Abstract
INTRODUCTION:
Despite enormous efforts during the past decades, pancreatic adenocarcinoma (PAC) remains one of the most deleterious cancer entities. A useful biomarker for early detection or prognosis of PAC does not yet exist. The goal of our study was the characterization of β6-integrin (ITGB6) as a novel serum tumor marker for refined diagnosis and prognosis of PAC. Serum ITGB6 levels were analyzed in 3 independent PAC cohorts consisting of retrospectively and prospectively collected serum and/or (metastatic) PAC tissue specimens.
METHODS:
Using 2 independent cohorts, we measured serum ITGB6 concentrations in 10 chronic pancreatitis patients, 10 controls, as well as in 27 (cohort 1) and 24 (cohort 2) patients with PAC, respectively. In these patients, we investigated whether ITGB6 serum levels correlate with known clinical and prognostic markers for PAC and whether they might differ between patients with PAC or benign inflammatory diseases of the pancreas.
RESULTS:
We found that elevated serum ITGB6 levels (≥0.100 ng/mL) in patients suffering from metastasizing PAC presented an unfavorable prognostic outcome. By correlating the ITGB6 tissue expression in primary and metastatic PAC with clinical parameters, we found that positive ITGB6 expression in the tumor tissue is linked to increased serum ITGB6 levels in nonmetastatic PAC and correlates with carbohydrate antigen 19-9 and clinical outcome.
DISCUSSION:
Our findings suggest that ITGB6 might serve as a novel serum biomarker for early diagnosis and prognosis of PAC. Given the limited specificity and sensitivity of currently used carbohydrate antigen 19-9–based assays, ITGB6 may have the potential to improve the diagnostic accuracy for PAC.
INTRODUCTION
The diagnosis and management of pancreatic adenocarcinoma (PAC) remains a major clinical challenge since conventional cancer therapies are rarely curative, leading to approximately 30,000 deaths per year in the United States. Surgery potentially cures patients; however, only 15%–20% of patients present with resectable disease, of whom only 20% survive the initial 5 years (1,2). In addition, projections of cancer deaths suggest that PAC will become the second leading cause of cancer-related deaths within the next 10–15 years worldwide, thereby surpassing breast cancer and colorectal cancer (CRC) (3). Easily accessible biomarkers ideally facilitate early diagnosis of PAC in patients at risk, guide therapy, and reflect treatment response. To date, carbohydrate antigen 19-9 (CA19-9) is the only available serum tumor marker for PAC. In clinical practice, however, CA19-9–based assays are plagued by a moderate sensitivity and specificity (2,4), whereas newer blood tests combining tumor-specific circulating proteins with cell-free DNA require further validation before being implemented in clinical practice (5,6).
Until now, there is limited evidence that β6-integrin (ITGB6) plays a significant role in PAC pathogenesis (7). Expression and upregulation of ITGB6 in adult epithelial cells is exclusive to the epithelial-to-mesenchymal transition processes (e.g., wound healing, fibrosis, and carcinogenesis) (8,9). In addition, ITGB6 is strongly upregulated in PAC tissue (10), promoting malignant cellular behavior through the ERK-ETS pathway (7). Moreover, this upregulation of PAC tissue is associated with a significant reduction in the median survival of the patients (7).
We have recently shown that serum ITGB6 levels can predict CRC diagnosis and prognosis (11). We further hypothesized that serum ITGB6 levels may be similarly elevated in patients with PAC and correlate with survival and clinical parameters. To investigate whether serum levels of ITGB6 are of diagnostic and/or prognostic relevance for patients with PAC, we examined serum ITGB6 levels in 2 cohorts of prospectively and retrospectively enrolled patients. In addition, we examined the expression pattern of ITGB6 in human PAC tissue as a function of clinical outcomes.
