Abstract
Background:
Cervical cancer remains a major public health issue for the Uyghur women and other women living mainly in rural areas of Xinjiang. This study aims to investigate the distribution of human papillomavirus (HPV) infection and cervical cancer in rural areas of Xinjiang, China.
Methods:
Cervical cancer screening was performed on rural women aged 35 to 64 years from Xinjiang, China in 2017 through gynecological examination, vaginal discharge smear microscopy, cytology, and HPV testing. If necessary, colposcopy and biopsy were performed on women with suspicious or abnormal screening results.
Results:
Of the 216,754 women screened, 15,518 received HPV testing. The HPV-positive rate was 6.75% (1047/15,518). Compared with the age 35–44 years group, the odds ratios (ORs) of HPV positivity in the age 45–54 years and 55–64 years groups were 1.18 (95% confidence interval [CI]: 1.02–1.37) and 1.84 (95% CI: 1.53–2.21), respectively. Compared with women with primary or lower education level, the ORs for HPV infection rates of women with high school and college education or above were 1.37 (95% CI: 1.09–1.72) and 1.62 (95% CI: 1.23–2.12), respectively. Uyghur women were less likely to have HPV infection than Han women, with an OR (95% CI) of 0.78 (0.61–0.99). The most prevalent HPV types among Xinjiang women were HPV 16 (24.00%), HPV 33 (12.70%), and HPV 52 (11.80%). The detection rate of cervical intraepithelial neoplasia (CIN)2+ was 0.14% and the early diagnosis rate of cervical cancer was 85.91%. The detection rates of vaginitis and cervicitis were 19.28% and 21.32%, respectively.
Conclusions:
The HPV infection rate in Xinjiang is low, but the detection rate of cervical cancer and precancerous lesions is higher than the national average level. Cervical cancer is a prominent public health problem in Xinjiang, especially in southern Xinjiang.
Keywords: Cervical cancer, Cervical precancerous lesions, Genotype, Human papillomavirus
Introduction
Cervical cancer is the sixth most common female cancer in China and the eighth leading cause of death among women.[1] In 2015, the age-standardized incidence of cervical cancer was 11.78 per 100,000 women, and the age-standardized death was 3.29 per 100,000 women in China, accounting for 18.7% and 11.5% of all cervical cancer cases and deaths worldwide, respectively.[2] Cervical cancer is disproportionately prevalent in developing nations. The vast majority (around 85%) of the global cervical cancer burden lies in less developed regions. Factors such as inadequate and infrequent screening can lead to the detection of cancer at late stages. In addition, inadequate lifestyle conditions in developing regions, such as inaccessibility of physical contraceptives, and, poor living conditions and personal hygiene, are thought to exacerbate the burden of cervical cancer.[3] Epidemiological study shows that human papillomavirus (HPV) is the main cause of cervical cancer and precancerous lesions.[4] Persistent infection with high-risk HPV (HR-HPV) is related to the occurrence of cervical intraepithelial neoplasia (CIN) and cervical cancer.[4,5] HR-HPV genotypes include HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68, of which HPV 16 and 18 are the most common types in the world.[6,7] The World Health Organization recommends HPV DNA testing as a primary cervical cancer screening method in areas with adequate health resources,[8,9] because HPV infection predicts the risk of subsequent CIN.[10,11]
The Xinjiang Uyghur Autonomous Region, located in northwestern China, is a vast and remote area composing of a variety of ethnic communities such as Uyghur, Han, Kazakh, and other peoples. Factors, such as misunderstandings of the importance of HPV testing and prevention, and inadequate hygiene, as well as the sheer vastness of the region, hinder the development of effective diagnostic programs in Xinjiang. Therefore, cervical cancer remains a major public health issue for the Uyghur women and other women living mainly in rural areas of Xinjiang. However, there are currently limited data on HPV infection rates and cervical cancer burden in rural areas of Xinjiang.
Here, we examined the prevalence of HPV infection and the cervical cancer burden among women in Xinjiang based on data collected from national breast and cancer screening programs in rural areas. The participants were categorized by ethnicity, age, region of Xinjiang, and educational level. These data will provide epidemiological evidence to support the development of a comprehensive and appropriate cervical cancer prevention program in Xinjiang. We also aim to highlight the need for comprehensive data collection to increase our understanding of HPV infection rates in Xinjiang.
