Supplemental Digital Content is available in the text.
Keywords: phthalates, ADHD, Attention Deficit Hyperactivity Disorder, biomarkers, prenatal, gestational, MoBa, The Norwegian Mother, Father and Child Cohort Study
Abstract
Prenatal phthalate exposure has been linked to altered neurobehavioral development in both animal models and epidemiologic studies, but whether or not these associations translate to increased risk of neurodevelopmental disorders is unclear. We used a nested case-cohort study design to assess whether maternal urinary concentrations of 12 phthalate metabolites at 17 weeks gestation were associated with criteria for Attention Deficit Hyperactivity Disorder (ADHD) classified among 3-year-old children in the Norwegian Mother, Father and Child Cohort Study (MoBa). Between 2007 and 2011, 260 children in this substudy were classified with ADHD using a standardized, on-site clinical assessment; they were compared with 549 population-based controls. We modeled phthalate levels both linearly and by quintiles in logistic regression models adjusted for relevant covariates and tested for interaction by child sex. Children of mothers in the highest quintile of di-iso-nonyl phthalate (∑DiNP) metabolite levels had 1.70 times the odds of being classified with ADHD compared with those in the lowest quintile (95% confidence interval [CI] = 1.03 to 2.82). In linear models, there was a trend with the sum of di-2-ethylhexyl phthalate metabolites (∑DEHP); each natural log-unit increase in concentration was associated with 1.22 times the odds of ADHD (95% CI = 0.99 to 1.52). In boys, but not girls, mono-n-butyl phthalate exposure was associated with increased odds of ADHD (odds ratio [OR] 1.42; 95% CI = 1.07 to 1.88). Additional adjustment for correlated phthalate metabolites attenuated estimates. These results suggest gestational phthalate exposure may impact the behavior of children as young as 3 years.
What this study adds
Epidemiologic evidence suggests prenatal exposure to phthalates is associated with emotional and behavioral difficulties in children. This is the first study of prenatal phthalate exposure to use a standardized, validated diagnostic interview to ascertain ADHD-like symptoms in preschool-aged children. Our results support prior research showing a positive association between gestational exposure to DEHP metabolites and risk of ADHD in childhood. We are the first to report an association between gestational exposure to DiNP metabolites and children’s neurobehavioral development. This research is an important step toward identifying modifiable risk factors for ADHD with important public health consequences.
Introduction
Phthalates are high-production volume chemicals used primarily as plasticizers in a broad array of consumer products.1,2 Sources of human exposure to phthalates vary by individual chemical. Some phthalates, including di(2-ethylhexyl) phthalate (DEHP), and increasingly, diisononyl phthalate (DiNP), are primarily consumed through contaminated food and drinking water.3,4 Others like diethyl phthalate (DEP), di-n-butyl phthalate (DnBP), diiosbutyl phthalate (DiBP), and butylbenzyl phthalate (BBzP) are typically found in consumer goods and personal care products.5,6
Because of the pervasive use of phthalates, and the ease with which they are leached from products into the environment, human exposure to phthalates is nearly ubiquitous in many countries.7,8 In North America, phthalate metabolites are consistently detected in nationally representative populations.7,9 In Norway, phthalates have been found at levels within ranges reported in studies worldwide, including among pregnant women.10–14 Of particular concern is that phthalate exposure in pregnant women can result in exposure to the developing fetus, as phthalates are known to cross the placenta.15
There is growing epidemiologic evidence linking prenatal phthalate exposure to emotional and behavioral difficulties in children.16–23 In prospective birth cohort studies, prenatal maternal phthalate levels have been associated with internalizing behaviors,19–22,24–27 externalizing behaviors,20,22,24,28,29 attention problems,24,29 and social or peer relationship problems in the child,19,21,25,30,31 with some studies showing stronger adverse effects among boys20,21,27,29,32 and one among girls.27 However, not all studies find associations between prenatal phthalates and adverse behavioral outcomes,26,30,33,34 and there is a lack of consistency across studies on the specific phthalate implicated and on the existence and direction of sex-specific associations.
We previously reported that Norwegian mothers in the highest quintile of gestational DEHP exposure had nearly three times the odds of having a child registered with hyperkinetic disorder (HKD) based on ICD-10 codes in the Norwegian Patient Registry (NPR).35 However, HKD requires the presence of hyperactive and inattentive symptoms, and thus is most similar to combined-type Attention Deficit Hyperactivity Disorder (ADHD) based on Diagnostic and Statistical Manual (DSM) criteria.36,37 Although NPR registration ensures rigor in the clinical standards applied to diagnosis, referrals are likely to be biased toward more severe cases with a larger degree of impairment.38,39 Moreover, clinical referral has been shown to depress the extent to which girls meeting diagnostic criteria are identified.40,41 Indeed, in our prior investigation that used NPR registration for case finding,35 girls comprised less than 30% of all ADHD cases, which may have undermined power to examine effect measure modification by child sex.
Clinically significant ADHD-like symptoms, which can result in substantial impairment and predict long-term functioning, often debut during the preschool period.42,43 The Norwegian Mother, Father and Child Cohort (MoBa) preschool ADHD substudy was established to examine social, environmental, and behavioral factors that may be etiological determinants of preschool ADHD. Nested within a large, population-based birth cohort, this study utilized the MoBa 36-month questionnaire to ascertain child ADHD-like symptoms that may be suggestive of maladaptive behavioral development, subsequently inviting these children in for a clinical evaluation.44,45 This approach may be less affected by referral biases in case-identification that result in underidentification of girls as well as less severe cases. We leveraged this high-quality assessment to examine the extent to which gestational phthalate exposure increased risk for preschool ADHD in a nested case-cohort subset of MoBa.
Methods
Study population
MoBa is an ongoing prospective population-based cohort study of over 100,000 mother-child pairs, enrolled between 1999 and 2008, conducted by the Norwegian Institute of Public Health.46,47 Pregnant women were recruited at their first ultrasound appointment, at approximately 17 weeks’ gestation, and responded to questionnaires at three time points during pregnancy (17, 22, and 30 weeks gestation). MoBa is also linked to the Medical Birth Registry of Norway (MBRN), providing information on pregnancy and birth records. Maternal biologic samples, including urine, were collected at approximately 17 weeks’ gestation.48 Questionnaires covering child development were obtained at multiple points after delivery.
Preschool ADHD substudy
The MoBa preschool ADHD substudy was initiated to ascertain prenatal and early childhood risk factors for this disorder. Eligibility for the preschool ADHD substudy was restricted to children who were born between April 2004 and January 2008 and who lived proximate to or within a direct flight to Oslo. Included in the 36-month MoBa questionnaire were 11 items about symptoms related to ADHD, including six items from the Child Behavior Checklist/1.5–549 and five items from the DSM-IV-TR criteria for ADHD.37 Item-specific numeric scores were assigned to responses and summed to form a quantitative index. All children meeting the eligibility criteria and scoring at or above the 90th percentile on the quantitative index (n = 2798), as well as a smaller group of randomly selected children from the eligible cohort (n = 654) were invited to participate in an on-site assessment of preschool ADHD. Among those invited, 1195 children (35%) aged 3.1–3.8 years agreed to participate in the substudy and took part in a 1-day clinical assessment in Oslo, including a diagnostic interview (conducted primarily with mothers) between 2007 and 2011. Mothers of these children were slightly older, more highly educated, and had fewer children than those who chose not to participate.50 Of those, 870 also had available maternal gestational urine samples stored in the MoBa Biobank48 (Figure 1).
Figure 1.
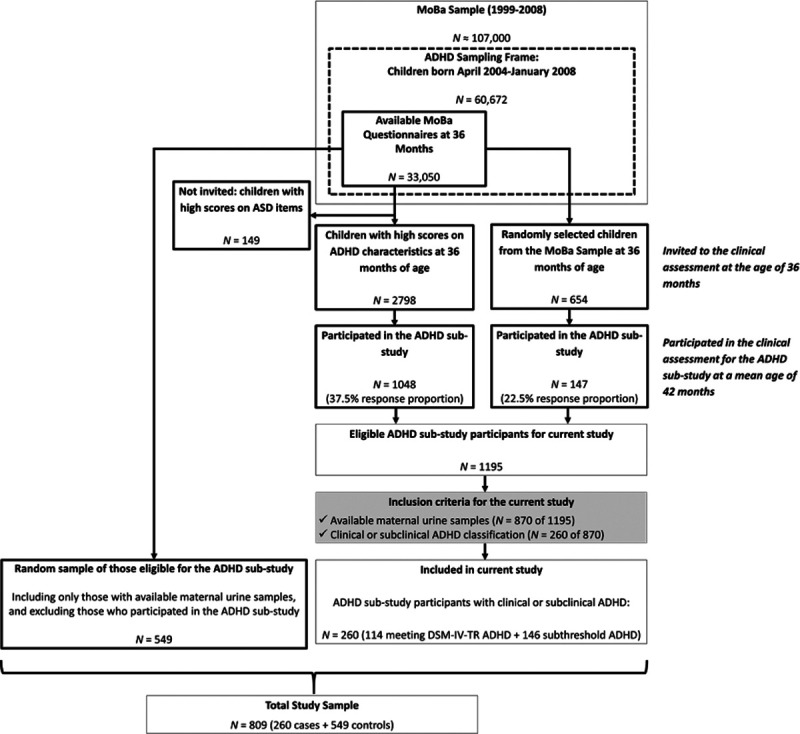
ADHD Substudy population selection diagram.
