Abstract
Background
There is limited clinical patient data comparing the first and second waves of the coronavirus disease 2019 (COVID-19) in the United States and the effects of a COVID-19 resurgence on different age, racial and ethnic groups. We compared the first and second COVID-19 waves in the Bronx, New York, among a racially and ethnically diverse population.
Methods
Patients in this retrospective cohort study were included if they had a laboratory-confirmed SARS-CoV-2 infection by a real-time PCR test of a nasopharyngeal swab specimen detected between March 11, 2020, and January 21, 2021. Main outcome measures were critical care, in-hospital acquired disease and death. Patient demographics, comorbidities, vitals, and laboratory values were also collected.
Findings
A total of 122,983 individuals were tested for SARS-CoV-2 infection, of which 12,659 tested positive. The second wave was characterized by a younger demographic, fewer comorbidities, less extreme laboratory values at presentation, and lower risk of adverse outcomes, including in-hospital mortality (adj. OR = 0·23, 99·5% CI = 0·17 to 0·30), hospitalization (adj. OR = 0·65, 99·5% CI = 0·58 to 0·74), invasive mechanical ventilation (adj. OR = 0·70, 99·5% CI = 0·56 to 0·89), acute kidney injury (adj. OR = 0·62, 99·5% CI = 0·54 to 0·71), and length of stay (adj. OR = 0·71, 99·5% CI = 0·60 to 0·85), with Black and Hispanic patients demonstrating most improvement in clinical outcomes.
Interpretation
The second COVID-19 wave in the Bronx exhibits improved clinical outcomes compared to the first wave across all age, racial, and ethnic groups, with minority groups showing more improvement, which is encouraging news in the battle against health disparities.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Resurgence, Race, Ethnicity, Mortality, Bronx
Research in context.
Evidence before this study: There is limited clinical patient data comparing the first and second waves of the coronavirus disease 2019 (COVID-19) in the United States and the effects of a COVID-19 resurgence on different age, racial and ethnic groups. Studies from other countries have observed that the second wave was characterized by a younger cohort with fewer comorbidities and improved clinical outcomes.
Added value of this study: In the largest cohort to date, our study included a racially and ethnically diverse population and compared a large number of clinical variables and outcomes between the first and second COVID-19 waves.
Implications of all the available evidence: The second COVID-19 wave in the Bronx exhibits improved clinical outcomes compared to the first wave across all age, racial, and ethnic groups with minority groups showing more improvement. This is encouraging news in the battle against health disparities and may reflect improved public health measures, large scale PCR testing, earlier diagnosis, and new therapies.
Alt-text: Unlabelled box
1. Introduction
Less than a year into the coronavirus disease 2019 (COVID-19) pandemic [1,2], the United States (US) has reached a grim and once-unthinkable milestone of 500,000 COVID-19 deaths with more than 25 million Americans infected by SARS-CoV-2 (https://coronavirus.jhu.edu, Feb 22, 2021). Many COVID-19 survivors continue to have short- to intermediate-term health issues, and some may experience long-term sequela, imposing a heavy health and socioeconomic burden for years to come [3]. COVID-19 has also highlighted and exacerbated health and healthcare disparities [4], [5], [6].
On January 9, 2020, when the World Health Organization (WHO) announced a coronavirus-related pneumonia outbreak in Wuhan, China, little was known about this new infectious disease. Preventative measures, SARS-CoV-2 PCR and antibody tests, and targeted therapies were limited, resulting in the first wave of widespread global infection. During the second and subsequent waves, reliable testing was more widely available, contact tracing more efficient, experimental therapies under investigation, amongst others, enabling healthcare providers to better treat the disease resulting in improved clinical outcomes.
Remarkable progress has been made in our understanding of SARS-CoV-2’s pathogenicity and transmissibility. Public health measures (i.e., wearing masks, hand hygiene, social distancing, etc.) are effective in curbing the spread of SARS-CoV-2 [7], and new and effective treatments and vaccines are becoming available. The first anti-viral COVID-19 treatment, remdesivir, was approved by the Food and Drug Administration on October 22, 2020, and the first COVID-19 vaccine was approved on December 11, 2020, with a third vaccine recently approved for use in the US at the time of this writing.
Despite many measures to combat COVID-19, multiple resurgences are occurring around the world [8], [9], [10], [11], reflecting variable successes in controlling SARS-CoV-2 infection. Because future resurgences of this virus and its variants are likely, it is crucial to understand how this affects disease outcomes. Asia and Europe [8], [9], [10], [11] experienced a second wave several weeks to several months ahead of the US but few studies to date have characterized resurgences with respect to clinical variables in details and most studies had small sample sizes.
COVID-19 disproportionally affects racial and ethnic minority groups [4,12]. Living conditions, household density, occupational exposure, and access to quality care, amongst others, might have contributed to increased vulnerability to SARS-CoV-2 infection and poorer outcomes in underserved populations [13]. Understanding how clinical outcomes evolve differently amongst racial and ethnic groups across the COVID-19 pandemic could better inform public policy and outreach initiatives.
The Montefiore Health System (MHS), one of the largest healthcare systems in New York City (NYC) with 15 hospitals in the Bronx environs, was hit hard by the first wave of COVID-19, which peaked in April 2020 [14]. After a relatively long quiescent period, a distinct second COVID-19 wave followed. The MHS serves a large low-income, and racially and ethnically diverse population providing an opportunity to study the effects of the second COVID-19 wave in a unique patient population.
