Abstract
Although a less well-known consequence of alpha-1 antitrypsin deficiency (AATD) liver disease is the second leading cause of death among patients with the condition. The alpha-1 antitrypsin (AAT) protein is produced by hepatocytes within the liver, which retain pathological variants of AAT instead of secreting the proteinase inhibitor into the systemic circulation. This intracellular retention is caused by inefficient folding and polymerization of mutant AAT and the accumulation of these AAT aggregates leads to diverse manifestations of liver disease, which can present differently in both children and adults. The progression from hepatocyte apoptosis to liver inflammation, fibrosis and cirrhosis, and liver failure is still not fully understood, but in older patients, liver disease can surpass lung disease as the principal cause of death. Liver function tests (LFTs) can measure plasma levels of liver enzymes to assess liver function but require careful interpretation. Non-invasive tests are being developed that can detect early liver disease, but liver biopsy is still the gold standard for assessing liver fibrosis once abnormal LFTs have been detected in a patient. Currently, there is no licensed treatment for AATD-related liver disease (intravenous AAT therapy is not indicated for this purpose), but liver transplantation is associated with positive outcomes and may even slow emphysema progression. Therefore, new strategies are being developed to address treatment of AATD-related liver disease, such as accelerating degradation of mutant AAT and assisting hepatocytes in the folding and secretion of mutant AAT, but these approaches remain at early stages of development.
Keywords: alpha-1 antitrypsin, alpha-1 antitrypsin deficiency, cirrhosis, hepatocellular carcinoma, liver disease, liver transplant
Introduction
Liver disease is a less well-known consequence of alpha-1 antitrypsin deficiency (AATD), but it is the second leading cause of death among both adults and children with AATD.1 Liver disease is thought to affect around 10% of individuals with AATD and is also the cause of death in approximately 10% of individuals with AATD.2–4 As is the case with lung disease, AATD is under-diagnosed in patients with liver disease.5 In this chapter, the pathophysiology, presentation, and diagnosis/management of liver disease related to AATD will be discussed, as well as the current open research questions and future developments in the management of AATD-associated liver disease.
Pathophysiology and genetics
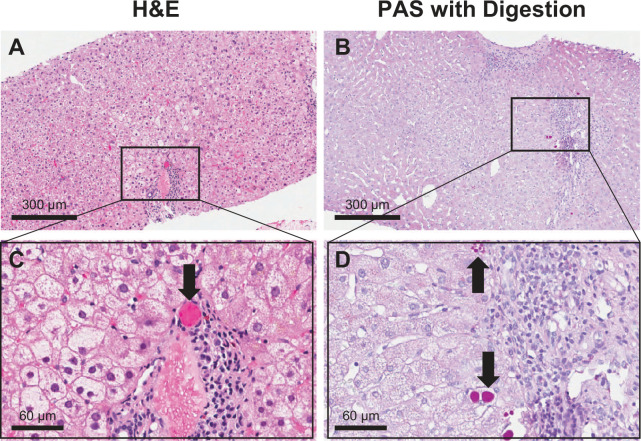
Hepatocytes are responsible for producing the majority of the body’s alpha-1 antitrypsin (AAT) protein, which functions to protect the lungs by inhibiting a range of pro-inflammatory proteases.6 The wild-type AAT protein, referred to as the M type, is normally synthesized in the hepatocyte and rapidly secreted by the endoplasmic reticulum (ER) in large quantities. In contrast, the Z variant folds inefficiently into its final conformation and polymerizes, resulting in 85% ER retention through protein quality control pathways.7 Liver disease from AATD arises due to this polymerization behaviour associated with certain AAT protein variants, such as the Z and S variants, as well as the rare MMalton variant.8,9 With these AAT variants, the mutated AAT protein is not secreted from hepatocyte ER due to misfolding during synthesis and is intracellularly retained. The polymerized AAT is subjected to intracellular proteolysis rather than being secreted into the systemic circulation where it functions as an enzymatic inhibitor.10 These misfolded mutant proteins aggregate in the ER of the hepatocytes and form large polymers/globules, which can be identified microscopically by periodic acid-Schiff staining with diastase digestion on liver biopsy tissue samples (Figure 1). This abnormal accumulation of mutated AAT in the liver has been shown to cause mitochondrial autophagy and caspase activation in hepatocytes,11 which can further lead to hepatocellular apoptosis, liver inflammation, liver fibrosis and cirrhosis, as well as end-stage liver disease.12 Emerging data suggest that circulating extracellular vesicles produced as a result of hepatic injury in AATD patients contain pro-fibrinogenic factors that may promote liver fibrosis. Whether this occurs in situ is yet to be determined.13
Figure 1.