METHODS
Serum and tissue cohorts
Serum specimens were collected prospectively at the Department of Gastroenterology and Hepatology, University Hospital Zurich, Switzerland, between February 2018 and June 2019 (cohort 1, see Supplementary Table 1, http://links.lww.com/CTG/A661) and retrospectively at the Department of Medicine I, Freiburg University Medical Center, Germany, between December 2015 and January 2018 (cohort 2, see Supplementary Table 2, http://links.lww.com/CTG/A662). The tissue-based cohort (cohort 3, see Supplementary Table 3, http://links.lww.com/CTG/A663) was collected by the Department of Pathology and Molecular Pathology, University Hospital Zurich, Switzerland, between January 2007 and June 2016. All patients with chronic pancreatitis (cP) received either a computed tomography (n = 4), an MRI (n = 4), or endosonography (n = 2) to rule out suspicious pancreatic lesions.
Ethical consideration
The studies were approved by the local ethics committees (Switzerland; Approval No. EK-1755 and BASEC No. 2017-01319) and by the ethics committee (Freiburg, Germany: Approval No. 46/18). For this study, all participants gave written informed consent. Patients' data were pseudonymized, and all analyses were performed in accordance with the Helsinki declaration.
Human ITGB6 and CA19-9 detection
According to manufacturer's instructions, serum ITGB6 levels were assessed with the human ITGB6 enzyme-linked immunosorbent assay kit (E92099Hu; USCN Life Science, Wuhan, China). Serum CA19-9 levels were analyzed with certified standard assays in the clinical routine laboratory of the Institute of Clinical Chemistry, University Hospital Zurich, Switzerland (see Supplementary Figure, http://links.lww.com/CTG/A664).
Immunohistochemistry
A tissue microarray (TMA) with formalin-fixed paraffin-embedded PAC tissue specimens from 83 patients was automatically stained with a human anti-ITGB6 polyclonal antibody raised in rabbit (HPA023626, dilution 1:100; Atlas Antibodies AB, Bromma, Sweden) using a validated standard protocol on a Ventana automat (Ventana Medical System, Tucson, AZ). The stained TMA was evaluated by 2 independent pathologists of the Department of Pathology and Molecular Pathology, University Hospital Zurich (see Supplementary Figure, http://links.lww.com/CTG/A664).
Statistical analyses
Data sets are reported according to the remark criteria (12) and statistical analyses using IBM SPSS statistics 25.0 (SPSS, Chicago, IL). More details on the statistical analyses are provided in the supporting information (see Supplementary Figure, http://links.lww.com/CTG/A664).
RESULTS
Higher serum ITGB6 levels are associated with PAC
To investigate whether ITGB6 is detectable in the serum of patients with PAC, we examined serum ITGB6 levels in a prospective cohort of 27 consecutive patients of all tumor stages (cohort 1, see Supplementary Table 1, http://links.lww.com/CTG/A661). We found that ITGB6 was detectable in the serum of the majority of the study population with a mean concentration of 1.03 ± 0.398-ng/mL ITGB6 (ranging from 0 to 10.86 ng/mL; Figure 1a). By contrast, all healthy controls showed a significantly lower serum ITGB6 level (0.24 ± 0.102-ng/mL ITGB6) compared with patients with PAC (P = 0.019; Figure 1a). In patients with cP, moderately elevated levels of ITGB6 were detected (cP; 0.40 ± 0.105-ng/mL ITGB6); however, no significant differences were observed when compared with patients with PAC or controls, respectively (Figure 1a). Patients with a serum ITGB6 level ≥0.100 ng/mL were more likely to be diagnosed with PAC than patients with low serum ITGB6 levels (<0.100 ng/mL; ns; odds ratio [OR] 1.438 [0.220–9.405]). Patients with serum ITGB6 levels ≥0.100 ng/mL displayed a risk ratio (RR) of 1.113 (0.609–2.033) for diagnosis of PAC, which was rising up to 1.429 (1.130–1.806) for patients with a serum ITGB6 level ≥1.0 ng/mL. By contrast, the risk of a patient to be diagnosed with cP while presenting a serum ITGB6 level ≥0.100 ng/mL was only 0.774 (0.215–2.783), and no risk of cP diagnosis was detected with a serum ITGB6 concentration ≥1.0 ng/mL.
