Introduction
In the U.S., more than one in five new HIV diagnoses occurred in young people aged 13–24 in 2016.1 Seventy percent were young men who have sex with men (MSM), especially those of color. Young people, particularly those of color, also have high rates of sexually transmitted infections (STIs) that can enhance acquisition of HIV.2 Adolescence is a dynamic construct, usually defined as the period from puberty to adulthood, typically categorized as ages 10–18 years, although some scholars view it as continuing until 26 years of age.3 For the purposes of this paper, we define adolescents as those less than 18 years. The Centers for Disease Control does not separate data from adolescents under age 18 from data from other youth, instead reporting HIV and AIDs statistics by age categories of less than 13, 13–14 and 15–19.4 However, adolescents clearly account for a sizable number of young people affected by the virus. The likelihood of adolescents acquiring HIV is related primarily to sexual risk behavior. Adolescence is a time of exploration and experimentation, especially in sexuality and intimate relationships.5 According to the Youth Risk Behavior Surveillance System, by the age of 16, one-third of students have engaged in vaginal intercourse.6 Among sexually active students, 46.2% did not use a condom at last sex and 18.8% of students used alcohol or drugs with sex.7 Research is urgently needed that tests behavioral, community, and biomedical interventions to reduce STIs, including HIV incidence in adolescence.
However, critical ethical questions arise concerning whether studies of new behavioral and biomedical HIV preventive interventions, such as Pre-Exposure Prophylaxis (PrEP), among adolescents should require parental permission for participation.8 Research studies on new prevention technologies such as PrEP (Truvada: emtricitabine/tenofovir disoproxil fumarate), long-acting PrEP, intravaginal rings, and broadly neutralizing HIV-1 antibodies (bNAbs) are now recruiting adolescents, notably high-risk adolescents, posing ethical, legal, and regulatory concerns about study participation.9 Novel biomedical devices and medications may pose different, increased, or unknown risks to young research participants. Behavioral interventions that use online and social media approaches may also present risks, including challenges to privacy and confidentiality that are difficult to anticipate, especially for lesbian, gay, bisexual, transgender, and queer (LGBTQ) youth. PrEP holds significant promise for preventing HIV acquisition in young people, and now that the Federal Drug Administration (FDA) has approved this intervention for adolescents aged 15–17, a growing number of studies may recruit adolescents. Increasingly, researchers and Institutional Review Boards (IRBs) are thus confronting the scientific and ethical dilemmas involved.
Adolescent minors are defined as those younger than legal age of consent, typically, 18 years old in the U.S. Currently, IRB regulations require parental permission for minor youth to participate in research, but the Common Rule (45-CFR-46) permits waivers for the protection of human subjects under certain circumstances.10 IRBs usually waive parental permission only in minimal-risk research. FDA regulations do not have a comparable waiver of parental permission for marketing approval of bio-medical products.
This paper presents issues that arose in the meeting as well as others that have emerged in the literature. We examine here first the relevant regulations and sources of ethical guidance concerning parental waiver that influenced our analyses. We then explore arguments for and against waiving parental permission. Finally, we present areas for future research, and recommendations regarding when parental permission should be waived, and when researchers should adopt special protections for adolescents who consent for themselves.
Researchers generally request a waiver of parental permission for one or more of several reasons: when requiring such permission might result in disclosure of youths’ sexual behavior or identity with possible punitive results; when requiring parent permission would significantly reduce participation rates and bias sample characteristics, affecting study validity; or when parents are not available. These reasons clearly differ. The issue that requiring parental permission may put adolescents at risk relates to the best interest of the teen. The objection that requiring parental permission may limit adolescent participation, which in turn jeopardizes the study’s findings’ validity, relates to science advancement. If researchers ask to waive parental consent in order to more quickly advance science, IRBs should carefully balance this request against the potential benefits and risks to individual participants. Such waivers might be reasonable to consider in certain cases and not others.
Researchers know that when parental permission is required in research studies about sexual behavior in adolescents, participation is considerably lower, and the resulting sample may be a biased subset of the population as a whole.11 But whether parental waivers are warranted for HIV prevention studies that include a medical or any other type of intervention is unclear. Even in minimal risk research, researchers, IRBs, and other stakeholders need to address when to permit adolescents to decide on their own about study or intervention participation. The considerations that must be addressed include adolescent factors (e.g., autonomy, cognitive and emotional maturity, and familiarity with research), the study’s potential risks and benefits, the value of the research to adolescents in general, whether parental involvement would be protective, potentially harmful or unknown, and whether alternative protective strategies (e.g., ombudsmen or youth advocates) should be used for adolescents enrolled without parental involvement. Depending on the study, stakeholders need to assess whether adolescents can sufficiently assess the risk/benefit ratio without parental oversight. If parental permission is waived, young people may be exposed to research risks they do not anticipate or understand. Researchers often experience a moral conflict over this issue, struggling to determine when to obtain parental permission, knowing it may reduce the study’s scientific value, and having to assess IRB regulations, state laws, institutional risk avoidance, respect for the parental role, and concerns or misconceptions about adolescent capacity to make decisions. These factors can all influence ethical decisions regarding the design and conduct of the research.
To address this critical question, the HIV Center for Clinical and Behavioral Studies at the New York State Psychiatric Institute and Columbia University recently held an invitational meeting entitled, “Obtaining parental permission in HIV prevention research with adolescent minors: Whose risk, whose benefit, whose judgment?” (list of participants provided in Acknowledgements). The reason for this meeting emerged from several participants’ experiences reviewing grant proposals on adolescent studies. Some proposals required parental permission and others did not. Reviewers did not always agree with each other or with the investigators’ decisions on the need for parental permission.
The day-long HIV Center meeting aimed to help researchers and IRBs make decisions about the need for parent permission in adolescent HIV prevention studies. Scientists, ethicists, developmental psychologists, pediatricians, and governmental representatives collaborated to identify the relevant legal and regulatory contexts (e.g., the Common Rule, and individual state laws); the factors that enhance or hinder the likelihood that parental permission would be waived; the characteristics (or subgroups) of young people that might influence this decision-making; the nature of risk conceptually and practically, and ways it should be identified, characterized and minimized; and the future research needed to address the major outstanding questions. We developed conclusions and recommendations for future HIV prevention research among adolescents.
This paper presents issues that arose in the meeting as well as others that have emerged in the literature. We examine here first the relevant regulations and sources of ethical guidance concerning parental waiver that influenced our analyses. We then explore arguments for and against waiving parental permission. Finally, we present areas for future research, and recommendations regarding when parental permission should be waived, and when researchers should adopt special protections for adolescents who consent for themselves.
While several aspects of ethical and regulatory issues related to research on youth in general have been explored,12 we focus here on challenges and questions that arise in applying these guidelines and regulations specifically to the important case of researcher and IRB decision-making about waiving parent permission in adolescent HIV prevention research.
