ocn) on the 7th day of osteogenic induction in each group. (B) Representative ALP staining results on the 7th day of osteogenic induction in each group. All data are presented as the mean ± SD, *p < 0.05.
Quercetin Reduced OS and Increased the Expression of NRF2 in vivo
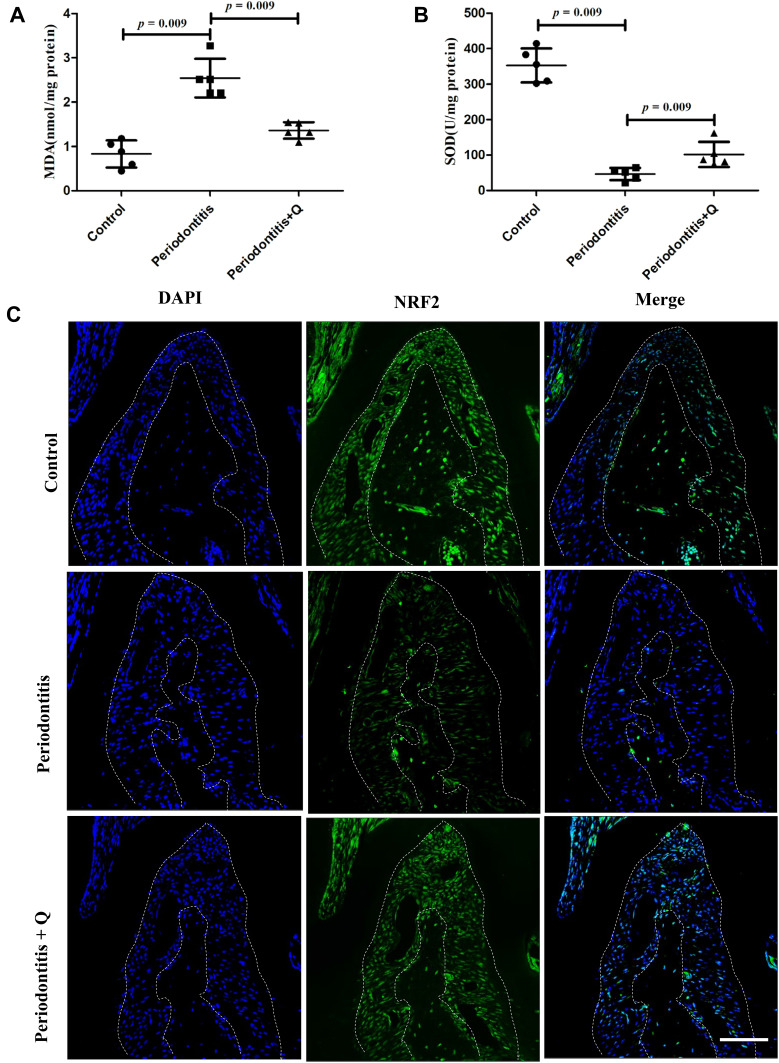
To investigate the effects of quercetin on OS levels caused by periodontitis, we detected two OS biomarkers, MDA and SOD, in an in vivo study. We found that the increased MDA level in serum and periodontal tissues of the mice with periodontitis was reversed by quercetin (*p<0.05) (Figure S1A, Figure 5A). The SOD activities in the quercetin treatment groups were higher than those in the periodontitis group (*p<0.05) (Figure 5B). These results indicated that the mice with periodontitis had elevated serum levels of OS and that quercetin reduced oxidative damage caused by periodontitis. Moreover, we examined the expression of NRF2 in the periodontal ligaments. Immunofluorescence staining revealed that quercetin enhanced the expression of NRF2 compared with that in the periodontitis group (Figure 5C). Then, NRF2 and NRF2-mediated downstream genes (HO-1,NQO-1,Gpx3 and CAT) of periodontal tissues in different groups were also detected. The RT-qPCR results indicated that quercetin also upregulated the mRNA expression of above genes in vivo (Figure S1B) (*p<0.05) Accordingly, quercetin reduced OS levels by activating the expression of NRF2 in vivo.
Figure 5.
Quercetin alleviates OS and activates NRF2 signaling in vivo. (A) MDA levels in serum (n=5 each group). (B) SOD levels in serum (n=5 each group). (C) Representative immunofluorescence staining images of NRF2 in each group. Scale bar: 50 μm. All data are presented as the mean ± SD and specific p values are indicated on the graphs.