Methods
Ethical approval
This study was approved by the Ethics Committee of Xinjiang Medical University (approval No. K-2020019). Informed consent was waived due to the retrospective nature of this study.
Study population
This study was conducted based on the national project titled “National Breast and Cervical Cancer Screening in Rural Areas” (or “two-cancer screening”). In this study, we mainly focused on the data from cervical cancer screening in the year 2017. The data used here were derived from the monthly and quarterly reports of cervical cancer screening as well as the case registration cards from the “National Women and Children Major Public Health Service Information Direct Reporting System.” In brief, female residents aged 35 to 64 years from the rural areas of Xinjiang Uyghur Autonomous Region, without a history of hysterectomy or mastectomy, not currently pregnant, and willing to complete all screening procedures were considered eligible for the study. A total of 216,754 eligible women were screened in 2017 in the present study.
Screening procedure
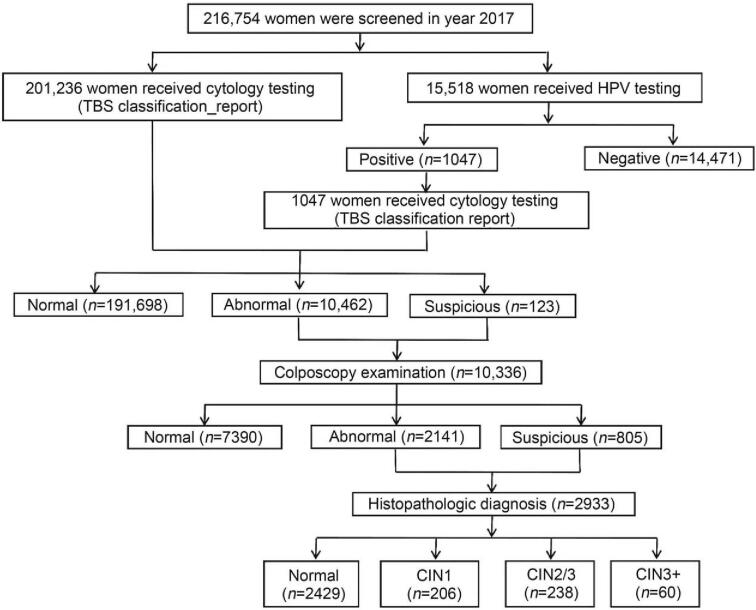
Women were invited to complete the questionnaire regarding basic demographic characteristics. Then, each woman underwent a pelvic examination, and cervical exfoliated cells were collected for cytology testing or careHPV testing (care™ HPV; Qiagen Inc., Gaithersburg, MD, USA). Vaginal secretions were also collected for genital tract infection testing. Women with cytological results of undefined or more severe atypical squamous cells (ASC-US+) were referred to colposcopy and biopsy if indicated. The study flow procedure is shown in Figure 1.
Figure 1.
Flowchart of participant enrollment. CIN: Cervical intraepithelial neoplasia.
Laboratory examinations
Cytology
Most women (N = 201,236) received cytology-based screening using traditional Pap smear or liquid-based cytology testing (AUTOPrep; TIB, Fuzhou, China). The cytology testing results were interpreted by experienced cytopathologists. Cytological diagnoses were evaluated according to the Bethesda system criteria.[12] The terms used in this study were as follows: atypical squamous cells of undetermined significance (ASC-US), atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesions (HSIL) (ASC-H), low-grade squamous intraepithelial lesions, HSIL, and squamous cell carcinoma.
HPV DNA test
A total of 15,518 women finally received the HPV-based screening procedure with cytology triage. The careHPV was used as the primary HPV screening test, which is a signal-amplification, rapid batch diagnostic test for 14 HR-HPV DNA detection. In addition, polymerase chain reaction-based HPV genotyping (HybriMax, HybriBio Ltd., Chaozhou, China) was performed for HPV-positive women. This assay can determine 14 high-risk types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), five low-risk types (6, 11, 42, 43, and 44), and two unknown-risk types (53 and CP8304) by the flow-through hybridization technique.