Key Guidelines, Regulations and Laws Concerning Adolescent Participation in Research and Parental Waiver
The Belmont Report
The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research of Biomedical and Behavioral Research issued 17 reports, including the Belmont Report13 and a report on Research Involving Children.14 The Belmont Report was written to identify the basic ethical principles that should underlie the conduct of biomedical and behavioral research involving human subjects.15 The Report identified three principles to guide ethical decision-making: Autonomy/Respect for Persons, Beneficence, and Justice.
Autonomy/Respect for Persons
Participants should be treated as autonomous individuals capable of making informed decisions; additional protections should be provided only to those with diminished autonomy.16 With adolescent HIV prevention research, questions therefore arise as to whether youth should be respected to make autonomous decisions about research participation. The Institute of Medicine (IOM) in 2004,17 and The Society for Adolescent Medicine (now the Society for Adolescent Health and Medicine or SAHM)18 in 2003 reviewed the literature on adolescent development and capacity to consent to research participation. The SAHM report concludes that for adolescents aged 14 years old or older, understanding of research and the cognitive ability to make decisions about research participation are similar to these abilities in adults.19 Several studies, for instance, on competency among adolescents, found that 12 or 14 year olds were as competent as adults in providing research consent.20
Beneficence
Researchers should maximize the potential benefits, and minimize the potential risks to study participants. However, requiring parental permission in adolescent research can alter the risks and benefits. By asking parental permission, a researcher might unintentionally harm teens by disclosing their sexual behavior or sexual identity to parents, exposing them to emotional or physical harm, discrimination, and stigma. Such disclosure could violate their privacy and confidentiality. On the other hand, not requiring parental permission assists teenagers in keeping secrets from parents, removes an important protection against potential risks of research participation and source of social support, and interferes with parental rights over their children’s care and activities.21 Thus, when evaluating the risk/benefit ratio of a research study, parent permission needs to be considered in the equation. HIV research does not always provide clear direct benefits to participants. In general, a primary goal of clinical trials is to advance scientific knowledge, particularly related to treatment and patient wellbeing, but it may not necessarily benefit any current participants. Nonetheless, participants in studies without direct benefits may gain from the study either directly or indirectly. For instance, comparative effectiveness of studies on HIV and other conditions may compare two treatments, each of which is known to be effective. Participants in each arm would thus receive some benefit from the study. However, in other protocols (e.g., those using placebo), a least some participants may not receive any direct benefit.
Justice
The principle of justice requires that procedures and outcomes in selection of research subjects be fair, and that the benefits and burdens of research be equitably distributed across groups.22 Vulnerable groups should neither be exploited nor excluded from the benefits.23 At the study protocol review meetings mentioned earlier, the justice principle appeared important in successful arguments for studies to include adolescents without parental permission. IRBs and other bureaucratic entities may impede research that could improve the health of vulnerable youths; these decisions may be inconsistent with the principle of justice, which requires that researchers should not include in studies only those individuals who are easier to recruit or in a compromised position, or easily manipulated.
When research fosters the development of new treatments, procedures, or devices, justice demands both that these advances be provided to those who can benefit from them, and that research include persons from groups who are likely to benefit from applications of the research. Limiting study access to those who are easily accessible results in compromised science and hence abridges the study’s social benefit. Some observers even suggest that overprotective IRBs are “complicit in the creation of health inequities” when they prevent some communities from receiving potential benefits of participating in research.24
IRBs’ reluctance to permit adolescents to self-consent to research without parental involvement has impeded critical advances in HIV prevention among adolescent MSM.25 This reluctance is abetted by assumptions regarding parental rights and youth decision-making capacity that may be inaccurate or not apply to all youth and all parents.26
The National Commission’s Report on Research Involving Children
In its 1977 report Research Involving Children27, The National Commission for the Protection of Human Subjects of Biomedical Behavioral Research specified categories that warrant a waiver of parent permission, which many IRBs draw on as well: (1) if adolescents can obtain treatment for the diseases or conditions being studied without parental permission; (2) if the research involves “mature minors,”28 and poses no more than minimal risk; (3) the research addresses the needs of children designated by their parents as “in need of supervision;” and (4) research involving children whose parents are legally or functionally incompetent. The notion of “mature minor” is sometimes used in common law in the U.S. and England.29 The National Commission states that “assent of such mature minors should be considered sufficient,” particularly when they can legally consent to treatment outside of research for the health conditions being studied.30 When an adolescent’s assent is insufficient, the Commission suggested that a surrogate be appointed (e.g., a social worker, pediatric nurse, or physician) to assure informed consent and assure the right to withdraw.
The Common Rule
The Code of Federal Regulations (45-CFR-46), known as The Common Rule,31 was developed based on the National Commissions’ reports,32 and governs research ethics in the U.S. It allows a waiver of permission by parents or guardians as long as specific criteria are met, including: (1) the research involves no more than minimal risk to the subjects; (2) the waiver or alteration will not adversely affect the rights and welfare of the subjects; (3) the research could not practicably be carried out without the waiver or alteration; and (4) whenever appropriate, the subjects will be provided with additional pertinent information after participation.
Local and State Laws
All states and the District of Columbia allow most minors to receive STI testing and treatment without parental permission.33 Thirty-two states allow minors to self-consent to HIV testing, and 27 allow both HIV testing and treatment.34 Culp and Caucci found, in an analysis of statutes and regulations nationwide, that access to PrEP by minors without parent permission was inconsistent and unclear.35 In April 2017, New York State reclassified HIV as a Group B STI and clarified that local health departments must provide, directly or through referral, diagnosis and treatment, including prevention services to persons with or at risk of a listed STI regardless of age.36 The amendment also prohibited the release of a minor patient’s medical and billing records to his or her parent/guardian without the minor’s consent. Thus, teenagers in New York State have the right to obtain HIV prophylaxis such as PrEP and post-exposure prophylaxis (PEP) and treatment without parental/guardian involvement.
According to the Office for Human Research Protection (OHRP), if adolescents are legally able to obtain contraception and PrEP, they are not considered “children” and they can provide their own consent for research. The NIH Adolescent Trials Network, Adolescent MSM PrEP trial (ATN113) used this rationale to request parental waiver; however, some IRBs did not approve the protocol, based on their own state laws which did not specify HIV prevention services.37 For similar reasons, some IRBs have refused requests for parental waivers for other studies among adolescent MSM.38
Adolescent Age and the Law
How should the adolescent’s age influence decisions about the need for parental permission? Although some past academic articles have defined adolescents as individuals 10–18 years old, the HIV Center meeting workshop focused on individuals 12–17 years-old — still a large age range. Ethical issues involved in working with 16–17 year olds are very different from those involved in working with 12–14 year olds.
In general, the age of majority — the age at which adolescents typically attain many of the legal rights and privileges of adulthood — is age 18. Emancipation is the achievement of adult legal status before the age of majority by marriage, motherhood, or military service. Some states have “minor consent laws” that allow access to some health care independently before the age of majority.39 The term “mature minor” derives from common law, and means the capacity to make independent judgements when faced with specific life decisions. Deciding whether an adolescent is a mature minor cognitively, socially, or emotionally is a challenge; clearly it cannot rely on age alone.