Quercetin Alleviated Alveolar Bone Resorption in the Mice with Periodontitis
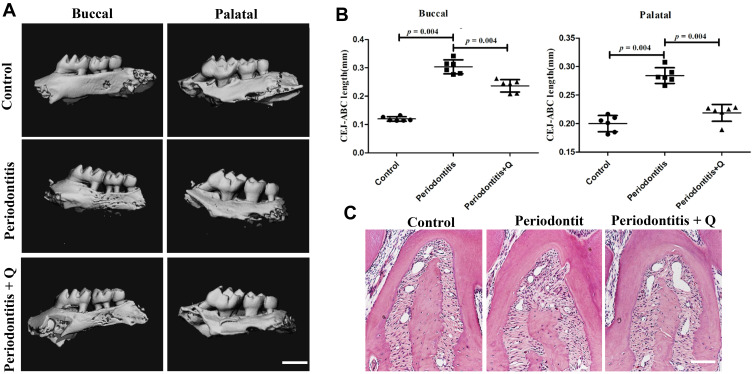
To assess whether quercetin could decrease alveolar bone loss in vivo, we examined the effects of quercetin on a mouse model of ligature-induced experimental periodontitis. Micro-CT showed that alveolar bone loss on both the buccal and palatal sides of the maxillary second molars was significantly increased in the periodontitis group compared with the control group and was alleviated through oral gavage of quercetin (*p<0.05) (Figure 6A). Quantitative analyses of the distance between the CEJ and the ABC led to the same conclusions (*p<0.05) (Figure 6B). H&E staining also verified the above results (Figure 6C). Therefore, quercetin could protect against alveolar bone loss. Moreover, no obvious histological changes were found in H&E staining in the heart, liver, spleen, stomach and kidney of mice after administering of quercetin for 10 days (Figure S2).
Figure 6.
Quercetin prevents against alveolar bone resorption in mice with periodontitis. (A) Micro-CT reconstruction images, including the buccal and palatal sides of the maxillaries, in the control, periodontitis, and periodontitis + quercetin groups (n=6 each group). Scale bar: 1 mm. (B) The distance from the CEJ to the ABC of the maxillary second molars was analyzed based on the micro-CT results. (C) Representative H&E staining images of each group. Scale bar: 50 μm. All data are presented as the mean ± SD and specific p values are indicated on the graphs.
Discussion
Periodontitis is a common dental disease associated with plaque biofilm colonization and the host immune response. Recently, increasing evidence has shown that this condition is also closely associated with excess OS.26 Excessive accumulation of ROS in periodontal tissues is an important cause of tissue injury during periodontitis.27 Moreover, OS mediates the relationship between periodontitis and systemic diseases, including cardiovascular diseases.26,28 Thus, amelioration of OS damage is important for the treatment of periodontitis.
PDLCs are cells with regenerative potential in periodontal tissues, whose primary functions are support and protection of teeth from injury by mechanical loading.9 Previous studies have revealed that OS could affect the proliferation and osteogenic differentiation of PDLCs.29,30 Therefore, protection PDLCs from OS is important to maintain the dynamic stability of periodontal tissues. In the present study, we stimulated hPDLCs with H2O2 to mimic the elevated OS levels in periodontitis according to previous studies.13,14 Then, we assessed the effects of an antioxidant, quercetin, on OS in hPDLCs. Quercetin could enhance the proliferative ability of hPDLCs under OS. Then, we found that ROS production was inhibited and the biomarker of oxidative damage, MDA, were decreased. MDA is an important marker for evaluation of OS and is produced by lipid peroxidation of the cell membrane by free radicals; this molecule can destroy the structure of the cell membrane.31 We found that quercetin enhanced the expression of antioxidant enzyme-related genes, including HO-1, NQO-1, GPx3 and CAT and elevated SOD activity in an in vitro study. Among these genes, HO-1 is a rate-limiting enzyme for heme degradation and a cytoprotective enzyme with antioxidant properties. The NQO1 enzyme is a flavinase that provides electron catalysis for the reduction of various quinone compounds to hydroquinones via NADH or NAD(P)H.32 GPx3 can catalyze the reduction of hydrogen peroxide and lipid hydrogen peroxide by reducing glutathione.33 CAT is an enzyme that metabolizes hydrogen peroxide.34 SOD can catalyze superoxide anion radical disproportionation to produce hydrogen peroxide and oxygen.35 These molecules are members of the antioxidant enzyme system in the body, which is important in relieving OS.