Colposcopy and pathological diagnosis
Colposcopy was performed on participants with abnormal cytological results. A directed biopsy was performed for a satisfactory colposcopy but with clinically indicated lesions. For unsatisfactory colposcopy impression, an endocervical curettage was performed. All specimens were interpreted by the senior pathologist of Xinjiang Tumor Hospital according to the pathological diagnostic standard criteria. The results of this study were classified as normal, CIN1, CIN2, CIN3, and cervical cancer.
Quality control
Before the initiation of the screening program, all healthcare workers involved in each stage of the screening process received annual training by experienced professionals from higher-level units to standardize the screening quality. The training included the training of communication skills while recruiting the eligible women, giving health education, conducting questionnaires, and so on. Besides, senior gynecological experts from the higher unit trained the on-site gynecologists to perform speculum examination, collection of cervical specimens, and colposcopy examination according to the uniform standard. The histology results were interpreted by the senior pathologist of Xinjiang Tumor Hospital. The database used in this project was the national “Information reporting system for major public health services for women and children.” To ensure the accuracy of the data, the information reporting system set up several quality control processes at the county (district) level, prefecture-level, provincial-level, and national-level. Each unit shall be responsible for examining and verifying the original form, identify the wrong or missing items, check the logical errors and other situations, and notify the reporter to check them if necessary.
Statistical analysis
The prevalence of HPV infection, different cervical lesions, and other common gynecological diseases were calculated. Besides, the early diagnostic rate (%) of cervical cancer was determined by the following formula: (number of CIN2, CIN3, carcinoma in situ, and microscopic invasive carcinoma (stage Ia)/number of all detected CIN2+) × 100%. Socio-demographic characteristics were described using frequencies and proportions, and the odds ratio (OR) with 95% confidence interval (CI) was calculated for each explanatory variable. SPSS 19.0 (IBM, Armonk, NY, USA) was used for statistical analyses. All statistical analyses were two-sided and a P value < 0.05 was determined as statistically significant.
Results
HPV prevalence and related risk factors
Among 15,518 women who underwent HPV testing, the HPV-positive rate was 6.75% (1047/15,518). The most prevalent HPV types in Xinjiang were HPV 16 (24.00%), HPV 33 (12.70%), and HPV 52 (11.80%). Compared with women aged 35 to 45 years, women aged 45 to 55 years and 55 to 64 years had significantly higher HPV infection rates, with ORs of 1.18 (95% CI: 1.02–1.37) and 1.84 (95% CI: 1.53–2.21), respectively. Interestingly, HPV infection rates were positively correlated with the highest level of education of the participants. Compared with women with the education level of primary school or below, the ORs (95% CI) of HPV infection among women with an education level of senior high school and college degree or above were 1.37 (1.09–1.72) and 1.62 (1.23–2.12), respectively. Compared with Han women, Uyghur women were at a lower risk of HPV infection with the OR of 0.78 (95% CI: 0.61–0.99). We also found that the HPV infection rate was highest among women in Eastern Xinjiang (7.9%), which was followed by Northern Xinjiang (5.8%). Women from Southern Xinjiang had the lowest HPV infection rate of 4.1%.
Prevalence of common gynecological diseases and different cervical lesions in Xinjiang
The prevalence of common gynecological diseases, as well as cervical cancer and precancerous lesions, were analyzed according to region, and the results are shown in Table 2. Regarding common gynecological diseases, Northern Xinjiang had the highest prevalence of vaginitis (26.10%, P < 0.001) and genital condyloma acuminata (0.20%), whereas cervicitis was more prevalent in women from Southern Xinjiang (26.40%, P < 0.001), which was followed by Eastern Xinjiang (20.50%) and Northern Xinjiang (11.60%). For regional distribution, Southern and Eastern Xinjiang had a higher prevalence (0.01%) of microinvasive carcinoma. The detection rate of CIN2+ was 0.15%, 0.10%, and 0.14% in Southern, Northern, and Eastern Xinjiang, respectively (P = 0.018). The early diagnosis rate of cervical cancer in women from Southern, Northern, and Eastern Xinjiang was 88.41%, 79.17%, and 84.21%, respectively, without significant differences (P = 0.148).
Table 2.