The goal of this retrospective cohort study was to compare demographic data, clinical characteristics, and clinical outcomes of individuals with SARS-CoV-2 infection between the first and second wave of the pandemic in a large New York City health system, and to evaluate the potential differential impact of the second surge across age, racial and ethnic groups. To our knowledge, this is the largest cohort to date comparing the first and second COVID-19 waves amongst a large racially and ethnically diverse population.
2. Method
This study was approved by the institutional review board of the Albert Einstein College of Medicine, and informed consent was waived because this was a retrospective cohort study with deidentified patient data. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies.
2.1. Data source
All data originated from MHS and were made available for research after standardization to the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) version 6. OMOP CDM represents healthcare data from diverse sources, which are stored in standard vocabulary concepts, [15] allowing for the systematic analysis of disparate observational databases, including data from the electronic medical record, administrative claims, and disease classifications systems (e.g., ICD-10, SNOWMED, LOINC, etc.). ATLAS, a web-based tool developed by the Observational Health Data Sciences and Informatics (OHDSI) community that enables navigation of patient-level, observational data in the CDM format, was used to search vocabulary concepts and facilitate cohort building. Data were subsequently imported into an SQLite database (www.sqlite.org) and queried using the DB Browser (version 3·12·0).
2.2. Study population
A total of 122,983 patients were tested for COVID-19. The first wave included summary data from March 11, 2020, to August 15, 2020, and the second wave included summary data from August 16, 2020, to January 21, 2021. In the first wave, 49,403 patients were tested for SARS-CoV-2, and in the second wave, 73,580 were tested for COVID-19 infection using real-time polymerase chain reaction test for SARS-CoV-2 on a nasopharyngeal swab specimen. All individuals with lab confirmed SARS-CoV-2 infection during the study time frame, including incidental cases, were included in this study.
2.3. COVID-19 outcomes and clinical variables
Primary outcome was mortality. Secondary outcomes were emergency department (ED) visits, hospitalization, intensive care unit (ICU) admission, need for invasive mechanical ventilation (IMV), length of stay (LOS), and acute kidney injury (AKI). AKI was defined by KDIGO standards [16,17] either a 0·3 mg/dl increase within 48 h or 1·5 times the lowest reading during hospitalization due to lack of data prior to hospitalization.
Other tabulated clinical variables included demographics, chronic comorbidities, and clinical/laboratory tests at presentation. Demographic data were self-identified by the patient during clinical visits and included age, gender, ethnicity and race categorized as Hispanic, non-Hispanic Black, non-Hispanic White, Asian, other (comprising non-Hispanic patients indicating their race as multiple selected, American Indian or Alaska Native, or some other race) or unknown/declined. Median household incomes by zip codes (84·5% were from Bronx County) were also tabulated.
2.4. Statistical analysis
All statistical analysis were performed using Stata statistical software (version 13·1, StataCorp, College Station, TX). We used descriptive statistics to report patient demographic characteristics, including mean ± standard deviation age, proportion of female patients, proportion of racial and ethnic groups, and prevalence of pre-existing conditions for each of the two cohorts: (1) individuals with SARS-CoV-2 infection detected during the first wave (i.e., March 11, 2020, to August 15, 2020) and (2) individuals with SARS-CoV-2 infection detected during the second wave of the pandemic (i.e. August 16, 2020, to January 21, 2021). We reported vital signs and laboratory values as proportion of individuals who exceeded normal ranges. Comparisons of COVID-19 outcomes (i.e., ED visits, hospitalization, LOS, ICU admission, IMV use, AKI, overall death, and in-hospital death) between first and second COVID-19 wave cohorts were reported as risk ratios, risk differences, and age-adjusted odds ratios along with 99·5% confidence intervals given large sample size and multiple outcomes of interest.
2.5. Role of the funding source
No funds.
3. Results
3.1. Patient demographics, pre-existing conditions, and laboratory variables
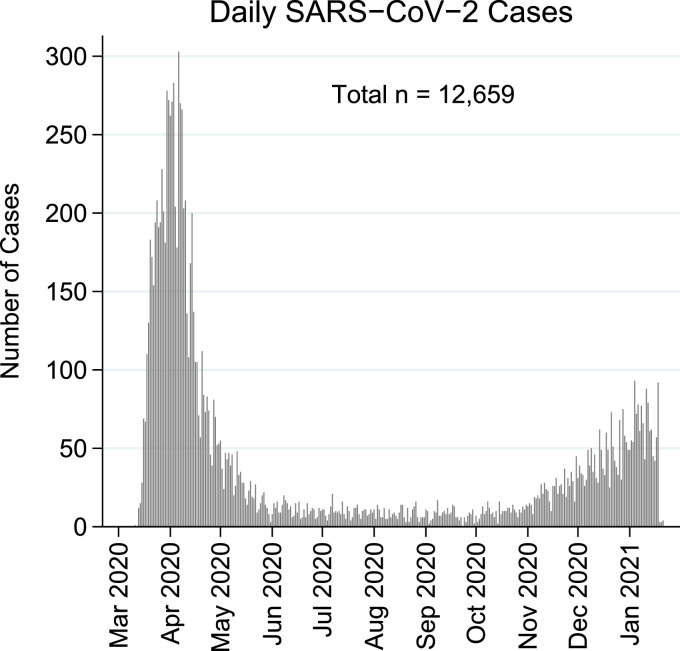
Between March 11, 2020, and January 21, 2021, there were two distinct waves of COVID-19 positive cases (Fig. 1). The first wave peaked in April 2020 followed by a low infection rate period during the summer and fall of 2020, and a distinct resurgence peaked in January 2021. The second wave had a slower rise and lower peak compared to the first wave.
Fig. 1.
Daily SARS-CoV-2 positive cases in the Bronx.