Liver biopsy histology from a PI*ZZ alpha-1 antitrypsin deficiency (AATD) patient.
Hematoxylin and eosin (H&E) staining (A and C) and periodic acid-Schiff (PAS) with digestion staining (B and D) showing positive globules indicative of alpha-1 antitrypsin (AAT) polymerization/accumulation within hepatocytes (arrows).
AATD is one of the three most frequently occurring genetic liver disorders, along with Wilson’s disease and hemochromatosis.14 Although there are many genotypes associated with AATD, liver disease is more strongly associated with the PI*ZZ and PI*SZ genotypes, and has some association with the PI*MZ genotype.15–18 The PI*ZZ genotype has been known to be associated with liver disease for some time, but recent studies have revealed an association between the PI*SZ genotype and liver disease in both adults and children.17 These cases are often less prevalent and less clinically severe than in patients with the PI*ZZ genotype.17 The PI*MZ genotype has also recently been confirmed as a risk factor for the development of liver disease,19,20 and an association has been noted with the rare MMalton variant, which also causes AAT protein polymerization.9 Nevertheless, further data are required on the prevalence of liver disease in AATD, the risk of liver disease associated with different AATD genotypes, and the impact of aggravating factors (e.g. hepatotoxic drugs, alcohol).
Disease presentation
Much like the presentation of lung disease in AATD, the potential manifestations of liver injury are diverse and include chronic hepatitis, cirrhosis, cholestatic jaundice, fulminant hepatic failure, and hepatocellular carcinoma (HCC).21 The typical presentation of liver disease also differs between adults and children with AATD.12
In children, the age of diagnosis varies significantly. AATD-related liver disease has been identified in children between the ages of 1 month and 15 years,16,22 although the most well-known presentation in children is neonatal hepatitis syndrome, which is characterized by severe and prolonged jaundice.21 However, multiple birth screening studies in Europe and North America suggest that most PI*ZZ children are healthy and therefore go undiagnosed in childhood.23,24 A small number of PI*ZZ neonates develop cholestatic hepatitis (neonatal hepatitis syndrome), which manifests as elevated levels of conjugated serum bilirubin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels.25,26 The majority of neonates with cholestatic hepatitis improve spontaneously,27,28 although some PI*ZZ neonates exhibit severe complications of liver disease and go on to develop cirrhosis, portal hypertension and liver failure at a variety of ages, from pre-school to early adulthood.4,27,28 In addition, a subsection of PI*ZZ children who do not exhibit neonatal cholestasis go on to develop liver failure later in childhood. The exact factors influencing progression to liver failure are still unknown, although several new studies are shedding more light on this.
Scarring of the liver, that is, hepatic cirrhosis, usually progresses slowly in patients with AATD and is more commonly seen in adult patients over 50 years of age. The prognosis for these patients is typically poor,29 and liver disease surpasses lung disease as the principal cause of death in older patients, particularly in patients who have never smoked.1 The progression of liver disease into HCC is not well understood, with suggested possible mechanisms including saturation of autophagy, and altered regulation of genes that promote cellular proliferation and tumorigenesis.30 In heterozygous individuals (i.e. those with the PI*MZ genotype), development of HCC appears to be dependent on the presence of pre-existing viral hepatitis infection,31 and in homozygous patients, HCC has been found to develop independently of viral infection and/or pre-existing liver damage.32
Liver disease diagnosis in AATD
Analysis of a patient’s serum AAT protein phenotype and SERPINA1 genotype are considered the gold standard tests for diagnosing AATD.1 Protein phenotype gel analysis (isoelectric focusing electrophoresis) requires technical expertise and is therefore best performed in a reference laboratory with experience in this technique.33 Diagnosis of liver disease via liver biopsy is not necessarily required for AATD diagnosis, but can be useful in assessing the degree and extent of liver injury, as well as fibrosis and cirrhosis.