Prevalence of common gynecological diseases, cervical cancer, and precancerous lesions in various regions of Xinjiang.
| Prevalence of common gynecological diseases (n (%)) | Prevalence of cervical cancer and precancerous lesions (n (%)) | |||||||||
| Regions | N | Vaginitis | Genital condyloma acuminata | Cervicitis | CIN1 | CIN2/3 | Microinvasive carcinoma | Invasive cancer | CIN2+ detection rate (%) | Early diagnosis rate of cervical cancer (%)∗ |
| Southern Xinjiang | 134,308 | 21,809 (16.24) | 177 (0.13) | 35,407 (26.36) | 132 (0.10) | 166 (0.12) | 17 (0.01) | 24 (0.02) | 0.15 | 88.41 |
| Northern Xinjiang | 68,702 | 17,965 (26.15) | 115 (0.17) | 7978 (11.61) | 63 (0.09) | 57 (0.08) | 0 (0.00) | 15 (0.02) | 0.10 | 79.17 |
| Eastern Xinjiang | 13,744 | 2010 (14.62) | 5 (0.04) | 2822 (20.53) | 11 (0.08) | 15 (0.11) | 1 (0.01) | 3 (0.02) | 0.14 | 84.21 |
| Total | 216,754 | 41,784 (19.28) | 297 (0.14) | 46,207 (21.32) | 206 (0.10) | 238 (0.11) | 18 (0.01) | 42 (0.02) | 0.14 | 85.91 |
| P value | <0.001 | 0.002 | <0.001 | 0.906 | 0.077 | 0.035 | 0.936 | 0.018 | 0.148 | |
Early diagnosis rate of cervical cancer (%)∗ = (number of CIN2/3 and microinvasive carcinoma/total number of CIN2+) × 100%. CIN1: Cervical intraepithelial neoplasia grade 1; CIN2/3: Cervical intraepithelial neoplasia grade 2/3.
Distribution of cervical precancerous and cancer lesions by demographic characteristics
Women with a histological diagnosis of CIN and cervical cancer were grouped into distinctive social categories, and the relative rates of cervical cancer and precancerous lesions were compared and analyzed [Table 3]. We found that women aged 55 to 64 years had the highest proportion (22.95%) of invasive cancer. CIN distribution differed significantly according to education levels (P < 0.05). The relative proportions of CIN1 in women with the educational level of primary school, junior high school, senior high school, and college degree or above, were 36.46%, 45.87%, 12.50%, and 0, respectively, whereas the relative proportions of CIN2/3 were 45.86%, 43.12%, 62.50%, and 81.82%, respectively. This result illustrates the uneven severity of the cervical cancer burden among women of different social status within Xinjiang. Although women with higher education are more likely to develop this disease [Table 1], they also have access to more health resources and a positive prognosis.
Table 3.
Distribution of patients according to age, education level, and cervical cancer and precancerous lesions, n (%).
| Characteristics | N | CIN1 | CIN2/3 | Microinvasive carcinoma | Invasive cancer |
| Age | |||||
| 35–44 years | 118 | 42 (35.59) | 60 (50.85) | 4 (3.39) | 12 (10.17) |
| 45–54 years | 146 | 55 (37.67) | 73 (50.00) | 1 (0.68) | 17 (11.64) |
| 55–64 years | 61 | 22 (36.07) | 21 (34.43) | 4 (6.56) | 14 (22.95) |
| P value | 0.937 | 0.079 | 0.054 | 0.043 | |
| Education level | |||||
| Primary school or below | 181 | 66 (36.46) | 83 (45.86) | 4 (2.21) | 28 (15.47) |
| Junior high school | 109 | 50 (45.87) | 47 (43.12) | 2 (1.83) | 10 (9.17) |
| Senior high school | 24 | 3 (12.50) | 15 (62.50) | 3 (12.50) | 3 (12.50) |
| College degree or above | 11 | 0 | 9 (81.82) | 0 | 2 (18.19) |
| P value | 0.001 | 0.039 | 0.097 | 0.390 | |
| Ethnicity | |||||
| Han | 86 | 28 (32.56) | 43 (50.00) | 1 (1.16) | 14 (16.28) |
| Uyghur | 205 | 71 (34.63) | 100 (48.78) | 7 (3.41) | 27 (13.17) |
| Others | 34 | 20 (58.82) | 11 (32.35) | 1 (2.94) | 2 (5.88) |
| P value | 0.017 | 0.176 | 0.582 | 0.317 | |
CIN1: Cervical intraepithelial neoplasia grade 1; CIN2/3: Cervical intraepithelial neoplasia grade 2/3.