Between March 11, 2020, and January 21, 2021, there were 12,659 positive SARS-CoV-2 cases in the Montefiore Health System. Data demonstrates a bimodal distribution of infections with its first peak in April 2020, a subsequent period with low infections during the summer followed by a second rise in the winter.
Patient demographic characteristics and comorbidities for first and second wave COVID-19 cohorts are presented in Table 1. There were 8,759 SARS-CoV-2 positive cases in the first wave and 3,900 SARS-CoV-2 positive cases in the second wave. Compared to the first wave (mean age ± standard deviation = 56·3 ± 19·1 years, range = 0 to 103 years; 51·7% female; 38·6% Hispanic; 10·3% White, 33·8% Black; 3·2% Asian) the second wave cohort was younger (mean age ± standard deviation = 50·9 ± 21·8 years, range = 0 to 101 years) had more Hispanic (47·3%) and White patients (13·8%), fewer Black patients (23·9%), and about the same proportion Asian patients (3·4%) and female sex (53·2%).
Table 1.
Sample characteristics of individuals with SARS-CoV-2 infection in the Montefiore Health System during the first and second wave of the pandemic.
| First Waven = 8,759 | Second Waven = 3,900 | |
|---|---|---|
| Demographics | ||
| Age in years, mean ± SD | 56·3 ± 19·1 | 50·9 ± 21·8 |
| Female sex, n (%) | 4,526 (51·7) | 2,073 (53·2) |
| Race, n (%) | ||
| Asian | 276 (3·2) | 132 (3·4) |
| Black | 2,963 (33·8) | 930 (23·9) |
| White | 900 (10·3) | 537 (13·8) |
| Other | 3,651 (41·9) | 1,953 (50·5) |
| Unknown | 948 (10·8) | 331 (8·5) |
| Ethnicity, n (%) | ||
| Hispanic | 3,382 (38·6) | 1,846 (47·3) |
| Non-Hispanic | 4,334 (49·5) | 1,688 (43·3) |
| Unknown | 1,043 (11·9) | 366 (9·4) |
| Comorbidities, n (%) | ||
| Chronic kidney disease | 562 (6·4) | 193 (5·0) |
| COPD and asthma | 740 (8·5) | 316 (8·1) |
| Coronary artery disease | 482 (5·5) | 216 (5·5) |
| Diabetes | 1,538 (17·6) | 543 (13·9) |
| Heart failure | 542 (6·2) | 181 (4·6) |
| Hypertension | 2,128 (24·3) | 797 (20·4) |
| Stroke | 181 (2·1) | 57 (1·5) |
Abbreviations: COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Second wave patients had proportionally fewer pre-existing conditions, including hypertension, stroke, type 2 diabetes, chronic kidney disease, and heart failure.
Compared to first wave patients, proportionally fewer second wave patients exceeded normal range laboratory values and vitals at presentation (Table 2) with the greatest differences in lactate dehydrogenase (difference = -15·4%, 99·5% CI = -19·4 to -11·5%), blood urea nitrogen (difference = -15·0%, 99·5% CI = -18·6 to -11·5%), and D-dimer (difference = -8·2%, 99·5% CI = -10·9 to -5·5%).
Table 2.
Individuals with SARS-CoV-2 infection who exceeded normal range laboratory values and vitals at presentation.
| Exceeded normal range n (%) |
Second wave vs first wave |
||||
|---|---|---|---|---|---|