Children meeting criteria for ADHD or subthreshold ADHD were included as cases. Under the supervision of a child psychologist or psychiatrist, trained graduate psychology students conducted diagnostic interviews based on the Preschool Age Psychiatric Assessment (PAPA), designed to evaluate children aged 2–6 years of age and well-validated for use with preschoolers.51,52 Using the PAPA, ADHD symptoms were defined as present when reported by parents to be pervasive across at least two settings.53 Only symptoms lasting ≥3 months were counted as present. A separate rater, blind to the parent and teacher ratings, rescored audiotapes of 79 randomly selected assessment interviews. The average intraclass correlation (ICC) was 0.98 for the total number of ADHD symptoms. In addition, impairment or impact of symptoms in six functional domains (family relationships; friends; learning; play/leisure activities; child’s quality of life; and family burden) was scored on a four-point Likert scale. The functional domains gave a total impairment scale score (range 0–18). Scores of ≥2 indicated presence of impairment. ADHD was defined by the presence of both (a) ≥6 symptoms on the PAPA that met DSM-IV-TR37 criteria and (b) impairment. Children with six or more ADHD symptoms but without clear evidence of impairment, or with 3–5 ADHD symptoms and evidence of impairment, were classified as having subthreshold preschool ADHD. Among the clinically evaluated children (n = 1195), with available urine samples (n = 870), 114 children met the criteria for preschool ADHD, and an additional 146 children met the criteria for subthreshold symptoms of preschool ADHD. Although the ADHD classifications defined by the PAPA are not equivalent to a clinical ADHD diagnosis, which would require a more intensive assessment of multiple sources of information, children meeting criteria for either above threshold or subthreshold symptoms of ADHD are included as “cases” in this study (n = 260), hereafter referred to as “preschool ADHD cases.”
MoBa reference population “controls”
The controls in this nested case-cohort study were a stratified random sample of 556 children from among the 27,347 who were both eligible for the ADHD substudy and also whose mothers had available urine samples, frequency matched to preschool ADHD cases on year of birth. Because controls were randomly selected independent of their scores on the 3-year MoBa questionnaire and were not required to have completed an on-site ADHD clinical assessment, in theory, they reflect the distributions of phthalate metabolites and measured and unmeasured confounding factors among all those eligible for the ADHD substudy.54,55 Among our randomly sampled controls, seven were also preschool ADHD cases. Given this minimal overlap, for the purpose of this study, these individuals were treated as cases only, for a total reference population of 549 noncase children.
Phthalate metabolite measurements
A detailed description of urine collection and analysis methods have been previously published.35 Briefly, maternal urines collected during pregnancy were shipped to and processed in the MoBa Biobank.48 Collection and processing methods have been previously validated for phthalate metabolite analysis.11 Phthalate metabolites were analyzed at the Norwegian Institute of Public Health, using methods that have previously been described.56 We measured 12 phthalate metabolites: monoethyl phthalate (MEP), a metabolite of DEP; mono-iso-butyl phthalate (MiBP), a metabolite of DiBP; mono-n-butyl phthalate (MnBP), a metabolite DnBP; monobenzyl phthalate (MBzP), a metabolite of BBzP; mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxoyhexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), and mono-2-methylcarboxyhexyl phthalate (MMCHP), metabolites of DEHP; and mono-4-methyl-7-hydroxyoctyl phthalate (OH-MiNP), mono-4-methyl-7oxooctyl phthalate (oxo-MiNP), and mono-4-methyl-7-carboxyheptyl phthalate (cx-MiNP), metabolites of DiNP.
A complete description of quality control procedures has been previously described.35 Briefly, 4–6 laboratory blinded quality control (QC) samples of pooled urine were included in every analytic batch to assess batch-to-batch variability and assay precision, along with unblinded laboratory QC materials. Cases and controls were randomly allocated to batch. To account for urinary dilution, specific gravity was measured using a pocket refractometer (PAL-10S) from Atago. The coefficient of variation was <0.1% for the in-house control urine samples. In laboratory blinded QC samples, average batch coefficients of variation were less than 5%.
Phthalate metabolite concentrations for each participant were adjusted for specific gravity and batch as previously described.35 Following specific gravity and batch adjustment, molar sums of DEHP and DiNP metabolites were computed, and concentrations were expressed as μmol/L DEHP and ∑DiNP. Because the distributions of metabolite concentrations were heavily right-skewed, adjusted measures were subsequently natural log (ln)-transformed following standard practice. Finally, one implausibly high value (16 and 185 times the second and third highest values, respectively) for MnBP was removed. The specific gravity and batch-adjusted phthalate metabolite measures are used in all tables, figures, and statistical models.
Statistical models
Potential confounders of the relationship between gestational phthalate exposure and preschool ADHD were selected a priori using directed acyclic graphs (see eFigure 1; http://links.lww.com/EE/A142, which provides an example) and previous literature regarding covariates that could influence both the exposure and outcome. These covariates were child sex, maternal age at delivery, parity, maternal education, marital status, self-reported maternal smoking during the first trimester of pregnancy, maternal depression in early pregnancy, and maternal ADHD-like symptoms. Maternal depression was evaluated using the SCL-5 (short Symptom Checklist) questions from the 17-week maternal questionnaire.57 The mean score of the depression-related questions were dichotomized so that a mean score of two or greater indicated the presence of depressive symptoms.57–60 Maternal symptoms of ADHD were evaluated in the MoBa 36-month questionnaire using six items from the Adult ADHD Self-Report Scale, based on DSM-IV criteria, with a mean score ≥4 considered indicative of ADHD-like symptoms.61 The level of maternal education was reported as “other education” for 19 individuals. This category was combined with “>college completed,” the group to which it was the most similar in association magnitude in models in which ADHD was regressed against all categories of education (data not shown). We further conducted sensitivity analyses excluding those in the “other education” category. Owing to concerns with nonpositivity, the adjustment set was further reduced if removal improved model fit (by reducing the Akaike Information Criterion value) and changed the primary estimate by <10%. The final adjustment set included child sex, maternal age, parity, maternal education, maternal depression, and maternal ADHD-like symptoms.
Sex-specific estimates for linear models were created using the augmented product term method.62 The threshold for statistical significance of interaction product terms was set at P < 0.20 a priori. Starting with a fully augmented product term model, sex*covariate interactions were removed if the P value for the interaction term was >0.20. The final models for all phthalates included interaction terms between sex and phthalate and between child sex and maternal education. We examined results of a fully augmented product term model, with interaction terms between sex and every covariate, in sensitivity analyses. Sex-specific results were not estimated for quintile models owing to concerns about power and positivity.63
Phthalate exposure was examined both continuously and categorically (to allow detection of nonlinear and nonmonotonic associations), with thresholds defined by quintiles of individual-specific gravity and batch-adjusted phthalate concentrations in the randomly sampled control population.54 Logistic regression models were used to calculate odds ratios (ORs) for estimates of the associations between phthalates and preschool ADHD, adjusted for covariates described above. Restricted cubic splines with knots at 20th, 40th, 60th, and 80th percentiles and Wald tests were used to assess the statistical significance of nonlinear associations. For primary analyses, phthalates were analyzed as individual exposures in single-phthalate models. In sensitivity analyses, we additionally examined models coadjusted for correlated phthalates (Pearson correlation coefficient was ≥0.40) to examine the potential for confounding by coexposures. The current analysis is based on version 9 of the MoBa quality-assured data files.
Results
Demographic characteristics of the study population are presented in Table 1. Fifty-six percent of the 260 preschool ADHD cases were boys, whereas sex distribution was even among the 549 noncases. Mothers of children classified with preschool ADHD were more likely to be younger at delivery, less highly educated, primiparous, and self-report smoking during early pregnancy than mothers in the reference population. A total of 16% of mothers of preschool ADHD case children had a mean depression score ≥2 on the SCL-5 indicating the presence of depressive symptoms, compared with 6% of mothers of noncases. Thirteen percent of mothers of case children had significant symptoms of ADHD compared with 4% of mothers of noncases.
Table 1.
