Abstract
Objectives
To assess how maternal body mass index and gestational weight gain are related to on fetal venous liver flow and birthweight in pregnancies with pre-gestational diabetes mellitus.
Methods
In a longitudinal observational study, 49 women with pre-gestational diabetes mellitus were included for monthly assessments (gestational weeks 24–36). According to the Institute Of Medicine criteria, body mass index was categorized to underweight, normal, overweight, and obese, while gestational weight gain was classified as insufficient, appropriate or excessive. Fetal size, portal flow, umbilical venous flow and distribution to the fetal liver or ductus venosus were determined using ultrasound techniques. The impact of fetal venous liver perfusion on birthweight and how body mass index and gestational weight gain modified this effect, was compared with a reference population (n = 160).
Results
The positive association between umbilical flow to liver and birthweight was more pronounced in pregnancies with pre-gestational diabetes mellitus than in the reference population. Overweight and excessive gestational weight gain were associated with higher birthweights in women with pre-gestational diabetes mellitus, but not in the reference population. Fetuses of overweight women with pre-gestational diabetes mellitus had higher umbilical (p = 0.02) and total venous liver flows (p = 0.02), and a lower portal flow fraction (p = 0.04) than in the reference population. In pre-gestational diabetes mellitus pregnancies with excessive gestational weight gain, the umbilical flow to liver was higher than in those with appropriate weight gain (p = 0.02).
Conclusions
The results support the hypothesis that umbilical flow to the fetal liver is a key determinant for fetal growth and birthweight modifiable by maternal factors. Maternal pre-gestational diabetes mellitus seems to augment this influence as shown with body mass index and gestational weight gain.
Introduction
In pregnancies with pre-gestational diabetes mellitus (PGDM), the risk of adverse perinatal outcome is increased [1], and complications are often associated with large for gestational age neonates [2,3]. Since hyperglycemia may cause accelerated fetal growth, optimal glycemic control is a cornerstone in the clinical follow-up [4,5]. However, in PGDM populations with apparently good glycemic control the incidence of large neonates remains high [6]. Recent improvements in glucose monitoring demonstrate that reduced glucose excursions/variability improve pregnancy outcomes [5].
These women have on average higher pre-pregnancy body mass index (BMI) and more gestational weight gain than women without diabetes mellitus [7,8]. Overweight and obesity add significantly to the risk of large for gestational age offspring in these pregnancies [7], and excess gestational weight gain is linked to risk for neonatal macrosomia independent of glycemic control in women with type 1 diabetes [8]. Thus, women with PGDM are advised to aim for pre-pregnancy BMI in the normal range, less gestational weight gain than women without diabetes, and strict glycemic control [5,8,9].
A known mechanism regulating fetal growth is the distribution of umbilical venous blood to the fetal liver (Fig 1) [10,11]. This blood, high in nutrition and oxygen, is directed either to the fetal liver or shunted through the ductus venosus supplying the fetal heart and brain (Fig 1). In low-risk pregnancies, at average 70–80% of the umbilical venous return is distributed to the liver [12–14]. Experimentally increased umbilical flow to the fetal liver induces hepatic cell proliferation and production of IGF-1 and -2 that is followed by augmented growth of heart, skeletal muscle and kidneys [15]. In humans, a higher umbilical flow to the liver is associated with newborn adiposity [16]. The distribution of the umbilical blood is influenced by maternal BMI in pregnancies without diabetes [17]. In normal weight women the maternal-fetal glucose gradient was found to correlate with the distribution of the umbilical flow to the fetal liver, while in overweight mothers no such correlation was found [18]. In pregnancies with PGDM, we found that the proportion of umbilical venous return distributed to the fetal liver was graded according to maternal HbA1C [19]. However, whether maternal BMI and gestational weight gain in women with PGDM influence this distributional mechanism is not known.
Fig 1. The fetal umbilical venous circulation schematic.
Well-oxygenated and nutrient rich blood (red) from the placenta reaches the fetus through the umbilical vein (UV). This blood is distributed either to the fetal liver (arrows within the liver) or shunted through the ductus venosus (DV) to supply the heart and brain. The portal vein (PV) carries low-oxygenated blood (blue) from the visceral organs and blends in with the umbilical blood from the left portal branch (LPV) to supply the right liver lobe.
The aim of the present study was to assess the relation between fetal venous liver flow and birthweight in PGDM pregnancies, and how this relation is modified by BMI and gestational weight gain.
Materials and methods
The present prospective longitudinal observational study was part of the project DiaDoppler investigating fetal hemodynamics in pregnancies with PGDM. We have previously reported the development of the ductus venosus, umbilical and portal blood flows during the second half of pregnancy in this population [19,20]. Here we assess whether maternal BMI and gestational weight gain are associated with modification of the venous perfusion of the fetal liver and birthweight.
Subjects
In our region, all pregnant women with PGDM are referred to the tertiary center at Haukeland University Hospital for follow-up by a multidisciplinary team. All women with PGDM and singleton pregnancies during the period August 2013 to May 2016 were invited to participate in the study. The study protocol was approved by the Regional Committee for Medical Research Ethics (REK vest 2011/2030), and 52 women (74% of those invited) gave informed written consent. All participants used insulin treatment during pregnancy. Forty-four participants had type 1 and eight had type 2 diabetes. Three participants with type 2 diabetes withdrew after the first visit, thus 49 women with PGDM constituted our study population.
Information on maternal height and pre-pregnancy weight was self-reported and collected from medical records. Pre-pregnancy BMI (weight (kg)/height (m)2) was categorized according to the Institute Of Medicine (IOM) guidelines: underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9) and obese (≥30) [21]. Weekly gestational weight gain was calculated by subtracting pre-pregnancy weight from the last weight measured before delivery, divided by gestational age at the last weighing. Weekly gestational weight gain was categorized according to pre-pregnancy BMI and the IOM guideline as insufficient, appropriate or excessive [21].
Gestational age was determined by measuring the crown rump length [22], using a vaginal ultrasound probe (Vivid 7, GE Healthcare Vingmed Ultrasound, E8C, 8 MHz) around week 9 of pregnancy. HbA1C was measured at inclusion in the first trimester. Birthweight z-scores were calculated according to gestational age at delivery [23]. Information on maternal HbA1C, birthweight, neonatal acidosis at birth, mode of delivery, Apgar score and transfer to the neonatal ward was collected from clinical records.
Flow variables
The ultrasound and Doppler examinations were performed at gestational weeks 24, 28, 32 and 36. Using an abdominal transducer (Vivid 7, GE Healthcare Vingmed Ultrasound, Horten, Norway) (M4S, 2.0–4.3 MHz), the fetal vein diameters and blood flow velocities were measured to calculate the blood flow volumes. Measurement techniques and formulas used for the calculations are reported previously [24,25].
Statistics
BMI, weekly maternal weight gain and fetal flows in the study population were compared with reference ranges (obtained in a longitudinal study of 160 healthy pregnancies using identical methods by our research group) [14,24,25]. We tested whether HbA1C differed between the BMI and gestational weight gain groups.