| Laboratory values and vitals | Normal range | First Wave | Second Wave | Difference, % | 99·5% CI |
| Albumin, g/dL | 3·5 to 5·0 | 20 (0·4) | 4 (0·2) | -0·2 | -0·6 to 0·2 |
| AST:ALT ratio | < 1 | 3,625 (77·6) | 1,327 (71·0) | -6·6 | -10·0 to -3·2 |
| Blood urea nitrogen, mg/dL | 7 to 20 | 2,654 (50·9) | 735 (35·8) | -15·0% | -18·6 to -11·5 |
| C-reactive protein, mg/dL | < 1 | 3,767 (89·1) | 1,378 (82·0) | -7·1% | -10·0 to -4·1 |
| Creatinine, mg/dL | 0·84 to 1·21 | 3,765 (72·2) | 1,367 (66·7) | -5·5 | -8·9 to -2·1 |
| D-dimer, µg/mL | < 0·4 | 3,681 (93·0) | 1,452 (84·8) | -8·2 | -10·9 to -5·5 |
| Lactate dehydrogenase, U/L | 140 to 280 | 3,262 (74·4) | 924 (58·9) | -15·4 | -19·4 to -11·5 |
| Lymphocytes, x109/L | 1·0 to 4·0 | 2,196 (42·6) | 839 (40·7) | -1·9 | -5·5 to 1·7 |
| Oxygen saturation, % | > 95% | 1,078 (18·4) | 253 (10·8) | -7·6 | -9·9 to -5·3 |
| Oral temperature, F | 97·6 to 99·6 | 1,401 (26·1) | 419 (19·5) | -6·6 | -9·5 to -3·6 |
3.2. COVID-19 outcomes
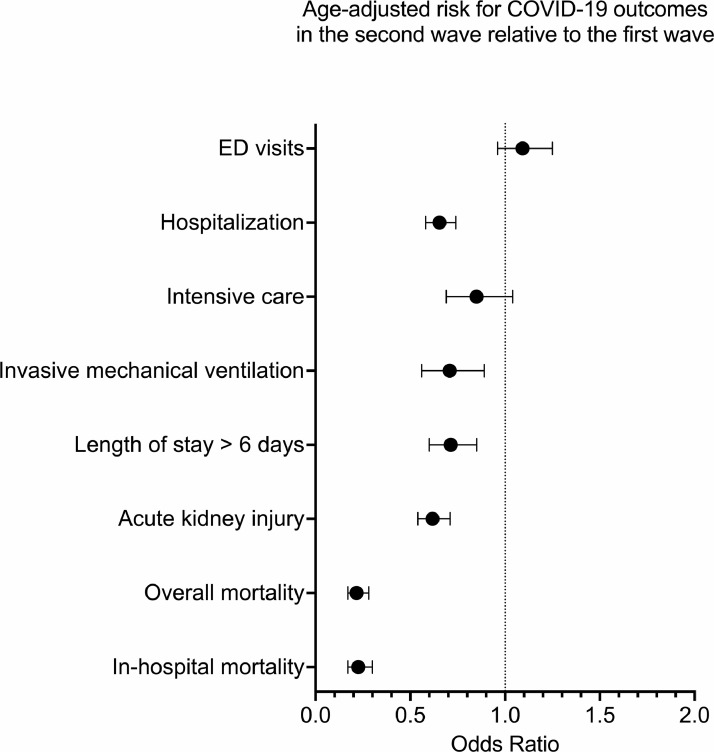
Comparisons of COVID-19 outcomes between first and second wave cohorts are presented in Fig. 2 and Table 3. Compared to the first wave, the second wave cohort had lower risk for all clinical outcomes (risk differences = -1·76% to -16·59%) with most pronounced reduction in mortality (overall mortality: adj. OR = 0·22, 99·5% CI = 0·17 to 0·28; in-hospital mortality: adj. OR = 0·23, 99·5% CI = 0·17 to 0·30), acute kidney injury (adj. OR = 0·62, 99·5% CI = 0·54 to 0·71), and hospitalization (adj. OR = 0·65, 99·5% CI = 0·58 to 0·74). Least pronounced cohort differences in clinical outcomes were noted for ED visits (adj. OR = 1·09, 99·5% CI = 0·96 to 1·25) and ICU admission (adj. OR = 0·85, 99·5% CI = 0·69 to 1·04).
Fig. 2.
Age-adjusted odds ratios for COVID-19 related clinical outcomes in the second wave cohort relative to the first wave cohort.
Logistic Regression showed lower risk for adverse COVID-19 outcomes in the second wave (i.e., OR < 1, and 99·5% confidence intervals < 1), including hospitalization, need for invasive mechanical ventilation, length of stay, acute kidney injury, overall mortality and in-hospital mortality, but not emergency department (ED) visits or intensive care unit admission. Error bars represent 99·5% confidence intervals.
Table 3.
Clinical outcomes of individuals with SARS-CoV-2 infection in the Montefiore Health System during the first and second wave of the pandemic.
| First Wave n = 8,759 | Second Wave n = 3,900 | Second wave vs first wave |
||||||
|---|---|---|---|---|---|---|---|---|
| Clinical outcomes | Absolute risk, n (%) | Risk ratio | 99·5% CI | Risk difference, % | 99·5% CI | Odds Ratiob | 99·5% CI | |
| ED visits | 6,689 (76·37) | 2,878 (73·79) | 0·97 | 0·94 to 1·00 | -2·53 | -4·89 to -0·18 | 1·09 | 0·96 to 1·25 |
| Hospitalization | 5,060 (57·77) | 1,707 (43·77) | 0·76 | 0·72 to 0·80 | -14·00 | -16·68 to -11·32 | 0·65 | 0·58 to 0·74 |