Furthermore, the mechanism involving upregulation of the expression of these antioxidant genes was investigated in this study. We observed that quercetin can activate NRF2 signaling in the nucleus of PDLCs both in vivo and in vitro. NRF2, as a key transcriptional regulator, could bind to antioxidant response elements (AREs) to regulate antioxidant gene expression. NRF2 binds to the cytoplasmic protein Keap1 to inhibit its activity under normal conditions. In contrast, this molecule was separated from Keap1 and then enters the nucleus to regulate the expression of antioxidant genes during OS.36 Periodontitis is a disease associated with increased OS. The expression level of NRF2 is closely related to the dynamic balance of periodontal tissues. Studies have found that NRF2 expression is decreased in periodontitis tissue.37 Enhanced NRF2 signaling in PDLCs can improve their antioxidant capacity and maintain functional stability.38 However, NRF2−/- mice showed more severe periodontitis than control mice.39 Antioxidant therapy effectively alleviated periodontitis-related bone loss by activating NRF2 signaling.40,41 Quercetin, as a natural antioxidant, is often combined with dasatinib to eliminate senescent cells.42 This compound can activate NRF2 signaling when used alone.43,44 However, few studies have linked quercetin’s role in activating NRF2 signaling to its therapeutic effect on periodontitis.
In an in vitro study, we found that the levels of the critical cellular senescence pathway p53/p21 and the DNA damage biomarker γH2AX were significantly increased in the H2O2-treated hPDLCs and that this effect was reversed by quercetin. OS is one of the triggers of premature cellular senescence through DNA damage, which causes the DNA damage response (DDR).45 Histone H2AX is rapidly phosphorylated at a DNA double-stranded break site (γH2AX) to form a focal point and recruit injury repair-related proteins for repair in DDR.46 The persistence of the DDR activates p53/p21 pathway.47 Aquino-Martinez et al48 found that senescent osteocytes in the periodontal tissue of mice with periodontitis contribute to the destruction of periodontal tissue in old age. Unfortunately, senescent cells were not observed in the periodontal tissues in this study. Further studies are needed.
Based on the in vitro results, we further studied the therapeutic effects of quercetin on periodontitis in vivo using a classical ligature-induced mouse model of periodontitis. Micro-CT and H&E staining showed that this treatment was effective in relieving bone loss in periodontitis. Moreover, quercetin alleviated oxidative damage by decreasing MDA levels and elevating SOD activity in serum. This antioxidant effect is achieved through activating NRF2 signaling, which was observed to be enhanced in the periodontal ligaments of the quercetin-treated group. This result confirmed that quercetin has promising applications in the treatment of periodontitis.
However, our study has some limitations. NRF2 signal and its downstream genes were activated after quercetin treatment both in vivo and in vitro experiments, but the specific mechanism between quercetin and NRF2 signaling remains to be further confirmed. Moreover, with low bioavailability, low water solubility and inactivity of metabolites of quercetin, the optimal dose and drug-delivery way of quercetin for clinical treatment of periodontitis should be further explored.