Table 1.
Multivariate unconditional logistic regression analysis between HPV infection and potential high-risk factors.
| Characteristics | N | HPV prevalence, n (%) | OR (95% CI) |
| Age | |||
| 35–44 years | 6929 | 397 (5.7) | 1 (reference) |
| 45–54 years | 6526 | 445 (6.8) | 1.182 (1.023–1.367) |
| 55–64 years | 2063 | 205 (9.9) | 1.836 (1.525–2.211) |
| Education level | |||
| Primary school or below | 7319 | 441 (6.0) | 1 (reference) |
| Junior high school | 5884 | 409 (7.0) | 1.126 (0.961–1.320) |
| Senior high school | 1504 | 119 (7.9) | 1.366 (1.088–1.715) |
| College degree or above | 811 | 78 (9.6) | 1.618 (1.233–2.124) |
| Nationality | |||
| Han | 7286 | 580 (8.0) | 1 (reference) |
| Uyghur | 5665 | 298 (5.3) | 0.775 (0.609–0.985) |
| Kazakh | 1461 | 91 (6.2) | 0.803 (0.636–1.013) |
| Others | 1106 | 78 (7.1) | 0.903 (0.703–1.159) |
| Regions | |||
| Eastern Xinjiang | 8610 | 679 (7.9) | 1 (reference) |
| Northern Xinjiang | 4922 | 286 (5.8) | 0.721 (0.625–0.831) |
| Southern Xinjiang | 1986 | 82 (4.1) | 0.503 (0.340–0.636) |
| Total | 15,518 | 1047 (6.7) | - |
HPV: Human papillomavirus; OR: Odds ratio; -: Not applicable..
Discussion
HPV infection is a necessary condition for the occurrence and development of cervical cancer, which can be detected in 99.7% of cervical cancer lesions with an risk ratio of 250 and a percentage of attributable risk of >98%.[4] In this cross-sectional population-based study of women in Xinjiang, we found the overall HR-HPV prevalence was 6.75%. These results are consistent with those reported by Sui et al,[13] which showed that the HPV prevalence among Xinjiang Uyghur women was 7.25%. Both studies showed a lower infection rate than the national average. Zhao et al[8,14] reported that the HPV incidence in China was 16.8%, in Shenyang was 17%, in Shenzhen was 18%, and in Jiangsu was 19%. The most frequent HPV genotypes identified in our study were HPV 16, 33, and 52, which differed from studies performed elsewhere in China and worldwide.[15–18] The results were also different from those of a high-risk region-based study of Uyghur women conducted by Mijit et al,[19] who reported that the most frequent HPV types were HPV 16, 58, and 39. In China, HPV 16 and 18 are the predominant types, followed by HPV 58, 31, and 52.[14] A proportion of 71.4% of HPV-positive high-grade lesions (CIN2+) was attributed to HPV 16 and 18 infection, and 24.1% were attributed to HPV 33, 52, and 58 infection.[20] However, in our study, the HPV 18 infection rate in Xinjiang was low. This was consistent with results previously reported in Xinjiang.[13,19] The HPV infection rate in Xinjiang is not high, and whether this phenomenon is related to the menopausal status needs to be determined in future studies.
The overall HPV infection rate of rural women in Xinjiang showed a gradual upward trend with age, although no significant differences in peak ages of infection were observed. The HPV infection rates in the different age groups were consistent with the results of previous studies.[19,21] However, after menopause, the latent HPV may be reactivated due to impaired immune response in HPV-positive women.
One unusual but previously shown trend in our study was the increased prevalence of HPV among women with higher levels of education. This trend was also reported by Abulizi et al,[3] contrary to the results of previous studies in other parts of China and the world.[22–24] This might be explained by the correlation between the number of sexual partners and the sexual hygiene in a woman's lifetime. Women with a lower education level are more likely to marry earlier and have a fixed sexual partner, whereas those with a higher educational level tend to have more sexual partners and then settle down into monogamous marital relationships later in life.