PI*ZZ patients should theoretically not produce AAT protein levels in the near-normal range. However, experience from the authors suggest that PI*SZ patients with active liver disease and inflammation occasionally show serum AAT levels within the normal range, and systemic inflammation has been shown to mask the presence of the PI*MZ variant.34 Therefore, testing serum AAT levels should be interpreted carefully and should not be used alone for diagnosing AATD. Serum AAT levels are often higher in neonates and then rapidly decrease over the first few months of life, which may not be reflected in the reference ranges of many laboratories. If a patient has recently received a plasma transfusion, measuring serum AATD levels or phenotype testing should be avoided, as results will reflect the donor’s plasma levels rather than the patient’s own plasma levels. Similarly, this is true for patients with emphysema who regularly receive AAT protein replacement therapy.
Monitoring and management of liver disease in AATD
There are several methods used to detect liver disease in AATD. One easy and simple method is to measure plasma levels of liver enzymes using standard blood tests (liver function tests; LFTs). PI*ZZ and PI*SZ patients have been shown to have increased ALT and AST levels compared to control patients, although plasma levels may still be within the normal range.35,36 However, levels of liver enzymes should be interpreted carefully as enzyme levels can be influenced by the use of contraceptive medication, alcohol use and increased body weight with liver steatosis, and therefore should not solely be relied on for diagnosing liver disease.35,36 These factors also add to problems in defining the ‘normal’ reference ranges for these LFTs. The presence of elevated liver enzymes may not accurately reflect ongoing liver injury with AATD and therefore will require further investigation.
Liver biopsy is the gold standard for assessing liver fibrosis and could be used to detect AATD-associated liver disease after the detection of abnormal LFTs, but due to the invasiveness, procedural pain, complications and diagnostic accuracy concerns, non-invasive imaging-based methods for assessing liver disease are preferred. Elastography methods such as magnetic resonance elastography (MRE), acoustic radiation force impulse (ARFI) quantification and two-dimensional shear wave elastography (2D-SWE) have the potential to be suitable imaging tools for the assessment of AATD-related liver fibrosis as they can detect early liver fibrosis before the development of cirrhosis, but require further investigation with larger patient populations.37–39 FibroScan is another non-invasive method that has proved to be accurate in diagnosing liver fibrosis through liver stiffness measurements.19,40 FibroScan can give an estimation of liver fibrosis staging from blood fibrosis test results without the need for liver biopsy,40 and has been validated in AATD patients, with the proportion of invalid scans in children decreasing with age.41,42 Blood tests may also be performed for markers of liver injury, for example, reduced thrombopoietin, or more specific parameters may be assessed using HepaScore and FibroTest. If blood test and elastography results are discordant, liver biopsy may be considered.39
AATD patients with advanced liver disease (cirrhosis or failure) may ultimately require liver transplantation, which is associated with positive outcomes in terms of survival in both children and adult patients.15,22 Liver transplantation may also slow the progression of emphysema, owing to the return to normal levels of AAT protein post-transplant.15,43 However, in some PI*ZZ and PI*SZ patients, levels of forced expiratory volume in 1 s continue to decline unexpectedly post-liver transplant.15
Currently, there is no licensed pharmacological treatment for AATD-related liver disease and liver transplantation is the only way of resolving advanced liver cirrhosis. Therefore, as with liver cirrhosis of any etiology, avoidance of non-steroidal anti-inflammatory drugs (NSAIDs) is typically advised.44,45 Animal model studies of AATD suggest that NSAIDs can be toxic to a PI*ZZ liver uniquely, even in the absence of cirrhosis. The mechanism for NSAID toxicity in PI*ZZ individuals is thought to be due to the prostaglandin production blockade induced by NSAIDs, which can increase AAT synthesis, and therefore, increase misfolded protein accumulation.46 This pattern of injury by NSAIDs in a PI*ZZ liver has only been reported in animal models and has not yet been shown in humans. Therefore, in case of fever or pain in PI*ZZ patients, many authorities suggest overall NSAID avoidance in favour of moderate doses of acetaminophen.
