Abstract
Background:
Perioperative hyperglycemia can have an even more detrimental effect on postoperative outcomes in patients without diabetes than in patients with diabetes, but it has not been established if the treatment of patients without diabetes is safe and effective. We hypothesized that sliding-scale insulin for severe postoperative hyperglycemia (glucose ≥180 mg/dL) could lower mean postoperative glucose levels and minimize short-term complications in patients without diabetes undergoing major joint replacement.
Methods:
In a prospective study group, 1,398 consecutive patients, with and without diabetes, undergoing joint replacement were monitored and treated for hyperglycemia and were compared with 886 historical, less frequently monitored controls. The primary outcome was the mean glucose level in patients with and without diabetes within 48 hours after the surgical procedure. Two secondary outcomes could be examined only in the prospective study group, which, by design, had much more frequent glucose sampling and insulin use than the historical controls. First, the contribution of comorbidities and procedural factors to postoperative hyperglycemia in patients without diabetes was assessed with multivariable linear regression. Second, the ability of insulin treatment to reduce complications in patients without diabetes who developed hyperglycemia was evaluated.
Results:
In comparison with 886 historical controls, enhanced glucose management lowered the mean glucose (and standard deviation) from 129 ± 28 mg/dL to 123 ± 23 mg/dL for patients without diabetes (p = 0.041). Multivariable linear regression revealed factors that contributed to elevated mean glucose in patients without diabetes: preoperative fasting glucose (p < 0.001), perioperative steroid use (p < 0.001), general anesthesia (p < 0.001), procedure duration (p = 0.003), and transfusion (p 0.008). Of 968 patients without diabetes, 203 developed severe hyperglycemia. The recommended insulin coverage was given to 129 of these patients, and 74 patients did not receive it for various clinical reasons. Insulin treatment reduced the frequency of positive cultures from any site (p = 0.025) and a composite of positive cultures and readmissions (p = 0.006) in comparison with no insulin treatment. No patient without diabetes who received insulin experienced mild or severe hypoglycemia.
Conclusions:
Postoperative hyperglycemia is frequent in patients without diabetes after orthopaedic surgery, but an enhanced glucose management program can lower mean postoperative glucose levels. The treatment of hyperglycemia in patients without diabetes reduced short-term complications and was associated with minimal side effects.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
Hyperglycemia is a potentially modifiable risk factor for a variety of complications after a surgical procedure, including infections1. Both the World Health Organization (WHO) and U.S. Centers for Disease Control and Prevention (CDC) recommend perioperative glucose control2,3, but there is debate over which surgical patients should be treated and what protocol would maximize benefits and minimize side effects. For many surgical specialties, perioperative glucose control was envisioned initially as important for patients with diabetes.
Recently, a stronger link between postoperative hyperglycemia and adverse outcomes in patients without diabetes than in patients with diabetes has become evident, suggesting that patients without diabetes may benefit especially from glucose control4-6. Whether insulin can counteract the effects of postoperative hyperglycemia in patients without diabetes has not been established.
Studies on patients after major joint surgery have demonstrated a worrisome level of perioperative hyperglycemia in patients without diabetes. Jämsen et al. noted that the predominant risk factor for postoperative hyperglycemia was the preoperative metabolic status of the patient and that hyperglycemia was a common occurrence. Up to 40% of orthopaedic patients developed glucose values of >7.8 mmol/L on 2 occasions, with 25% of patients demonstrating values of >10 mmol/L, levels consistent with stress (140 to 179 mg/dL) and severe postoperative hyperglycemia (≥180 mg/dL)7. Unless surveillance is thorough, meaningful elevations in glucose after major joint surgery might go unnoticed8.
Because studies have demonstrated adverse outcomes in orthopaedic patients associated with hyperglycemia9-11, we hypothesized that the outcomes of orthopaedic patients without diabetes might be improved with stricter glucose control.
In this study, patients with and without diabetes underwent comprehensive surveillance and treatment of postoperative hyperglycemia. The primary outcome was a comparison of mean glucose, within the first 48 hours after the surgical procedure, between a prospective interventional study group treated with enhanced glucose management and a group of historical controls treated with standard practice.