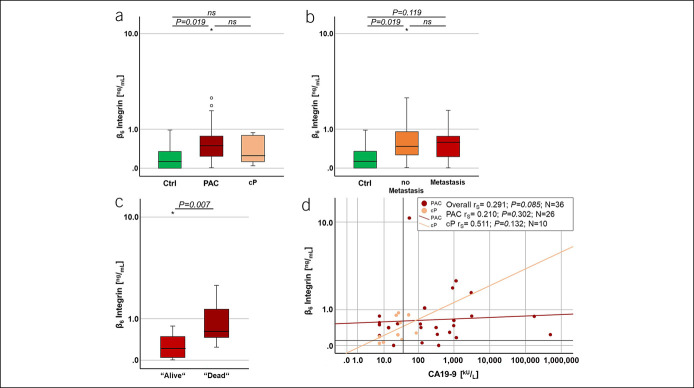
Figure 1.
Validation of ITGB6 as possible biomarker for PAC. (a) Serum ITGB6 levels were assessed in a prospective study cohort of 27 patients with PAC (cohort 1, N = 27). As control served 9 healthy volunteers (Ctrl; N = 9) and 10 patients with cP (N = 10). Significant differences in ITGB6 levels were observed between patients with PAC and Ctrl (P = 0.019). (b) Comparing patients with or without distant metastatic PAC, serum ITGB6 levels were not significantly different. However, a significant increase in ITGB6 levels was observed between Ctrl and patients with nonmetastatic PAC (P = 0.019). (c) To assess the prognostic value of serum ITGB6 levels, patients with PAC were plotted against their status of survival at time of blood assessment. A significant difference in ITGB6 concentration was observed between patients with PAC with status alive vs dead (P = 0.007). (d) Two-dimensional scatterplots depict serum ITGB6 levels in relation to the serum CA19-9 levels from each cP (rs = 0.511; P = 0.132) and patient with PAC (rs = 0.210; P = 0.302). In (a–c), Mann–Whitney U Exact and Sig. 2-Tailed test were performed, and in (d), Spearman's rho correlation (rs) and Sig. 2-tailed test were performed. (d) Red lines indicate ITGB6 cutoff value at 0.1 ng/mL and black line CA19-9 cutoff value at 37.0 kU/L, respectively. cP, chronic pancreatitis; Ctrl, control; ITGB6, β6-integrin; PAC, pancreatic adenocarcinoma; rs, Spearman's rho correlation. *Means P < 0.05.
Taken together, our data suggest that serum ITGB6 levels may help to distinguish between patients suffering from PAC or cP.
ITGB6 as predictor of PAC and worse survival in patients with PAC
To determine whether ITGB6 reflects metastatic PAC, we analyzed the correlation of serum ITGB6 levels with the presence of distant metastases (Figure 1b). Independent of the presence of metastases, serum ITGB6 levels were increased in patients with PAC when compared with controls (Ctrl vs nonmetastatic PAC, P = 0.019; Ctrl vs metastatic PAC, P = 0.119). Furthermore, patients with PAC and an ITGB6 serum level of ≥0.100 ng/mL were found to have a high probability and relative risk to be diagnosed with metastatic PAC (OR 1.750 [0.129–23.703]; RR 1.400 [0.259–7.582]; without metastases: RR 0.800 [0.316–2.027]) at time of blood sampling. The same was true for lymph node (LN) metastases (LNM; data not shown). Patients with an ITGB6 serum level ≥0.100 ng/mL showed an increased probability and relative risk to be diagnosed with LNM (OR 8.400 [0.701–100.595], RR 2.947 [0.529–16.435]; without LNM: RR 0.351 [0.137–0.899]).