Regarding alcohol consumption, data are currently lacking in PI*ZZ patients without any evidence of liver damage. However, the PI*MZ genotype was found to increase the risk of developing cirrhosis in alcohol misusers, although the risk for the PI*MS genotype is less clear.20,47,48 According to guidelines from the American Association for the Study of Liver Diseases (AASLD) for adults with hepatitis C infection, there is no known safe level of alcohol use, and therefore, all patients should be advised to abstain from alcohol;49 this recommendation becomes more critical in hepatitis C-positive patients with AATD. However, it is currently unclear if a similar strategy should be recommended for patients with AATD and without hepatitis C infection.
Intravenous AAT augmentation therapy is not recommended for the treatment of AATD-related liver disease as clinical experience suggests that liver disease is unaffected by this therapy.50 Augmentation therapy is also not recommended for AATD treatment in patients that have undergone liver transplantation, as a successful liver transplant should lead to normal circulating levels of the AAT protein in the patient post-transplant.50
Future developments
New strategies are required for the development of pharmacological interventions for the treatment of AATD-related liver disease and many new approaches are currently being examined. Extensive studies have recently been published using in vitro analyses of the molecular structure of the PI*ZZ protein, and more than 10 different compounds have been shown to block liver injury in the PI*ZZ mouse model of AATD-related liver disease, although none are yet approved for use in humans.51–54 Several gene repair technologies,55–58 and the use of chemical chaperones,59,60 are also being investigated to improve proper folding of mutant AAT proteins.
Several applications of RNA interference (RNAi) technology are also being examined to prevent mutant PI*ZZ protein synthesis and thereby prevent toxic hepatic accumulation and liver injury. In the PI*ZZ mouse model, these methods have been shown to eliminate liver injury, reverse liver disease, and prevent liver disease in young mice, therefore representing a promising therapy for AATD-related liver disease.61 Phase I/II and phase II/III human trials using silencing RNA (siRNA) technology to inhibit PI*ZZ protein synthesis as a therapy are now underway in Europe and the United States, respectively.62,63 The use of in silico or ‘cell-free’ systems has also been examined for designing therapeutic strategies for the disruption of mutant AAT protein polymerisation, which is likely to be an event distal to the protein retention signal.54,64 However, the predicted effect of many compounds has not yet been reported in follow-up cell culture models, and the process of chemically creating medicinal molecules for trials in animal models has proved challenging. Another type of therapy, currently undergoing phase II trials, is being led by Vertex Pharmaceuticals. This therapy utilises small molecular chaperones to help reduce PI*ZZ AAT polymerization, which in theory could benefit AATD lung and liver disease simultaneously.65
Methods to accelerate intracellular degradation of mutant AAT proteins as a potential treatment for the liver have also been investigated. Several successful experiments in both cell culture and mouse models have demonstrated that up-regulating the autophagy degradation pathway can reduce the burden of the mutant AAT protein in the liver and reduce the associated liver injury.21,27,66 Autophagy is an intracellular degradation pathway that is primarily important for balancing sources of energy during critical points of development and in response to nutrient stress. Autophagy is also important for removing misfolded/aggregated proteins and damaged organelles and is known to be activated in response to the accumulation of misfolded AAT proteins in the liver.66 Enhancing autophagy using compounds such as sirolimus, carbamazepine and ursodeoxycholic acid, as well as genetic approaches to induce the expression of key autophagy regulators, have all been shown to decrease mutant AAT protein accumulation within cells and reduce liver cell injury.51,52,67,68 However, excessively high doses of all of these agents were required to show any affect. A human trial is currently underway for the use of low doses of carbamazepine in PI*ZZ patients with liver cirrhosis.69
Conclusions
The manifestations of liver disease related to AATD are extremely diverse, and the etiology and exact prevalence are still not fully understood. As liver disease can manifest at any age, patients who develop chronic liver disease without any clear etiology, such as alcohol abuse or viral hepatitis, should be tested for AATD.50 There is currently no specific treatment for AATD-related liver disease, and so effective pharmacological therapies to reduce the need for liver transplantation and disease progression are urgently needed. New technologies such as gene therapy and RNAi may hold promise for the treatment of liver disease caused by AATD, but further research and human trials are still required.