Demographic characteristics of study population.
| Preschool ADHD | MoBa controls | |||
|---|---|---|---|---|
| N = 260 | N = 549 | |||
| N or Mean | % or SDa | N or Mean | % or SDa | |
| Child sex | ||||
| Male | 145 | 56% | 275 | 50% |
| Female | 115 | 44% | 274 | 50% |
| Child age at clinical assessment (months) | 41.6 | 1.2 | ||
| Maternal age at delivery (years)b | 30.0 | 4.1 | 30.9 | 4.2 |
| ≤25 | 32 | 12% | 46 | 8% |
| 26–30 | 111 | 43% | 206 | 38% |
| 31–35 | 99 | 38% | 218 | 40% |
| >35 | 18 | 7% | 77 | 14% |
| Missing | 2 | |||
| Maternal educationb | ||||
| <College completed | 92 | 36% | 121 | 22% |
| College completed | 108 | 42% | 237 | 44% |
| >College completed | 59 | 23% | 184 | 34% |
| Missing | 1 | 7 | ||
| Marital statusb | ||||
| Married | 113 | 44% | 287 | 53% |
| Cohabitating | 131 | 51% | 244 | 45% |
| Other | 15 | 6% | 13 | 2% |
| Missing | 1 | 5 | ||
| Primiparousb | 156 | 60% | 270 | 49% |
| Missing | 2 | |||
| Self-reported smoking during pregnancyb | 62 | 24% | 76 | 14% |
| Missing | 1 | 6 | ||
| Maternal self-reported symptoms during pregnancy | ||||
| Significant symptoms of depressionb,c | 42 | 16% | 34 | 6% |
| Missing | 5 | 14 | ||
| Significant ADHD-like symptomsb,d | 33 | 13% | 21 | 4% |
| Missing | 2 | 12 | ||
aPercentages may not add to 100% due to rounding.
bComparing reference population to all cases, significant (P < 0.05) using t-test for continuous variables, chi-squared test for categorical variables, or Fisher’s exact test for categorical variables with sparse cells.
cMean score ≥2 indicative of presence of depression.
dScores ≥4 indicative of ADHD-like symptoms.
The distribution of gestational phthalate metabolite concentrations, stratified by case/noncase status, is presented in Table 2. The geometric mean of phthalate metabolite concentrations was similar or slightly higher among mothers of preschool ADHD cases as compared to mothers of noncases (Table 2). There were moderately strong correlations observed between some phthalates, particularly between ln-transformed MiBP, MnBP, and MBzP (Pearson r’s between 0.48 and 0.61; see eFigure 2; http://links.lww.com/EE/A143, which illustrates correlations between ln-transformed phthalate metabolite measures). Ln-transformed ∑DEHP was also moderately correlated with ln-transformed ∑DiNP (r = 0.40).
Table 2.
Distribution of gestational phthalate concentrationsa (µg/L) among preschool ADHD cases and MoBa controlsb.
| N | Geometric mean | Geometric SD | Min | 25% | 50% | 75% | Max | LOQc | % >LOQ | |
|---|---|---|---|---|---|---|---|---|---|---|
| MEP (µg/L) | 0.5 | 100.0 | ||||||||
| cases | 260 | 113 | 4.37 | 3.92 | 38.8 | 108 | 288 | 7530 | ||
| MoBa controls | 554 | 99.7 | 4.32 | 2.32 | 32.6 | 97.6 | 296 | 6760 | ||
| MiBP (µg/L) | 0.5 | 100.0 | ||||||||
| cases | 260 | 19.7 | 2.12 | 2.74 | 12.0 | 19.4 | 31.4 | 180 | ||
| MoBa controls | 555 | 18.3 | 2.47 | 1.68 | 9.62 | 16.6 | 32.2 | 562 | ||
| MnBP (µg/L) | 0.5 | 100.0 | ||||||||
| cases | 260 | 20.0 | 2.22 | 3.02 | 12.1 | 19.8 | 33.2 | 379 | ||
| MoBa controls | 554 | 18.1 | 2.17 | 2.00 | 11.4 | 17.2 | 31.0 | 4170d | ||
| MBzP (µg/L) | 0.2 | 100.0 | ||||||||
| cases | 260 | 5.40 | 2.49 | 0.46 | 2.84 | 5.08 | 9.87 | 114 | ||
| MoBa controls | 555 | 4.65 | 2.47 | 0.56 | 2.56 | 4.26 | 7.87 | 103 | ||
| MEHP (µg/L) | 0.5 | 100.0 | ||||||||
| cases | 260 | 12.1 | 2.12 | 1.96 | 7.51 | 11.1 | 16.6 | 871 | ||
| MoBa controls | 555 | 11.4 | 2.11 | 2.23 | 7.12 | 10.4 | 17.4 | 812 | ||
| MEHHP (µg/L) | 0.4 | 100.0 | ||||||||
| cases | 260 | 15.6 | 2.54 | 2.01 | 9.05 | 14.0 | 22.1 | 1490 | ||
| MoBa controls | 555 | 14.0 | 2.45 | 1.93 | 8.13 | 12.6 | 20.6 | 1700 | ||
| MEOHP (µg/L) | 0.4 | 100.0 | ||||||||
| cases | 260 | 10.6 | 2.55 | 1.36 | 5.90 | 9.40 | 15.4 | 1190 | ||
| MoBa controls | 555 | 9.44 | 2.42 | 1.24 | 5.51 | 8.47 | 14.1 | 807 | ||
| MECPP (µg/L) | 2.0 | 100.0 | ||||||||
| cases | 260 | 23.1 | 2.12 | 7.39 | 14.7 | 19.0 | 28.3 | 2020 | ||
| MoBa controls | 555 | 20.9 | 1.94 | 4.96 | 13.9 | 18.6 | 25.8 | 768 | ||
| MMCHP (µg/L) | 2.0 | 100.0 | ||||||||
| cases | 260 | 22.6 | 2.03 | 7.94 | 14.5 | 18.2 | 30.0 | 1110 | ||
| MoBa controls | 555 | 21.0 | 1.85 | 5.23 | 14.0 | 18.1 | 26.3 | 372 | ||
| ∑DEHP (µmol/L) | NA | NA | ||||||||
| cases | 260 | 0.29 | 2.13 | 0.08 | 0.18 | 0.25 | 0.37 | 22.4 | ||
| MoBa controls | 555 | 0.27 | 2.00 | 0.07 | 0.18 | 0.23 | 0.34 | 14.9 | ||
| oh-MiNP (µg/L) | 0.2 | 100.0 | ||||||||
| cases | 260 | 1.12 | 2.23 | 0.31 | 0.72 | 0.97 | 1.36 | 138 | ||
| MoBa controls | 554 | 1.07 | 2.01 | 0.20 | 0.69 | 0.96 | 1.43 | 60.7 | ||
| oxo-MiNP (µg/L) | 0.2 | 98.5 | ||||||||
| cases | 260 | 1.30 | 2.58 | 0.27 | 0.72 | 1.04 | 1.76 | 122 | ||
| MoBa controls | 555 | 1.22 | 2.34 | 0.18 | 0.70 | 1.04 | 1.76 | 201 | ||
| cx-MiNP (µg/L) | 1.0 | 100.0 | ||||||||
| cases | 260 | 3.80 | 1.83 | 1.27 | 2.51 | 3.37 | 5.15 | 49.7 | ||
| MoBa controls | 555 | 3.65 | 1.71 | 1.14 | 2.50 | 3.49 | 4.74 | 141 | ||
| ∑DiNP (µmol/L) | NA | NA | ||||||||
| cases | 260 | 0.02 | 2.04 | 0.01 | 0.01 | 0.02 | 0.03 | 0.96 | ||
| MoBa controls | 554 | 0.02 | 1.86 | 0.01 | 0.01 | 0.02 | 0.03 | 1.07 |
aStandardized to specific gravity and adjusted for analytic batch. Quintiles were calculated using the distribution of phthalates in reference population.
bFor the purpose of evaluating population distributions of phthalates only, 7 preschool ADHD cases were not excluded from the control group
cLimit of quantification.
dA value of 70,164 µg/L was discarded as implausibly high.
After adjustment for covariates, children of mothers with higher levels of exposure to some phthalates had greater odds of preschool ADHD (Table 3). The association between ∑DiNP and preschool ADHD was significantly nonlinear (Wald P for nonlinearity < 0.05). Mothers at the highest quintile of ∑DiNP had 1.70 times the odds of a child with preschool ADHD compared with mothers at the lowest quintile (95% CI = 1.03 to 2.82), although mothers at the second quintile of ∑DiNP had 2.07 times the odds of those at the lowest quintile of having a child with preschool ADHD (95% CI = 1.27 to 3.37). We identified nonmonotonicity in the association between gestational MiBP and preschool ADHD (Wald P < 0.05). Specifically, although there appeared to be monotonic elevation in ORs with increasing quintile of exposure up to the fourth quintile, the odds dropped substantially in the fifth quintile. There were also positive trends between increasing levels of ∑DEHP and odds of preschool ADHD in both linear and quintile models. However, confidence intervals were wide. Each ln-unit increase in ∑DEHP was associated with 1.22 times the odds of preschool ADHD (95% CI = 0.99 to 1.52), although children of mothers at the highest quintile of ∑DEHP had 1.58 times the odds of preschool ADHD compared with children whose mothers were in the lowest quintile of ∑DEHP exposure (95% CI = 0.96 to 2.16). We identified no associations with MEP metabolites. Excluding children of mothers with education level of “other” somewhat strengthened estimates for MBzP and ∑DEHP, and general conclusions remained the same (see eTable 1; http://links.lww.com/EE/A144), which provides estimates from these sensitivity analyses).
Table 3.