As a secondary outcome, we evaluated the risk factors for postoperative hyperglycemia, and the effects of insulin treatment on the prevention of complications in patients without diabetes who developed hyperglycemia. Secondary outcomes were necessarily confined to the prospective study group because our normal practice during the historical control period was not to monitor patients without diabetes for hyperglycemia or routinely treat them with insulin.
Materials and Methods
The historical controls consisted of 886 consecutive patients who underwent joint replacement surgery from August 1, 2016, to December 21, 2017. There was no standardized institutional approach for the evaluation of hyperglycemia in patients without diabetes, and only patients with diabetes were monitored and treated.
Enhanced Glucose Management
From January 16, 2018, until March 16, 2020, 1,398 patients were treated with the enhanced glucose management. All patient charts were screened preoperatively, and patients who were suspected to have undiagnosed or poorly controlled diabetes were referred to the endocrinology service and were included in this study. Hemoglobin A1C (HbA1C) tests were not routinely performed but were often available in the patient’s history. Two clinical pharmacists administered the program.
Patients had a fasting glucose measurement taken on the morning of the surgical procedure. A patient was considered to have diabetes if there was a preoperative history of diabetes, if the HbA1C value was ≥6.5%, or if the fasting blood glucose was >125 mg/dL. A patient was considered to have prediabetes if the HbA1C was between 5.7% and 6.4%, or if the fasting blood glucose was between 100 and 125 mg/dL. A patient with a normal metabolic state had a normal HbA1C, if measured, and a fasting glucose of <100 mg/dL. Classifying patients as having or not having diabetes with this definition, instead of using a history of diabetes, increased the percentage of patients with diabetes to 31.1% from 22% in the protocol patients and to 27.7% from 22% in the historical controls.
Patients with insulin-dependent diabetes were given instructions on how to adjust the insulin in the preoperative period. Similarly, patients with non-insulin-dependent diabetes had their oral medications adjusted consistent with recent recommendations12.
Point-of-care finger-stick glucose samples were taken in the post-anesthesia care unit (PACU), pre-dinner, at bedtime, and in the morning after the surgical procedure. Sliding-scale insulin (lispro) was used to treat severe hyperglycemia in patients without diabetes. Patients with non-insulin-dependent diabetes were readministered their oral medications and were also given sliding-scale insulin for glucose values of ≥180 mg/dL. Patients with insulin-dependent diabetes received basal/bolus insulin injections. This approach is fashioned after an Emory University program, and we refer to it as enhanced glucose management (see the Appendix for details)12.
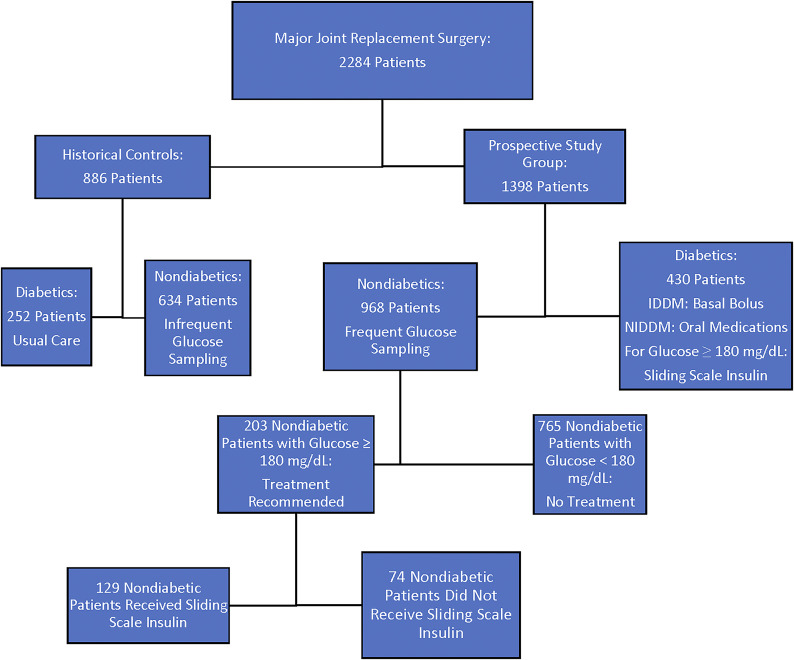
A flowchart of the study design is seen in Figure 1.