Acknowledgments
Medical writing assistance was provided by Ben McDermott and Steven Foster of Meridian HealthComms Ltd., Plumley, UK, in accordance with good publication practice (GPP3), funded by CSL Behring.
Footnotes
Author contributions: Both authors contributed to the writing of the manuscript, reviewed the manuscript, and approved the manuscript for submission.
Conflict of interest: DP reports no conflicts of interest. JT reports grants from NIH, Alpha-1 Foundation, Vertex, Dicerna, Camp4, Arrowhead, KorroBio, and Gilead; and consulting fees from Alpha-1 Foun-dation, Vertex, Arrowhead, Dicerna, RestoreBio, Takeda, Retrophin, GLG, BioMarin, and Boehringer.
Contributor Information
Dhiren Patel, Department of Pediatrics, Division of Gastroenterology, Hepatology and Nutrition, St Louis University School of Medicine, St Louis, MO, USA.
Jeffrey Teckman, Department of Pediatrics and Department of Biochemistry and Molecular Biology, St Louis University School of Medicine, St Louis, MO, USA.
References
- 1.American Thoracic Society and European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2003; 168: 818–900. [DOI] [PubMed] [Google Scholar]
- 2.Strange C, Stoller JK, Sandhaus RA, et al. Results of a survey of patients with alpha-1 antitrypsin deficiency. Respiration 2006; 73: 185–190. [DOI] [PubMed] [Google Scholar]
- 3.Tanash HA, Nilsson PM, Nilsson JA, et al. Survival in severe alpha-1-antitrypsin deficiency (PiZZ). Respir Res 2010; 11: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsend SA, Edgar RG, Ellis PR, et al. Systematic review: the natural history of alpha-1 antitrypsin deficiency, and associated liver disease. Aliment Pharmacol Ther 2018; 47: 877–885. [DOI] [PubMed] [Google Scholar]
- 5.Shah RS, Alsuleiman B, Bena J, et al. Alpha-1 antitrypsin deficiency is under-recognized in individuals with cirrhosis undergoing liver transplantation. Eur J Gastroenterol Hepatol. Epub ahead of print 30 November 2020. DOI: 10.1097/meg.0000000000002005 [DOI] [PubMed] [Google Scholar]
- 6.Janciauskiene SM, Bals R, Koczulla R, et al. The discovery of α1-antitrypsin and its role in health and disease. Respir Med 2011; 105: 1129–1139. [DOI] [PubMed] [Google Scholar]
- 7.Patel D, Teckman JH.Alpha-1-antitrypsin deficiency liver disease. Clin Liver Dis 2018; 22: 643–655. [DOI] [PubMed] [Google Scholar]
- 8.Ferrarotti I, Baccheschi J, Zorzetto M, et al. Prevalence and phenotype of subjects carrying rare variants in the Italian registry for alpha1-antitrypsin deficiency. J Med Genet 2005; 42: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joly P, Guillaud O, Hervieu V, et al. Clinical heterogeneity and potential high pathogenicity of the Mmalton Alpha 1 antitrypsin allele at the homozygous, compound heterozygous and heterozygous states. Orphanet J Rare Dis 2015; 10: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teckman JH, Burrows J, Hidvegi T, et al. The proteasome participates in degradation of mutant alpha 1-antitrypsin Z in the endoplasmic reticulum of hepatoma-derived hepatocytes. J Biol Chem 2001; 276: 44865–44872. [DOI] [PubMed] [Google Scholar]
- 11.Teckman JH, An JK, Blomenkamp K, et al. Mitochondrial autophagy and injury in the liver in alpha 1-antitrypsin deficiency. Am J Physiol Gastrointest Liver Physiol 2004; 286: G851–G862. [DOI] [PubMed] [Google Scholar]
- 12.Teckman JH.Liver disease in alpha-1 antitrypsin deficiency: current understanding and future therapy. COPD 2013; 10 (Suppl. 1): 35–43. [DOI] [PubMed] [Google Scholar]
- 13.Khodayari N, Oshins R, Holliday LS, et al. Alpha-1 antitrypsin deficient individuals have circulating extracellular vesicles with profibrogenic cargo. Cell Commun Signal 2020; 18: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarrilli F, Elce A, Scorza M, et al. An update on laboratory diagnosis of liver inherited diseases. Biomed Res Int 2013; 2013: 697940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey EJ, Iyer VN, Nelson DR, et al. Outcomes for recipients of liver transplantation for alpha-1-antitrypsin deficiency-related cirrhosis. Liver Transpl 2013; 19: 1370–1376. [DOI] [PubMed] [Google Scholar]