Quintile of phthalate and odds of preschool ADHD
| Model 1 crudea | Model 2adjusted for covariatesb | Model 3adjusted for covariates and correlated phthalate metabolitesc | |||
|---|---|---|---|---|---|
| Phthalate | Quintile | N casesd | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| MEP | 1. <26.0 µg/L | 44 | ref. | ref. | |
| 2. 26.0–62.2 µg/L | 44 | 0.96 (0.59 to 1.56) | 0.91 (0.54 to 1.53) | ||
| 3. 62.2–148 µg/L | 57 | 1.28 (0.80 to 2.05) | 1.19 (0.72 to 1.95) | ||
| 4. 148–380 µg/L | 55 | 1.26 (0.79 to 2.01) | 1.24 (0.76 to 2.03) | ||
| 5. >380 µg/L | 53 | 1.18 (0.74 to 1.90) | 1.05 (0.63 to 1.75) | ||
| Linear modele | 253 | 1.06 (0.96 to 1.17) | 1.03 (0.93 to 1.15) | ||
| MiBPf | 1. <8.76 µg/L | 32 | ref. | ref. | ref. |
| 2. 8.76–13.2 µg/L | 41 | 1.30 (0.77 to 2.20) | 1.37 (0.79 to 2.39) | 1.61 (0.87 to 2.97) | |
| 3. 13.2–20.8 µg/L | 65 | 2.07 (1.26 to 3.39) | 1.88 (1.12 to 3.18) | 2.21 (1.19 to 4.10) | |
| 4. 20.8–38.9 µg/L | 75 | 2.32 (1.43 to 3.78) | 2.37 (1.41 to 3.99) | 2.77 (1.46 to 5.25) | |
| 5. >38.9 µg/L | 40 | 1.23 (0.73 to 2.09) | 1.19 (0.68 to 2.08) | 1.29 (0.65 to 2.57) | |
| Linear modele | 253 | 1.10 (0.93 to 1.30) | 1.08 (0.89 to 1.29) | 0.98 (0.78 to 1.24) | |
| MnBP | 1. <9.90 µg/L | 49 | ref. | ref. | ref. |
| 2. 9.90–14.6 µg/L | 41 | 0.83 (0.51 to 1.35) | 0.76 (0.45 to 1.27) | 0.54 (0.30 to 0.97) | |
| 3. 14.6–21.9 µg/L | 48 | 0.96 (0.60 to 1.55) | 0.92 (0.56 to 1.52) | 0.55 (0.29 to 1.03) | |
| 4. 21.9–34.0 µg/L | 55 | 1.17 (0.74 to 1.85) | 1.10 (0.67 to 1.80) | 0.59 (0.31 to 1.14) | |
| 5. >34.0 µg/L | 60 | 1.22 (0.77 to 1.93) | 1.15 (0.70 to 1.89) | 0.64 (0.32 to 1.30) | |
| Linear modele | 253 | 1.17 (0.97 to 1.41) | 1.18 (0.95 to 1.45) | 1.13 (0.84 to 1.52) | |
| MBzP | 1. <2.19 µg/L | 40 | ref. | ref. | ref. |
| 2. 2.19–3.46 µg/L | 40 | 1.06 (0.64 to 1.76) | 0.93 (0.54 to 1.59) | 0.93 (0.52 to 1.69) | |
| 3. 3.46–5.51 µg/L | 53 | 1.36 (0.84 to 2.22) | 1.27 (0.76 to 2.13) | 1.28 (0.71 to 2.30) | |
| 4. 5.51–9.60 µg/L | 54 | 1.40 (0.86 to 2.27) | 1.40 (0.83 to 2.33) | 1.41 (0.75 to 2.66) | |
| 5. >9.60 µg/L | 66 | 1.70 (1.06 to 2.72) | 1.39 (0.83 to 2.31) | 1.37 (0.71 to 2.64) | |
| Linear modele | 253 | 1.19 (1.01 to 1.40) | 1.13 (0.95 to 1.35) | 1.07 (0.85 to 1.35) | |
| ∑DEHP | 1. <0.16 µmol/L | 41 | ref. | ref. | ref. |
| 2. 0.16–0.21 µmol/L | 52 | 1.34 (0.83 to 2.17) | 1.26 (0.76 to 2.09) | 1.28 (0.76 to 2.15) | |
| 3. 0.21–0.27 µmol/L | 49 | 1.23 (0.75 to 2.01) | 1.23 (0.73 to 2.06) | 1.15 (0.68 to 1.97) | |
| 4. 0.27–0.38 µmol/L | 47 | 1.21 (0.74 to 1.97) | 1.23 (0.73 to 2.06) | 1.19 (0.70 to 2.03) | |
| 5. >0.38 µmol/L | 64 | 1.59 (0.99 to 2.54) | 1.58 (0.96 to 2.61) | 1.51 (0.89 to 2.56) | |
| Linear modelf | 253 | 1.17 (0.96 to 1.43) | 1.22 (0.99 to 1.52) | 1.18 (0.93 to 1.49) | |
| ∑DiNPf | 1. <0.012 µmol/L | 42 | ref. | ref. | ref. |
| 2. 0.012–0.016 µmol/L | 76 | 1.82 (1.15 to 2.88) | 2.07 (1.27 to 3.37) | 2.04 (1.25 to 3.33) | |
| 3. 0.016–0.020 µmol/L | 35 | 0.88 (0.53 to 1.47) | 0.89 (0.52 to 1.55) | 0.86 (0.50 to 1.50) | |
| 4. 0.020–0.027 µmol/L | 42 | 1.01 (0.61 to 1.66) | 1.13 (0.67 to 1.92) | 1.07 (0.62 to 1.82) | |
| 5. >0.027 µmol/L | 58 | 1.37 (0.85 to 2.20) | 1.70 (1.03 to 2.82) | 1.54 (0.91 to 2.61) | |
| Linear modele | 253 | 1.11 (0.89 to 1.39) | 1.18 (0.94 to 1.49) | 1.10 (0.85 to 1.42) |
aAdjusted for specific gravity, analytic batch.
bAdjusted for specific gravity, analytic batch, child sex and maternal age, education, parity, depression during pregnancy, and ADHD-like symptoms.
cAdjusted for specific gravity, analytic batch, child sex and maternal age, education, parity, depression during pregnancy, ADHD-like symptoms and additionally adjusted for correlated metabolites (a) MiBP, MnBP, and MBzP co-adjusted for one another; (b) ∑DEHP and ∑DiNP coadjusted for each other).
dNumber with no missing exposure or covariates in Model 2.
ePer 1 natural log-unit increase in biomarker.
fAssociated nonlinearly (Wald test P < 0.10) for MiBP and ∑DiNP when modeled using Model 2 adjustment set and restricted cubic splines with knots at 20th, 40th, 60th, and 80th percentiles.
Given the patterns of phthalate correlations described previously, we examined two groups of multi-phthalate models: (1) MiBP, MnBP, MBzP, coadjusted for one another; and (2) ∑DEHP, ∑DiNP, coadjusted for each other. Coadjustment for ∑DEHP and ∑DiNP somewhat attenuated estimates of association for these phthalates, and confidence intervals were wider (in part, at least, owing to lower model precision), but trends were generally similar (Table 3). Adjustment for MnBP and MBzP appeared to strengthen the nonmonotonic associations between MiBP and preschool ADHD (Table 3).
We further examined sex-specific effects using augmented product term models, with sex interaction product terms for each phthalate and for maternal education (Table 4). There were largely null findings, with the exception of statistically significant modification by child sex of the linear association between MnBP and preschool ADHD (interaction P = 0.05). Among boys, one ln-unit increase in maternal gestational MnBP was associated with 1.42 times the odds of preschool ADHD (95% CI = 1.07 to 1.88). This association persisted after additional adjustment for MBzP and MiBP. There was no evidence of an association with MnBP in girls. Adding interaction terms between sex and all other covariates did not materially change results (see eTable 2; http://links.lww.com/EE/A145), which provides estimates from these sensitivity analyses).
Table 4.