How should the adolescent’s age influence decisions about the need for parental permission? Although some past academic articles have defined adolescents as individuals 10–18 years old, the HIV Center meeting workshop focused on individuals 12–17 years-old — still a large age range. Ethical issues involved in working with 16–17 year olds are very different from those involved in working with 12–14 year olds.
In defining “children,” the SAHM guidelines40 and IOM report41 rely on the definition of children in the U.S. federal regulations. Specifically, 45 CFR 46 defines children as “persons who have not attained the legal age for consent to treatments or procedures involved in the research, under the applicable law of the jurisdiction in which the research will be conducted.”42 Thus, the definition of a child, for research purposes, varies considerably, based on state laws. Few U.S. states have laws defining the age at which persons can consent to participate in research. Most states define the age of majority as 18 years, but recognize a younger age for persons to consent to general or specialty health care. SAHM states, “If an adolescent is allowed to consent to treatments or procedures involved in the research, they should not be considered children.”43 Under federal regulations, adolescents who are not children do not require parental permission to participate in research.44
Where Do IRBs Stand on Parental Waiver?
IRBs vary in their thresholds for determining the risk/benefit ratio of study participation and whether to waive parental permission. Gilbert et al. described issues that the Adolescent Trials Network Protocol 113 (ATN113) faced in obtaining IRB approval for a multisite trial of PrEP with 15–17 year old MSM at sites in 12 states.45 IRB concerns about approving adolescent self-consent in the study included: (a) adolescents might not have a realistic view of PrEP’s possible benefits and might underestimate risks; (b) they might be overly influenced by monetary reimbursements; and (c) they may dismiss or underestimate risks of study participation to their health.46 Evidence that adolescents had the legal right to self-consent for routine health care did not influence the IRB’s decision-making. Deliberations focused particularly on the principle of beneficence, and challenges to balancing potential benefit (mainly prevention of HIV) with possible harms, including coercion, privacy issues related to recruitment on social media, and worry about potential drug resistance that could result from persistent nonadherence. The IRBs did consult state minor consent laws, most of which allow minors to consent to diagnosis or treatment of STIs, but which did not clearly address self-consent for preventive services.47 Two IRBs referenced almost identical statutory language but reached opposite conclusions.48 IRBs often claim to differ in their decisions based on local culture and values, but this is not typically the reason.49 Thus, inconsistent applications of the law raise concerns, and suggest potential advantages of national standards on adolescent participation in research.
A related study was done of Principal Investigators (PIs) at these same 13 sites for the ATN113 multisite study to understand their dilemmas in undertaking this PrEP trial which relied on self-consent of minors.50 The data showed that PIs experienced moral tensions about conflicting duties, one to the participants, who deserved protection, and one to science, which must be valid (also see Merritt51), as shown in the following quotes:
IRB barriers to self-consent deprive adolescent MSM of their right to participate in trials that will protect them from receiving developmentally untested, inappropriate, and unsafe interventions and is a clear case of scientific inequity driving health inequities.52
It is also an instance of losing sight of the forest for the trees. Study-by-study, IRBs have sought to minimize risk to the institution and to [Adolescent] MSM participants by disapproving waivers of parental permission; in doing so, those individual decisions add up to a systemic injustice.53
Governmental resources do not assist IRBs in interpreting state law, adding to inconsistencies across IRBs, and indicating a much-needed area of work, particularly regarding HIV, for which biomedical prevention are rapidly advancing, and adolescents increasingly bear the brunt of the epidemic.
Rationale for Waiving Parental Permission
For multiple reasons, investigators may wish to waive parental permission in their prevention studies. These reasons include both threats to adolescent well-being and quality and validity of the science.
Requiring Parental Permission May Put Adolescents at Risk
IRB regulations generally treat adolescents as children, a protected class with diminished autonomy. The requirement of parental permission assumes that parents will act in the best interest of their child and have the cognitive maturity to make good decisions on his or her behalf. However, in studies that involve recruitment of sexual minority or sexually active youth, obtaining parental permission may involve youth disclosing information to a parent that they have kept secret and that can put adolescents at risk for emotional and even physical harm.
Requiring Parental Permission May Jeopardize Scientific Validity
Many youth, if required to disclose sensitive information to a parent in order to obtain permission, may choose not to participate, resulting in biased samples, and hence jeopardizing study results and generalizability of findings. As a barrier to their participation in a study of PrEP adherence, sexual and gender minority youth 14–17-years old have cited the requirement for guardian permission.54 Requiring parental permission can lead to underrepresentation of some youth, including those most at risk (e.g., African-American MSM) who may refuse study participation at higher rates, in part because discussions of sex and sexual identify are taboo, and parents may not accept the youths’ sexual orientation.55 When parental permission is required, systematic bias in subject recruitment can cause underrepresentation of adolescent MSM56 and misleading data and interventions that do not meet the needs of the most vulnerable or at risk populations because these teenagers are insufficiently represented in trials.
Reasons for Opposing Waivers of Parental Permission
Opposition to parental waiver is sometimes based on the fact that risk in research is not all knowable. Unanticipated risks can occur, especially in HIV prevention studies that include pharmaceutical interventions that have received relatively little prior investigation in humans. In addition, studies on HIV prevention almost always collect data on sexual behavior and sexual identity. When sensitive data are collected, or interventions are part of the research, some believe that parental permission should be obtained.
Adolescent Decision-Making is “Too Immature”
Decisions about whether to obtain parental permission need to consider adolescents’ psychological maturity and cognitive development. Some adolescent experts have raised concerns about relying on adolescent consent because of ongoing brain development that continues to occur through adolescence. Teenagers have immature impulse and emotional control, in part because the pre-frontal cortex, which is critical in cognition regarding future planning, abstract reasoning and response inhibition has not yet fully developed.57 Teenagers are thus more responsive to emotionally-loaded content than are adults, and more likely to seek sensation and possibly minimize research risks.58 Though adolescents are capable of abstract thought and deductive reasoning,59 which are necessary for decision-making, the emotional reward system matures later. In conditions of strong emotional meaning or “hot cognition” (which involves emotionally-charged social interactions with peers, such as when drinking or engaging in risk taking behaviors), decision-making may be compromised and more impulsive.60 In contrast, in conditions that are not emotionally charged, adolescents’ “cold cognition” decision-making is similar to adults.61 Adolescent decision-making and executive functioning are also influenced by age, social context, emotional context, adverse social events and environments, previous life experiences, emotional states, and the risk and complexity of a particular study. In 1989, The United Nation’s Convention on the Rights of the Child (UNCRC) introduced two constructs — evolving capacities, and what is in the child’s best interest — that help in understanding adolescents’ ability to consent for themselves in HIV research.62 “Evolving capacities” suggests that autonomous decision-making develops with age and maturity, and affects the ability of an adolescent to provide informed consent, suggesting needs to carefully assess individual adolescents’ capacity.63 “Best interest” recognizes the personhood of the child, and the need to both provide protection and promote the human rights of the child.64 Though not ratified by the U.S. Senate, the UNCRC provides important conceptualizations and remains a vital guide in many countries.