Another intriguing result was the higher incidence of Han women with positive HPV relative to their Uyghur or Kazakh counterparts. This discrepancy could be a result of several factors, including the typical average number of lifetime sexual partners for each respective ethnicity. Uyghurs and Kazakhs have relatively traditional culture, and generally have a very conservative view regarding sexual promiscuity. The discrepancy in the numbers of lifetime sexual partners between these ethnic groups could; therefore, be explained through a cultural framework. However, Abulizi et al[3] reported that approximately 50% (48.5%) of Uyghur women remarried at least once, and 6.5% married more than three times. This might compete with lifetime partners in Han women, although the data remain unclear.
Currently recognized risk factors for cervical cancer, in addition to HPV infection, are synergistic risk factors. These include biological factors, including bacterial infections from species such as Chlamydia, as well as viral infections. The present study showed that the detection rate of vaginitis in rural areas of Xinjiang was 19.28%, and that of cervicitis was 21.32%. These rates are higher than the national average rates in China (vaginitis: 4.39–11.17; cervicitis: 5.77–15.53).[25,26] The detection rates of vaginitis and external genital condyloma acuminata were also calculated, and the regional distribution of these diseases was compared. The Northern region had higher rates of both diseases compared with other areas, whereas the Southern region had a relatively high incidence of cervicitis. According to Abulizi et al,[3] most farmers in the rural areas of southern Xinjiang used cloth and clods for wiping after using the toilet, cleaned menstrual period blood with a cloth and substandard toilet paper substitutes, and had sex after cleaning the vagina using fingers. Certain lifestyle habits that cause malnutrition in male and female genitals can also pose a risk to women with general vaginal illnesses. Patients with cervicitis and condyloma acuminata may face an increased risk of developing cervical cancer. These people should be the focus of future census research and enhanced surveillance programs in Xinjiang, especially in the Northern region, where the detection rate of genital warts is high. Special investigations have identified key counties (cities) and high-risk groups that should be the focus of early intervention programs.
The detection rate of cervical cancer in rural Xinjiang women was 0.14%, and the early diagnosis rate was 79.9%. This detection rate is much higher than the national average, which was reported at 0.02% in the 2013 national “two cancer” screening data. However, the early diagnosis rate is lower than the national average of 89.57%. The high detection rate and low early diagnosis rate suggest that cervical cancer is a prominent, yet poorly managed, a public health problem that greatly affects rural women in Xinjiang. On the other hand, these results underscore the need to improve the rate of early diagnoses and the level of participation among rural Xinjiang women.
The relative detection rate of cervical cancer in rural areas of Xinjiang showed an imbalanced regional distribution compared with that in more populated areas. The detection rate of cervical cancer among women in Southern Xinjiang was higher than that of other areas, suggesting that Southern Xinjiang bearded the heaviest burden of cervical cancer in Xinjiang. Uyghur women in the area displayed the highest rates of cervical cancer and precancerous lesions compared with those of other ethnicities. Precancerous lesions primarily occurred in 45- to 55-year-old women who received an education through or up to college degree or above, and the incidence of cervical cancer was higher in women aged 55 to 64 years and below. This was consistent with the 2015 Xinjiang Cancer Registry Report[27] and a previous study of cervical cancer incidence in Xinjiang.[28] The factors affecting these results are related to the population, economy, medical care, religion, and culture in Southern Xinjiang. As of the end of 2020, the Xinjiang Statistical Yearbook reported that the total population of Xinjiang was 25,852,345. Among them, the Uyghur population accounted for 11.62 million, accounting for 44.96%.[29] Uyghur people mainly live in Southern Xinjiang, and their per capita gross domestic product is lower than that of Northern and Eastern Xinjiang. Rural Southern Xinjiang women are vulnerable to HPV-activating and the long-term existence of chronic cervicitis, and poor health and sexual hygiene habits. Such lifestyle habits self-perpetuate in a vicious cycle as long as they remain unaddressed and are perpetuated in the region.
This is the study with such a large sample size to comprehensively report the epidemiological characteristics of HPV infection and cervical (pre)cancer in Xinjiang. Although the study did not include the sampling process, and also due to that the screening practice was conducted by different health workers from rural areas of the Xinjiang region, the representativeness of the study population and the quality assurance might be suboptimal. However, considering the fact that although the national cervical cancer screening project has been launched in 2008, the related publications that summarize the screening outcomes and performance in the Xinjiang region is very limited. Thus, based on available data, we devote to provide a comprehensive blueprint of cervical cancer epidemiology in Xinjiang, which is of great importance for future cervical cancer prevention strategy in this region.