Next, we investigated whether serum ITGB6 levels could serve as a prognostic factor for patient survival. We found that serum ITGB6 levels show a significant association with the status of survival (rs = 0.522; P = 0.005). Noteworthy, the median ITGB6 concentration was 0.62 ± 0.184 ng/mL for patients with PAC who were deceased at the time of analysis of the data (P = 0.007; compared with those with status ‟alive”: 0.22 ± 0.886 ng/mL; Figure 1c).
Overall, our data indicate that serum ITGB6 levels can be used to discriminate between patients with PAC with or without metastatic disease, as well as to identify patients with poor prognosis.
Combination of ITGB6 with serum CA19-9 levels may improve diagnostic accuracy of detecting PAC
We further investigated whether serum ITGB6 levels in combination with the commonly used CA19-9 serum marker could facilitate the diagnosis of patients with PAC. We found that CA19-9 is significantly elevated in patients with PAC compared with cP patients (26,348.14 ± 19,918.721 kU/L and 30.38 ± 7.688 kU/L, respectively; P = 0.023; data not shown). Furthermore, patients with serum CA19-9 levels ≥37.0 kU/L showed a higher probability to suffer from PAC (OR 9.000 [1.550–52.266], P = 0.011; diagnosis PAC: RR 1.800 [1.079–3.001]), whereas the risk of cP diagnosis was reduced (RR 0.200 [0.049–0.814]). In line with this, we found a weak correlation (rs = 0.210; ns; Figure 1d) between serum ITGB6 and CA19-9 concentrations for patients with PAC. Patients with PAC tended to have higher levels of ITGB6 (≥0.100 ng/mL) and CA19-9 (>37.0 kU/L) (P = 0.060; OR 6.400 [1.124–36.437], RR 1.600 [1.026–2.495]). This was not true for cP patients who were mainly grouped below 37.0-kU/L CA19-9 and presented lower serum ITGB6 levels overall (rs = 0.511; ns; Figure 1d). Such patients showed a low-risk cP diagnosis when presenting high serum levels of ITGB6 and CA19-9 (≥0.100 ng/mL and ≥37.0 kU/L; RR 0.250 [0.061–1.019]). Furthermore, patients with PAC with serum ITGB6 and CA19-9 levels above their cutoff values showed an increased probability to be assessed deceased at the time of data analysis (OR 5.133 [0.922–28.570], RR 2.292 [0.841–6.246]) (ns).
Our data show that serum levels of CA19-9 (<37.0 kU/L) and ITGB6 (<0.100 ng/mL) below a certain threshold were indicative of cP disease. By contrast, serum levels of CA19-9 of ≥37.0 kU/L together with elevated serum ITGB6 levels ≥0.100 ng/mL were highly indicative of PAC disease and present a possible tool to distinguish between cP and PAC at early diagnosis.