Phthalate metabolite concentration and odds of preschool ADHD by child sex, using augmented product term models, with sex-interaction products for phthalate and for maternal education.
| Adjusted for covariatesa | Adjusted for covariates and correlated phthalatesb | |||||||
|---|---|---|---|---|---|---|---|---|
| Casesc | Boys | Girls | Boys | Girls | ||||
| Phthalate | Boys | Girls | OR (95% CI)d | OR (95% CI)d | P e | OR (95% CI)d | OR (95% CI)d | P e |
| MEP | 142 | 111 | 1.03 (0.89 to 1.19) | 1.03 (0.88 to 1.21) | 0.97 | |||
| MiBP | 142 | 111 | 1.17 (0.92 to 1.50) | 0.97 (0.73 to 1.29) | 0.33 | 0.98 (0.71 to 1.35) | 1.00 (0.71 to 1.41) | 0.93 |
| MnBP | 142 | 111 | 1.42 (1.07 to 1.88) | 0.93 (0.68 to 1.28) | 0.05 | 1.43 (0.97 to 2.10) | 0.82 (0.51 to 1.31) | 0.07 |
| MBzP | 142 | 111 | 1.20 (0.95 to 1.53) | 1.06 (0.82 to 1.37) | 0.47 | 1.01 (0.74 to 1.39) | 1.17 (0.84 to 1.65) | 0.53 |
| ∑DEHP | 142 | 111 | 1.32 (1.00 to 1.74) | 1.10 (0.78 to 1.55) | 0.42 | 1.28 (0.95 to 1.73) | 1.03 (0.70 to 1.51) | 0.37 |
| ∑DiNP | 142 | 111 | 1.22 (0.88 to 1.68) | 1.17 (0.83 to 1.64) | 0.86 | 1.10 (0.78 to 1.55) | 1.15 (0.79 to 1.69) | 0.85 |
aAdjusted for specific gravity, analytic batch, child sex and maternal age, education, parity, depression during pregnancy, and maternal ADHD-like symptoms.
bAdjusted for specific gravity, analytic batch, child sex and maternal age, education, parity, depression during pregnancy, maternal ADHD-like symptoms, and additionally adjusted for correlated metabolites (a) MiBP, MnBP, and MBzP coadjusted for one another; (b) ∑DEHP and ∑DiNP coadjusted for each other.
cNumber with no missing exposure or covariates.
dPer 1 natural log-unit increase in biomarker.
eP for sex*phthalate interaction in linear regression models.
Discussion and conclusions
The purpose of this study was to evaluate the relationship between maternal gestational urinary concentrations of phthalate metabolites and the risk of preschool ADHD, using a high quality on-site assessment. In this nested case-cohort study, preschool-aged children of mothers with the highest quintile of gestational ∑DiNP and ∑DEHP were at increased risk of being classified with preschool ADHD, although associations were not always monotonic. The association between MnBP levels and preschool ADHD was significantly modified by child sex, with no evidence of an association among girls, and a significant positive linear relationship among boys. Finally, we observed some evidence that increasing exposure to MiBP may increase risk of ADHD; however, estimates in the highest quintile of exposure exhibited a downward trend, which may be suggestive of nonlinear effects, or alternatively, of uncontrolled confounding. Adjustment for correlated phthalates did not materially alter our observations.
These results build upon our prior research in MoBa.35 Using ADHD diagnoses registered in the NPR, we previously reported monotonically increasing risk of ADHD in relation to gestational DEHP exposure. In this current study, we find increased risk of preschool ADHD, although of a somewhat lesser magnitude (OR per log increase in DEHP in Engel et al [2018] 1.47 [95% CI = 1.09 to 1.94]; current study OR 1.22 [95% CI = 0.99 to 1.52]). In both studies, we find no substantial modification of the DEHP association by child sex. However, in this current study, we find evidence of sex-specific effects of MnBP exposure among boys, as well as some evidence of increased risk in the highest quintile of DiNP exposure. There are several key differences in the design of these studies. First, the current study has a much more even representation of girls and boys among children classified with preschool ADHD—44% of the current cases were girls compared with fewer than 30% of those in the NPR subset. Thus we had better power to examine effect measure modification by child sex. Second, the NPR cases were on average shifted toward earlier birth years because DEHP exposure exhibits a temporally declining trend,64–66 NPR cases were on average gestationally exposed to higher levels of DEHP than our preschool ADHD cases, which may explain why our effect estimates are somewhat attenuated in this study. During this time, DiNP was frequently used as a substitute for DEHP67 and levels of its metabolites have increased in pregnant women in the United States and Europe.68 The structural similarities that make DiNP a useful substitute may partially explain why these two chemicals show similarly strong associations with preschool ADHD in this study. Third, NPR cases are likely a biased subset of ADHD cases as a whole, representing a higher degree of impairment, underidentifying girls, and requiring both hyperactive and inattentive symptoms.36,38–41 Moreover, some cases identified in the preschool period will experience a decline in symptom intensity over time.42,69,70 Thus, ADHD cases identified in these studies may comprise slightly different, although overlapping, populations of affected children. The alignment in our overall results is therefore notable and suggests that prenatal exposure is associated with both clinically significant ADHD symptoms in the preschool period and ADHD diagnosis later in childhood.
Children with ADHD often have impaired executive functions.71 One mechanism through which phthalates could increase risk of ADHD diagnosis is through impacts on executive functions, which begin to develop early in life and continue development through adolescence.72,73 Choi et al74 examined the relationships between prenatal phthalate exposure and preschool-aged executive functions among the subset of MoBa children who returned for the preschool ADHD clinical assessment. Similar to results presented in this current paper, they found that increased MnBP levels during pregnancy were associated with deficits in executive functions among boys but not girls. However, the most consistent associations in Choi et al were for adverse effects of MBzP across all measures of executive function and for both sexes. Although we observed increased odds of preschool ADHD with increasing levels of gestational MBzP, adjustment for covariates attenuated estimates, particularly in the highest quintile of exposure. In contrast to our findings and to those of Engel et al,35 Choi et al74 did not observe consistently adverse associations with DEHP or DiNP.
A number of prospective birth cohort studies have examined associations between perinatal exposure to phthalate metabolites and childhood behavior. Although some recent systematic reviews of the existing body of evidence have concluded that phthalates have an overall negative effect on various aspects of neurobehavioral development,16,18 others have concluded that the lack of consistency across studies prevents firm conclusions from being drawn.75 A complicating feature of the phthalate literature is that exposure was often measured at different time points in pregnancy, and outcome assessment approaches have been varied. There have been very few studies that have had access to clinical assessments of children. Rather, the majority of studies have relied on parent-reported behavioral inventories that may imperfectly capture developmental problems. In contrast, we leveraged a standardized, high quality, on site assessment for preschool ADHD that used a validated diagnostic interview appropriate for preschoolers. The only other study to our knowledge with prenatal exposure and a clinical ADHD diagnosis is our prior study in MoBa.35 These studies together support the possibility that prenatal DEHP exposure may have a long-term impact on behavior in children. However, the literature has been inconsistent as to whether associations for any given phthalate exhibit consistent evidence of sexual dimorphism. For MnBP in particular, several studies have found associations that are stronger in boys,20,21,23,27,29,32 whereas others have not.22,25,33,35 We cannot exclude the possibility that interactions by sex are owing to chance, particularly since there is no specific rationale to support sex interactions for MnBP alone as opposed to any of the other phthalates that were measured.
Despite a considerable amount of epidemiologic research on prenatal phthalate exposure and behavioral outcomes in children,16 there is a more limited body of experimental evidence in animals.76 The majority of experimental model studies have focused on DEHP,77 with limited research on other phthalates, and have found that phthalate exposure can increase anxiety-like behaviors,78–83 impair memory,80,83,84 and cause hyperactivity.85,86 However, the mechanisms underlying these effects are unclear. Phthalates are endocrine-disrupting chemicals, several of which have antiandrogenic properties.87,88 A number of phthalates, including DEHP, DBP, and MBP, have been linked to thyroid disruption in both animal models and human epidemiologic studies.89–97 Maternal thyroid sufficiency is critical for fetal neurodevelopment,98 and both maternal hyper- and hypothyroidism have been associated with ADHD.99 In addition, evidence from pregnancy cohorts suggests phthalates perturb normal sex steroid levels in pregnant women.92–97,100,101 Sex steroids act throughout the brain to govern various aspects of neurodevelopment and cognition,102 and early life hormonal exposures may influence neurobiological differences in brain structural and functional development.103–105 Given the complex nature of these hormones and the importance of normal hormone function during pregnancy on fetal brain development,106 phthalate-induced hormone disruption during pregnancy could have long-term effects on the developing child.107
Our study has a number of limitations. The half lives of phthalates are relatively short, ranging from a few hours to a few days,108–111 and the intraclass correlation coefficients (ICCs) for repeated measures of prenatal phthalate metabolite concentrations reported in the literature are low to moderate.112–115 Thus, a single spot urine sample, which was used for exposure assessment in this study, is unlikely to accurately reflect a woman’s exposure to phthalates across her entire pregnancy. If the putative sensitive window was not 17 weeks’ gestation when the prenatal urine was collected, this lack of reproducibility in phthalate exposures over time may result in bias in the estimated exposure-outcome association. It is unknown whether there is any specific sensitive window for phthalate exposure, as the development of the brain begins very early in gestation and continues into postnatal life. However, the prenatal period in general, and the second trimester specifically, is a relevant window of vulnerability to perturbations in fetal growth that can impact long-term neurodevelopmental outcomes. Additionally, evaluating behavioral outcomes during preschool years is challenging, as some symptoms required for diagnosis of ADHD may in fact be developmentally appropriate.53,116 However, our study leveraged a high-quality assessment with diagnostic interviews supervised by psychiatrists/psychologists, and our assessment was optimized for this period. To maximize ascertainment of preschool ADHD cases, selection for clinical assessment was primarily owing to parentally reported symptoms on the MoBa 36-month questionnaire. Additionally, a small subset of children without symptoms was randomly selected for an on-site clinical interview, six of whom were found to have subclinical or clinically significant symptoms of preschool ADHD. This suggests both a potential undercount of preschool ADHD cases and the possibility that there are members of our subcohort random sample who are also undetected preschool ADHD cases. The presence of cases in our random sample is expected and does not represent a bias in our estimates, as the purpose of the subcohort is to represent the phthalate exposure distribution in the source population. However, our preschool ADHD cases may underrepresent children whose symptoms are not as noticeable to their caretakers. Finally, there is likely some bias that is attributable to self-selection into MoBa and its follow-up studies, which could influence our results.117 Statistical approaches for addressing self-selection often rely on the availability of population-level estimates of exposure in the target population, which are not available for phthalates in Norway. We did, however, include in our statistical model adjustment for important predictors of exposure and selection into the follow-up study that may influence findings, including maternal age, education, and parity. Statistical adjustment may mitigate any residual bias in our findings attributable to these factors.