Conclusion
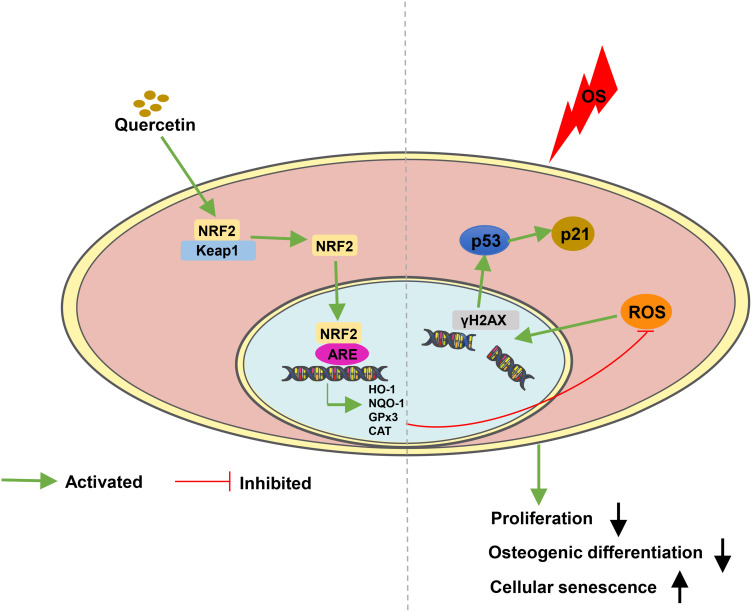
In conclusion, the present study revealed that quercetin ameliorated the oxidative damage and improved the antioxidative ability of PDLCs by activating the NRF2 signaling pathway in vitro and in vivo (Figure 7). In addition, quercetin could prevent alveolar bone absorption in periodontitis. Therefore, quercetin may be a potential therapeutic candidate for treatment of periodontitis.
Figure 7.
A model illustrating quercetin protects PDLCs against OS via activation of the NRF2 signaling pathway. The ability of proliferate and osteogenic differentiation in PDLCs are impaired under OS. In addition, increased ROS can also damage the DNA double strands and induce cellular senescence through the p53/p21 signaling pathway. Moreover, quercetin protects PDLCs against OS, through increasing the expression and nuclear localization of NRF2, which combines with antioxidant response element (ARE) to promote the expression of antioxidant enzymes or substances such as HO-1,NQO-1, GPx3, CAT and SOD.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 81470716), the Natural Science Foundation of Shanghai (grant no.19ZR462000) and the Science and Technology Committee Foundation of Shanghai (grant no. 14411967200).
Data Sharing Statement
All data included in this study are available upon request by contact with the corresponding author.
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sczepanik FSC, Grossi ML, Casati M, et al. Periodontitis is an inflammatory disease of oxidative stress: we should treat it that way. Periodontol. 2020;2000(84):45–68. doi: 10.1111/prd.12342 [DOI] [PubMed] [Google Scholar]
- 3.Hirschfeld JWP, Milward MR, Cooper PR, Chapple ILC. Modulation of neutrophil extracellular trap and reactive oxygen species release by periodontal bacteria. Infect Immun. 2017;85:e00297–00217. doi: 10.1128/IAI.00297-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Andrukhov O, Rausch-Fan X. Oxidative stress and antioxidant system in periodontitis. Front Physiol. 2017;8:1–13. doi: 10.3389/fphys.2017.00910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Cai W, Zhao S, et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2019;46:608–622. doi: 10.1111/jcpe.13112 [DOI] [PubMed] [Google Scholar]
- 6.Almerich-Silla JM, Montiel-Company JM, Pastor S, Serrano F, Puig-Silla M, Dasi F. Oxidative Stress parameters in saliva and its association with periodontal disease and types of bacteria. Dis Markers. 2015;2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Mo L, Niu Y, Li X, Zhou X, Xu X. The role of reactive oxygen species and autophagy in periodontitis and their potential linkage. Front Physiol. 2017;8:1–13. doi: 10.3389/fphys.2017.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanzaki H, Wada S, Narimiya T, et al. Pathways that regulate ROS scavenging enzymes, and their role in defense against tissue destruction in periodontitis. Front Physiol. 2017;8:1–8. doi: 10.3389/fphys.2017.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomokiyo A, Wada N, Maeda H. Periodontal ligament stem cells: regenerative potency in periodontium. Stem Cells Dev. 2019;28(15):974–985. doi: 10.1089/scd.2019.0031 [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Huang X, Fu C, et al. Recombinant klotho protects human periodontal ligament stem cells by regulating mitochondrial function and the antioxidant system during H2O2-induced oxidative stress. Oxid Med Cell Longev. 2019;2019:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei YM, Li L, Wang XQ, et al. AGEs induces apoptosis and autophagy via reactive oxygen species in human periodontal ligament cells. J Cell Biochem. 2019;121(8–9):3764–3779. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Ji Y, Jin X, et al. Mitochondrial abnormalities are involved in periodontal ligament fibroblast apoptosis induced by oxidative stress. Biochem Biophys Res Commun. 2019;509:483–490. doi: 10.1016/j.bbrc.2018.12.143 [DOI] [PubMed] [Google Scholar]