Multilevel regression was used to calculate the main outcome fetal blood flow by gestational age [14,23]. We used log-likelihood test to assess whether adding BMI or gestational weight gain categories significantly influenced the longitudinal development of flow by gestational age. Since only two participants with PGDM were underweight, this group was excluded from the log-likelihood analyses. Flow variable categories (tertiles) were defined by the distribution in the low-risk reference population. Differences in birthweight between flow tertiles, and between BMI and weekly gestational weight gain categories, were estimated using analysis of variance. Relations between birthweight z-scores and the exposures, BMI and gestational weight gain were assessed as continuous variables in regression analyses.
The statistical analyses were performed with the Statistical Package for the Social Sciences (version 24, SPSS, Chicago, IL) and the MLWin program (version 2.35, Centre of Multilevel Modeling, University of Bristol, UK). P-values <0.05 were considered significant.
Results
Characteristics of the study and reference populations at inclusion are shown in Table 1 and have been described previously [14,19]. The birthweight z-score distributions by BMI and gestational weight gain categories are presented in Table 2.
Table 1. Maternal and neonatal characteristics and outcomes in the study population of 49 pregnancies with pregestational diabetes mellitus.
| Number | Percent | |
|---|---|---|
| Type 1 diabetes mellitus | 44 | 89.8 |
| Type 2 diabetes mellitus | 5 | 10.2 |
| Maternal diabetic complications | 9 | 18.4 |
| Hypothyroidism | 9 | 18.4 |
| Chronic hypertension | 7 | 14.3 |
| Preeclampsia | 3 | 6.1 |
| Preterm birth* | 15 | 30.6 |
| Cesarean section | 22 | 44.9 |
| Metabolic acidosis at birth † | 1 | 2 |
| 5-min Apgar score <7 | 1 | 2 |
| Transfer to neonatal intensive care ward | 20 | 40.8 |
| Perinatal death ‡ | 1 | 2 |
| Malformation § | 2 | 4 |
*Preterm birth, gestational age <37 weeks
† Metabolic acidosis defined as an umbilical arterial pH of <7.0 and a base deficit of >12.
‡Intrauterine fetal death at gestational week 36.
§One neonate with sagittal craniosynostosis and one with congenital heart defect (anomalous left coronary artery from the pulmonary artery).
Table 2. Distribution of BMI and GWG categories and birthweight z-scores in the healthy reference and the PGDM populations.
| Reference Median (range) | PGDM Median (range) | ||||||
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 23.0 (17.0–41.0) | 25.4 (19.8–44.1) | |||||
| GWG/week (kg/week) | 0.37 (0.01–0.73) | 0.46 (-0.14–0.95) | |||||
| Category | % | Mean BW z-score | n | % | Mean BW z-score | ||
| BMI | normal weight | 101 | 63.1 | -0.11 | 22 | 44.9 | 0.62 |
| overweight | 43 | 26.9 | 0.17 | 14 | 28.6 | 2.02 | |
| obese | 9 | 5.6 | -0.52 | 11 | 22.4 | 0.59 | |
| p * | 0.224 | 0.001* | |||||
| GWG | insufficient | 47 | 29.4 | -0.16 | 6 | 12.2 | 0.31 |
| appropriate | 61 | 38.1 | -0.08 | 16 | 32.7 | 0.60 | |
| excessive | 47 | 29.4 | 0.10 | 27 | 55.1 | 1.48 | |
| p * | 0.556 | 0.008* | |||||
| Total group | 155 | -0.06 (-3.02–1.81) | 49 | 1.05 (-2.15–5.82) | |||
PGDM, pregestational diabetes; Body Mass Index, BMI; BMI categories were defined as: normal weight (18.5–25), overweight (25–30), obese (≥30); Gestational Weight Gain, GWG; GWG categories were defined as: insufficient, appropriate, excessive
* Mean birthweight z-score difference between categories tested by univariate linear regression (one-way ANOVA)
At inclusion median HbA1C was 6.70% (50 mmol/L) (range 4.90–12% (30–108 mmol/L)) and median duration of diabetes 17 years (range 1–37 years). The mean difference between measured weight at inclusion (at median gestational age 9.4 weeks) and the self-reported pre-pregnancy weight in the study population was 2.0 kg. There was no difference in HbA1C between the various BMI or gestational weight gain categories, p = 0.72 and p = 0.35 respectively. The gestational age at birth was lower in the study population than in the reference population, 37.8 weeks and 40.3, respectively [14].
Fetal venous flow and birthweight
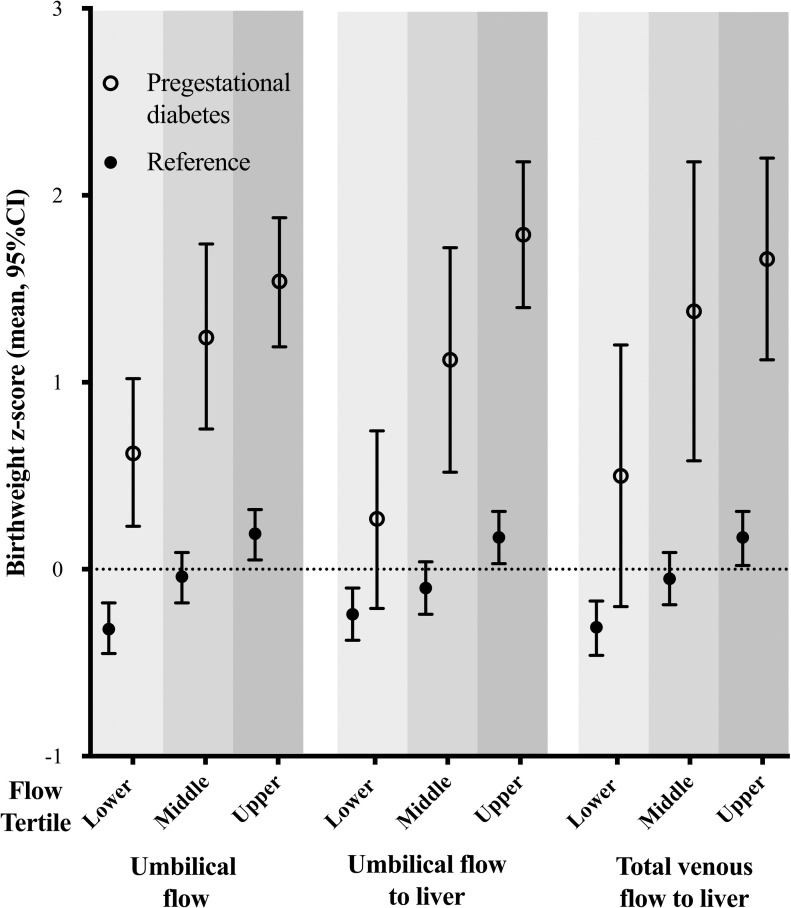
In both the reference and PGDM populations, fetal venous liver flow was positively related to birthweight, but the association to birthweight was more pronounced in pregnancies with PGDM (Fig 2 and Table 3).