Prognostic value of serum ITGB6 levels in patients with PAC
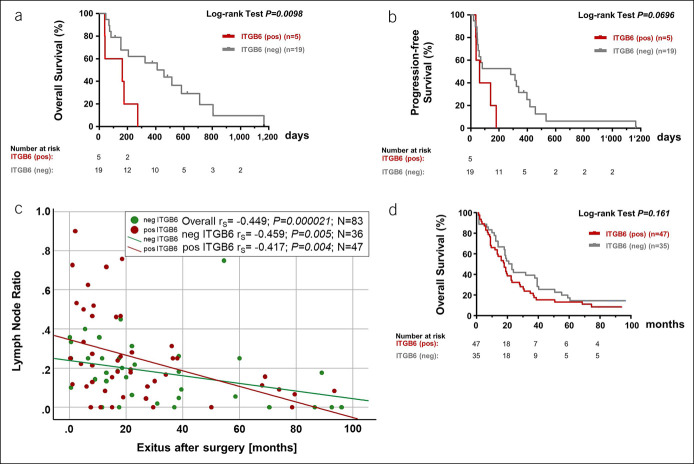
We then analyzed the prognostic impact of serum ITGB6 levels with respect to overall survival (OS) in patients with (metastatic) PAC. Before initiation of systemic therapy, we found a significantly reduced OS in patients with PAC with serum ITGB6 levels > 0.100 ng/mL (ITGB6-positive patients, 0.42 ± 0.194 ng/mL; median OS 163 days; hazard ratio 8.17 [1.659–40.22]), compared with patients with low serum ITGB6 levels (ITGB6-negative patients, log-rank P = 0.0098; 0.172 ± 0.104 ng/mL; OS 458 days; cohort 2; Figure 2a and Supplementary Table 2, http://links.lww.com/CTG/A662). Moreover, there was a strong tendency toward a reduced progression-free survival (PFS) in ITGB6-positive patients (median PFS of 64 days; hazard ratio 3.61 [0.9022–14.46]), compared with patients with PAC with negative serum ITGB6 levels (log-rank P = 0.0696; median PFS of 285 days; cohort 2; Figure 2b).
Figure 2.
Serum ITGB6 levels may predict overall and progression-free survival in patients with PAC. (a) Survival rates and (b) progression-free survival of patients with PAC who underwent systemic therapy are plotted according to serum ITGB6 changes after initiation (N = 24; cohort 2). (c) Two-dimensional scatterplots depict lymph node ratio (LNR) in relation to exits after surgery (months) for each patient in accordance to the ITGB6 protein expression pattern. Hazard ratio (HR) was calculated with the Cox regression. (d) Overall survival in AU12 patients with PAC (N582; cohort 3) was plotted according to ITGB6 protein expression pattern. Kaplan-Meier estimation was performed and plotted accordingly. The log-rank P values are indicated. ITGB6, β6-integrin; PAC, pancreatic adenocarcinoma.
In summary, these data indicate that serum ITGB6 levels might be a suitable biomarker for prognosis in patients with PAC.
Protein expression of ITGB6 in PAC tissue specimens
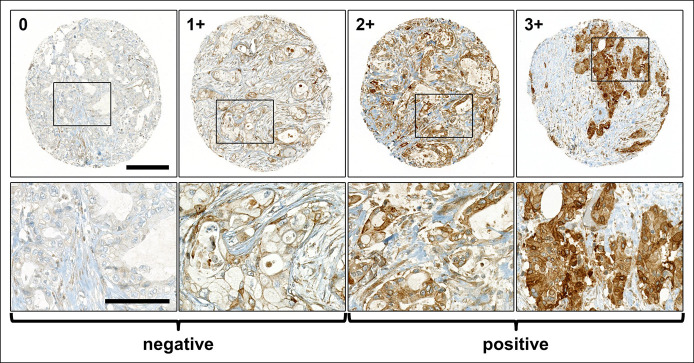
We next assessed the protein expression pattern of ITGB6 by immunohistochemistry in an independent TMA of primary PAC specimens and their corresponding LNM (cohort 3; see Supplementary Table 3, http://links.lww.com/CTG/A663), as well as in available PAC tissue samples from cohort 1. In all the assessed tissue specimens, the expression pattern of ITGB6 was cytoplasmic and/or membranous (Figure 3).
Figure 3.
ITGB6 protein expression in PAC tissue specimens by IHC. Representative TMA specimens (from cohort 3, N = 83) illustrate ITGB6-negative (no [0] or mild [1+] expression) and ITGB6-positive PAC specimens (moderate [2+] or strong [3+] expression; scale bars: upper panel 200 μm; lower panel 100 μm). IHC, immunohistochemistry; ITGB6, β6-integrin; PAC, pancreatic adenocarcinoma; TMA, tissue microarray.