| Intensive care | 785 (8·96) | 281 (7·21) | 0·80 | 0·67 to 0·97 | -1·76 | -3·20 to -0·31 | 0·85 | 0·69 to 1·04 |
| Invasive mechanical ventilation | 714 (8·15) | 207 (5·31) | 0·65 | 0·53 to 0·81 | -2·84 | -4·14 to -1·54 | 0·70 | 0·56 to 0·89 |
| Length of stay > 6 daysa | 2,406 (47·55) | 532 (31·17) | 0·66 | 0·59 to 0·73 | -16·38 | -20·10 to -12·67 | 0·71 | 0·60 to 0·85 |
| Acute kidney injury | 2,769 (31·61) | 793 (20·41) | 0·64 | 0·58 to 0·71 | -11·28 | -13·56 to -9·00 | 0·62 | 0·54 to 0·71 |
| Overall mortality | 1,302 (14·86) | 144 (3·69) | 0·25 | 0·20 to 0·32 | -11·17 | -12·54 to -9·81 | 0·22 | 0·17 to 0·28 |
| In-hospital mortalitya | 1,219 (24·09) | 128 (7·50) | 0·31 | 0·24 to 0·40 | -16·59 | -19·05 to -14·13 | 0·23 | 0·17 to 0·30 |
Among hospitalized patients
Age-adjusted odds ratio
3.3. COVID-19 outcomes by age
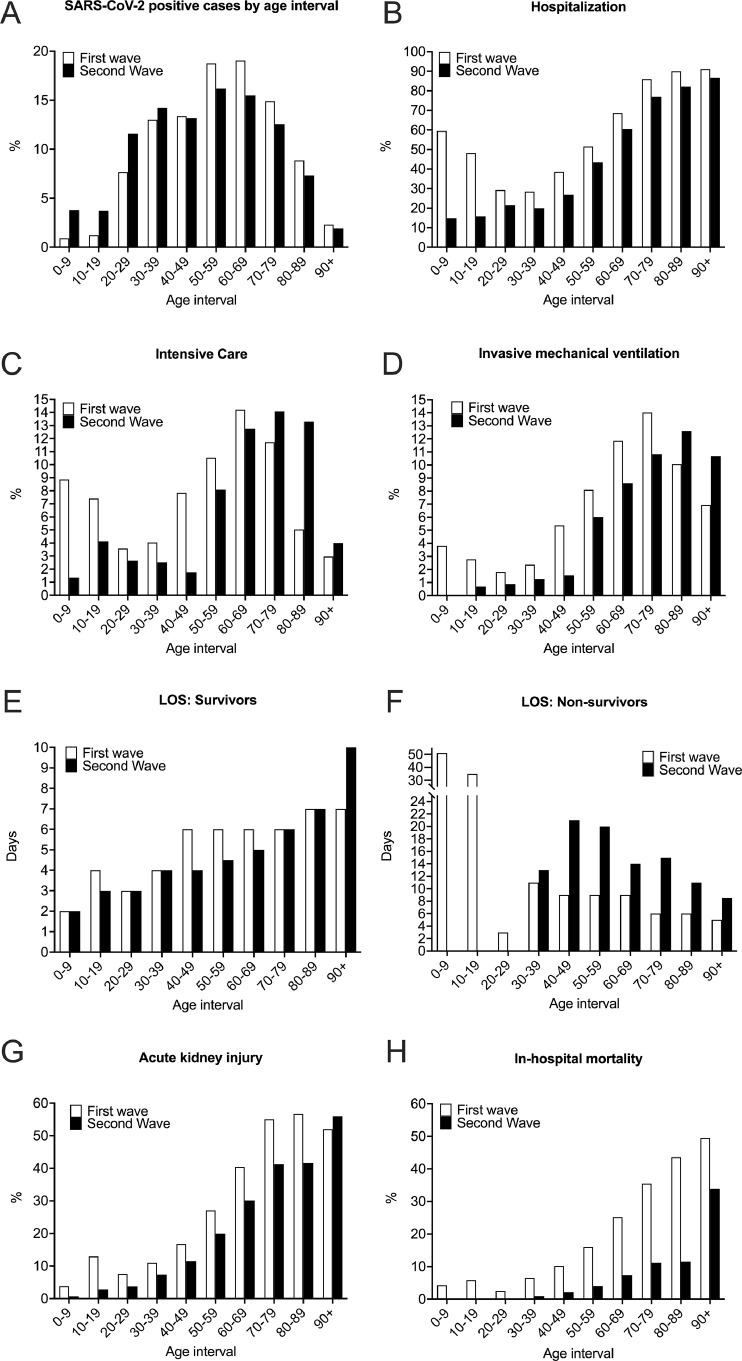
Clinical outcomes of the first and second waves are plotted for different age groups (Fig. 3). In the second wave, the proportion of positive cases increased in the age groups under 40 years old and decreased in the older age groups. Hospitalization rate was lower for each age group. ICU admission was lower for each age group, except 70+ age groups. Invasive mechanical ventilator use was lower for each age group except in the 80+ age groups. Median hospital length of stay was shorter for survivors, but longer for non-survivors. Incidence of acute kidney injury and in-hospital mortality were lower for all age groups.
Fig. 3.
COVID-19 outcomes by 10-year age intervals.
Compared to the first wave, there were relatively more SARS-CoV-2 infections under age 40, and relatively fewer infections over age 50 in the second wave (A). Hospitalization rate was lower for all age groups (B). Intensive care admission was lower for all age groups, except 70+ (C). Invasive mechanical ventilator use was lower for all age groups, except for 80+ age groups (D). Hospital median length of stay (LOS) was shorter for survivors (E), but longer for non-survivors (F). Incidence of acute kidney injury (G) and in-hospital mortality (H) were lower for all age groups in the second wave.
3.4. COVID-19 outcomes by racial and ethnic groups
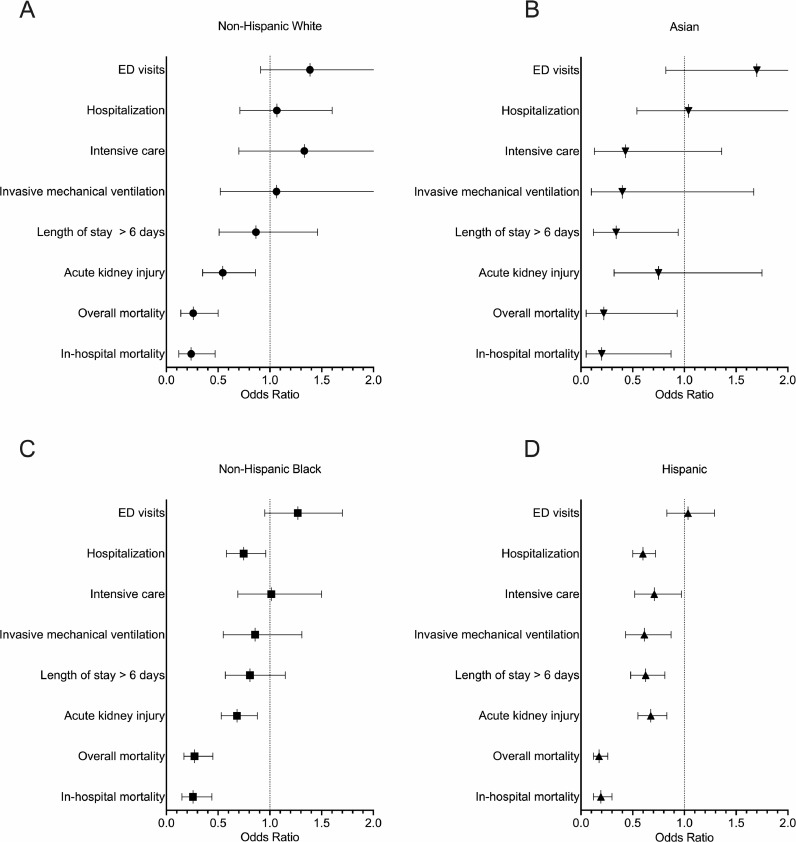
Age-adjusted odds ratios for COVID-19 clinical outcomes in the second wave relative to the first wave, stratified by race and ethnicity, are presented in Fig. 4 and Supplemental Table 1. All racial and ethnic groups had significantly lower mortality in the second wave (adj. OR's ≤ 0·27), but unlike White and Asian patients, Black and Hispanic patients had more clinical outcomes, including hospitalization, invasive mechanical ventilation, ICU admission, and hospital duration with lower risk (adj. OR's < 1). A detailed summary of proportions escalated care, in-hospital acquired disease, and death in first and second wave COVID-19 by racial and ethnic groups can be found in Supplemental Table 2.