Fig 2. Birthweight z-scores in fetal flow tertiles in the study population with pregestational diabetes mellitus (PGDM) and the reference group.
Flow variables were divided into tertiles defined by the distribution in the reference group.
Table 3. Birthweight z-scores according to fetal flow tertiles in the reference and the pregestational diabetes mellitus population (160 and 49 participants, respectively).
| Flow tertiles | Birthweight z-scores | p-value† | ||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Pregestational diabetes | |||||||
| N | mean | CI | n | mean | CI | |||
| Umbilical flow | lower | 191 | -0.32 | -0.45 –-0.18 | 66 | 0.62 | 0.23–1.02 | <0.0001 |
| middle | 192 | -0.04 | -0.18–0.09 | 41 | 1.24 | 0.75–1.74 | <0.0001 | |
| upper | 191 | 0.19 | 0.05–0.32 | 85 | 1.54 | 1.19–1.88 | <0.0001 | |
| p * | <0.001 | 0.003 | ||||||
| Umbilical flow to liver | lower | 185 | -0.24 | -0.38 –-0.10 | 40 | 0.27 | -0.21–0.74 | 0.007 |
| middle | 185 | -0.10 | -0.24–0.04 | 25 | 1.12 | 0.52–1.72 | <0.0001 | |
| upper | 185 | 0.17 | 0.03–0.31 | 58 | 1.79 | 1.40–2.18 | <0.0001 | |
| p * | <0.001 | <0.001 | ||||||
| Ductus venosus flow | lower | 181 | 0.18 | 0.04–0.32 | 62 | 1.28 | 0.86–1.70 | <0.0001 |
| middle | 181 | -0.12 | -0.26–0.23 | 25 | 0.85 | 0.19–1.51 | <0.0001 | |
| upper | 181 | -0.25 | -0.39 –-0.11 | 51 | 1.14 | 0.68–1.61 | <0.0001 | |
| p * | <0.001 | 0.548 | ||||||
| Ductus venosus fraction | lower | 178 | -0.07 | -0.22–0.07 | 62 | 1.47 | 1.06–1.88 | <0.0001 |
| middle | 178 | -0.01 | -0.15–0.14 | 28 | 1.05 | 0.44–1.66 | <0.0001 | |
| upper | 178 | -0.09 | -0.24–0.05 | 33 | 0.66 | 0.10–1.22 | 0.001 | |
| p * | 0.671 | 0.067 | ||||||
| Left portal vein blood velocity¥ | lower | 184 | -0.26 | -0.40 - -0.12 | 38 | 0.62 | 0.10–1.13 | <0.0001 |
| middle | 185 | 0.03 | -0.12–0.17 | 51 | 0.84 | 0.39–1.28 | <0.0001 | |
| upper | 184 | 0.07 | -0.07–0.22 | 113 | 1.44 | 1.14–1.74 | <0.0001 | |
| p * | 0.002 | 0.009 | ||||||
| Portal vein flow | lower | 186 | -0.41 | -0.55 - -0.27 | 35 | 1.45 | 0.89–2.00 | <0.0001 |
| middle | 186 | -0.01 | -0.14–0.14 | 19 | 0.77 | 0.02–1.53 | 0.003 | |
| upper | 186 | 0.20 | 0.07–0.34 | 40 | 1.26 | 0.74–1.78 | <0.0001 | |
| p * | <0.001 | 0.364 | ||||||
| Portal vein fraction | lower | 174 | -0.12 | -0.26–0.03 | 34 | 1.74 | 1.17–2.31 | <0.0001 |
| middle | 173 | -0.05 | -0.20–0.10 | 9 | 0.73 | -0.38–1.83 | 0.021 | |
| upper | 173 | -0.05 | -0.20–0.10 | 33 | 0.91 | 0.33–1.49 | <0.0001 | |
| p * | 0.761 | 0.085 | ||||||
| Total venous flow to liver | lower | 175 | -0.31 | -0.46 - -0.17 | 22 | 0.499 | -0.20–1.20 | 0.001 |
| middle | 175 | -0.05 | -0.19–0.09 | 17 | 1.380 | 0.58–2.18 | <0.0001 | |
| upper | 175 | 0.17 | 0.02–0.31 | 37 | 1.656 | 1.12–2.20 | <0.0001 | |
| p * | <0.001 | 0.037 | ||||||
Flow variables were divided into tertiles defined by the distribution in the reference population (upper, middle, lower), n; total number of observations
*Birthweight z-score difference between fetal blood flow tertiles tested by ANOVA within each population (table read vertically)
† Birthweight z-score difference between the reference and study populations in flow tertiles tested by independent sample T-test (table read horizontally); CI, confidence interval; Flow, volume blood flow (mL/min); z-score, standard deviation score
¥ Flow velocity, time-averaged maximum blood velocity (cm/sec).
BMI, gestational weight gain and birthweight
In women with PGDM, overweight and excessive weight gain were associated with higher birthweight, which was not evident in the reference population (Table 4). In the PGDM population, 39% of the neonates had developed macrosomia (birthweight >90th percentile), and 8% were small for gestational age (<10th percentile), compared with 7 and 14%, respectively, in the reference population [23].
Table 4. Distribution of BMI categories, gestational weight gain categories, and birthweight z-scores in the reference and the pregestational diabetes mellitus population (160 and 49 participants, respectively).
| Reference Median (range) | PGDM Median (range) | ||||||
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 23.0 (17.0–41.0) | 25.4 (19.8–44.1) | |||||
| GWG/week (kg/week) | 0.37 (0.01–0.73) | 0.46 (-0.14–0.95) | |||||
| Category | n | % | Mean BW z-score | n | % | Mean BW z-score | |
| BMI | Underweight | 7 | 4.4 | -0.15 | 2 | 4.1 | 1.47 |
| Normal weight | 101 | 63.1 | -0.11 | 22 | 44.9 | 0.62 | |
| Overweight | 43 | 26.9 | 0.17 | 14 | 28.6 | 2.02 | |
| Obese | 9 | 5.6 | -0.52 | 11 | 22.4 | 0.59 | |
| p * | 0.224 | 0.001* | |||||
| GWG | Insufficient | 47 | 29.4 | -0.16 | 6 | 12.2 | 0.31 |
| Appropriate | 61 | 38.1 | -0.08 | 16 | 32.7 | 0.60 | |
| Excessive | 47 | 29.4 | 0.10 | 27 | 55.1 | 1.48 | |
| p * | 0.556 | 0.008* | |||||
| Total group | 160 | -0.06 (-3.02–1.81) | 49 | 1.05 (-2.15–5.82) | |||
PGDM, pregestational diabetes; BMI, body mass index (kg/m2); BMI categories defined by Institute Of Medicine guidelines: BMI; underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9), obese (≥30); BW, birthweight; GWG, weekly gestational weight gain; GWG category defined by Institute Of Medicine: insufficient, appropriate, excessive; z-score, standard deviation score
* p<0.05, difference between BMI and GWG categories within the reference and the PGDM populations tested by ANOVA.