Fig. 1.
Flowchart of the study patients. IDDM = insulin-dependent diabetes mellitus, and NIDDM = non-insulin-dependent diabetes mellitus.
Outcome Measures
The primary outcome was the mean glucose within 48 hours, or until discharge, if earlier, of the historical controls and the prospective study group, in patients with and without diabetes.
The secondary outcomes were risk factors for postoperative hyperglycemia and the effect of insulin treatment on complications (positive clinical cultures, 90-day readmissions, 90-day emergency room [ER] visits) in patients without diabetes in the prospective study group.
All culture reports were extracted from the electronic medical record (Epic) starting the day after the surgical procedure through 30 days. A result was considered positive if pathogenic bacteria were identified, not normal flora. Mixed urogenital organisms were not considered to be positive cultures. The end point was a patient with ≥1 positive culture, which categorized a patient as having a positive clinical culture.
A patient was considered to have a complication of a readmission or ER visit if there were 1 or more readmissions or ER visits within 90 days of discharge.
The study was approved by the institutional review board.
Statistical Analysis
Data for continuous variables were described as the mean and the standard deviation. Two-sample t and z tests, and tests for 2 proportions, were used to compare groups. A repeated-measures analysis of variance (ANOVA) was used to examine the differences in mean glucose values over time. Linear and logistic regression were used to assess variables for their association with postoperative hyperglycemia, positive clinical cultures, and readmissions or ER visits.
Source of Funding
There was no source of funding for this study.
Results
The patient and surgical procedure characteristics of the prospective study group and historical controls are described in Table I.
TABLE I.
Patient and Procedure-Related Characteristics of Historical Control and Glucose Protocol Groups
| Historical Control Group (N = 886) | Glucose Protocol Group (N = 1,398) | P Value | |
|---|---|---|---|
| Patient characteristics | |||
| Male sex* | 45.50% | 38.30% | 0.003 |
| Age† (yr) | 66 ± 10.5 | 69 ± 9.5 | <0.0001 |
| History of diabetes* | 21.70% | 22.50% | 0.69 |
| Body mass index† (kg/m2) | 33 ± 6.9 | 33 ± 7.5 | 0.76 |
| HbA1C† (%) | 6.22 ± 1.0 | 6.19 ± 1.0 | 0.976 |
| Hematocrit† (vol%) | 39.8 ± 5.0 | 38.8 ± 4.8 | <0.0001 |
| Creatinine† (mg/dL) | 0.95 ± 0.4 | 0.96 ± 0.4 | 0.68 |
| Surgery characteristics | |||
| Total knee replacement‡ | 545 | 874 | 0.63 |
| Knee revisions‡ | 38 | 56 | 0.74 |
| Total hip replacement‡ | 294 | 445 | 0.5 |
| Hip revisions‡ | 9 | 23 | 0.21 |
| Procedure duration† (min) | 153 ± 43 | 142 ± 45 | <0.0001 |
| Mean transfusion† (mL) | 38 ± 305 | 35 ± 261 | 0.79 |
| Steroids for nausea* | 49% | 61% | <0.0001 |
| General anesthesia* | 27% | 31% | 0.05 |
The values are given as the percentage of patients.
The values are given as the mean and the standard deviation.
The values are given as the number of patients.
A Comparison of the Prospective Study Group and Historical Controls
Mean Glucose Values
The mean glucose values for the patients in the prospective study group were lower than those for the historical controls (Fig. 2). The difference was significant at all time points (p < 0.001), including the mean over 48 hours. However, the protocol treatment had a significant effect (p = 0.041) only in patients without diabetes, who had a 48-hour mean level of 123 ± 23 mg/dL, compared with a level of 129 ± 28 mg/dL in historical controls. In contrast, the 48-hour mean glucose values in patients with diabetes were similar (p = 0.140) in the prospective study group (162 ± 38 mg/dL) and the historical controls (167 ± 44 mg/dL). Even though patients with diabetes had a less impressive reduction in glucose overall with enhanced glucose management, glucose values were lower on the morning after the surgical procedure (p < 0.001), a time point when hyperglycemia has been associated with increased infection risk10. See the Appendix for detailed glucose values.
Fig. 2.