- 13.Xiong Y, Zhao B, Zhang W, Jia L, Zhang Y, Xu X. Curcumin promotes osteogenic differentiation of periodontal ligament stem cells through the PI3K/AKT/Nrf2 signaling pathway. Iran J Basic Med Sci. 2020;23:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa FPD, Puty B, Nogueira LS, et al. Piceatannol increases antioxidant defense and reduces cell death in human periodontal ligament fibroblast under oxidative stress. Antioxidants (Basel). 2019;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019;24:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–271. doi: 10.1016/j.fitote.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Raj K, Singh S. Neuroprotective effect of quercetin in combination with piperine against rotenone- and iron supplement-induced Parkinson’s disease in experimental rats. Neurotox Res. 2020;37:198–209. doi: 10.1007/s12640-019-00120-z [DOI] [PubMed] [Google Scholar]
- 18.Khan H, Ullah H, Aschner M, Cheang WS, Akkol EK. Neuroprotective Effects of quercetin in Alzheimer’s disease. Biomolecules. 2019;10:1–20. doi: 10.3390/biom10010059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong G, Ji W, Wang F, et al. Quercetin inhibits inflammatory response induced by LPS from porphyromonas gingivalis in human gingival fibroblasts via suppressing NF-kappaB signaling pathway. Biomed Res Int. 2019;2019:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napimoga MH, Clemente-Napimoga JT, Macedo CG, et al. Quercetin inhibits inflammatory bone resorption in a mouse periodontitis model. J Nat Prod. 2013;76:2316–2321. doi: 10.1021/np400691n [DOI] [PubMed] [Google Scholar]
- 21.Chandra A, Lagnado AB, Farr JN, et al. Targeted reduction of senescent cell burden alleviates focal radiotherapy-related bone loss. J Bone Miner Res. 2020;35(6):1119–1131. doi: 10.1002/jbmr.3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan C, Liu J, Wang H, Song J, Tan L, Zhao H. Porphyromonas gingivalis can invade periodontal ligament stem cells. BMC Microbiol. 2017;17:38. doi: 10.1186/s12866-017-0950-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu J, Hao L, Tian Y, Liu Y, Gu Y, Wu J. miR-199a-3p is involved in estrogen-mediated autophagy through the IGF-1/mTOR pathway in osteocyte-like MLO-Y4 cells. J Cell Physiol. 2018;233:2292–2303. doi: 10.1002/jcp.26101 [DOI] [PubMed] [Google Scholar]
- 24.Wu W, Fu J, Gu Y, Wei Y, Ma P, Wu J. JAK2/STAT3 regulates estrogen-related senescence of bone marrow stem cells. J Endocrinol. 2020;245:141–153. doi: 10.1530/JOE-19-0518 [DOI] [PubMed] [Google Scholar]
- 25.Ai Z, Wu Y, Yu M, Li J, Li S. Theaflavin-3, 3ʹ-Digallate suppresses RANKL-induced osteoclastogenesis and attenuates ovariectomy-induced bone loss in mice. Front Pharmacol. 2020;11:1–12. doi: 10.3389/fphar.2020.00803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Díaz CM, Bullon B, Ruiz‐Salmerón RJ, et al. Molecular inflammation and oxidative stress are shared mechanisms involved in both myocardial infarction and periodontitis. J Periodontal Res. 2020;55:519–528. doi: 10.1111/jre.12739 [DOI] [PubMed] [Google Scholar]
- 27.Zukowski P, Maciejczyk M, Waszkiel D. Sources of free radicals and oxidative stress in the oral cavity. Arch Oral Biol. 2018;92:8–17. doi: 10.1016/j.archoralbio.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 28.Kumar J, Teoh SL, Das S, Mahakknaukrauh P. Oxidative stress in oral diseases: understanding its relation with other systemic diseases. Front Physiol. 2017;8:693. doi: 10.3389/fphys.2017.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian J, Gu L, Adams A, Wang X, Huang R. Pellino-1 protects periodontal ligament stem cells against H2O2-induced apoptosis via activation of NF-kappaB signaling. Mol Biotechnol. 2018;60:533–538. doi: 10.1007/s12033-018-0067-6 [DOI] [PubMed] [Google Scholar]