In PGDM, the relation between BMI and birthweight had an inverted U-shape, with the highest mean birthweight z-score in the overweight group (Fig 3). Within the PGDM population, neonates of obese women weighed less than those in the overweight group. Still, these neonates had a larger birthweight z-score than the obese of the reference group (mean z-scores difference 1.11, p = 0.045) (Table 1).
Fig 3. Relation between body mass index and gestational weight gain and birthweight z-score in the reference and pregestational diabetes populations (160 and 49 participants, respectively).
In the PGDM population, there was a positive linear relation between weekly gestational weight gain and z-scores of birthweights (Fig 3). In contrast, no such relation was found in the reference population (Table 4).
BMI, gestational weight gain and fetal venous liver flow
In the study population, pre-pregnancy BMI and gestational weight gain substantially modified fetal venous liver flow, compared with what was seen in the low-risk reference population (Fig 4, and Tables 5 and 6).
Fig 4. Development of umbilical flow to the fetal liver and its association with BMI or gestational weight gain in pregnancies with pregestational diabetes mellitus (n = 49) compared with that of the reference pregnancies (n = 160).
Table 5. Fetal venous liver flow according to pre-pregnancy BMI categories in the reference and pregestational diabetes mellitus populations (160 and 49 participants, respectively).
| Flow z-score | |||||||
|---|---|---|---|---|---|---|---|
| BMI category | Reference population | Pregestational diabetes population | |||||
| n | mean | CI | n | mean | CI | ||
| Umbilical flow | normal | 363 | 0.016 | -0.09–0.12 | 91 | 0.228 | -0.09–0.55 |
| overweight | 155 | -0.121 | -0.28–0.04 | 58 | 0.703 | 0.30–1.11 | |
| obese | 30 | 0.327 | -0.04–0.69 | 43 | 0.206 | -0.26–0.67 | |
| p | 0.130 | 0.144 | |||||
| Umbilical flow to liver | normal | 353 | 0.028 | -0.08–0.13 | 59 | 0.101 | -0.31–0.52 |
| overweight | 150 | -0.143 | -0.30–0.02 | 40 | 0.906 | 0.40–1.41 | |
| obese | 26 | 0.218 | -0.17–0.61 | 24 | 0.063 | -0.59–0.71 | |
| p | 0.190 | 0.033* | |||||
| Ductus venosus flow | normal | 344 | 0.132 | -0.26–0.52 | 70 | -0.145 | -0.59–0.30 |
| overweight | 147 | 0.092 | -0.01–0.20 | 42 | -0.234 | -0.81–0.34 | |
| obese | 27 | -0.171 | -0.33 - -0.01 | 26 | -0.870 | -1.60 - -0.14 | |
| p | 0.005* | 0.237 | |||||
| Ductus venosus flow fraction | normal | 340 | -0.072 | -0.52–0.28 | 59 | -0.158 | -0.56–0.24 |
| overweight | 143 | 0.207 | -0.18–0.04 | 40 | -0.759 | -1.25 - -0.27 | |
| obese | 26 | 0.088 | -0.30–0.48 | 24 | -0.550 | -1.18–0.08 | |
| p | 0.042* | 0.160 | |||||
| Left portal vein flow velocity | normal | 349 | -0.178 | -0.57–0.21 | 100 | 0.591 | 0.35–0.84 |
| overweight | 149 | 0.002 | -0.10–0.11 | 54 | 0.844 | 0.51–1.18 | |
| obese | 30 | 0.037 | -0.12–0.20 | 48 | 0.507 | 0.15–0.86 | |
| p | 0.801 | 0.343 | |||||
| Portal vein flow | normal | 354 | -0.014 | -0.12–0.09 | 51 | 0.315 | -0.25–0.88 |
| overweight | 149 | 0.062 | -0.10–0.22 | 30 | .0385 | -0.35–1.12 | |
| obese | 30 | 0.195 | -0.17–0.56 | 13 | -0.157 | -1.27–0.96 | |
| p | 0.660 | 0.706 | |||||
| Portal vein fraction | normal | 332 | -0.043 | -0.15–0.07 | 42 | 0.066 | -0.80–0.61 |
| overweight | 140 | 0.163 | -0.01–0.33 | 25 | -0.446 | -1.15–0.26 | |
| obese | 26 | -0.090 | -0.48–0.30 | 9 | 0.102 | -1.08–1.28 | |
| p | 0.159 | 0.491 | |||||
| Total venous flow to liver | normal | 333 | 0.012 | -0.10–0.12 | 42 | 0.304 | -0.18–0.78 |
| overweight | 142 | -0.115 | -0.28–0.05 | 25 | 1.087 | 0.47–1.71 | |
| obese | 26 | 0.238 | -0.15–0.63 | 9 | -0.160 | -1.19–0.88 | |
| P | 0.213 | 0.061 | |||||
n, total number of observations in reference (n = 160) and study population (49)
* p-value <0.05, Fetal flow z-score according to body mass index (BMI) categories within each population tested by ANOVA; n, number of observations; Flow (mL/min); Flow velocity, time-averaged maximum velocity (cm/sec); BMI categorized as: normal (18.5–24.9), overweight (25–29.9) or obese (≥30) (underweight BMI category was excluded).
UV flow to liver = UV flow- DV flow.
Total venous flow to liver = UV flow to liver + PV flow.
Ductus venosus flow fraction = DV flow/UV flow*100.
Portal vein fraction = PV flow/ Total venous liver flow*100.
Table 6. Fetal venous liver flow according to gestational weight gain categories in the reference and pregestational diabetes mellitus populations (160 and 49 participants, respectively).