Our study also had many important strengths. This case-cohort study was nested within a well-characterized, population-based cohort. ADHD case ascertainment relied on a standardized, on-site clinical assessment of children, an improvement in outcome measurement as compared to previous research. Previous studies of ADHD symptoms in preschoolers have relied primarily on ratings by parents, which may be less reliable. In our previous research of ADHD diagnoses, cases were determined from registry records, and any variation in diagnostic tendencies between providers could not be accounted for. In contrast, the interrater reliability of this research-quality clinical assessment was very high. Further, a number of important confounders of the relationship between maternal prenatal phthalate exposure and preschool behavior were included in statistical models, including maternal self-reported symptoms of both ADHD and depression during pregnancy, which have not been available in prior studies.
In summary, we found evidence that gestational exposure to some phthalates, including DiNP, DEHP, MiBP, and in males, MnBP, may increase the risk of ADHD-like symptoms in the preschool period. This is the first study to report an association between prenatal exposure to metabolites of DiNP, an industry substitute for DEHP, and neurodevelopmental outcome in children. ADHD represents an important health burden both to the patient118,119 and society,120–122 as reflected by lower than average educational attainment and future income among ADHD cases. Despite considerable research, relatively few modifiable risk factors for ADHD have been identified.123,124 This research is an important step toward identifying modifiable risk factors for ADHD with important public health consequences.
Supplementary Material
Footnotes
Published online 1 July 2021
Analytic code used for the present analysis may be obtained from the corresponding author. All inquires related to obtaining data from the Norwegian Mother, Father and Childbirth cohort (MoBa) should be directed to the MoBa executive officer at the Norwegian Institute of Public Health (mobaadm@fhi.no).
The authors declare that they have no conflicts of interest with regard to the content of this report.
The Norwegian Mother, Father and Child Cohort Study (MoBa) is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558), NIH/NINDS (grant no. 1 UO1 NS 047537-01 and grant no. 2 UO1 NS 047537-06A1), and the Norwegian Research Council/FUGE (grant no. 151918/S10). The Preschool ADHD study, a substudy to MoBa, is supported by funds and grants from the Norwegian Ministry of Health, The Norwegian Health Directorate, The South Eastern Health Region, G&PJ Sorensen Fund for Scientific Research, and from The Norwegian Centre of Expertise for Neurodevelopmental Disorders and Hypersomnias. This study was supported in part by the National Institute of Environmental Health Sciences (NIEHS) (R01-ES021777, T32-ES007018, P30ES010126), the Intramural Research Program of the National Institute of Health (NIH), and The Norwegian Institute of Public Health. We are grateful to all the participating families in Norway who take part in this ongoing cohort study.
The establishment of MoBa and initial data collection was based on a license from the Norwegian Data protection agency and approval from The Regional Committees for Medical and Health Research Ethics. The MoBa cohort is based on regulations based on the Norwegian Health Registry Act. The current study was approved by The Regional Committees for Medical and Health Research Ethics (ref. no. 2012/985-1) and the Institutional Review Board at UNC Chapel Hill.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.EPA Phthalates Action Plan (Revised). Available at: https://www.epa.gov/sites/production/files/2015-09/documents/phthalates_actionplan_revised_2012-03-14.pdf. Accessed 12 August 2019.
- 2.Rodgers KM, Rudel RA, Just AC. Phthalates in food packaging, consumer products, and indoor environments. In: Snedeker SM, ed. Toxicants in Food Packaging and Household Plastics. Molecular and Integrative Toxicology. 1 ed. London: Springer-Verlag, 2014 [Google Scholar]
- 3.Zota AR, Phillips CA, Mitro SD. Recent fast food consumption and Bisphenol A and phthalates exposures among the U.S. population in NHANES, 2003-2010. Environ Health Perspect. 2016; 124:1521–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colacino JA, Harris TR, Schecter A. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ Health Perspect. 2010; 118:998–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014; 24:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harley KG, Kogut K, Madrigal DS, et al. Reducing phthalate, paraben, and phenol exposure from personal care products in adolescent girls: findings from the HERMOSA Intervention Study. Environ Health Perspect. 2016; 124:1600–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010. Environ Health Perspect. 2014; 122:235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwedler G, Seiwert M, Fiddicke U, et al. Human biomonitoring pilot study DEMOCOPHES in Germany: contribution to a harmonized European approach. Int J Hyg Environ Health. 2017; 220:686–696 [DOI] [PubMed] [Google Scholar]
- 9.Haines DA, Saravanabhavan G, Werry K, Khoury C. An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007-2019. Int J Hyg Environ Health. 2017; 2202 pt A13–28 [DOI] [PubMed] [Google Scholar]
- 10.Haug LS, Sakhi AK, Cequier E, et al. In-utero and childhood chemical exposome in six European mother-child cohorts. Environ Int. 2018; 121pt 1751–763 [DOI] [PubMed] [Google Scholar]
- 11.Ye X, Pierik FH, Angerer J, et al. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa). Int J Hyg Environ Health. 2009; 212:481–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovanoulis G, Alves A, Papadopoulou E, et al. Evaluation of exposure to phthalate esters and DINCH in urine and nails from a Norwegian study population. Environ Res. 2016; 151:80–90 [DOI] [PubMed] [Google Scholar]
- 13.Sakhi AK, Sabaredzovic A, Cequier E, Thomsen C. Phthalate metabolites in Norwegian mothers and children: levels, diurnal variation and use of personal care products. Sci Total Environ. 2017; 599-600:1984–1992 [DOI] [PubMed] [Google Scholar]
- 14.Katsikantami I, Sifakis S, Tzatzarakis MN, et al. A global assessment of phthalates burden and related links to health effects. Environ Int. 2016; 97:212–236 [DOI] [PubMed] [Google Scholar]
- 15.Mose T, Mortensen GK, Hedegaard M, Knudsen LE. Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reprod Toxicol. 2007; 23:83–91 [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Chen XZ, Huang X, Wang M, Wu J. The association between prenatal exposure to phthalates and cognition and neurobehavior of children-evidence from birth cohorts. Neurotoxicology. 2019; 73:199–212 [DOI] [PubMed] [Google Scholar]
- 17.Lee DW, Kim MS, Lim YH, Lee N, Hong YC. Prenatal and postnatal exposure to di-(2-ethylhexyl) phthalate and neurodevelopmental outcomes: a systematic review and meta-analysis. Environ Res. 2018; 167:558–566 [DOI] [PubMed] [Google Scholar]
- 18.Ejaredar M, Nyanza EC, Ten Eycke K, Dewey D. Phthalate exposure and childrens neurodevelopment: a systematic review. Environ Res. 2015; 142:51–60 [DOI] [PubMed] [Google Scholar]
- 19.Hyland C, Mora AM, Kogut K, et al. Prenatal exposure to phthalates and neurodevelopment in the CHAMACOS cohort. Environ Health Perspect. 2019; 127:107010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.England-Mason G, Martin JW, MacDonald A, et al. Similar names, different results: consistency of the associations between prenatal exposure to phthalates and parent-ratings of behavior problems in preschool children. Environ Int. 2020; 142:105892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel S, Balalian AA, Insel BJ, et al. Prenatal and early childhood exposure to phthalates and childhood behavior at age 7 years. Environ Int. 2020; 143:105894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, Papandonatos GD, Calafat AM, et al. Gestational and childhood exposure to phthalates and child behavior. Environ Int. 2020; 144:106036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans SF, Raymond S, Sethuram S, et al. Associations between prenatal phthalate exposure and sex-typed play behavior in preschool age boys and girls. Environ Res. 2021; 192:110264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang HB, Kuo PH, Su PH, Sun CW, Chen WJ, Wang SL. Prenatal and childhood exposure to phthalate diesters and neurobehavioral development in a 15-year follow-up birth cohort study. Environ Res. 2019; 172:569–577 [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Eom S, Kim HJ, et al. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2years of age- CHECK cohort study. Sci Total Environ. 2018; 624:377–384 [DOI] [PubMed] [Google Scholar]
- 26.Philippat C, Nakiwala D, Calafat AM, et al. EDEN Mother–Child Study Group Prenatal exposure to nonpersistent endocrine disruptors and behavior in boys at 3 and 5 years. Environ Health Perspect. 2017; 125:097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whyatt RM, Liu X, Rauh VA, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect. 2012; 120:290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lien YJ, Ku HY, Su PH, et al. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort Study. Environ Health Perspect. 2015; 123:95–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engel SM, Miodovnik A, Canfield RL, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010; 118:565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jankowska A, Polańska K, Hanke W, et al. Prenatal and early postnatal phthalate exposure and child neurodevelopment at age of 7 years - Polish Mother and Child Cohort. Environ Res. 2019; 177:108626. [DOI] [PubMed] [Google Scholar]