Fig. 4.
Stratified by racial and ethnic groups, age-adjusted odds ratios for primary COVID-19 outcomes in the second wave cohort relative to the first wave cohort.
In the second wave, all racial and ethnic groups (A-D) had lower risk of mortality (OR < 1, 99·5% CI < 1) relative to the first wave. However, Black (C) and Hispanic (D) COVID-19 patients had more improved COVID-19 outcomes, including lower risk of hospitalization and acute kidney injury than White (A) and Asian (B) COVID-19 patients. More detailed information with exact odds ratios and 99·5% confidence intervals can be found in Supplemental Table 1.
4. Discussion
The second COVID-19 wave across fifteen hospitals in the Bronx and surrounding NYC areas was characterized by a slower rise, lower peak, younger cohort, fewer comorbidities, less extreme laboratory values at admission, lower hospitalization rate, shorter hospital duration, lower incidence of acute kidney injury, and markedly lower mortality rate across all ages. Clinical outcomes also improved across all racial and ethnic groups, in particular among Black and Hispanic patients, which is encouraging news in the battle against health disparities.
After the first peak in April 2020, a relatively quiescent period followed between June and November 2020, which was likely due to effective public health messaging, behavioural changes (i.e., social distancing, mask wearing, hand hygiene, quarantining, and contact tracing), partial lockdowns, seasonal changes, some degree of herd immunity, and a controlled re-opening in NYC areas that collectively mitigated disease spread. The resurgence that peaked in January 2021, coincided with the preceding holiday season and colder weather that resulted in more frequent indoor gatherings, which might have contributed to increased rates in transmissions.
The second wave COVID-19 patients were younger, which could be due to the younger population returning to school and work activities and/or better preventive measures to protect the older population, especially in nursing homes and assisted living facilities [19]. It is also possible that some of the older vulnerable patients died in the first wave, resulting in a subsequent shift towards a younger demographic in the second wave.
Lower hospitalization rate might be the result of increased COVID-19 testing, which would lead to identification of milder cases not meeting criteria for hospitalization. This idea is supported by the fact that ED visit rate remained high in the second wave. Changes in hospital duration (i.e., shorter LOS for survivors and longer LOS for non-survivors), likely reflect improved disease management and available treatment options [20].
Mortality rates showed stark contrast between the first and second waves, likely the results of lessons learned, improved prevention, treatments and medical management [20]. Also, changes in testing policy may have enabled early detection and timely intervention, which likely contributed to reduction in severe disease. The improved clinical outcomes in the second wave were consistent with admission blood tests that indicated less severe disease at admission [21], [22], [23], [24].
Systematic documentation of admission criteria and hospital capacity between the first and second waves were not readily available across all hospitals and clinics in the Montefiore Health System. The practice and capacity across multiple hospitals likely varied. However, we speculate that during the first wave of the pandemic when our Health System reached maximum capacity, stricter admission criteria could have led to only the most severe people being admitted, whereas during the second wave, our Health System was less burdened and likely admitted less severe cases.
Montefiore is a private, non-profit healthcare organization with an integrated academic delivery system and a multi-county ambulatory network. Montefiore is affiliated with the Albert Einstein College of Medicine, a premier institution for medical education, basic research and clinical investigation. We do not know how public or private healthcare system status could have affected COVID-19 outcomes, which would likely vary.
Historically, epidemics have occurred in phases often with more severe subsequent waves. For example, during the 1898 and 1918 influenza pandemics, second and third waves were much more fatal than the first [25,26] possibly due to mutated virus strains and military operations during World War I, which facilitated virus spread [27]. In our study, the second COVID-19 wave was less deadly and had a slower rise and lower peak compared to the first wave. While this finding is in line with COVID-19 resurgences observed in other parts of the world [8,9,28,29], many countries experience a more traditional pattern. For example, COVID-19 resurgences are reportedly much worse in India, Taiwan, Turkey and other countries (https://graphics.reuters.com/world-coronavirus-tracker-and-maps/). This temporal variability in COVID-19 severity across countries may be partially due to pathogen evolution, behavioural changes, public health interventions, vaccination rates, comorbidity burden, and socio-economic factors [30,31].