Yet the notion that by mid-adolescence, all youth can never provide informed, rational, and voluntary consent is flawed and may result from misapplication of research on only adolescent brain development, while ignoring other data (rates of learning equivalent to adults’). Such reductionist conclusions ignore the large body of empirical data indicating that youth as young as age 14 can make research consent decisions at adult levels when information is presented at an age-appropriate level and in contexts in which stress is minimized.65 In fact, research has demonstrated that LGBTQ youth aged 14–17 years adequately understand the rationale for, and voluntary nature of, participation in PrEP adherence trials, including a rational weighing of risks and benefits.66
Adolescents display cognitive abilities for agency, judgment, and self-protection that are fundamental to the capacity to provide informed consent and to make decisions about research participation. Even young adolescents are generally able to make sensible decisions about research and health care in the absence of coercion and unhealthy influences such as peer pressure. Data on 12 to 14 year olds show that they are as capable as adults of understanding multiple viewpoints and considering conflicting information.67
IRBs should include professionals who work with adolescents and understand adolescent development, and consider whether investigators assess capacity, if parental permission is waivered.
Parents’ Rights to Protect Their Children
Research that allows adolescents to self-consent may conspire with them to hide key aspects about their behavior from their parents.68 Adolescents may be conflicted about aspects of their behavior; with support, they might welcome and benefit from open discussion with their parents. Investigators who began their relationship with adolescents by assuming that parents should be excluded may discourage valuable intra-familial communication, and inadvertently convey to youths that researchers will collaborate with them in deceiving their parents. Researchers need ways of assessing whether an adolescent desires open communication with his or her parents — and avoiding creation of artificial barriers to communication.69 Some critics may, as an underlying value, prioritize parental rights over the rights of a youth to act on behalf of his or her own health. Parents have a moral, and even legal, authority over their children’s care and activities, which should generally be respected. Moreover, in many families, parents can serve a significant supportive role, particularly for youth undergoing stress and discrimination due to sexual, gender, and/or ethnic minority status. Parents often have a strong sense of their children’s needs, fears, mental health issues, and executive function capacities.
There are exceptions to this moral authority in mature minor laws, as well as in case law that lets physicians to abrogate parental rights in making medical decisions if the child’s health is in jeopardy.70 Parents may coerce or unduly influence an adolescent to make decisions contrary to his or her best interests, concerning, for instance, whether or not to marry, to work instead of attending school, or to continue or abort a pregnancy. Laws allowing adolescents to seek health care independently from parents recognize the potential for parental conflicts of interest and for conflicts between parents and adolescents. As discussed above, IRBs are authorized to waive parental permission when it would not be protective under certain circumstances. Assessment of parent-adolescent relationships can also be important in some studies as part of the participation process, even when parent permission waived.
Parental Permission May be Particularly Important in Certain Studies
Parental permission may be especially important in several particular situations and types of studies. First, minimal risk studies may pose minimal risks to health, but in order to qualify as minimal risk, these potential dangers must be no more than adolescents would experience in daily life or “in a routine medical, psychological, or educational examinations, tests, or procedures of the general population.”71 Health risks may, however, be known to be minimal for adults, but remain unknown for adolescents, who may not fully appreciate the potential differences. For instance, a drug that is safe in adults may not be for teenagers. What happens when adolescents experience any of these risks in studies that included parental waiver? Adolescents may misunderstand the ramifications of study participation, or underestimate the possibility of negative psychological or health risks. If children need medical care, unanticipated disclosure to parents may then occur.
Second, studies vary in their risk of violations of confidentiality. Adolescents may, for example, be very comfortable using online resources or texting in a social media study, but discount or misunderstand their vulnerability to potential breaches of confidentiality.
Third, “privacy” may have different meanings for different adolescents and mean more to some than others. Parents may also value their child’s privacy and perceive its violation as a more important risk than the child does. Thus, even the definition of risk/benefit, in the absence of a parent’s permission, should be clearly and broadly defined. Researchers must distinguish between “likelihood” and “severity” of a risk such as breach of confidentiality. In the case of gender or sexual minorities, these issues may be particularly important, given stigma and possible discrimination and harm that may ensue.
Fourth, studies generally offer incentives and inducements to adolescents to provide data and participate in interventions. Depending on the amount of incentive, the level of financial need, and the onerousness of research requirements, adolescents may minimize risk in favor of participation, a decision parents might not share. Significant incentives may sway all prospective participants, but especially adolescents.
Recommendations on When to Waive Parental Permission
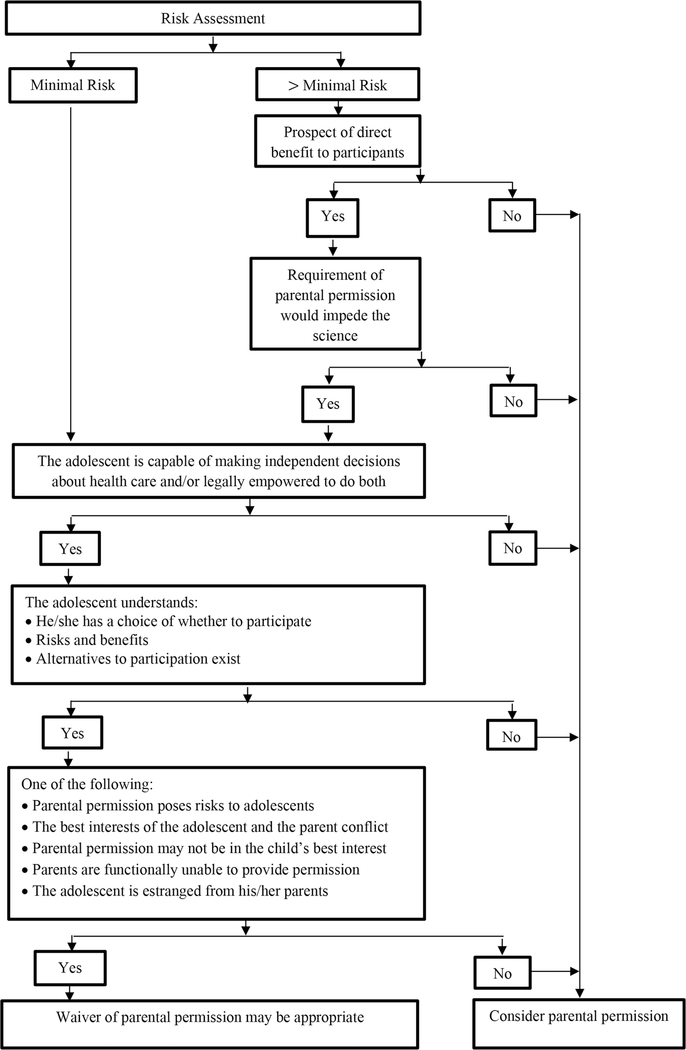
Many investigators focus on getting a waiver of parental permission because of concerns that they will otherwise fail to obtain an adequate or representative sample. However, that concern should not trump the risk to adolescents of trial participation. Creative approaches to research design might foster research that has both parent permission and adolescent assent. However, HIV prevention research with adolescents will likely often require waivers of parental permission. Based on research to date, we believe that most adolescents have the cognitive abilities for agency, judgment, and self-protection, which are fundamental skills that demonstrate the capacity to provide informed consent and to make decisions about research participation. As illustrated in Figure 1, studies should thus consider requesting a waiver of parental permission under the following circumstances:
-
1
Research poses only minimal risks to the adolescent or poses more than minimal risk but offers the prospect of direct benefit, and lack of participation might have a negative impact on the adolescent and scientific advances.