A line graph showing the mean glucose values (mg/dL) of the prospective study group and of the historical controls at different time points within the first 24 hours after the surgical procedure. The star symbols represent p < 0.05. The mean and the standard deviation is shown for each time point.
Differences in Insulin Treatment
Postoperative insulin use was common for patients without diabetes in the prospective study group, and it was rare in the historical controls, as seen in Figure 3. With glucose management, there was a significant increase in insulin use in both patients with and without diabetes (p < 0.0001).
Fig. 3.
A bar graph showing the percentage of patients with and without diabetes who received insulin in the historical controls compared with the prospective study group.
There was no difference in the overall short-term complications between the prospective study group and the historical controls (Table II). However, it was not possible to define a subgroup of patients without diabetes with severe2 hyperglycemia in the historical control group because of less frequent glucose sampling. Glucose sampling in the prospective study group occurred at 75.7% of the prescribed time points, in comparison with 18.4% in the historical control group (p < 0.0001).
TABLE II.
Complications in Patients with and without Diabetes
| Historical Control Group* | Glucose Management Protocol Group* | P Value | |
|---|---|---|---|
| Patients without diabetes | |||
| No. of patients | 634 | 968 | |
| Readmissions within 90 days | 51 (8.0%) | 74 (7.6%) | 0.771 |
| ER visits within 90 days | 94 (14.8%) | 129 (13.3%) | 0.396 |
| Positive clinical cultures | 13 (2.1%) | 26 (2.7%) | 0.420 |
| Total complications | 158 (24.9%) | 229 (23.7%) | 0.563 |
| Patients with diabetes | |||
| No. of patients | 252 | 430 | |
| Readmissions within 90 days | 30 (11.9%) | 41 (9.5%) | 0.328 |
| ER visits within 90 days | 48 (19.0%) | 74 (17.2%) | 0.545 |
| Positive clinical cultures | 7 (2.8%) | 16 (3.7%) | 0.510 |
| Total complications | 85 (33.7%) | 131 (30.5%) | 0.376 |
The values are given as the number of patients, with or without the percentage in parentheses.
The Prospective Study Group
Determinants of Postoperative Hyperglycemia
The prospective study group allowed a more complete examination of perioperative glucose changes in patients without diabetes. The perioperative glucose levels in the patients in the prospective study group within the first 24 hours after the surgical procedure are shown in Figure 4. A 1-way repeated-measures ANOVA showed that glucose values were significantly different at the 4 time points (p < 0.0001). The time point with the highest mean glucose was bedtime, but even in the morning after the surgical procedure, the mean glucose was still higher than the preoperative value.
Fig. 4.
A box-and-whisker plot showing the glucose values from the prospective study group. Postoperative sampling in the historical controls was too infrequent to be used in this type of analysis. The time points are during the preoperative fasting and at the 4 sampling times in the first 24 hours after the surgical procedure. The line within the box represents the median, and the x represents the mean. The whiskers extend from the box for a distance of 1.5 times the interquartile range. The circles indicate outliers. The glucose values are of all of the patients in the prospective study group: the patients with normal glucose metabolism, patients with prediabetes, and patients with diabetes. The glucose values were significantly different at each time point (p < 0.0001).
Even though the mean postoperative glucose in the patients without diabetes was much lower than in the patients with diabetes, a worrisome number of patients without diabetes developed severe hyperglycemia. Sixteen percent of patients with normal preoperative glucose levels and 26% of patients with prediabetes developed glucose values of ≥180 mg/dL. Also, >30% of patients with normal preoperative glucose levels and patients with prediabetes experienced at least 1 glucose value in the range of 140 to 179 mg/dL (Fig. 5).
Fig. 5.
The percentage of patients in the normal, prediabetic, and diabetic groups who developed postoperative hyperglycemia. The expected postoperative glucose was <140 mg/dL, mild postoperative hyperglycemia was glucose ≥140 mg/dL but <180 mg/dL, and severe postoperative hyperglycemia was glucose ≥180 mg/dL.