Asia and Europe [8], [9], [10], [11] experienced a second wave several weeks to several months ahead of the US but few studies to date have characterized resurgences with respect to clinical variables in details. A study using a Japanese public registry of 5,194 patients [8] found that the second wave had a younger demographic, fewer comorbidities, fewer severe patients at admission, and reduced mortality. Limited clinical and laboratory variables were analyzed. A study from Spain of 468 hospitalized COVID-19 patients found that their second wave cohort was younger, had shorter LOS, needed fewer invasive mechanical ventilation, and had lower mortality in the second wave [10]. An Italian study with 200 Caucasian males over the age of 50 reported lower in-hospital mortality in the second wave [9]. In contrast, a study from France reported no survival difference between 50 first and second wave critically ill COVID-19 patients [32]. Another study from Houston, Texas observed the first wave in April 2020 followed closely by a resurgence in July 2020 and reported lower mortality and a shift toward younger demographics [29]. Our findings are in general agreement that the second surge had a younger demographic and lower mortality rate. In contrast to previous studies, our study has the largest cohort to date, consists of a large population of racial and ethnic minorities, and compares many clinical variables and outcomes between the two waves.
4.1. Differences amongst racial and ethnic subgroups
Black and Hispanic patients were more likely than White and Asian patients to visit the ED and to be hospitalized especially during the first wave, consistent with previous reports [5,33]. Historical distrust in the healthcare system [34], being less informed about COVID-19 risks [12], and/or language barriers [35] might have delayed seeking timely medical attention for COVID-19 symptoms and contributed to higher ED visit and hospitalization rate.
However, we found no evidence that Black and Hispanic patients with COVID-19 had higher mortality rates in the first and second COVID-19 wave in the Bronx and its environs compared to White and Asian patients. Mortality rates could be affected by the number of patients tested. If, due to unequal access, individuals were only tested when they show severe symptoms , the estimated mortality rate for COVID-19 might be different from a situation with equal testing opportunity. We also found no evidence of median household incomes by zip codes affecting mortality rates (Supplemental Table 3). Taken together, these findings suggest that, within the study population, there was no observed elevated burden of this disease among minority populations. Nonetheless, continuing outreach efforts to racial and ethnic minority groups are important in light of the relatively high ED and hospital visits among these groups, as well as challenges in vaccine access and distribution in underserved communities [36].
5. Limitations
As with any retrospective study, there could be unintentional patient selection bias. Although our data came from 15 hospitals, this cohort came predominantly from the Bronx and its environs. These findings need to be replicated at other hospitals to achieve broader generalizability. Data on COVID-19 treatments or clinical trials – possible explanatory variables in terms of improved outcomes – were not readily available for extraction in this study. Another limitation is related to the study population definition, which includes potential misclassification due to: (i) evolving test accuracy (some test results might have been false negative early in the pandemic), and (ii) evolving test policy and availability to population at large (testing was less available early in the pandemic). Thus, percentage of positive rate, percentage of hospitalization, and percentage of worse hospital outcomes need to be interpreted with caution. Median household income based on zip code was used in this study and future studies will need to compare clinical outcomes with socioeconomic status. Finally, this study only reported on in-hospital outcomes. It is also important to investigate the long-term outcomes of COVID-19 survivors among different racial and ethnicity subgroups.
6. Conclusions
The second COVID-19 wave in the Bronx exhibits improved clinical outcomes compared to the first wave across all age, racial, and ethnic groups, with minority groups showing more improvement. This is encouraging news in the battle against health disparities and may reflect improved public health measures, large scale PCR testing, earlier diagnosis, and new therapies. Reducing health disparities is important to minimize the overall socioeconomic and health burden of future COVID-19 resurgences.
Funding
None.
Data sharing statement
Deidentified data may be available upon request. Please contact the corresponding authors.
Contributors
Wouter S. Hoogenboom: Formal analysis, Data curation, Visualization, Writing – original draft, Writing – review & editing. Antoine Pham: Visualization, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Harnadar Anand: Visualization, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Roman Fleysher: Visualization, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Alexandra Buczek: Visualization, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Selvin Soby: Formal analysis, Data curation. Parsa Mirhaji: Formal analysis, Data curation, Writing – review & editing, Supervision. Judy Yee: Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Supervision. Tim Q. Duong: Formal analysis, Data curation, Visualization, Writing – original draft, Writing – review & editing, Supervision.
Declaration of Interest
None.
Acknowledgments
We would like to acknowledge the contributions of the Montefiore Einstein Center for Health Data Innovations; and Dr. Kenny Ye, biostatistician at Albert Einstein College of Medicine, Department of Epidemiology & Population Health, for his assistance with the statistical analysis.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lana.2021.100041.
Contributor Information
Wouter S. Hoogenboom, Email: wouter.hoogenboom@einsteinmed.org.
Tim Q. Duong, Email: tim.duong@einsteinmed.org.