ITGB6 immunohistochemistry was positive in 47 and negative in 36 PAC specimens, respectively (Figure 3). Here, we found a high likelihood that patients with ITGB6-positive PAC presented ITGB6-positive LNM. Of 37 available LNM, 26 were positive and 11 negative for ITGB6 expression. Of these LNM, 24 matched their corresponding primary tumors with respect to ITGB6 staining (P = 0.036; OR 6.136 [1.101–34.214]; ITGB6-positive LNM: RR 1.604 [1.041–2.473]), whereas 11 PAC specimens were assessed ITGB6-negative and their corresponding LNM were ITGB6-positive. In 2 TMA specimens, we found the opposite pattern.
Similarly, we found a higher probability that patients with ITGB6-positive PAC presented already detectable LNM in cohort 1. Here, 9 of 12 patients with ITGB6-positive PAC already had LNM (ns; OR 6.000 [0.704–51.102] and RR 1.909 [0.776–4.694]), whereas 4 of 6 ITGB6-negative PAC showed no detectable LNM at diagnosis (RR 0.318 [0.078–1.301]).
Next, we studied the association between ITGB6 expression in TMA specimens and clinicopathological parameters. ITGB6 expression in TMA specimens was associated with male sex (P = 0.050; men: OR 2.471 [1.014–6.019]; RR 1.532 [0.984–2.384]; women: RR 0.620 [0.388–0.992]), but not with tumor location, TNM staging, or grading (see Supplementary Table 3, http://links.lww.com/CTG/A663). Interestingly, we also found a weak correlation between the LN ratio (LNR) and OS after surgery (rs = −0.449; P = 0.000021; Figure 2c). Patients with ITGB6-positive expression in the tumor tissue showed a steeper correlation of high LNR with reduced OS (rs = −0.417; P = 0.004) compared with patients with ITGB6-negative PAC (rs = −0.459; P = 0.005; Figure 2c). This was further reflected in the status of survival at analysis. Patients with PAC with ITGB6-positive primary tumors showed a higher likelihood to be assessed deceased at time point of analysis (ns; OR 1.734 [0.430–6.986], RR 1.062 [0.908–1.244]), had a significantly increased LNR (0.267 ± 0.231; P = 0.048) and a reduced OS (P = 0.000022; 18.60 ± 16.48 months), compared with patients with status alive (LNR: 0.077 ± 0.064; and OS 80.123 ± 10.09 months). Similar tendencies were found for patients with negative ITGB6 expression (deceased: LNR 0.201 ± 0.162 and OS 22.70 ± 17.51 months vs alive: LNR 0.035 ± 0.079 [P = 0.007] and OS 86.90 ± 9.85 months [P = 0.000005], respectively). Although not significant, the prognosis of these patients with ITGB6-positive staining in their primary tumors was reduced compared with patients with ITGB6-negative PAC (log-rank P = 0.161, Figure 2d).
In summary, our data show that ITGB6 protein expression pattern correlates with the LNR and with the prognosis of patients suffering from PAC.
DISCUSSION
PAC is one of the most lethal human cancers because of its high resistance to most conventional treatment options, the significant molecular interpatient heterogeneity (13), and its delayed diagnosis, partly because of the lack of appropriate serum biomarkers (14). In PAC specimens, ITGB6 has already been identified as an unfavorable prognostic indicator (7); however, to the best of our knowledge, serum ITGB6 levels in patients with PAC were not investigated so far.
With respect to the lack of predictive and reliable biomarkers for PAC disease, the goal of this proof-of-concept study was to characterize ITGB6 as a suitable serum tumor marker for diagnosis and prognosis of patients suffering from PAC.
Overall, our data suggest that serum ITGB6 levels might be of prognostic value with respect to survival in patients with PAC. In 2 independent cohorts, we observed that increased serum ITGB6 levels were associated with significantly reduced OS in patients suffering from PAC. Similar observations were found for PFS, which was significantly reduced in patients with positive serum ITGB6 levels.