Linear regression was performed so that predictors of elevated postoperative glucose could be identified for the patients with and without diabetes. Demographic and procedure-related independent predictors were included in both models. The multivariate regressions are seen in Tables III and IV. The most important variable predicting elevated postoperative glucose for both patients with diabetes and patients without diabetes was preoperative fasting glucose (p < 0.001 for both). In patients without diabetes, hyperglycemia was also significantly associated with general anesthesia (p < 0.001), the use of steroids to control postoperative nausea (p < 0.001), procedure duration (p < 0.003), and transfusions (p < 0.008). The explanatory factors for hyperglycemia were similar in patients with diabetes, except for a closer association with HbA1C and less of an effect from general anesthesia.
TABLE III.
Multiple Linear Regression Demonstrating Variables Associated with the Mean Glucose for Patients without Diabetes
| Variable | β* | P Value† |
|---|---|---|
| Preoperative glucose (mg/dL) | 0.64 (0.52 to 0.75) | <0.001 |
| Procedure duration (min) | 0.05 (0.02 to 0.09) | 0.003 |
| Blood transfusion (mL) | 0.01 (0.00 to 0.02) | 0.008 |
| Steroid for nausea control | 12.19 (9.44 to 14.94) | <0.001 |
| General anesthesia | 6.54 (3.49 to 9.59) | <0.001 |
The values are given as the β coefficient, with the 95% confidence interval in parentheses.
R2 = 0.23.
TABLE IV.
Multiple Linear Regression Demonstrating Variables Associated with the Mean Glucose for Patients with Diabetes
| Variable | β* | P Value† |
|---|---|---|
| Preoperative glucose (mg/dL) | 0.48 (0.37 to 0.58) | <0.001 |
| HbA1C (%) | 13.41 (9.25 to 17.58) | <0.001 |
| Blood transfusion (mL) | 0.01 (0.00 to 0.02) | 0.02 |
| Steroid for nausea control | 13.69 (6.84 to 20.54) | <0.001 |
| General anesthesia | 5.27 (−2.05 to 12.59) | 0.16 |
The values are given as the β coefficient, with the 95% confidence interval in parentheses.
R2 = 0.40.
The Effect of Insulin on Clinical Outcomes
In the prospective study group, the protocol called for supplemental insulin to be given to patients without diabetes with severe hyperglycemia (glucose ≥180 mg/dL). In practice, one-third of patients did not receive insulin, due to a combination of clinical factors. A significant reduction (p < 0.025) in the frequency of positive cultures from any site occurred when patients without diabetes but with severe hyperglycemia were given insulin, compared with those in this group who did not receive insulin (Fig. 6). Logistic regression with backward elimination was used to adjust for confounders, and insulin usage had the largest relative effect size in this population on the outcome of positive clinical cultures, followed by preoperative anemia (Table V). The effect of insulin was then compared with other potential predictors of positive clinical cultures in all patients without diabetes. Insulin usage had a larger effect on reducing positive cultures than the avoidance of general anesthesia (Table VI), which has been associated with infections after joint surgery13,14.
Fig. 6.
A bar graph showing the percentage of patients without diabetes in the prospective study group who developed any positive clinical culture. These patients were categorized according to their degree of postoperative hyperglycemia: expected glucose (glucose <140 mg/dL), mild hyperglycemia (glucose ≥140 mg/dL but <180 mg/dL), or severe hyperglycemia (glucose ≥180 mg/dL). The patients in the severe group were divided into those who did or did not receive insulin.
TABLE V.
Multiple Logistic Regression Demonstrating Variables Associated with Positive Clinical Cultures in Patients without Diabetes with Severe Hyperglycemia
| Variable | Adjusted Odds Ratio* | P Value |
|---|---|---|
| Hematocrit (vol%) | 0.840 (0.703 to 0.991) | 0.04 |
| Insulin | 0.059 (0.003 to 0.385) | 0.01 |
The values are given as the adjusted odds ratio, with the 95% confidence interval in parentheses.
TABLE VI.
Multiple Logistic Regression Demonstrating Variables Associated with Positive Clinical Cultures for All Patients without Diabetes
| Variable | Adjusted Odds Ratio* | P Value |
|---|---|---|
| Age (yr) | 1.040 (0.997 to 1.088) | 0.08 |
| Hematocrit (vol%) | 0.886 (0.815 to 0.963) | 0.004 |
| Peak postoperative glucose (mg/dL) | 1.008 (0.998 to 1.018) | 0.08 |
| Procedure duration (min) | 0.994 (0.983 to 1.004) | 0.26 |
| General anesthesia | 2.532 (1.081 to 5.930) | 0.03 |
| Insulin | 0.095 (0.005 to 0.567) | 0.03 |
The values are given as the adjusted odds ratio, with the 95% confidence interval in parentheses.