| Flow z-score | |||||||
|---|---|---|---|---|---|---|---|
| GWG category | Reference population | Pregestational diabetes population | |||||
| n | Mean | CI | n | Mean | CI | ||
| Umbilical flow | insufficient | 172 | -0.078 | -0.23–0.08 | 19 | -0.613 | -1.31–0.08 |
| appropriate | 218 | 0.071 | -0.07–0.21 | 63 | 0.440 | 0.06–0.82 | |
| excessive | 164 | 0.017 | -0.14–0.08 | 110 | 0.494 | 0.21–0.78 | |
| p | 0.364 | 0.015 | |||||
| Umbilical flow to liver | insufficient | 171 | -0.068 | -0.22–0.09 | 14 | -1.087 | -1.91–0.26 |
| appropriate | 209 | 0.075 | -0.06–0.21 | 40 | 0.425 | -0.06–0.92 | |
| excessive | 157 | -0.025 | -0.18–0.13 | 69 | 0.608 | 0.24–0.98 | |
| p | 0.367 | 0.001 | |||||
| Ductus venosus flow | insufficient | 172 | 0.071 | -0.08–0.22 | 17 | -0.437 | -1.34–0.47 |
| appropriate | 205 | 0.031 | -0.11–0.17 | 45 | -0.556 | -1.11–0.01 | |
| excessive | 151 | -0.114 | -0.28–0.47 | 76 | -0.133 | -0.56–0.30 | |
| p | 0.229 | 0.474 | |||||
| Ductus venosus fraction | insufficient | 171 | -0.009 | -0.16–0.14 | 14 | 0.272 | -0.55–1.10 |
| appropriate | 200 | 0.061 | -0.20–0.08 | 40 | -0.583 | -1.07 - -0.09 | |
| excessive | 148 | 0.091 | -0.07–0.26 | 69 | -0.484 | -0.86 - -0.11 | |
| p | 0.381 | 0.196 | |||||
| Left portal vein flow velocity | insufficient | 170 | -0.083 | -0.24–0.07 | 24 | 0.683 | 0.19–1.18 |
| appropriate | 210 | 0.053 | -0.08–0.19 | 66 | 0.287 | -0.01–0.59 | |
| excessive | 153 | 0.057 | -0.10–0.22 | 112 | 0.836 | 0.61–1.07 | |
| p | 0.340 | 0.017 | |||||
| Portal vein flow | insufficient | 173 | -0.110 | -0.26–0.04 | 12 | 0.030 | -1.13–1.12 |
| appropriate | 208 | 0.050 | -0.09–0.19 | 31 | 0.587 | -0.13–1.31 | |
| excessive | 158 | 0.132 | -0.02–0.29 | 51 | 0.138 | -0.42–0.70 | |
| p | 0.077 | 0.564 | |||||
| Portal vein fraction | insufficient | 170 | -0.037 | -0.19–0.12 | 10 | 1.002 | -0.08–2.01 |
| appropriate | 193 | -0.046 | -0.19–0.10 | 24 | 0.083 | -0.62–0.78 | |
| excessive | 144 | 0.111 | -0.06–0.28 | 42 | -0.463 | -0.99–0.07 | |
| p | 0.315 | 0.050 | |||||
| Total venous flow to liver | insufficient | 171 | -0.066 | -0.22–0.09 | 10 | -0.887 | -1.84–0.07 |
| appropriate | 193 | 0.053 | -0.09–0.20 | 24 | 0.917 | 0.30–1.53 | |
| excessive | 146 | 0.012 | -0.15–0.18 | 42 | 0.604 | 0.14–1.07 | |
| p | 0.536 | 0.008 | |||||
Fetal flow z-scores according to weekly gestational weight gain (GWG) categories within each population tested by ANOVA; n, total number of observations; Flow (mL/min); Flow velocity, time-averaged maximum velocity (cm/sec); Gestational weight gain (GWG) categories defined by the institute of medicine: insufficient, appropriate or excessive.
UV flow to liver = UV flow- DV flow.
Total venous flow to liver = UV flow to liver + PV flow.
Ductus venosus flow fraction = DV flow/UV flow*100.
Portal vein fraction = PV flow/ Total venous liver flow*100.
In the study population, the overweight group had the highest umbilical flow to liver, left portal vein blood velocity, and thus the highest total venous flow to liver, but the lowest relative portal contribution (Fig 4 and Table 5).
Further, in the study population, gestational weight gain was significantly associated with fetal venous flow. Women with excessive gestational weight gain had the highest umbilical flow, umbilical flow to liver, and left portal vein velocity, while the total venous flow to liver was highest in the appropriate weight gain group (Fig 4 and Table 6). Those with appropriate and excessive gestational weight gain had the highest umbilical flow to liver (Fig 4).
Discussion
We found that in PGDM pregnancies, high birthweight was related to increased umbilical flow to the fetal liver. For similar volumes of umbilical flow to the liver, the association of flow with birthweight was stronger in PGDM pregnancies compared with the reference. Interestingly, with increasing BMI and gestational weight gain the umbilical flow to the liver increased, but at extreme BMI, (obesity), this relation seemed to break down as both flows (Fig 4) and birthweights were lower (Fig 3).
The results are in line with experimental studies showing that increased umbilical flow to the fetal liver, leads to increased insulin-like growth factor 1 and 2 production and a correspondingly augmented somatic growth of the fetus [10,15]. This concept is supported by human studies showing that the fetal liver, with its umbilical venous supply, plays a key role in fetal growth regulation and fat deposition, even in accelerated fetal growth of non-diabetic mothers [11,15,16]. In our study of PGDM pregnancies, these mechanisms were augmented and powerfully modified by maternal BMI and gestational weight gain.
The present findings are also in agreement with the previously reported synergism between high BMI, excessive gestational weight gain and PGDM leading to increased risk of large for gestational age offspring [7,26]; here we have added to the understanding of the pathophysiology that these mechanisms seem, to a large extent to operate through the fetal venous liver circulation. Furthermore, the impact of gestational weight gain on birthweight is independent of glycemic control and BMI in women with PGDM [8,27]. This is in line with our study, where glycemic control (HbA1C) did not differ between the BMI or gestational weight gain categories. Rather, it seemed to be through augmentation of umbilical flow to the liver that BMI and weight gain affected birthweight (Figs 2 and 4).
The level of glucose exposure influences fetal growth, via modulation of blood flow to the fetal liver [28]. In low-risk pregnancies, a maternal oral glucose load increased umbilical and venous liver flows and the response was associated with large fetal abdominal circumference [29]. The maternal metabolic status seems to influence the fetal response to a maternal meal: in a healthy population, increased umbilical flow to liver was observed in normal weight, but not in overweight mothers [17]. Further, the maternal-fetal glucose gradient correlated negatively with umbilical flow to liver in pregnancies of normal weight, but not overweight women [18]. Inadequate glycemic control is more frequent in patients with type 1 diabetes with high BMI [30]. Although HbA1C was not higher in those with overweight or excessive weight gain in our study population, episodes of hyperglycemia are more frequent in these groups [31] and this could be related to the observed increased umbilical venous flow and higher birthweights [29,32]. Further, defect epinephrine counter-regulation during hypoglycemia in PGDM pregnancies contributes to excessive fetal growth [33], probably through compensatory bouts of calorie intake with subsequent fetal hyperinsulinemia.
In women with PGDM, gestational weight gain contributes to excessive fetal growth, independent of maternal BMI and glycemic control [8,34]. The mechanisms are not completely understood, but additional nutrients delivery (fatty acids and amino acids) and altered leptine levels are suggested to contribute to accelerated fetal growth [35,36]. In addition to nutritional and hormonal influence, the present study suggests fetal blood flow as a possible link between maternal GWG and increased birth weight: Our PGDM population with excessive weight gain had higher umbilical flow to the liver and also higher birthweights (Figs 2 and 4). In PGDM pregnancies the augmented venous liver flow in the fetus seems to enhance fetal growth and fat deposition, possibly as a combined effect of increased flow and increased glucose and lipid content [15,37].