-
2
The adolescent is capable of making independent decisions about medical or mental health care or social services and/or is legally empowered (or legally or functionally emancipated) to make these decisions;
Figure 1.
Decision Tree Concerning Whether to Waive Parental Permission in HIV Prevention Research Among Adolescents
AND any one of the following:
-
3
Parental permission poses risk to adolescents who would have to disclose to the parent private information, (e.g., sexual behavior or sexual identity);
-
4
The best interests of the adolescent and the parent conflict, or parental permission may not be in the child’s best interest;
-
5
Parents are functionally incapacitated, unavailable, or unable to provide permission or the adolescent is estranged from their parents.
Special Considerations for Researchers When Parental Permission is Waived
If a parental waver is granted, researchers should work to protect adolescent participants from study risks, including breach of confidentiality, loss of privacy, and/or coercion.
Assuring Adolescent Privacy and Confidentiality
Research that waives parental permission should include a safety plan to help adolescents avoid breaches of confidentiality or privacy. For example, studies should develop tools to guide minors to protect their privacy (e.g., clearing phone texts between study staff and the youth, helping adolescents with how to talk to parents).
Should Individual Adolescents be Screened for Capacity to Consent, and If So, How?
Researchers should carefully consider recruitment processes and establish a higher bar for participants to meet inclusion/exclusion criteria. A procedure should document that youth:
have capacity to consent;
- and understand that:
- they have a choice about whether to participate (i.e., participation is voluntary);
- the benefits of participation are not guaranteed (e.g., access to an HIV prevention method not yet widely available);
- the study may pose medical, social, and psychological risks, known and unknown, short- and long-term;
- there are alternatives to the study (i.e., there are other ways HIV can be prevented, such as safer sex).
As examples, a study requiring parental permission might be a phase I study of a monoclonal antibody treatment for HIV. As a Phase I study, the study is more than minimal risk. The prospect of direct benefit is relatively low, and requiring parental permission would not impede the science.
Parental permission might be waived in a minimal risk study for an anonymous descriptive, internet-based psychosocial study of HIV risk behaviors among 15–16-year-old LGBTQ youth.
Parental permission might be waived, even though the study is more than minimal risk, for a study among 16-year-old MSM of two forms of dosing of PrEP — daily versus event-driven (e.g., taking PrEP only on days when the participant thinks he may be engaging in high risk sexual activity). The study is more than minimal risk, but provides direct benefit to participants, since it is comparing two arms, both of which would provide benefit to the adolescent. Requiring parental permission may also bias the sample and thus impede the potential scientific benefit of the study.
When parental permission is waived, researchers should consider using a screening tool during the recruitment and screening processes to identify adolescents who lack capacity to consent. Tools can identify adolescents who should be excluded from the study because of researcher concerns about their cognitive and emotional capacity to provide consent or because risks of a breach confidentiality outweigh the benefits of research participation. Tools can also assess the degree to which an adolescent understands the study and its risks and benefits, as well as the individual’s cognitive capacity, and whether the parent knows of the teen’s interest in the study, or of the teen’s gender or sexual minority status. Researchers should also consider the adolescent’s use of substances prior to enrollment, as with adults.
Project staff should receive rigorous training on screening for eligibility, and specifically on the importance of assuring the adolescent’s ability to provide informed consent. Some researchers may see the study as an opportunity to encourage youth to communicate with their parents, and may only enroll youth who can safely talk to their parents, about research participation, regardless of whether parental permission is waived. When IRBs approve a waiver of parental permission, researchers should, prior to commencing enrollment, ask each youth if he or she will inform the parent of the study. Researchers should evaluate whether participants may have elevated risks due to parents learning in an unintended way about the child’s sexual risk behavior or sexual identity. If such elevated risk exists, the researcher should establish a safety plan that includes resources for adolescents, whether they choose to disclose or not disclose their participation to parents. In trials of interventions that require medication adherence, conversations around adherence support may also help.
Identifying Adolescents Who Are at Elevated Risk from Study Participation
Not all adolescents will be able to make a considered decision about research participation that is in their own best interests. Some may be in a situation in which a power differential increases the likelihood of coercion or undue influence. Certain subgroups are especially at risk. It may be unclear, for instance, who has the right to consent for children in foster care. Some youth are more or less developmentally mature. Youth who live in more-protected rather than less-protected environments might divulge private information without understanding privacy risks, which may or may not make them more vulnerable to breach of confidentiality. Younger adolescents, those with cognitive deficits, those in high toxic stress environments, and those who have psychiatric illness or use alcohol or drugs may face more obstacles.
On the other hand, in a study of 60 sexual and gender-nonconforming youth age 14–16 who were being recruited into a PrEP adherence study, researchers evaluated capacity to consent and found that youth generally understood benefits, potential medical side effects, confidentiality risks, and random assignment, and felt comfortable with the study procedures and/or felt comfortable declining participation.72 The researchers concluded that adolescents had the capacity to consider health risks and benefits, assess their ability to take pills every day, and understand requirements of the trial, and were able to self-consent.
Conclusions
The issues raised here suggest several areas for future research and recommendations for researchers, IRBs, and other stakeholders.
In short, enrollment of adolescents in studies of STI/HIV prevention, such as PrEP, is vital, but poses a series of questions that IRBs, researchers and other stakeholders need to consider. The development, refinement, and implementation of several specific types of instruments and guidelines, as suggested here, can help. This discussion can spur additional work in this area. Further such efforts over time can help ensure that research is conducted that can aid adolescents at risk for HIV, while minimizing risks, and protecting these individuals’ rights as much as possible.
A Research Agenda
A series of research needs emerge for future attention:
“Risk assessment tools” should be developed to inform the IRB, sponsor, and researchers about the nature, intensity, reversibility, and likelihood of risk to minors of a given STI/HIV prevention strategy study.
Research is needed to better understand parents’ attitudes toward allowing their children to participate in research in general, and in research on sexual risk and HIV. How do parents identify and define research risks? Do they acknowledge the potential for benefit? Do they resist the idea that their child is sexually active?
Studies are needed on the effects of parents’ involvement in youth research participation. Does parental involvement have a positive impact on study participation or youth adherence to the trial intervention? Are there negative effects of parent learning about adolescent’s sexual behavior?
Research is required on what a safety plan should consist of as part of any research protocol when parental permission is waived, to assist the adolescent in minimizing risks from breach of confidentiality, and to ensure support, should interventions have any negative side effects.
Studies are needed to better understand how adolescents self-assess their safety when considering research participation.
Studies are needed on how adolescents view the risks and benefits of interventions, including PrEP.