- 16.Comba A, Demirbas F, Caltepe G, et al. Retrospective analysis of children with alpha-1 antitrypsin deficiency. Eur J Gastroenterol Hepatol 2018; 30: 774–778. [DOI] [PubMed] [Google Scholar]
- 17.McElvaney GN, Sandhaus RA, Miravitlles M, et al. Clinical considerations in individuals with α1-antitrypsin PI*SZ genotype. Eur Respir J 2020; 55: 1902410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz M, Lacaille F, Berthiller J, et al. Liver disease related to α1-antitrypsin deficiency in French children: the DEFI-ALPHA cohort. Liver Int 2019; 39: 1136–1146. [DOI] [PubMed] [Google Scholar]
- 19.Mandorfer M, Bucsics T, Hutya V, et al. Liver disease in adults with alpha1-antitrypsin deficiency. United European Gastroenterol J 2018; 6: 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strnad P, Buch S, Hamesch K, et al. Heterozygous carriage of the alpha1-antitrypsin Pi*Z variant increases the risk to develop liver cirrhosis. Gut 2019; 68: 1099–1107. [DOI] [PubMed] [Google Scholar]
- 21.Teckman JH, Mangalat N.Alpha-1 antitrypsin and liver disease: mechanisms of injury and novel interventions. Expert Rev Gastroenterol Hepatol 2015; 9: 261–268. [DOI] [PubMed] [Google Scholar]
- 22.Filipponi F, Soubrane O, Labrousse F, et al. Liver transplantation for end-stage liver disease associated with alpha-1-antitrypsin deficiency in children: pretransplant natural history, timing and results of transplantation. J Hepatol 1994; 20: 72–78. [DOI] [PubMed] [Google Scholar]
- 23.Laurell CB, Sveger T.Mass screening of newborn Swedish infants for alpha antitrypsin deficiency. Am J Hum Genet 1975; 27: 213–217. [PMC free article] [PubMed] [Google Scholar]
- 24.Volpert D, Molleston JP, Perlmutter DH.α1-Antitrypsin deficiency-associated liver disease progresses slowly in some children. J Pediatr Gastroenterol Nutr 2000; 31: 258–263. [DOI] [PubMed] [Google Scholar]
- 25.Fregonese L, Stolk J.Hereditary alpha-1 antitrypsin deficiency and its clinical consequences. Orphanet J Rare Dis 2008; 3: 16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monajemzadeh M, Shahsiah R, Vasei M, et al. Alpha 1 antitrypsin deficiency in infants with neonatal cholestasis. Iran J Pediatr 2013; 23: 501–507. [PMC free article] [PubMed] [Google Scholar]
- 27.Teckman JH, Rosenthal P, Abel R, et al. Baseline analysis of a young α-1-antitrypsin deficiency liver disease cohort reveals frequent portal hypertension. J Pediatr Gastroenterol Nutr 2015; 61: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sveger T, Eriksson S.The liver in adolescents with α-antitrypsin deficiency. Hepatology 1995; 22: 514–517. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson S.Alpha 1-antitrypsin deficiency and liver cirrhosis in adults. An analysis of 35 Swedish autopsied cases. Acta Med Scand 1987; 221: 461–467. [PubMed] [Google Scholar]
- 30.Topic A, Ljujic M, Radojkovic D.Alpha-1-antitrypsin in pathogenesis of hepatocellular carcinoma. Hepat Mon 2012; 12_suppl: e7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodges JR, Millward-Sadler GH, Barbatis C, et al. Heterozygous MZ alpha 1-antitrypsin deficiency in adults with chronic active hepatitis and cryptogenic cirrhosis. N Engl J Med 1981; 304: 557–560. [DOI] [PubMed] [Google Scholar]
- 32.Van Thiel DH, Ramadori G. Non-viral causes of hepatocellular carcinoma. J Gastrointest Cancer 2011; 42: 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greene DN, Elliott-Jelf MC, Straseski JA, et al. Facilitating the laboratory diagnosis of α1-antitrypsin deficiency. Am J Clin Pathol 2013; 139: 184–191. [DOI] [PubMed] [Google Scholar]