- 30.Kook SH, Lee D, Cho ES, et al. Activation of canonical Wnt/beta-catenin signaling inhibits H2O2-induced decreases in proliferation and differentiation of human periodontal ligament fibroblasts. Mol Cell Biochem. 2016;411:83–94. doi: 10.1007/s11010-015-2570-4 [DOI] [PubMed] [Google Scholar]
- 31.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021 [DOI] [PubMed] [Google Scholar]
- 32.Dinkova-Kostova AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang C, Worley BL, Phaeton R, Hempel N. Extracellular glutathione peroxidase GPx3 and its role in cancer. Cancers (Basel). 2020;12:1–19. doi: 10.3390/cancers12082197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glorieux C, Calderon PB. Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol Chem. 2017;398:1095–1108. doi: 10.1515/hsz-2017-0131 [DOI] [PubMed] [Google Scholar]
- 35.Borgstahl GEO, Oberley-Deegan RE. Superoxide Dismutases (SODs) and SOD mimetics. Antioxidants (Basel). 2018;7:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harder B, Jiang T, Wu T, et al. Molecular mechanisms of Nrf2 regulation and how these influence chemical modulation for disease intervention. Biochem Soc Trans. 2015;43:680–686. doi: 10.1042/BST20150020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Sun X, Zhang X, et al. Enhanced oxidative damage and Nrf2 downregulation contribute to the aggravation of periodontitis by diabetes mellitus. Oxid Med Cell Longev. 2018;2018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Yang H, Wen Y, et al. Nrf2 inhibits periodontal ligament stem cell apoptosis under excessive oxidative stress. Int J Mol Sci. 2017;18:1–16. doi: 10.3390/ijms18051076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sima C, Aboodi GM, Lakschevitz FS, Sun C, Goldberg MB, Glogauer M. Nuclear factor Erythroid 2-related factor 2 down-regulation in oral neutrophils is associated with periodontal oxidative damage and severe chronic periodontitis. Am J Pathol. 2016;186:1417–1426. doi: 10.1016/j.ajpath.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhattarai G, Poudel SB, Kook SH, Lee JC. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016;29:398–408. doi: 10.1016/j.actbio.2015.10.031 [DOI] [PubMed] [Google Scholar]
- 41.Jia L, Xiong Y, Zhang W, Ma X, Xu X. Metformin promotes osteogenic differentiation and protects against oxidative stress-induced damage in periodontal ligament stem cells via activation of the Akt/Nrf2 signaling pathway. Exp Cell Res. 2020;386:1–12. doi: 10.1016/j.yexcr.2019.111717 [DOI] [PubMed] [Google Scholar]
- 42.Partridge L, Fuentealba M, Kennedy BK. The quest to slow ageing through drug discovery. Nat Rev Drug Discov. 2020;19:513–532. [DOI] [PubMed] [Google Scholar]
- 43.Shao Y, Yang Y, Li M, Hang L, Xu X. A solid dispersion of quercetin shows enhanced Nrf2 activation and protective effects against oxidative injury in a mouse model of dry age-related macular degeneration. Oxid Med Cell Longev. 2019;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Granado-Serrano AB, Martin MA, Bravo L, Goya L, Ramos S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: involvement of p38. Chem Biol Interact. 2012;195:154–164. doi: 10.1016/j.cbi.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 45.Benkafadar N, Francois F, Affortit C, et al. ROS-induced activation of DNA damage responses drives senescence-like state in postmitotic cochlear cells: implication for hearing preservation. Mol Neurobiol. 2019;56:5950–5969. doi: 10.1007/s12035-019-1493-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28:436–453. doi: 10.1016/j.tcb.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 47.Herbig U, Chen B, Chen BPC, et al. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol Cell. 2004;14:501–513. doi: 10.1016/S1097-2765(04)00256-4 [DOI] [PubMed] [Google Scholar]
- 48.Aquino-Martinez R, Eckhardt BA, Rowsey JL, et al. Senescent cells exacerbate chronic inflammation and contribute to periodontal disease progression in old mice. J Periodontol. 2020;1–13. doi: 10.1002/JPER.20-0529 [DOI] [PMC free article] [PubMed] [Google Scholar]