Research is required on how teens define privacy and confidentiality, how important it is to them, and what the risks of disclosure might be to them, their parents, and their peers.
We need to study how incentives influence adolescents’ decision to participate in research. When are incentives coercive? When do they unduly influence a decision to participate? How do we assure that participants enroll in research voluntarily and not because the financial benefit was too attractive? What are differences between adolescents and adults that may influence types and value of incentives? Will youth with a secret (e.g., MSM) participate for financial gain despite risks of having this information revealed to their parents?
Studies are needed on how requirements for parental permission affect the risks of breach of confidentiality. With the exception of LGBTQ children, data is not conclusive about how often conducting studies with parental permission is associated with such breaches.
Research is needed to identify subsets of adolescents for whom parental permission should or should not be waived.
Studies are needed on how often, when waivers are granted, adolescents talk to their parents about the study or issues involved. How do the talks go? Do the parents find out? If so, does the adolescent initiate the disclosure?
Research is needed to elicit youth and parent perspectives on parental permission, and to compare the effectiveness of different procedures to improve informed consent. What can we do to maximize youth understanding of procedures and minimize potential risks?
Research is needed to empirically test and refine, if needed, the “decision tree,” specifying when waiver of parental permission would be appropriate or not.
Development of Principles for Research with Minors on HIV Prevention Research
It would be helpful to develop consensus about principles that can guide decisions concerning HIV prevention research with minors. Proposed principles can include, for example:
Adolescents should be involved in HIV prevention research.
When parental permission is waived, extra care is needed to assure that adolescents understand the risks and benefits of research. Research standards should be different for adolescents only when there is something unique about being an adolescent. Competence to consent is the same for adults and adolescents.
If parental permission is waived, researchers should not be de facto parents. However, studies should add extra protections to youth, especially to avoid breaches of confidentiality and ensure support for youth, related to study participation.
Parents are not always best at making the decision about their child’s research participation. Parent permission is a commonly used norm, but in fact, parents may not be the best deciders.
Many teens are competent to make the decision to participate in research independently.
Adolescents who provide informed consent directly should be asked whether they want to seek the advice of parents, other family members, or trusted adults, when doing so doesn’t pose risk for them. Obtaining support for participation in intervention studies, in particular, may be helpful, especially when medications are involved that may have side effects.
Researchers and IRBs should give special consideration and concern to both the screening and the consent processes.
The consent process should include a risk assessment to inform adolescents about the risks they may encounter from study participation and loss of confidentiality.
Long and often hard-to-interpret consent forms can impede the consent process.73 Consent forms should be simplified so that adolescents understand the key elements of these studies, including the risks.
To ensure respect for persons, enrollment should be coupled with assent procedures that assess decisional capacity and understanding of risk.
In short, enrollment of adolescents in studies of STI/HIV prevention, such as PrEP, is vital, but poses a series of questions that IRBs, researchers and other stakeholders need to consider. The development, refinement, and implementation of several specific types of instruments and guidelines, as suggested here, can help. This discussion can spur additional work in this area. Further such efforts over time can help ensure that research is conducted that can aid adolescents at risk for HIV, while minimizing risks, and protecting these individuals’ rights as much as possible.
Acknowledgements
This work was supported by a center grant from the National Institute Mental Health to the HIV Center for Clinical and Behavioral Studies at the New York State Psychiatric Institute and Columbia University (P30MH43520; Center PI: Robert H Remien, Ph.D.).Specifically, this grant funded the invitational meeting, “Obtaining Parental Permission in HIV Prevention Research with Adolescent Minors: Whose Risk, Whose Benefit, Whose Judgment?” held June 15, 2017 at The HIV Center for Clinical and Behavioral Studies at the New York State Psychiatric Institute and Columbia University, New York, NY. We acknowledge the participants at the meeting, listed alphabetical order: Susannah Allison, Ph.D., Program Officer, Division of AIDS Research, NIMH; Laurie Bauman, Ph.D., Professor of Pediatrics, Director of the Prevention Intervention Research Center and Professor of Pediatrics and Psychiatry and Behavioral Sciences, Science Core of Einstein-Rockefeller-CUNY-CFAR at the Albert Einstein College of Medicine, Director of the Biomedical Core, HIV Center for Clinical and Behavioral Studies; Melissa Epstein, Ph.D., M.B.E., C.I.P., Director, Office of Human Research Affairs, Albert Einstein College of Medicine/Montefiore Medical Center; Robert E. Fullilove, Ed.D., Associate Dean for Community and Minority Affairs, Professor of Clinical Sociomedical Sciences, Mailman School of Public Health, Columbia University; Donna Futterman, M.D., Professor of Pediatrics, Albert Einstein College of Medicine, Director, Adolescent AIDS Program, Montefiore; Adrian Guzman, J.D., M.P.H., Acting Director of External Affairs/Policy Analyst, Bureau of HIV/AIDS Prevention and Control (BHIV), NYC Health Department; Robert Klitzman, M.D., Professor of Psychiatry in the Vagelos College of Physicians and Surgeons and Mailman School of Public Health and Director of the Masters of Bioethics Program, Columbia University; Sonia Lee, Ph.D., Program Officer, Maternal and Pediatric Infectious Disease Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH; Carol Levine, M.A., Director, Families and Health Care Project, United Hospital Fund; Claude Ann Mellins, Ph.D., Professor of Medical Psychology (in Psychiatry and Sociomedical Sciences), Co-Director, HIV Center for Clinical and Behavioral Studies, Division of Gender, Sexuality, and Health, Co-Director, Office of Clinical Psychology, New York State Psychiatric Institute and Columbia University; Susan L. Rosenthal, Ph.D., A.B.P.P., Professor of Medical Psychology (in Pediatrics and Psychiatry), Vice Chair for Faculty Development, Department of Pediatrics, Director, Division of Child and Adolescent Health, Department of Pediatrics at Columbia University Irving Medical Center – Vagelos College of Physicians and Surgeons, New York Presbyterian Morgan Stanley Children’s Hospital; John Santelli, M.D., Professor, Population and Family Health and Pediatrics, Columbia University Mailman School of Public Health; Michele Ybarra, M.P.H., Ph.D., Center for Innovative Public Health Research (CiPHR).
Funding was provided by the National Institute of Mental Health (NIMH) to the HIV Center for Clinical and Behavioral Studies (#P30-MH45320; PI: Robert H. Remien, Ph.D.).
Contributor Information
Laurie J. Bauman, Psychiatry and Behavioral Sciences at the Albert Einstein College of Medicine, where she also serves as Director of the Preventive Intervention Research Center and of the Behavioral Science Core of the Einstein-Rockefeller-CUNY Center for AIDS Research. She is also Director of the Bio-Behavioral Core of the HIV Center for Clinical and Behavioral Studies. Her research addresses HIV prevention among adolescents, including behavioral interventions and adoption of PrEP..
Claude Ann Mellins, Professor of Medical Psychology in Psychiatry and Sociomedical Sciences at the HIV Center for Clinical and Behavioral Studies at the New York State Psychiatric Institute and Columbia University. She is the co-director of the HIV Center with 28 years of clinical and research experience working globally with youth and families affected by HIV..