Moreover, serum ITGB6 levels may help to discriminate between PAC and cP. After introducing the cutoff value of 0.100-ng/mL ITGB6 (11), we found that patients with serum levels above that threshold presented a higher probability of being diagnosed with metastatic PAC compared with patients with cP. The combination of ITGB6 with serum CA19-9 levels improved the diagnostic accuracy of PAC in our study cohort. This is important because elevated serum CA19-9 levels are likewise observed in patients with benign inflammatory pancreatic disease (15,16).
Finally, we confirmed that ITGB6 expression in PAC specimens represents an unfavorable prognostic indicator (7), although the association of clinical and pathological parameters with positive ITGB6 expression was partly different compared with the study of Li et al. (7). In contrast to the latter study (7), in our tissue cohort of European population men, sex (P = 0.050) was associated with positive ITGB6 expression in the primary tumor. However, additional studies that investigate potential ethnical differences in ITGB6 expression would be of great interest.
Because CA19-9 is not a suitable tumor marker among patients with a Lewis blood group antigen-negative phenotype (approximately 7%–10% of population) (17,18), ITGB6 may be especially valuable when applied in Lewis antigen-negative PAC patients.
One of the major limitations of this study is the small number of patients in all 3 independent study cohorts. A study with a large prospective cohort stratified according to stage with additionally large, age-matched control group and longitudinal follow-up would be of great interest. Such a cohort would also help determine the role of ITGB6 in patients not expressing the Lewis blood group antigen and elucidate whether ITGB6 expression is predictive with respect to certain chemotherapies. Nonetheless, because this study was intended to serve as a proof-of-principle, establishing such a prospective cohort would have been beyond the scope of this study.
Nevertheless, our findings suggest that ITGB6 may be a promising novel serum biomarker for improved diagnosis and prognosis of patients suffering from PAC. These results are in line with our observation that relapse or metastasis in patients with CRC is accompanied by a surge of serum ITGB6 levels (11). Furthermore, together with CA19-9, serum ITGB6 levels may allow for a more rigorous discrimination between patients suffering from PAC or cP. This is of paramount importance because specificity and sensitivity of serum CA19-9–based assays are limited.
CONFLICTS OF INTEREST
Guarantor of the article: Michael Scharl, MD.
Specific author contributions: Daniela Lenggenhager, MD, Susan Bengs, PhD, contributed equally to this work. D.L.: interpretation of data and writing of the report. S.B.: analysis of data and writing of the report. R.F., S.H., P.B., F.O.T., and C.G.: collection and interpretation of data and drafting of manuscript. K.E.: collecting and analysis of data. M.S. and B.M.: planning/conducting of the study, interpreting data, and drafting manuscript. All authors have approved the final draft.
Financial support: The work was supported by a grant from the Stiftung Experimentelle Biomedizin to M.S., a joint grant from the German Research Foundation/Swiss National Science Foundation to M.S. (320030E_190,969 Lead Agency DFG) and a grant from the Swiss National Science Foundation to MS (320,030_184,753). The work was independent of the funding.
Potential competing interests: None to report.
Data availability statement: The data that support the findings of this study are available from the corresponding author on reasonable request: michael.scharl@usz.ch.
Study Highlights.
WHAT IS KNOWN
✓ Useful serum biomarkers for diagnosis of pancreatic adenocarcinoma (PAC) are limited.
✓ This study investigates β6-integrin (ITGB6) serum levels and protein expression in tumor tissue specimens from patients suffering from PAC.
WHAT IS NEW HERE
✓ The authors investigated ITGB6 serum levels and protein expression in tumor tissue specimens from patients suffering from PAC.
✓ Serum ITGB6 levels and protein expression in primary and metastatic tumor tissue were associated with prognosis and allowed for a more careful distinction between PAC and chronic pancreatitis.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A661, http://links.lww.com/CTG/A662, http://links.lww.com/CTG/A663, http://links.lww.com/CTG/A664, http://links.lww.com/CTG/A665.