To conclude, our study indicates that the prevalence of HPV infection in Xinjiang women is relatively low, and the most prevalent HPV genotypes are HPV 16, HPV 33, and HPV 52. HPV infection status is positively correlated with age and educational level. The detection rates of cervical cancer and precancerous lesions in Xinjiang rural women are higher than the average national rates, whereas the early diagnostic rates are lower than the average national rates. Cervical cancer is a prominent public health problem among rural women in Xinjiang, especially in Southern Xinjiang. Programs focusing on the prevention and control of cervical cancer should be strengthened in this high-risk population.
Acknowledgements
The authors would like to thank the participants and the local health workers from Xinjiang Uyghur Autonomous Region for their contributions to the study.
Funding
This study was supported by the Tianshan Youth Project Foundation of Xinjiang, China (No. 2017Q056).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang Y, Cai YB, James W, Zhou JL, Rezhake R, Zhang Q. Human papillomavirus distribution and cervical cancer epidemiological characteristics in rural population of Xinjiang, China. Chin Med J 2021;134:1838–1844. doi: 10.1097/CM9.0000000000001441
References
- 1.Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, et al. Report of cancer epidemiology in China, 2015 (in Chinese). Chin J Oncol 2019; 41:19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Abulizi G, Li H, Mijiti P, Abulimiti T, Cai J, Gao J, et al. Risk factors for human papillomavirus infection prevalent among Uyghur women from Xinjiang, China. Oncotarget 2017; 8:97955–97964. doi: 10.18632/oncotarget.18901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19. doi: 10.1002/(sici)1096-9896(199909)189:1<12::Aid-path431>3.0.Co;2-f. [DOI] [PubMed] [Google Scholar]
- 5.Zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians 1999; 111:581–587. doi: 10.1046/j.1525-1381.1999.99723.x. [DOI] [PubMed] [Google Scholar]
- 6.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 7.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012; 131:2349–2359. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 8.Zhao FH, Lin MJ, Chen F, Hu SY, Zhang R, Belinson JL, et al. Performance of high-risk human papillomavirus DNA testing as a primary screen for cervical cancer: a pooled analysis of individual patient data from 17 population-based studies from China. Lancet Oncol 2010; 11:1160–1171. doi: 10.1016/s1470-2045(10)70256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Comprehensive Cervical Cancer Control: A Guide to Essential Practice. Available from: http://www.who.int/cancer/publications/9241547006/en/. [Accessed April 24, 2015] [Google Scholar]
- 10.Dillner J, Rebolj M, Birembaut P, Petry K-U, Szarewski A, Munk C, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smelov V, Elfstrom KM, Johansson AL, Eklund C, Naucler P, Arnheim-Dahlstrom L, et al. Long-term HPV type-specific risks of high-grade cervical intraepithelial lesions: a 14-year follow-up of a randomized primary HPV screening trial. Int J Cancer 2015; 136:1171–1180. doi: 10.1002/ijc.29085. [DOI] [PubMed] [Google Scholar]
- 12.Qinjing P. Retrospective and new interpretation of TBS cervical cytology report system (in Chinese). Chin J Clin Obstet Gynecol 2017; 18:95–96. doi: 10.13390/j.issn.1672-1861.2017.01.038. [Google Scholar]
- 13.Sui S, Jiao Z, Niyazi M, Sulaiya S, Lu P, Qiao YL. Genotype distribution and behavioral risk factor analysis of human papillomavirus infection in Uyghur women. Asian Pac J Cancer Prev 2013; 14:5861–5865. doi: 10.7314/apjcp.2013.14.10.5861. [DOI] [PubMed] [Google Scholar]