Robert Klitzman, Vagelos College of Physicians and Surgeons and the Mailman School of Public Health, and the Director of the online and in-person Bioethics Masters and Certificate Programs at Columbia University. He has published over 150 scientific journal articles and nine books on ethical issues concerning HIV, genetics, research, and other areas..
References
- 1.Centers for Disease Control and Prevention, HIV Among Youth, available at <https://www.cdc.gov/hiv/group/age/youth/index.html> (last visited February 24, 2020); Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of HIV/AIDS Prevention, HIV Among Youth, available at <https://www.cdc.gov/nchhstp/> (last visited February 24, 2020).
- 2.Hosek SG et al. , “Safety and Feasibility of Antiretroviral Pre-exposure Prophylaxis for Adolescent Men Who Have Sex With Men Aged 15 to 17 Years in the UnitedStates,” JAMA Pediatrics 171, no. 11 (2017): 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis AC, “Defining Adolescence,” Journal of Adolescent and Family Health 7, no. 2 (2015): 1–39. [Google Scholar]
- 4.Centers for Disease Control and Prevention, HIV Surveillance Report: Diagnoses of HIV Infection in the United States and Dependent Areas 2017, available at <https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html> (last visited February 24, 2020); See Centers for Disease Control and Prevention, supra note 1, CDC reference 2.
- 5.Silver E and Bauman L, “The Association of Sexual Experience with Attitudes, Beliefs, and Risk Behaviors of Inner-City Adolescents,” Journal of Research on Adolescence 16, no. 1 (2006): 29–45; D.K. Eaton et al., Youth Risk Behavior Surveillance - United States, 2009, available at <https://www.cdc.gov/mmwr/preview/mmwrhtml/ss5905a1.htm> (last visited February 24, 2020); L. Kann et al., Youth Risk Behavior Surveillance — United States, 2017, available at <https://www.cdc.gov/mmwr/volumes/67/ss/ss6708a1.htm> (last visited February 24, 2020); W.D. Mosher, A. Chandra, and J. Jones, Sexual Behavior and Selected Health Measures: Men and Women 15–44 Years of Age, United States, 2002, Centers for Disease Control. Advance Data no. 362 (2005): 1–55; J. Côté, “Identity Formation and Self Development in Adolescence,” in Handbook of Adolescent Psychology Volume 1: Individual Bases of Adolescent Development, ed. R.L.L. Steinberg (Hoboken, N.J.: John Wiley & Sons, 2009), 266–304. [Google Scholar]
- 6.Abma JC, Martinez GM, Mosher WD, and Dawson BS, “Teenagers in the United States: Sexual Activity, Contraceptive Use, and Childbearing, 2002,” Vital and Health Statistics Series 23, no. 24 (2004): 1–48. [PubMed] [Google Scholar]
- 7.SeeKann et al. , supra note 5.
- 8.SeeMosher et al. , supra note 5.
- 9.Pace JE, Siberry GK, Hazra R, and Kapogiannis BG, “Pre-exposure Prophylaxis for Adolescents and Young Adults at Risk for HIV infection: Is an Ounce of Prevention Worth a Pound of Cure?” Clinical Infectious Diseases 56, no. 8 (2013): 1149–1155; K.H. Mayer and B.C. Zanoni, “HIV Chemoprophylaxis for Adolescents: Educable Moment, Not Magic Bullet,” Clinical Infectious Diseases 56, no. 8 (2013): 1156–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Office of Human Research Protections, Federal Policy for the Protection of Human Subjects (“Common Rule”), available at <https://www.hhs.gov/ohrp/regulations-and-policy/regulations/common-rule/index.html> (last visited February 24, 2020).
- 11.Macapagal K et al. , “‘I Won’t Out Myself Just to Do a Survey’: Sexual and Gender Minority Adolescents’ Perspectives on the Risks and Benefits of Sex Research,” Archives of Sexual Behavior 46, no. 5 (2017): 1393–1409; C.B. Fisher et al., “‘Free Testing and PrEP Without Outing Myself to Parents:’ Motivation to Participate in Oral and Injectable PrEP Clinical Trials Among Adolescent Men Who Have Sex with Men,” PloS One 13, no. 7 (2018): e0200560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field MJ and Berman RE, eds., The Ethical Conduct of Clinical Research Involving Children (Washington, DC: National Academies Press, 2004); J.S. Santelli et al., “Guidelines for Adolescent Health Research. A Position Paper of the Society for Adolescent Medicine,” Journal of Adolescent Health 33, no. 5 (2003): 396–409. [PubMed] [Google Scholar]
- 13.U.S. Department of Health, Education, and Welfare, The Belmont Report, available at <https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report> (last visited February 24, 2020).
- 14.Office for Human Research Protection, Special Protections for Children as Research Subjects, available at <https://www.hhs.gov/ohrp/regulations-and-policy/guidance/special-protections-for-children/index.html> (last visited February 24, 2020).
- 15.The Belmont Report, supra. See. note 13.
- 16.Mustanski B, “Ethical and Regulatory Issues with Conducting Sexuality Research with LGBT Adolescents: A Call to Action for a Scientifically Informed Approach,” Archives of Sexual Behavior 40, no. 4 (2011): 673–686. [DOI] [PubMed] [Google Scholar]
- 17.SeeField and Berman, supra note 12.
- 18.SeeSantelli, supra note 12.
- 19.SeeSantelli, supra note 12.
- 20.Weithorn LA, “Children’s Capacities to Decide About Participation in Research,” IRB 5, no. 2 (1983): 1–5; I.M. Hein et al., “Informed Consent Instead of Assent is Appropriate in Children from the Age of Twelve: Policy Implications of New Findings on Children’s Competence to Consent to Clinical Research,” BMC Medical Ethics 16, no. 1 (2015): 76; I.M. Hein et al., “Accuracy of the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR) for Measuring Children’s Competence to Consent to Clinical Research,” JAMA Pediatrics 168, no. 12 (2014): 1147–1153. [PubMed] [Google Scholar]
- 21.Levine RJ, “Research Involving Adolescents as Subjects: Ethical Considerations,” Annals of the New York Academy of Sciences 1135, no. 1 (2008): 280–286. [DOI] [PubMed] [Google Scholar]
- 22.SeeMustanski, supra note 16.
- 23.SeeField and Berman, supra note 12.
- 24.SeeMustanski, supra note 16.
- 25.Hill BJ, “Medical Decision Making by and On Behalf of Adolescents: Reconsidering First Principles,” Journal of Health Care Law & Policy 15, no. 1 (2012): 37–73. [Google Scholar]
- 26.SeeMustanski, supra note 16; Hill, supra note 25.
- 27.The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, Research Involving Children: Report and Recommendations, available at <https://videocast.nih.gov/pdf/ohrp_research_involving_children.pdf> (last visited February 24, 2020).
- 28.SeeLevine, supra note 21.
- 29.Stenger R, “Exclusive or Concurrent Competence to Make Medical Decisions for Adolescents in the United States and United Kingdom,” Journal of Law and Health 14, no. 2 (1999): 209–241. [PubMed] [Google Scholar]
- 30.SeeNational Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, supra note 27.