The association between low umbilical flow and growth perturbation is well documented [38]. In our study, obesity was not associated with augmented fetal growth, in contrast to the fetuses of overweight women (Table 4 and Fig 3). Although lower birthweights in the obese women could seem advantageous (since several perinatal risks in PGDM pregnancies are associated with macrosomia [2]), we believe that lower birthweights in those with PGDM and obesity more likely reflect relative placental insufficiency added to the adverse effects of fetal hyperglycemia. The finding also corroborates the disadvantage of inflammation commonly shown in obesity and linked to placental changes with adverse outcome [39]. A clinical message emanates from these results; absence of macrosomia in PGDM pregnancies of obese women, does not exclude perinatal risks but calls for continued attentiveness [39].
In low-risk populations, low maternal BMI, low weight gain and low maternal skinfold thickness were associated with a compensating increase in umbilical flow to liver near term [14,16,40]. Such prioritization, in situations of restricted maternal nutritional supply, is thought to be a protective mechanism to enhance the offspring fat accretion [16,37]. In PGDM pregnancies however, such increase in umbilical flow to liver in combination with the hyperglycemic in-utero-metabolic environment, augments the fetal fat deposition [16].
The risks of metabolic syndrome, obesity and diabetes in individuals born from PGDM pregnancies, are not explained by genetic dispositions alone [41–43]. Important additional determinants are found in the in-utero metabolic programming that conditions health risks in postnatal life, increasingly supported by emerging epigenetic studies in the offspring of women with diabetes in pregnancy [44,45]. In this scenario, the fetal liver circulation stands out as an example of adaptive mechanisms in the interphase between umbilical blood flow and endocrine liver function, and metabolism sensitive to environmental cues, with possible consequences for child development and future health [32,46,47].
The strengths of this study are the unselected populations of low-risk (reference) and PGDM pregnancies [14], the identical and validated ultrasound and Doppler methods applied to both populations and the prospective longitudinal design.
Self-reported pre-pregnancy weight could introduce a recall bias but is widely used in research and allows comparison with other studies [8,48]. We consider the difference between the self-reported and measured weights at inclusion in the PGDM population (about 2 kilograms) to be plausible [49,50]. High BMI in our PGDM population hampered the ultrasound examination and reduced the success rate for the fetal flow measurements. A higher success rate in the leaner PGDM women may have skewed the study population towards normality, but this selection would reduce rather than increase the observed differences between the study- and reference populations. There were no differences in HbA1C between the group with missing and complete data, which makes selection bias by glycemic control less likely. In large population-based studies, pre-pregnancy BMI and gestational weight gain are associated with the risk of large for gestational age infants [51]. The absence of this association in our reference population might be due to the fact that the size of the association is too small for this sample size, or selection bias as the inclusions were healthy women, not random selection of the general population (Table 4). A possible limitation is that the study of the reference population was carried out almost ten years prior to the present study. Seven women in the study population used anti-hypertensive drugs which may influence maternal and feto-placental hemodynamics [52,53]. We considered the size of the study population too small for subgroup analyses of maternal ethnicity, the use of antihypertensive drugs or sex of the neonate.
In summary, increased umbilical flow to liver seems to be in the causal pathway to larger birthweights in PGDM pregnancies, and maternal overweight and excessive gestational weight gain augment this association. In obese women with PGDM however, birthweights in the normal range do not exclude perinatal risks as they are probably due to relatively blunted placental and metabolic resources.
Acknowledgments
We acknowledge bioengineer Carol Cook for her practical assistance in the study. We thank the women that participated in the study. The department of Obstetrics and Gynecology, Haukeland University Hospital provided facilities and equipment to conduct this research.
Abbreviations
- BMI
Body Mass Index
- GWG
Gestational Weight Gain
- IOM
Institute Of Medicine
- PGDM
Pre-gestational Diabetes Mellitus
Data Availability
The combination of detailed clinical information in our study could enable identification of specific participants. Therefore, data sharing must be approved by our ethics committee, even if the data are de-identified. The Regional Committee for Medical and Health Research Ethics (REK Vest) can be contacted referring to the number REK vest 2011/2030; post@helseforskning.etikkom.no. The rules and procedures can be found here: https://helseforskning.etikkom.no/reglerogrutiner/loverogregler?p_dim=34770&_ikbLanguageCode=us.
Funding Statement
This research was financed by the Western Norway Regional Health Authority, Helse Vest, project number 911765, https://helse-vest.no/vart-oppdrag/vare-hovudoppgaver/forsking/forskingsmidlar. This funded the PhD work for A.L., main author of the submitted paper. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: A large, population-based study. Diabetes Care. 2009;32(11):2005–9. doi: 10.2337/dc09-0656 ; PubMed Central PMCID: PMC2768194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Persson M, Pasupathy D, Hanson U, Norman M. Disproportionate body composition and perinatal outcome in large-for-gestational-age infants to mothers with type 1 diabetes. BJOG: an international journal of obstetrics and gynaecology. 2012;119(5):565–72. doi: 10.1111/j.1471-0528.2012.03277.x . [DOI] [PubMed] [Google Scholar]
- 3.Murphy HR, Howgate C, O’Keefe J, Myers J, Morgan M, Coleman MA, et al. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. The lancet Diabetes & endocrinology. 2021;9(3):153–64. Epub 2021/02/01. doi: 10.1016/S2213-8587(20)30406-X . [DOI] [PubMed] [Google Scholar]
- 4.Pedersen J. The Pregnant Diabetic and Her Newborn; Problems and Management. 1967 ed. Copenhagen: Munksgaard; 1967. [Google Scholar]