- 31.Miodovnik A, Engel SM, Zhu C, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011; 32:261–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobrosly RW, Evans S, Miodovnik A, et al. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6-10 years of age. Environ Health Perspect. 2014; 122:521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gascon M, Valvi D, Forns J, et al. Prenatal exposure to phthalates and neuropsychological development during childhood. Int J Hyg Environ Health. 2015; 218:550–558 [DOI] [PubMed] [Google Scholar]
- 34.Kim JI, Hong YC, Shin CH, Lee YA, Lim YH, Kim BN. The effects of maternal and children phthalate exposure on the neurocognitive function of 6-year-old children. Environ Res. 2017; 156:519–525 [DOI] [PubMed] [Google Scholar]
- 35.Engel SM, Villanger GD, Nethery RC, et al. Prenatal phthalates, maternal thyroid function, and risk of attention-deficit hyperactivity disorder in the Norwegian mother and child cohort. Environ Health Perspect. 2018; 126:057004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016; 387:1240–1250 [DOI] [PubMed] [Google Scholar]
- 37.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association, 2000 [Google Scholar]
- 38.Surén P, Bakken IJ, Aase H, et al. Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in Norwegian children. Pediatrics. 2012; 130:e152–e158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003; 2:104–113 [PMC free article] [PubMed] [Google Scholar]
- 40.Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997; 36:1036–1045 [DOI] [PubMed] [Google Scholar]
- 41.Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J Am Acad Child Adolesc Psychiatry. 2010; 49:217–28.e1 [PMC free article] [PubMed] [Google Scholar]
- 42.Breaux RP, Griffith SF, Harvey EA. Preschool neuropsychological measures as predictors of later attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2016; 44:1455–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leopold DR, Christopher ME, Olson RK, Petrill SA, Willcutt EG. Invariance of ADHD symptoms across sex and age: a latent analysis of ADHD and impairment ratings from early childhood into adolescence. J Abnorm Child Psychol. 2019; 47:21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohrer-Baumgartner N, Zeiner P, Egeland J, et al. Does IQ influence associations between ADHD symptoms and other cognitive functions in young preschoolers? Behav Brain Funct. 2014; 10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skogan AH, Zeiner P, Egeland J, et al. Inhibition and working memory in young preschool children with symptoms of ADHD and/or oppositional-defiant disorder. Child Neuropsychol. 2014; 20:607–624 [DOI] [PubMed] [Google Scholar]
- 46.Magnus P, Birke C, Vejrup K, et al. Cohort profile update: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2016; 45:382–388 [DOI] [PubMed] [Google Scholar]
- 47.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. MoBa Study Group Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2006; 35:1146–1150 [DOI] [PubMed] [Google Scholar]
- 48.Paltiel L, Anita H, Skjerden T, et al. The biobank of the Norwegian Mother and Child Cohort Study—present status. Norsk Epidemiologi. 2014; 24:29–35 [Google Scholar]
- 49.Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev. 2000; 21:265–271 [DOI] [PubMed] [Google Scholar]
- 50.Skogan AH, Zeiner P, Egeland J, Urnes AG, Reichborn-Kjennerud T, Aase H. Parent ratings of executive function in young preschool children with symptoms of attention-deficit/-hyperactivity disorder. Behav Brain Funct. 2015; 11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egger HL, Angold A. The Preschool Age Psychiatric Assessment (PAPA): A structured parent interview for diagnosing psychiatric disorders in preschool children. Handbook of Infant, Toddler, and Preschool Mental Health Assessment. New York, NY, US: Oxford University Press, 2004223–243 [Google Scholar]
- 52.Sterba S, Egger HL, Angold A. Diagnostic specificity and nonspecificity in the dimensions of preschool psychopathology. J Child Psychol Psychiatry. 2007; 48:1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Øvergaard KR, Oerbeck B, Friis S, et al. Attention-deficit/hyperactivity disorder in preschoolers: the accuracy of a short screener. J Am Acad Child Adolesc Psychiatry. 2018; 57:428–435 [DOI] [PubMed] [Google Scholar]
- 54.Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. III. Design options. Am J Epidemiol. 1992; 135:1042–1050 [DOI] [PubMed] [Google Scholar]
- 55.Langholz B. Use of cohort information in the design and analysis of case-control studies. Scand Stat Theory Appl. 2007; 34:120–136 [Google Scholar]
- 56.Sabaredzovic A, Sakhi AK, Brantsæter AL, Thomsen C. Determination of 12 urinary phthalate metabolites in Norwegian pregnant women by core-shell high performance liquid chromatography with on-line solid-phase extraction, column switching and tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2015; 1002:343–352 [DOI] [PubMed] [Google Scholar]
- 57.Tambs K, Røysamb E. Selection of questions to short-form versions of original psychometric instruments in MoBa. Norsk Epidemiologi. 2014; 24:195–201 [Google Scholar]
- 58.Strand BH, Dalgard OS, Tambs K, Rognerud M. Measuring the mental health status of the Norwegian population: a comparison of the instruments SCL-25, SCL-10, SCL-5 and MHI-5 (SF-36). Nord J Psychiatry. 2003; 57:113–118 [DOI] [PubMed] [Google Scholar]
- 59.Tambs K, Moum T. How well can a few questionnaire items indicate anxiety and depression? Acta Psychiatr Scand. 1993; 87:364–367 [DOI] [PubMed] [Google Scholar]
- 60.Gjerde LC, Eilertsen EM, Reichborn-Kjennerud T, et al. Maternal perinatal and concurrent depressive symptoms and child behavior problems: a sibling comparison study. J Child Psychol Psychiatry. 2017; 58:779–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005; 35:245–256 [DOI] [PubMed] [Google Scholar]
- 62.Buckley JP, Doherty BT, Keil AP, Engel SM. Statistical approaches for estimating sex-specific effects in endocrine disruptors research. Environ Health Perspect. 2017; 125:067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006; 60:578–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shu H, Jönsson BA, Gennings C, et al. Temporal trends of phthalate exposures during 2007-2010 in Swedish pregnant women. J Expo Sci Environ Epidemiol. 2018; 28:437–447 [DOI] [PubMed] [Google Scholar]
- 65.Tranfo G, Caporossi L, Pigini D, et al. Temporal trends of urinary phthalate concentrations in two populations: effects of REACH Authorization after Five Years. Int J Environ Res Public Health. 2018; 15:1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calafat AM, Valentin-Blasini L, Ye X. Trends in exposure to chemicals in personal care and consumer products. Curr Environ Health Rep. 2015; 2:348–355 [DOI] [PubMed] [Google Scholar]
- 67.Nagorka R, Koschorreck J. Trends for plasticizers in German freshwater environments - Evidence for the substitution of DEHP with emerging phthalate and non-phthalate alternatives. Environ Pollut. 2020; 262:114237. [DOI] [PubMed] [Google Scholar]
- 68.Shin HM, Dhar U, Calafat AM, Nguyen V, Schmidt RJ, Hertz-Picciotto I. Temporal trends of exposure to phthalates and phthalate alternatives in California pregnant women during 2007-2013: comparison with other populations. Environ Sci Technol. 2020; 54:13157–13166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barkley RA. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. 2nd ed. New York: Guilford Press, 1998 [Google Scholar]
- 70.Harvey EA, Youngwirth SD, Thakar DA, Errazuriz PA. Predicting attention-deficit/hyperactivity disorder and oppositional defiant disorder from preschool diagnostic assessments. J Consult Clin Psychol. 2009; 77:349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clark C, Prior M, Kinsella GJ. Do executive function deficits differentiate between adolescents with ADHD and oppositional defiant/conduct disorder? A neuropsychological study using the Six Elements Test and Hayling Sentence Completion Test. J Abnorm Child Psychol. 2000; 28:403–414 [DOI] [PubMed] [Google Scholar]
- 72.Garon N, Bryson SE, Smith IM. Executive function in preschoolers: a review using an integrative framework. Psychol Bull. 2008; 134:31–60 [DOI] [PubMed] [Google Scholar]
- 73.Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: four general conclusions. Curr Dir Psychol Sci. 2012; 21:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi G, Keil AP, Villanger GD, et al. Pregnancy exposure to common-detect organophosphate esters and phthalates and maternal thyroid function. Sci Total Environ. 2021; 782:146709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radke EG, Braun JM, Nachman RM, Cooper GS. Phthalate exposure and neurodevelopment: a systematic review and meta-analysis of human epidemiological evidence. Environ Int. 2020; 137:105408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palanza P, Paterlini S, Brambilla MM, et al. Sex-biased impact of endocrine disrupting chemicals on behavioral development and vulnerability to disease: of mice and children. Neurosci Biobehav Rev. 2021; 121:29–46 [DOI] [PubMed] [Google Scholar]