The effect of severe hyperglycemia on readmissions of patients without diabetes was also evaluated, and the reduction of readmissions with insulin use did not reach significance (p = 0.078) in this subgroup (Fig. 7). However, the combined end point of infections and readmissions was lower in the patients who were given insulin (p < 0.006). Multivariable logistic regression of patients without diabetes with postoperative glucose of ≥180 mg/dL did reveal that higher hematocrit and insulin use contributed to a significant reduction in readmissions (p = 0.02) (Table VII).
Fig. 7.
A bar graph showing the percentage of 90-day readmissions for patients without diabetes in the prospective study group. These patients were categorized according to their degree of postoperative hyperglycemia: expected glucose (glucose <140 mg/dL), mild hyperglycemia (glucose ≥140 mg/dL but <180 mg/dL), or severe hyperglycemia (glucose ≥180 mg/dL). The patients in the severe group were divided into those who did or did not receive insulin.
TABLE VII.
Multiple Logistic Regression Demonstrating Variables Associated with Readmission in Patients without Diabetes with Severe Hyperglycemia
| Variable | Adjusted Odds Ratio* | P Value |
|---|---|---|
| Hematocrit (vol%) | 0.829 (0.740 to 0.921) | 0.0007 |
| Insulin | 0.276 (0.090 to 0.795) | 0.02 |
The values are given as the adjusted odds ratio, with the 95% confidence interval in parentheses.
Hypoglycemia
Insulin use was associated with few serious side effects. There were no cases of severe hypoglycemia (<54 mg/dL) in the prospective study group or the control group. In the prospective study group, 10 patients with diabetes developed mild hypoglycemia (<70 mg/dL, but >54 mg/dL), with 8 patients being given insulin. Eighteen patients without diabetes developed mild hypoglycemia, and none were given insulin.
Discussion
An unproven rationale to justify increased postoperative glucose surveillance is that the outcomes of patients without diabetes might be improved with treatment. In this study, more thorough monitoring and treatment of postoperative hyperglycemia early after major joint surgery lowered mean postoperative glucose levels, predominantly in patients without diabetes, and caused no increase in hypoglycemia. In the prospective study group, we demonstrated that postoperative hyperglycemia and postoperative complications were lessened with insulin treatment in patients without diabetes.
Importance of Close Glucose Monitoring
Only 47% of surgical patients have any glucose sampling postoperatively, with patients without diabetes being less vigilantly monitored5. The infrequent sampling in our historical controls was similar, probably arising from the wish to avoid the discomfort of frequent finger sticks and patient anxiety regarding diabetes. With this background, a practical question for any surgical specialty is whether it is worth it to stratify the preoperative metabolic status of each patient and to monitor all patients for postoperative hyperglycemia. Our results suggest that it may be worth the effort. There may also be other advantages that were not studied. Identifying patients with prediabetes might help to persuade a high-risk group to make lifestyle changes to avoid diabetes. Finally, for patients taking oral antidiabetic medications alone, sliding-scale insulin can control glucose levels during the process of those medications being readministered postoperatively.
When postoperative hyperglycemia is recognized, there is no consensus for an optimal treatment protocol. This is understandable because a universal approach for glucose control after a surgical procedure is not practical. Glucose control in orthopaedic patients is challenging: a high percentage of patients have diabetes or prediabetes, resume an oral diet early after the surgical procedure, and are discharged from the hospital within 1 to 2 days. Short-acting insulin appears to be a suitable agent to treat the transient and unpredictable hyperglycemia in orthopaedic patients without diabetes, and its use might be less complex than other approaches to lowering glucose15.
Glucose Levels in Historical Controls and the Prospective Study Group
The increased insulin use lowered the mean glucose in patients without diabetes in the prospective study group compared with historical controls. A limitation of this comparison is that there was a less complete glucose sampling of the historical controls. However, given that most glucose samples in the historical control group were done in the PACU, where values were the lowest postoperatively, we suspect that more complete sampling would have revealed an even greater difference between the 2 groups.