- 34.Ottaviani S, Gorrini M, Scabini R, et al. C reactive protein and alpha1-antitrypsin: relationship between levels and gene variants. Transl Res 2011; 157: 332–338. [DOI] [PubMed] [Google Scholar]
- 35.Bernspang E, Carlson J, Piitulainen E.The liver in 30-year-old individuals with alpha(1)-antitrypsin deficiency. Scand J Gastroenterol 2009; 44: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 36.Clark VC, Dhanasekaran R, Brantly M, et al. Liver test results do not identify liver disease in adults with alpha(1)-antitrypsin deficiency. Clin Gastroenterol Hepatol 2012; 10: 1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiter R, Wetzel M, Hamesch K, et al. Comparison of non-invasive assessment of liver fibrosis in patients with alpha1-antitrypsin deficiency using magnetic resonance elastography (MRE), acoustic radiation force impulse (ARFI) quantification, and 2D-shear wave elastography (2D-SWE). PLoS One 2018; 13: e0196486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim RG, Nguyen P, Bettencourt R, et al. Magnetic resonance elastography identifies fibrosis in adults with alpha-1 antitrypsin deficiency liver disease: a prospective study. Aliment Pharmacol Ther 2016; 44: 287–299. [DOI] [PubMed] [Google Scholar]
- 39.Hamesch K, Strnad P.Non-invasive assessment and management of liver involvement in adults with alpha-1 antitrypsin deficiency. Chronic Obstr Pulm Dis 2020; 7: 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boursier J, Vergniol J, Guillet A, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol 2016; 65: 570–578. [DOI] [PubMed] [Google Scholar]
- 41.Guillaud O, Dumortier J, Traclet J, et al. Assessment of liver fibrosis by transient elastography (Fibroscan®) in patients with A1AT deficiency. Clin Res Hepatol Gastroenterol 2019; 43: 77–81. [DOI] [PubMed] [Google Scholar]
- 42.Shneider BL, Goodrich NP, Ye W, et al. Nonfasted liver stiffness correlates with liver disease parameters and portal hypertension in pediatric cholestatic liver disease. Hepatol Commun 2020; 4: 1694–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldberg DS, Fallon MB.Lung and heart disease secondary to liver disease. Clin Gastroenterol Hepatol 2015; 13: 2118–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandok N, Watt KD.Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc 2010; 85: 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rakoski M, Goyal P, Spencer-Safier M, et al. Pain management in patients with cirrhosis. Clin Liver Dis 2018; 11: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudnick DA, Shikapwashya O, Blomenkamp K, et al. Indomethacin increases liver damage in a murine model of liver injury from alpha-1-antitrypsin deficiency. Hepatology 2006; 44: 976–982. [DOI] [PubMed] [Google Scholar]
- 47.Abul-Husn NS, Cheng X, Li AH, et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med 2018; 378: 1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basyte-Bacevice V, Skieceviciene J, Valantiene I, et al. SERPINA1 and HSD17B13 gene variants in patients with liver fibrosis and cirrhosis. J Gastrointestin Liver Dis 2019; 28: 297–302. [DOI] [PubMed] [Google Scholar]
- 49.Ghany MG, Morgan TR.Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 2020; 71: 686–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandhaus RA, Turino G, Brantly ML, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis 2016; 3: 668–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hidvegi T, Ewing M, Hale P, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science 2010; 329: 229–232. [DOI] [PubMed] [Google Scholar]
- 52.Kaushal S, Annamali M, Blomenkamp K, et al. Rapamycin reduces intrahepatic alpha-1-antitrypsin mutant Z protein polymers and liver injury in a mouse model. Exp Biol Med 2010; 235: 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindblad D, Blomenkamp K, Teckman J.Alpha-1-antitrypsin mutant Z protein content in individual hepatocytes correlates with cell death in a mouse model. Hepatology 2007; 46: 1228–1235. [DOI] [PubMed] [Google Scholar]