- 14.Zhao FH, Lewkowitz AK, Hu SY, Chen F, Li LY, Zhang QM, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies. Int J Cancer 2012; 131:2929–2938. doi: 10.1002/ijc.27571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 16.Sundstrom K, Eloranta S, Sparen P, Arnheim Dahlstrom L, Gunnell A, Lindgren A, et al. Prospective study of human papillomavirus (HPV) types, HPV persistence, and risk of squamous cell carcinoma of the cervix. Cancer Epidemiol Biomarkers Prev 2010; 19:2469–2478. doi: 10.1158/1055-9965.Epi-10-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai M, Bao YP, Li N, Clifford GM, Vaccarella S, Snijders PJ, et al. Human papillomavirus infection in Shanxi Province, People's Republic of China: a population-based study. Br J Cancer 2006; 95:96–101. doi: 10.1038/sj.bjc.6603208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao R, Zhang WY, Wu MH, Zhang SW, Pan J, Zhu L, et al. Human papillomavirus infection in Beijing, People's Republic of China: a population-based study. Br J Cancer 2009; 101:1635–1640. doi: 10.1038/sj.bjc.6605351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mijit F, Ablimit T, Abduxkur G, Abliz G. Distribution of human papillomavirus (HPV) genotypes detected by routine pap smear in Uyghur-Muslim women from Karasay Township Hotan (Xinjiang, China). J Med Virol 2015; 87:1960–1965. doi: 10.1002/jmv.24240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang R, Velicer C, Chen W, Liaw KL, Wu EQ, Liu B, et al. Human papillomavirus genotype distribution in cervical intraepithelial neoplasia grades 1 or worse among 4215 Chinese women in a population-based study. Cancer Epidemiol 2013; 37:939–945. doi: 10.1016/j.canep.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Wang P, Ren Y, Du J, Jiang J, Jia X, et al. Prevalence of high-risk human papillomavirus (HR-HPV) genotypes and multiple infections in cervical abnormalities from Northern Xinjiang, China. PLoS One 2016; 11:e0160698.doi: 10.1371/journal.pone.0160698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Wang J, Fan J, Zhao W, Yang X, Wu L, et al. Risk factors for cervical intraepithelial neoplasia and cervical cancer in Chinese women: large study in Jiexiu, Shanxi Province, China. J Cancer 2017; 8:924–932. doi: 10.7150/jca.17416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Wu M, Wang J, Zhang S, Zhu L, Pan J, et al. A population-based study on the risks of cervical lesion and human papillomavirus infection among women in Beijing, People's Republic of China. Cancer Epidemiol Biomarkers Prev 2010; 19:2655–2664. doi: 10.1158/1055-9965.Epi-10-0212. [DOI] [PubMed] [Google Scholar]
- 24.Akinlotan M, Bolin JN, Helduser J, Ojinnaka C, Lichorad A, McClellan D. Cervical cancer screening barriers and risk factor knowledge among uninsured women. J Community Health 2017; 42:770–778. doi: 10.1007/s10900-017-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tianlin Y, Xiaochun L, Xiaoxia Y. Analysis of screening results of cervical cancer and breast cancer in rural women in Wuwei City in 2016 (in Chinese). Chin J Dis Control Prev 2018; 33:78–80. doi: 10.13215/j.cnki.jbyfkztb.1603066. [Google Scholar]
- 26.Yumei Z, Xianzhi X, Shuchun B, Geping Y. Epidemiological investigation and analysis of cervical cancer in 18365 elderly women in rural area of Dongying (in Chinese). Chin J Cancer Prev Treat 2014; 21:1774–1777. doi: 10.16073/j.cnki.cjcpt.2014.22.005. [Google Scholar]
- 27.Xiao L, Liu L, Zhang H, Zhang R, Gulibiye S, Liu P, et al. Analysis of the incidence of malignant tumors in the tumor registration area of Xinjiang Uygur Autonomous Region in 2013 (in Chinese). Chin J Health Stat 2015; 32:674–676. doi: CNKI:SUN:ZGWT.0.2015-04-039. [Google Scholar]
- 28.Jang S, Wang T, Tu S, Zhou J, Mai R, Xu X, et al. Epidemiological investigation of cervical cancer in Cele County of Xinjiang (in Chinese). Chin J Pract Gynecol Obstet 2006; 22:379–381. doi: 10.3969/j.issn.1005-2216.2006.05.025. [Google Scholar]
- 29.http://tjj.xinjiang.gov.cn/tjj/tjgn/202106/4311411b68d343bbaa694e923c2c6be0.shtml. Last Accessed June, 2021. [Google Scholar]