Hyperglycemia and Short-Term Outcomes
In the prospective study group, insulin reduced complications in patients without diabetes with severe hyperglycemia. This would imply that the patients without diabetes in the prospective study group should have had fewer complications than those in the historical control group, in whom little insulin was used. There may be several methodological reasons why this was not observed. First, the use of insulin would reasonably be expected to affect only patients without diabetes with hyperglycemia, which requires comprehensive postoperative sampling to define. It was not possible to directly compare the patients without diabetes with hyperglycemia from both groups because of less frequent glucose sampling in the historical control group. Second, insulin was administered only to the 21% of patients without diabetes who developed severe hyperglycemia; a comparison of all patients without diabetes might have obscured a possible difference in outcomes. Finally, because of less preoperative sampling in the historical controls, it was also more difficult to precisely separate patients without diabetes from those with diabetes.
Previous studies have indicated that the relationship between perioperative hyperglycemia and postoperative infection and readmission rates is different for patients with and without diabetes16. Patients with diabetes have a higher rate of postoperative infections and readmissions, but these complications are not mediated by perioperative hyperglycemia, possibly because insulin is given. In patients without diabetes, postoperative complication rates are lower, but, if insulin is not given, there is a dose-response relationship between glucose levels and complications5. In our prospective study, 2 contemporaneous groups of patients without diabetes with hyperglycemia were created by the practical difficulties in compliance with a protocol, and there was a significant reduction in complications in those patients who received insulin. The improvement in outcomes is consistent with observations in general surgical patients. Ramos et al. estimated that there was a 30% increase in infections for each 40-mg/dL increase in the first postoperative blood sample within a week of the surgical procedure17. In our study, we estimated that there was an approximately 60-mg/dL drop in glucose levels after insulin administration, which was associated with a significant drop in complications.
Complications from hyperglycemia in patients without diabetes might also be lessened by decreasing the use of factors that contribute to postoperative hyperglycemia, such as steroids for nausea control. Comprehensive perioperative glucose monitoring might help both in the avoidance and treatment of hyperglycemia.
Hypoglycemia
Protocols to identify and treat perioperative hyperglycemia can be complicated by hypoglycemia18. In this study population, and with this treatment protocol, severe hypoglycemia was uncommon, which makes it reasonable to attempt to avoid the complications from hyperglycemia19,20 even in patients without diabetes6,10.
Limitations
There were limitations to the study. First, the glucose levels of the prospective study group were compared with historical controls, in whom glucose sampling was less frequent. Second, the patients without diabetes in the study group were not randomized to receive or not receive insulin when they had hyperglycemia, but rather some patients did not receive insulin because of incomplete protocol compliance. Third, the clinical context may have been needed to interpret the meaning of positive clinical cultures. Finally, the effects of other institutional quality improvements to reduce infections may not have been applied equally across all patients. We interpret the evidence from our study as hypothesis-generating, but consistent with the idea that there is benefit from a more rigorous glucose control in patients without diabetes.
Conclusions
Multiple factors are associated with hyperglycemia after major joint replacement, some of which are modifiable. A program to comprehensively monitor glucose values lowers mean glucose levels and defines a subgroup of patients without diabetes with severe hyperglycemia. Treatment with insulin reduces complications in this subgroup. The low incidence of hypoglycemia and the apparent effect of insulin treatment justify continued interest in this area.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJSOA/A302).
Acknowledgments
Note: The authors thank Dain Chun for her help with statistical analysis.
Footnotes
Investigation performed at Bayhealth Medical Center, Dover, Delaware
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A301).