Appendix. Supplementary materials
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.19719. Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apea V.J., Wan Y.I., Dhairyawan R., et al. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: an observational cohort study. BMJ Open. 2021;11(1) doi: 10.1136/bmjopen-2020-042140. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golestaneh L., Neugarten J., Fisher M., et al. The association of race and COVID-19 mortality. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100455. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466–2467. doi: 10.1001/jama.2020.8598. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowling B.J., Aiello AE. Public health measures to slow community spread of coronavirus disease 2019. J Infect Dis. 2020;221(11):1749–1751. doi: 10.1093/infdis/jiaa123. 05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito S., Asai Y., Matsunaga N., et al. First and second COVID-19 waves in Japan: a comparison of disease severity and characteristics. J Infect. 2020 doi: 10.1016/j.jinf.2020.10.033. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghesi A., Golemi S., Carapella N., Zigliani A., Farina D., Maroldi R. Lombardy, Northern Italy: COVID-19 second wave less severe and deadly than the first? A preliminary investigation. Infect Dis. 2021:1–6. doi: 10.1080/23744235.2021.1884745. (Lond)Feb. [DOI] [PubMed] [Google Scholar]
- 10.Iftimie S.A., F.Vallverdú A., ImmaculadaHernàndez-Flix S., de Febrer G., SandraHernández-Aguilera A.R., Francesc J.J., Camps J., Castro A., Group R.S. First and second waves of coronavirus disease-19: a comparative study in hospitalized patients in Reus. Spain. preprint. 2020 doi: 10.1101/2020.12.10.20246959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaptein F.H.J., Stals M.A.M., Grootenboers M., et al. Incidence of thrombotic complications and overall survival in hospitalized patients with COVID-19 in the second and first wave. Thromb Res. 2020;199:143–148. doi: 10.1016/j.thromres.2020.12.019. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis-Jean J., Cenat K., Njoku C.V., Angelo J., Sanon D. Coronavirus (COVID-19) and racial disparities: a perspective analysis. J Racial Ethn Health Disparities. 2020;7(6):1039–1045. doi: 10.1007/s40615-020-00879-4. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabarriti R., Brodin N.P., Maron M.I., et al. Association of race and ethnicity with comorbidities and survival among patients with COVID-19 at an urban medical center in New York. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19795. 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadhera R.K., Wadhera P., Gaba P., et al. Variation in COVID-19 hospitalizations and deaths across New York City Boroughs. JAMA. 2020;323(21):2192–2195. doi: 10.1001/jama.2020.7197. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hripcsak G., Duke J.D., Shah N.H., et al. Observational health data sciences and informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015;216:574–578. [PMC free article] [PubMed] [Google Scholar]
- 16.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 17.Ad-hoc working group of E., Fliser D., Laville M., et al. A European renal best practice (ERBP) position statement on the kidney disease improving global outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27(12):4263–4272. doi: 10.1093/ndt/gfs375. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouslander J.G., Grabowski DC. COVID-19 in nursing homes: calming the perfect storm. J Am Geriatr Soc. 2020;68(10):2153–2162. doi: 10.1111/jgs.16784. 10. [DOI] [PubMed] [Google Scholar]
- 20.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry B.M., Aggarwal G., Wong J., et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38(9):1722–1726. doi: 10.1016/j.ajem.2020.05.073. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahu B.R., Kampa R.K., Padhi A., Panda AK. C-reactive protein: a promising biomarker for poor prognosis in COVID-19 infection. Clin Chim Acta. 2020;509:91–94. doi: 10.1016/j.cca.2020.06.013. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gungor B., Atici A., Baycan O.F., et al. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: a systematic review and meta-analysis. Am J Emerg Med. 2021;39:173–179. doi: 10.1016/j.ajem.2020.09.018. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J., Yan H., Chen H., et al. COVID-19 and coagulation dysfunction in adults: a systematic review and meta-analysis. J Med Virol. 2021;93(2):934–944. doi: 10.1002/jmv.26346. 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kempińska-Mirosławska B., Woźniak-Kosek A. The influenza epidemic of 1889-90 in selected European cities-a picture based on the reports of two Poznań daily newspapers from the second half of the nineteenth century. Med Sci Monit. 2013;19:1131–1141. doi: 10.12659/MSM.889469. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taubenberger J.K., Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12(1):15–22. doi: 10.3201/eid1201.050979. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oxford J.S., Sefton A., Jackson R., Innes W., Daniels R.S., Johnson NP. World war I may have allowed the emergence of "Spanish" influenza. Lancet Infect Dis. 2002;2(2):111–114. doi: 10.1016/s1473-3099(02)00185-8. Feb. [DOI] [PubMed] [Google Scholar]
- 28.Iftimie S., López-Azcona A., F.Vallverdú I., et al. First and second waves of coronavirus disease-19: a comparative study in hospitalized patients in Reus. Spain Preprint. 2020 doi: 10.1101/2020.12.10.20246959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vahidy F.S., Drews A.L., Masud F.N., et al. Characteristics and outcomes of COVID-19 patients during initial peak and resurgence in the houston metropolitan area. JAMA. 2020;324(10):998–1000. doi: 10.1001/jama.2020.15301. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorci G., Faivre B., Morand S. Explaining among-country variation in COVID-19 case fatality rate. Sci Rep. 2020;10(1):18909. doi: 10.1038/s41598-020-75848-2. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wessel L., Hua Y., Wu J., Moghadas SM. Public health interventions for epidemics: implications for multiple infection waves. BMC Public Health. 2011;11(Suppl 1):S2. doi: 10.1186/1471-2458-11-S1-S2. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contou D., Fraissé M., Pajot O., Tirolien J.A., Mentec H., Plantefève G. Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: no prognostic improvement during the second wave? Crit Care. 2021;25(1):3. doi: 10.1186/s13054-020-03449-6. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Killerby M.E., Link-Gelles R., Haight S.C., et al. Characteristics associated with hospitalization among patients with COVID-19 - Metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):790–794. doi: 10.15585/mmwr.mm6925e1. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell W., Richmond J., Mohottige D., Yen I., Joslyn A., Corbie-Smith G. Medical mistrust, racism, and delays in preventive health screening among African-American men. Behav Med. 2019;45(2):102–117. doi: 10.1080/08964289.2019.1585327. Apr-Jun 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortega P., Martínez G., Diamond L. Language and health equity during COVID-19: lessons and opportunities. J Health Care Poor Underserved. 2020;31(4):1530–1535. doi: 10.1353/hpu.2020.0114. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nephew LD. Systemic racism and overcoming my COVID-19 vaccine hesitancy. EClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2020.100713. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.