- 77.Engel SM, Patisaul HB, Brody C, et al. Neurotoxicity of ortho-phthalates: recommendations for critical policy reforms to protect brain development in children. Am J Public Health. 2021; 111:687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carbone S, Ponzo OJ, Gobetto N, et al. Antiandrogenic effect of perinatal exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate increases anxiety-like behavior in male rats during sexual maturation. Horm Behav. 2013; 63:692–699 [DOI] [PubMed] [Google Scholar]
- 79.Quinnies KM, Harris EP, Snyder RW, Sumner SS, Rissman EF. Direct and transgenerational effects of low doses of perinatal di-(2-ethylhexyl) phthalate (DEHP) on social behaviors in mice. PLoS One. 2017; 12:e0171977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barakat R, Lin PC, Park CJ, et al. Prenatal exposure to DEHP induces neuronal degeneration and neurobehavioral abnormalities in adult male mice. Toxicol Sci. 2018; 164:439–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma P, Liu X, Wu J, et al. Cognitive deficits and anxiety induced by diisononyl phthalate in mice and the neuroprotective effects of melatonin. Sci Rep. 2015; 5:14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan B, Guo J, Liu X, et al. Oxidative stress mediates dibutyl phthalate induced anxiety-like behavior in Kunming mice. Environ Toxicol Pharmacol. 2016; 45:45–51 [DOI] [PubMed] [Google Scholar]
- 83.Farzanehfar V, Naderi N, Kobarfard F, Faizi M. Determination of dibutyl phthalate neurobehavioral toxicity in mice. Food Chem Toxicol. 2016; 94:221–226 [DOI] [PubMed] [Google Scholar]
- 84.Lin H, Yuan K, Li L, et al. In utero exposure to diethylhexyl phthalate affects rat brain development: a behavioral and genomic approach. Int J Environ Res Public Health. 2015; 12:13696–13710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishido M, Masuo Y, Sayato-Suzuki J, Oka S, Niki E, Morita M. Dicyclohexylphthalate causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J Neurochem. 2004; 91:69–76 [DOI] [PubMed] [Google Scholar]
- 86.Masuo Y, Morita M, Oka S, Ishido M. Motor hyperactivity caused by a deficit in dopaminergic neurons and the effects of endocrine disruptors: a study inspired by the physiological roles of PACAP in the brain. Regul Pept. 2004; 123:225–234 [DOI] [PubMed] [Google Scholar]
- 87.Takeuchi S, Iida M, Kobayashi S, Jin K, Matsuda T, Kojima H. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors alpha and beta, and androgen receptor. Toxicology. 2005; 210:223–233 [DOI] [PubMed] [Google Scholar]
- 88.Boberg J, Christiansen S, Axelstad M, et al. Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol. 2011; 31:200–209 [DOI] [PubMed] [Google Scholar]
- 89.Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012; 355:240–248 [DOI] [PubMed] [Google Scholar]
- 90.Shen O, Wu W, Du G, et al. Thyroid disruption by Di-n-butyl phthalate (DBP) and mono-n-butyl phthalate (MBP) in Xenopus laevis. PLoS One. 2011; 6:e19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu C, Zhao L, Wei L, Li L. DEHP reduces thyroid hormones via interacting with hormone synthesis-related proteins, deiodinases, transthyretin, receptors, and hepatic enzymes in rats. Environ Sci Pollut Res Int. 2015; 22:12711–12719 [DOI] [PubMed] [Google Scholar]
- 92.Villanger GD, Drover SSM, Nethery RC, et al. Associations between urine phthalate metabolites and thyroid function in pregnant women and the influence of iodine status. Environ Int. 2020; 137:105509. [DOI] [PubMed] [Google Scholar]
- 93.Huang HB, Kuo PL, Chang JW, Jaakkola JJK, Liao KW, Huang PC. Longitudinal assessment of prenatal phthalate exposure on serum and cord thyroid hormones homeostasis during pregnancy - Tainan birth cohort study (TBCS). Sci Total Environ. 2018; 619-620:1058–1065 [DOI] [PubMed] [Google Scholar]
- 94.Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod. 2007; 22:2715–2722 [DOI] [PubMed] [Google Scholar]
- 95.Johns LE, Ferguson KK, Soldin OP, et al. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol. 2015; 13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Meeker JD. Associations between repeated measures of maternal urinary phthalate metabolites and thyroid hormone parameters during pregnancy. Environ Health Perspect. 2016; 124:1808–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Romano ME, Eliot MN, Zoeller RT, et al. Maternal urinary phthalate metabolites during pregnancy and thyroid hormone concentrations in maternal and cord sera: the HOME Study. Int J Hyg Environ Health. 2018; 221:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017; 342:68–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Drover SSM, Villanger GD, Aase H, et al. Maternal thyroid function during pregnancy or neonatal thyroid function and attention deficit hyperactivity disorder: a systematic review. Epidemiology. 2019; 30:130–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sathyanarayana S, Barrett E, Butts S, Wang C, Swan SH. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction. 2014; 147:401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cathey AL, Watkins D, Rosario ZY, et al. Associations of phthalates and phthalate replacements with CRH and other hormones among pregnant women in Puerto Rico. J Endocr Soc. 2019; 3:1127–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gore AC, Martien KM, Gagnidze K, Pfaff D. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr Rev. 2014; 35:961–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007; 62:847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997; 21:1185–1201 [DOI] [PubMed] [Google Scholar]
- 105.Cohen-Bendahan CC, Buitelaar JK, van Goozen SH, Cohen-Kettenis PT. Prenatal exposure to testosterone and functional cerebral lateralization: a study in same-sex and opposite-sex twin girls. Psychoneuroendocrinology. 2004; 29:911–916 [DOI] [PubMed] [Google Scholar]
- 106.Gore AC, Krishnan K, Reilly MP. Endocrine-disrupting chemicals: effects on neuroendocrine systems and the neurobiology of social behavior. Horm Behav. 2019; 111:7–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miranda A, Sousa N. Maternal hormonal milieu influence on fetal brain development. Brain Behav. 2018; 8:e00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koch HM, Angerer J. Di-iso-nonylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP. Int J Hyg Environ Health. 2007; 210:9–19 [DOI] [PubMed] [Google Scholar]
- 109.Koch HM, Christensen KL, Harth V, Lorber M, Brüning T. Di-n-butyl phthalate (DnBP) and diisobutyl phthalate (DiBP) metabolism in a human volunteer after single oral doses. Arch Toxicol. 2012; 86:1829–1839 [DOI] [PubMed] [Google Scholar]
- 110.Lessmann F, Schütze A, Weiss T, et al. Metabolism and urinary excretion kinetics of di(2-ethylhexyl) terephthalate (DEHTP) in three male volunteers after oral dosage. Arch Toxicol. 2016; 90:1659–1667 [DOI] [PubMed] [Google Scholar]
- 111.Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005; 79:367–376 [DOI] [PubMed] [Google Scholar]
- 112.Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014; 70:118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Adibi JJ, Whyatt RM, Williams PL, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008; 116:467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Braun JM, Smith KW, Williams PL, et al. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect. 2012; 120:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Casas M, Basagaña X, Sakhi AK, et al. Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environ Int. 2018; 121pt 1561–573 [DOI] [PubMed] [Google Scholar]
- 116.Connor DF. Preschool attention deficit hyperactivity disorder: a review of prevalence, diagnosis, neurobiology, and stimulant treatment. J Dev Behav Pediatr. 2002; 23suppl 1S1–S9 [DOI] [PubMed] [Google Scholar]
- 117.Biele G, Gustavson K, Czajkowski NO, et al. Bias from self selection and loss to follow-up in prospective cohort studies. Eur J Epidemiol. 2019; 34:927–938 [DOI] [PubMed] [Google Scholar]
- 118.Danckaerts M, Sonuga-Barke EJ, Banaschewski T, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2010; 19:83–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Erskine HE, Ferrari AJ, Polanczyk GV, et al. The global burden of conduct disorder and attention-deficit/hyperactivity disorder in 2010. J Child Psychol Psychiatry. 2014; 55:328–336 [DOI] [PubMed] [Google Scholar]
- 120.Le HH, Hodgkins P, Postma MJ, et al. Economic impact of childhood/adolescent ADHD in a European setting: the Netherlands as a reference case. Eur Child Adolesc Psychiatry. 2014; 23:587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Quintero J, Ramos-Quiroga JA, Sebastián JS, et al. Health care and societal costs of the management of children and adolescents with attention-deficit/hyperactivity disorder in Spain: a descriptive analysis. BMC Psychiatry. 2018; 18:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Daley D, Jacobsen RH, Lange AM, Sørensen A, Walldorf J. The economic burden of adult attention deficit hyperactivity disorder: a sibling comparison cost analysis. Eur Psychiatry. 2019; 61:41–48 [DOI] [PubMed] [Google Scholar]
- 123.Faraone SV, Asherson P, Banaschewski T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015; 1:15020. [DOI] [PubMed] [Google Scholar]
- 124.Thapar A, Cooper M, Eyre O, Langley K. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry. 2013; 54:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