References
- 1.Ban KA, Minei JP, Laronga C, Harbrecht BG, Jensen EH, Fry DE, Itani KM, Dellinger EP, Ko CY, Duane TM. American College of Surgeons and Surgical Infection Society: surgical site infection guidelines, 2016 update. J Am Coll Surg. 2017. Jan;224(1):59-74. Epub 2016 Nov 30. [DOI] [PubMed] [Google Scholar]
- 2.Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, Reinke CE, Morgan S, Solomkin JS, Mazuski JE, Dellinger EP, Itani KMF, Berbari EF, Segreti J, Parvizi J, Blanchard J, Allen G, Kluytmans JAJW, Donlan R, Schecter WP; Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017. Aug 1;152(8):784-91. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global guidelines on the prevention of surgical site infection. 2016. Accessed 2019 Aug 26. http://www.who.int/gpsc/ssi-prevention-guidelines/en/ [Google Scholar]
- 4.Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013. Jan;257(1):8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Dellinger EP, Farrokhi ET, Flum DR; SCOAP-CERTAIN Collaborative. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015. Jan;261(1):97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair BG, Neradilek MB, Newman SF, Horibe M. Association between acute phase perioperative glucose parameters and postoperative outcomes in diabetic and non-diabetic patients undergoing non-cardiac surgery. Am J Surg. 2019. Aug;218(2):302-10. Epub 2018 Oct 16. [DOI] [PubMed] [Google Scholar]
- 7.Jämsen E, Nevalainen PI, Eskelinen A, Kalliovalkama J, Moilanen T. Risk factors for perioperative hyperglycemia in primary hip and knee replacements. Acta Orthop. 2015. Apr;86(2):175-82. Epub 2014 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varady NH, Schwab PE, Jones T, Collins JE, Fitz W, Chen AF. Optimal timing of glucose measurements after total joint arthroplasty. J Arthroplasty. 2019. Jul;34(7S):S152-8. Epub 2019 Jan 9. [DOI] [PubMed] [Google Scholar]
- 9.Mraovic B, Suh D, Jacovides C, Parvizi J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011. Mar 1;5(2):412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kheir MM, Tan TL, Kheir M, Maltenfort MG, Chen AF. Postoperative blood glucose levels predict infection after total joint arthroplasty. J Bone Joint Surg Am. 2018. Aug 15;100(16):1423-31. [DOI] [PubMed] [Google Scholar]
- 11.Stryker LS. Modifying risk factors: strategies that work diabetes mellitus. J Arthroplasty. 2016. Aug;31(8):1625-7. Epub 2016 Mar 29. [DOI] [PubMed] [Google Scholar]
- 12.Duggan EW, Carlson K, Umpierrez GE. Perioperative hyperglycemia management: an update. Anesthesiology. 2017. Mar;126(3):547-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Ma C, Elkassabany N, Fleisher LA, Neuman MD. Neuraxial anesthesia decreases postoperative systemic infection risk compared with general anesthesia in knee arthroplasty. Anesth Analg. 2013. Oct;117(4):1010-6. Epub 2013 Sep 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholten R, Leijtens B, Hannink G, Kamphuis ET, Somford MP, van Susante JLC. General anesthesia might be associated with early periprosthetic joint infection: an observational study of 3,909 arthroplasties. Acta Orthop. 2019. Dec;90(6):554-8. Epub 2019 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher JM, Erich RA, Gattermeyer R, Beam KK. Postoperative hyperglycemia can be safely and effectively controlled in both diabetic and nondiabetic patients with use of a subcutaneous insulin protocol. JB JS Open Access. 2017. Feb 14;2(1):e0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones CE, Graham LA, Morris MS, Richman JS, Hollis RH, Wahl TS, Copeland LA, Burns EA, Itani KMF, Hawn MT. Association between preoperative hemoglobin A1c levels, postoperative hyperglycemia, and readmissions following gastrointestinal surgery. JAMA Surg. 2017. Nov 1;152(11):1031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos M, Khalpey Z, Lipsitz S, Steinberg J, Panizales MT, Zinner M, Rogers SO. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg. 2008. Oct;248(4):585-91. [DOI] [PubMed] [Google Scholar]
- 18.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ; NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009. Mar 26;360(13):1283-97. Epub 2009 Mar 24. [DOI] [PubMed] [Google Scholar]
- 19.Kang ZQ, Huo JL, Zhai XJ. Effects of perioperative tight glycemic control on postoperative outcomes: a meta-analysis. Endocr Connect. 2018. Dec 1;7(12):R316-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vries FEE, Gans SL, Solomkin JS, Allegranzi B, Egger M, Dellinger EP, Boermeester MA. Meta-analysis of lower perioperative blood glucose target levels for reduction of surgical-site infection. Br J Surg. 2017. Jan;104(2):e95-105. Epub 2016 Nov 30. [DOI] [PubMed] [Google Scholar]