- 5.Feig DS, Donovan LE, Corcoy R, Murphy KE, Amiel SA, Hunt KF, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. The Lancet. doi: 10.1016/S0140-6736(17)32400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evers IM, de Valk HW, Mol BW, ter Braak EW, Visser GH. Macrosomia despite good glycaemic control in Type I diabetic pregnancy; results of a nationwide study in The Netherlands. Diabetologia. 2002;45(11):1484–9. doi: 10.1007/s00125-002-0958-7 . [DOI] [PubMed] [Google Scholar]
- 7.Persson M, Pasupathy D, Hanson U, Westgren M, Norman M. Pre-pregnancy body mass index and the risk of adverse outcome in type 1 diabetic pregnancies: a population-based cohort study. BMJ open. 2012;2(1):e000601. doi: 10.1136/bmjopen-2011-000601; PubMed Central PMCID: PMC3282288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Secher AL, Parellada CB, Ringholm L, Asbjornsdottir B, Damm P, Mathiesen ER. Higher gestational weight gain is associated with increasing offspring birth weight independent of maternal glycemic control in women with type 1 diabetes. Diabetes Care. 2014;37(10):2677–84. doi: 10.2337/dc14-0896 . [DOI] [PubMed] [Google Scholar]
- 9.Kim SY, Sharma AJ, Sappenfield W, Salihu HM. Preventing large birth size in women with preexisting diabetes mellitus: The benefit of appropriate gestational weight gain. Preventive medicine. 2016;91:164–8. doi: 10.1016/j.ypmed.2016.08.026 ; PubMed Central PMCID: PMC5050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tchirikov M, Kertschanska S, Sturenberg HJ, Schroder HJ. Liver blood perfusion as a possible instrument for fetal growth regulation. Placenta. 2002;23Suppl A:S153–8. doi: 10.1053/plac.2002.0810 . [DOI] [PubMed] [Google Scholar]
- 11.Kessler J, Rasmussen S, Godfrey K, Hanson M, Kiserud T. Venous liver blood flow and regulation of human fetal growth: evidence from macrosomic fetuses. Am J Obstet Gynecol. 2011;204(5):429 e1–7. doi: 10.1016/j.ajog.2010.12.038 . [DOI] [PubMed] [Google Scholar]
- 12.Kiserud T, Rasmussen S, Skulstad S. Blood flow and the degree of shunting through the ductus venosus in the human fetus. Am J Obstet Gynecol. 2000;182(1 Pt 1):147–53. doi: 10.1016/s0002-9378(00)70504-7 . [DOI] [PubMed] [Google Scholar]
- 13.Haugen G, Kiserud T, Godfrey K, Crozier S, Hanson M. Portal and umbilical venous blood supply to the liver in the human fetus near term. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2004;24(6):599–605. Epub 2004/11/02. doi: 10.1002/uog.1744 . [DOI] [PubMed] [Google Scholar]
- 14.Kessler J, Rasmussen S, Godfrey K, Hanson M, Kiserud T. Longitudinal study of umbilical and portal venous blood flow to the fetal liver: low pregnancy weight gain is associated with preferential supply to the fetal left liver lobe. Pediatr Res. 2008;63(3):315–20. doi: 10.1203/pdr.0b013e318163a1de . [DOI] [PubMed] [Google Scholar]
- 15.Tchirikov M, Kertschanska S, Schroder HJ. Obstruction of ductus venosus stimulates cell proliferation in organs of fetal sheep. Placenta. 2001;22(1):24–31. doi: 10.1053/plac.2000.0585 . [DOI] [PubMed] [Google Scholar]
- 16.Ikenoue S, Waffarn F, Ohashi M, Sumiyoshi K, Ikenoue C, Buss C, et al. Prospective Association of Fetal Liver Blood Flow at 30 Weeks Gestation with Newborn Adiposity. Am J Obstet Gynecol. 2017. doi: 10.1016/j.ajog.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opheim GL, Henriksen T, Haugen G. The effect of a maternal meal on fetal liver blood flow. PloS one. 2019;14(6):e0216176. Epub 2019/06/13. doi: 10.1371/journal.pone.0216176; PubMed Central PMCID: PMC6561550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opheim GL, Moe Holme A, Blomhoff Holm M, Melbye Michelsen T, Muneer Zahid S, Paasche Roland MC, et al. The impact of umbilical vein blood flow and glucose concentration on blood flow distribution to the fetal liver and systemic organs in healthy pregnancies. FASEB J. 2020;34(9):12481–91. Epub 2020/07/31. doi: 10.1096/fj.202000766R . [DOI] [PubMed] [Google Scholar]
- 19.Lund A, Ebbing C, Rasmussen S, Kiserud T, Kessler J. Maternal diabetes alters the development of ductus venosus shunting in the fetus. Acta Obstet Gynecol Scand. 2018. Epub 2018/05/13. doi: 10.1111/aogs.13363. [DOI] [PubMed] [Google Scholar]
- 20.Lund A, Ebbing C, Rasmussen S, Kiserud T, Hanson M, Kessler J. Altered development of fetal liver perfusion in pregnancies with pregestational diabetes. PloS one. 2019;14(3):e0211788. Epub 2019/03/14. doi: 10.1371/journal.pone.0211788; PubMed Central PMCID: PMC6415794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmussen KM, Yaktine AL. Weight Gain During Pregnancy, Reexamining the Guidelines. Washington DC, USA: National Academic Press; 2009. [PubMed] [Google Scholar]
- 22.Robinson HP. Sonar measurement of fetal crown-rump length as means of assessing maturity in first trimester of pregnancy. British medical journal. 1973;4(5883):28–31. doi: 10.1136/bmj.4.5883.28 ; PubMed Central PMCID: PMC1587065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnsen SL, Rasmussen S, Wilsgaard T, Sollien R, Kiserud T. Longitudinal reference ranges for estimated fetal weight. Acta Obstet Gynecol Scand. 2006;85(3):286–97. doi: 10.1080/00016340600569133 . [DOI] [PubMed] [Google Scholar]
- 24.Kessler J, Rasmussen S, Kiserud T. The fetal portal vein: normal blood flow development during the second half of human pregnancy. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2007;30(1):52–60. doi: 10.1002/uog.4054 . [DOI] [PubMed] [Google Scholar]
- 25.Kessler J, Rasmussen S, Kiserud T. The left portal vein as an indicator of watershed in the fetal circulation: development during the second half of pregnancy and a suggested method of evaluation. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2007;30(5):757–64. doi: 10.1002/uog.5146 . [DOI] [PubMed] [Google Scholar]
- 26.Ray JG, Vermeulen MJ, Shapiro JL, Kenshole AB. Maternal and neonatal outcomes in pregestational and gestational diabetes mellitus, and the influence of maternal obesity and weight gain: the DEPOSIT study. Diabetes Endocrine Pregnancy Outcome Study in Toronto. QJM: monthly journal of the Association of Physicians. 2001;94(7):347–56. doi: 10.1093/qjmed/94.7.347 . [DOI] [PubMed] [Google Scholar]
- 27.Parellada CB, Asbjornsdottir B, Ringholm L, Damm P, Mathiesen ER. Fetal growth in relation to gestational weight gain in women with type 2 diabetes: an observational study. Diabetic medicine: a journal of the British Diabetic Association. 2014;31(12):1681–9. doi: 10.1111/dme.12558 ; PubMed Central PMCID: PMC4257095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerssen A, de Valk HW, Visser GH. Increased second trimester maternal glucose levels are related to extremely large-for-gestational-age infants in women with type 1 diabetes. Diabetes Care. 2007;30(5):1069–74. Epub 2007/05/01. doi: 10.2337/dc05-1985 . [DOI] [PubMed] [Google Scholar]