- 54.Mahadeva R, Dafforn TR, Carrell RW, et al. 6-Mer peptide selectively anneals to a pathogenic serpin conformation and blocks polymerization. Implications for the prevention of Z alpha(1)-antitrypsin-related cirrhosis. J Biol Chem 2002; 277: 6771–6774. [DOI] [PubMed] [Google Scholar]
- 55.Bjursell M, Porritt MJ, Ericson E, et al. Therapeutic genome editing with CRISPR/Cas9 in a humanized mouse model ameliorates α1 antitrypsin deficiency phenotype. EBioMedicine 2018; 29: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flotte TR, Trapnell BC, Humphries M, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum Gene Ther 2011; 22: 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loring HS, Flotte TR.Current status of gene therapy for α-1 antitrypsin deficiency. Expert Opin Biol Ther 2015; 15: 329–336. [DOI] [PubMed] [Google Scholar]
- 58.Shen S, Sanchez ME, Blomenkamp K, et al. Amelioration of alpha-1 antitrypsin deficiency diseases with genome editing in transgenic mice. Hum Gene Ther 2018; 29: 861–873. [DOI] [PubMed] [Google Scholar]
- 59.Burrows JA, Willis LK, Perlmutter DH.Chemical chaperones mediate increased secretion of mutant α1-antitrypsin (α1-AT) Z: a potential pharmacological strategy for prevention of liver injury and emphysema in α1-AT deficiency. Proc Natl Acad Sci USA 2000; 97: 1796–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teckman JH.Lack of effect of oral 4-phenylbutyrate on serum alpha-1-antitrypsin in patients with alpha-1-antitrypsin deficiency: a preliminary study. J Pediatr Gastroenterol Nutr 2004; 39: 34–37. [DOI] [PubMed] [Google Scholar]
- 61.Guo S, Booten SL, Aghajan M, et al. Antisense oligonucleotide treatment ameliorates alpha-1 antitrypsin-related liver disease in mice. J Clin Invest 2014; 124: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Institutes of Health. Safety, tolerability and effect on liver histologic parameters of ARO-AAT (SEQUOIA). Clinical trials.gov website. https://clinicaltrials.gov/ct2/show/results/NCT03945292 (2019, accessed 18 February 2020).
- 63.National Institutes of Health. A study of DCR-A1AT in healthy adult volunteers and patients with A1ATD-associated liver disease. Clinical trials. gov website. https://www.clinicaltrials.gov/ct2/show/NCT04174118 (2019, accessed 18 February 2020).
- 64.Parfrey H, Dafforn TR, Belorgey D, et al. Inhibiting polymerization: new therapeutic strategies for Z alpha1-antitrypsin-related emphysema. Am J Respir Cell Mol Biol 2004; 31: 133–139. [DOI] [PubMed] [Google Scholar]
- 65.National Institutes of Health. Evaluation of the efficacy and safety of VX-814 in subjects with the PiZZ genotype. Clinical trials.gov website. https://clinicaltrials.gov/ct2/show/NCT04167345 (2019, accessed 18 February 2020).
- 66.Teckman JH, Perlmutter DH.Retention of mutant alpha(1)-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am J Physiol Gastrointest Liver Physiol 2000; 279: G961–G974. [DOI] [PubMed] [Google Scholar]
- 67.Pastore N, Blomenkamp K, Annunziata F, et al. Gene transfer of master autophagy regulator TFEB results in clearance of toxic protein and correction of hepatic disease in alpha-1-anti-trypsin deficiency. EMBO Mol Med 2013; 5: 397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang Y, Fickert P, Trauner M, et al. Autophagy induced by exogenous bile acids is therapeutic in a model of α-1-AT deficiency liver disease. Am J Physiol Gastrointest Liver Physiol 2016; 311: G156–G165. [DOI] [PubMed] [Google Scholar]
- 69.National Institutes of Health. Carbamzepine in severe liver disease due to alpha-1 antitrypsin deficiency (CBZ). Clinical Trials.gov website. https://clinicaltrials.gov/ct2/show/NCT01379469 (2011, accessed 16 January 2020).