Contributor Information
Daniela Lenggenhager, Email: daniela.lenggenhager@usz.ch.
Susan Bengs, Email: susan.bengs@usz.ch.
Ralph Fritsch, Email: ralph.fritsch@usz.ch.
Saskia Hussung, Email: saskia.hussung@usz.ch.
Philipp Busenhart, Email: philipp.busenhart@usz.ch.
Katharina Endhardt, Email: k.endhardt@outlook.com.
Antonia Töpfer, Email: antonia.toepfer@usz.ch.
Frans Olivier The, Email: fransolivier.the@usz.ch.
Simon Bütikofer, Email: simon.buetikofer@luks.ch.
Christoph Gubler, Email: christoph.gubler@usz.ch.
Bernhard Morell, Email: bernhard.morell@usz.ch.
REFERENCES
- 1.Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet 2004;363:1049–57. [DOI] [PubMed] [Google Scholar]
- 2.Cwik G, Wallner G, Skoczylas T, et al. Cancer antigens 19-9 and 125 in the differential diagnosis of pancreatic mass lesions. Arch Surg 2006;141:968–74. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- 4.Pleskow DK, Berger HJ, Gyves J, et al. Evaluation of a serologic marker, CA19-9, in the diagnosis of pancreatic cancer. Ann Intern Med 1989;110:704–9. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018;359:926–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahrmann JF, Bantis LE, Capello M, et al. A plasma-derived protein-metabolite multiplexed panel for early-stage pancreatic cancer. J Natl Cancer Inst 2019;111:372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Lin P, Gao C, et al. Integrin beta6 acts as an unfavorable prognostic indicator and promotes cellular malignant behaviors via ERK-ETS1 pathway in pancreatic ductal adenocarcinoma (PDAC). Tumor Biol 2016;37:5117–31. [DOI] [PubMed] [Google Scholar]
- 8.Breuss JM, Gillett N, Lu L, et al. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem 1993;41:1521–7. [DOI] [PubMed] [Google Scholar]
- 9.Breuss JM, Gallo J, DeLisser HM, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 1995;108:2241–51. [DOI] [PubMed] [Google Scholar]
- 10.Sipos B, Hahn D, Carceller A, et al. Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology 2004;45:226–6. [DOI] [PubMed] [Google Scholar]
- 11.Bengs S, Becker E, Busenhart P, et al. Beta6-integrin serves as a novel serum tumor marker for colorectal carcinoma. Int J Cancer 2019;145:678–5. [DOI] [PubMed] [Google Scholar]
- 12.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor Marker prognostic studies (REMARK). Nat Clin Pract Urol 2005;2:416–2. [PubMed] [Google Scholar]
- 13.Akhoundova D, Hussung S, Fritsch RM. Precision oncology for hepato-pancreato-biliary (HPB) cancers: State of the art and future directions. Healthbook TIMES Onco Hema 2020;5:52–9. [Google Scholar]
- 14.Kleeff J, Korc M, Apte, et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- 15.Paganuzzi M, Onetto M, Marroni P, et al. CA 19-9 and CA 50 in benign and malignant pancreatic and biliary diseases. Cancer 1988;61:2100–8. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Kim MH, Myung SJ, et al. A new strategy for the application of CA19-9 in the differentiation of pancreaticobiliary cancer: Analysis using a receiver operating characteristic curve. Am J Gastroenterol 1999;94:1941–6. [DOI] [PubMed] [Google Scholar]
- 17.Lamerz R. Role of tumour markers, cytogenetics. Ann Oncol 1999;10:145–9. [PubMed] [Google Scholar]
- 18.Tempero MA, Uchida E, Takasaki H, et al. Relationship of carbohydrate antigen 19-9 and Lewis antiges in panreatic cancer. Cancer Res 1987;47:5501–3. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.