- 29.Haugen G, Bollerslev J, Henriksen T. Human fetoplacental and fetal liver blood flow after maternal glucose loading: a cross-sectional observational study. Acta Obstet Gynecol Scand. 2014;93(8):778–85. doi: 10.1111/aogs.12419 . [DOI] [PubMed] [Google Scholar]
- 30.Bae JP, Lage MJ, Mo D, Nelson DR, Hoogwerf BJ. Obesity and glycemic control in patients with diabetes mellitus: Analysis of physician electronic health records in the US from 2009–2011. Journal of diabetes and its complications. 2016;30(2):212–20. doi: 10.1016/j.jdiacomp.2015.11.016 . [DOI] [PubMed] [Google Scholar]
- 31.Scott EM, Feig DS, Murphy HR, Law GR, Group CC. Continuous Glucose Monitoring in Pregnancy: Importance of Analyzing Temporal Profiles to Understand Clinical Outcomes. Diabetes Care. 2020;43(6):1178–84. Epub 2020/03/27. doi: 10.2337/dc19-2527 ; PubMed Central PMCID: PMC7245356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy HR, Rayman G, Duffield K, Lewis KS, Kelly S, Johal B, et al. Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. Diabetes care. 2007;30(11):2785–91. Epub 2007/08/02. doi: 10.2337/dc07-0500 . [DOI] [PubMed] [Google Scholar]
- 33.Rosenn BM, Miodovnik M, Khoury JC, Siddiqi TA. Counterregulatory hormonal responses to hypoglycemia during pregnancy. Obstet Gynecol. 1996;87(4):568–74. Epub 1996/04/01. doi: 10.1016/0029-7844(95)00495-5 . [DOI] [PubMed] [Google Scholar]
- 34.Scifres CM, Feghali MN, Althouse AD, Caritis SN, Catov JM. Effect of excess gestational weight gain on pregnancy outcomes in women with type 1 diabetes. Obstet Gynecol. 2014;123(6):1295–302. doi: 10.1097/AOG.0000000000000271 . [DOI] [PubMed] [Google Scholar]
- 35.McGrath RT, Glastras SJ, Hocking SL, Fulcher GR. Large-for-Gestational-Age Neonates in Type 1 Diabetes and Pregnancy: Contribution of Factors Beyond Hyperglycemia. Diabetes Care. 2018;41(8):1821–8. Epub 2018/07/22. doi: 10.2337/dc18-0551 . [DOI] [PubMed] [Google Scholar]
- 36.Szabo AJ. Transferred maternal fatty acids stimulate fetal adipogenesis and lead to neonatal and adult obesity. Med Hypotheses. 2019;122:82–8. Epub 2018/12/30. doi: 10.1016/j.mehy.2018.10.022 . [DOI] [PubMed] [Google Scholar]
- 37.Godfrey KM, Haugen G, Kiserud T, Inskip HM, Cooper C, Harvey NC, et al. Fetal liver blood flow distribution: role in human developmental strategy to prioritize fat deposition versus brain development. PloS one. 2012;7(8):e41759. doi: 10.1371/journal.pone.0041759; PubMed Central PMCID: PMC3425554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiserud T, Ebbing C, Kessler J, Rasmussen S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2006;28(2):126–36. doi: 10.1002/uog.2832 . [DOI] [PubMed] [Google Scholar]
- 39.Desoye G. The Human Placenta in Diabetes and Obesity: Friend or Foe? The 2017 Norbert Freinkel Award Lecture. Diabetes care. 2018;41(7):1362–9. doi: 10.2337/dci17-0045 . [DOI] [PubMed] [Google Scholar]
- 40.Haugen G, Hanson M, Kiserud T, Crozier S, Inskip H, Godfrey KM. Fetal liver-sparing cardiovascular adaptations linked to mother’s slimness and diet. Circulation research. 2005;96(1):12–4. doi: 10.1161/01.RES.0000152391.45273.A2 . [DOI] [PubMed] [Google Scholar]
- 41.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340–6. doi: 10.2337/dc07-1596 . [DOI] [PubMed] [Google Scholar]
- 42.Hammoud NM, de Valk HW, van Rossem L, Biesma DH, Wit JM, Visser GH. Growth and BMI during the first 14 y of life in offspring from women with type 1 or type 2 diabetes mellitus. Pediatr Res. 2017;81(2):342–8. doi: 10.1038/pr.2016.236 . [DOI] [PubMed] [Google Scholar]
- 43.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49(12):2208–11. doi: 10.2337/diabetes.49.12.2208 . [DOI] [PubMed] [Google Scholar]
- 44.El Hajj N, Schneider E, Lehnen H, Haaf T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction. 2014;148(6):R111–20. doi: 10.1530/REP-14-0334 ; PubMed Central PMCID: PMC4241689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houshmand-Oeregaard A, Schrolkamp M, Kelstrup L, Hansen NS, Hjort L, Thuesen ACB, et al. Increased expression of microRNA-15a and microRNA-15b in skeletal muscle from adult offspring of women with diabetes in pregnancy. Human molecular genetics. 2018. doi: 10.1093/hmg/ddy085. [DOI] [PubMed] [Google Scholar]
- 46.Thorn SR, Baquero KC, Newsom SA, El Kasmi KC, Bergman BC, Shulman GI, et al. Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates. Diabetes. 2014;63(8):2702–13. doi: 10.2337/db14-0276 ; PubMed Central PMCID: PMC4113070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulay S, Philip A, Solomon S. Influence of maternal diabetes on fetal rat development: alteration of insulin receptors in fetal liver and lung. The Journal of endocrinology. 1983;98(3):401–10. doi: 10.1677/joe.0.0980401 . [DOI] [PubMed] [Google Scholar]
- 48.Egan AM, Dennedy MC, Al-Ramli W, Heerey A, Avalos G, Dunne F. ATLANTIC-DIP: excessive gestational weight gain and pregnancy outcomes in women with gestational or pregestational diabetes mellitus. The Journal of clinical endocrinology and metabolism. 2014;99(1):212–9. doi: 10.1210/jc.2013-2684 . [DOI] [PubMed] [Google Scholar]
- 49.Seijo M, Minckas N, Cormick G, Comande D, Ciapponi A, BelizAn JM. Comparison of self-reported and directly measured weight and height among women of reproductive age: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97(4):429–39. doi: 10.1111/aogs.13326 . [DOI] [PubMed] [Google Scholar]
- 50.Inskip H, Crozier S, Baird J, Hammond J, Robinson S, Cooper C, et al. Measured weight in early pregnancy is a valid method for estimating pre-pregnancy weight. J Dev Orig Health Dis. 2020:1–9. Epub 2020/10/14. doi: 10.1017/S2040174420000926 . [DOI] [PubMed] [Google Scholar]
- 51.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. Jama. 2017;317(21):2207–25. doi: 10.1001/jama.2017.3635 ; PubMed Central PMCID: PMC5815056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jouppila P, Kirkinen P, Koivula A, Ylikorkala O. Labetalol does not alter the placental and fetal blood flow or maternal prostanoids in pre-eclampsia. British journal of obstetrics and gynaecology. 1986;93(6):543–7. Epub 1986/06/01. doi: 10.1111/j.1471-0528.1986.tb07951.x . [DOI] [PubMed] [Google Scholar]
- 53.Erkinaro T, Kavasmaa T, Ylikauma L, Makikallio K, Haapsamo M, Acharya G, et al. Placental and fetal hemodynamics after labetalol or pindolol in a sheep model of increased placental vascular resistance and maternal hypertension. Reproductive sciences (Thousand Oaks, Calif). 2009;16(8):749–57. Epub 2009/04/22. doi: 10.1177/1933719109335068 . [DOI] [PubMed] [Google Scholar]