- 31.Office for Human Research Protections, 45 CFR 46, Pre-2018 Requirements, available at <https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45cfr46/index.html> (last visited July 31, 2019); Office for Human Research Protections, 45 CFR 46, 2018 Requirements, available at <https://www.hhs.gov/ohrp/regulations-andpolicy/regulations/45cfr46/index.html> (last visited July 31, 2019).
- 32.SeeNational Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, supra note 27.
- 33.Guttmacher Institute, An Overview of Minors’ Consent Law, available at <https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law> (last visited February 24, 2020).
- 34.SeeGuttmacher Institute, supra note 33.
- 35.Culp L and Caucci L, “State Adolescent Consent Laws and Implications for HIV Pre-exposure Prophylaxis,” American Journal of Preventive Medicine 44, no. 1 Suppl. 2 (2013): S119–S1124. [DOI] [PubMed] [Google Scholar]
- 36.New York State Department of Health, Frequently Asked Questions: New York State Public Health Law Article 23 and Title 10, New York Codes, Rules and Regulations — Section 23 Guidance for Local Health Departments (LHD) and Health Care Providers, available at <https://www.health.ny.gov/diseases/communicable/std/docs/faq_billing_consent.pdf> (last visited February 24, 2020).
- 37.Mustanski B and Fisher CB, “HIV Rates are Increasing in Gay/Bisexual Teens: IRB Barriers to Research Must be Resolved to Bend the Curve,” American Journal of Preventive Medicine 51, no. 2 (2016): 249–252; Q.L. Moore et al., “Legal Barriers to Adolescent Participation in Research about HIV and Other Sexually Transmitted Infections,” American Journal of Public Health 106, no. 1 (2016): 40–44; B. Mustanski, “Ethical and Regulatory Issues with Conducting Sexuality Research with LGBT Adolescents: A Call to Action for a Scientifically Informed Approach,” Archives of Sexual Behavior 40, no. 4 (2011): 673–686; A.L. Gilbert et al., “Adolescent Self-consent for Biomedical Human Immunodeficiency Virus Prevention Research,” Adolescent Health 57, no. 1 (2015): 113–1139; C.B. Fisher and B. Mustanski, “Reducing Health Disparities and Enhancing the Responsible Conduct of Research Involving LGBT Youth,” The Hastings Center Report 44, Suppl. 4 (2014): S28-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.supra. See. note 37 for Moore et al.; Gilbert et al.; Fisher and Mustanski.
- 39.SeeGuttmacher Institute, supra note 33.
- 40.SeeSantelli, supra note 12.
- 41.SeeField and Berman, supra note 12.
- 42.SeeOffice for Human Research Protections, supra note 31 (for 2018 Requirements)
- 43.SeeSantelli, supra note 12.
- 44.SeeOffice for Human Research Protections, supra note 31 (for both Pre and 2018 Requirements).
- 45.SeeGilbert et al. , supra note 37.
- 46.SeeFisher and Mustanski, supra note 37.
- 47.SeeFisher and Mustanski, supra note 37.
- 48.SeeFisher and Mustanski, supra note 37.
- 49.Klitzman R, The Ethics Police? The Struggle to Make Human Research Safe (New York: Oxford University Press, 2015). [Google Scholar]
- 50.Knopf AS et al. , “Adolescent Medicine Trials Network for HIV/AIDS Interventions. Moral Conflict and Competing Duties in the Initiation of a Biomedical HIV Prevention Trial with Minor Adolescents,” AJOB Empirical Bioethics 8, no. 3 (2017): 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merritt M, “Moral Conflict in Clinical Trials,” Ethics 115, no. 2 (2005): 306–330. [DOI] [PubMed] [Google Scholar]
- 52.SeeMustanski and Fisher, supra note 37.
- 53.SeeMustanski and Fisher, supra note 37.
- 54.SeeCulp and Caucci, supra note 35.
- 55.SeeMustanski and Fisher, supra note 37; Knopf et al., supra note 50.
- 56.SeeCulp and Caucci, supra note 35.
- 57.Arian M et al. , “Maturation of the Adolescent Brian,” Neuropsychiatric Disease and Treatment 9 (2013): 449–461; B.J. Casey and K. Caudle, “The Teenage Brain: Self Control,” Current Directions in Psychological Science 22, no. 2 (2013): 82–87; D. Yurgelun-Todd, “Emotional and Cognitive Changes During Adolescence,” Current Opinion in Neurobiology 17, no. 2 (2007): 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.SeeYurgelun-Todd, supra note 57.
- 59.Arnett JJ, “Cognitive Foundations,” in: Adolescence and Emerging Adulthood: A Cultural Approach. 4th edition, ed. by the author, 58–92. (Upper Saddle River, NJ: Prentice Hall; 2010). [Google Scholar]
- 60.Steinberg L, “A Social Neuroscience Perspective on Adolescent Risk-Taking,” Developmental Review 28, no. 1 (2008): 78–106; P. Grootens-Wiegers, I.M. Hein, J.M. van den Broek, and M.C. de Vries, “Medical Decision-Making in Children and Adolescents: Developmental and Neuroscientific Aspects,” BMC Pediatrics 17, no. 1 (2017): 120; See Casey and Caudle, supra note 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.SeeCasey and Caudle, supra note 56.
- 62.United Nations Human Rights Office of the High Commissioner, Convention on the Rights of the Child. Adopted and opened for signature, ratification and accession by General Assembly resolution 44/25 of 20 November 1989. Entry into force 2 September 1990, in accordance with article 49, available at <https://www.ohchr.org/EN/ProfessionalInterest/Pages/CRC.aspx> (last visited February 24, 2020); J.S. Santelli, S. Haerizadeh, and T. McGovern, Inclusion with Protection: Conducting Ethical Research with Adolescents, Innocenti Research Brief (2017), available at <https://www.unicef-irc.org/publications/877-inclusion-with-protection-obtaining-informed-consent-when-conducting-research-with.html> (last visited February 24, 2020).
- 63.SeeUN Human Rights Office of the High Commissioner, supra note 62.
- 64.SeeUN Human Rights Office of the High Commissioner, supra note 62.
- 65.Steinberg L, “Does Recent Research on Adolescent Brain Development Inform the Mature Minor Doctrine?” The Journal of Medicine and Philosophy 38, no. 3 (2013): 256–267. [DOI] [PubMed] [Google Scholar]
- 66.Fisher CB et al. , “Self-Consent for HIV Prevention. Research Involving Sexual and Gender Minority Youth: Reducing Barriers Through Evidence-based Ethics,” Journal of Empirical Research on Human Research Ethics 11, no. 1 (2016): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.SeeWeithorn, supra note 20.
- 68.SeeLevine, supra note 21.
- 69.The Belmont Report, supra. See. note 13.
- 70.SeeLevine, supra note 21; See Mustanski, supra note 16.
- 71.SeeOffice for Human Research Protections, supra note 31 (for both the Pre and 2018 Requirements).
- 72.SeeFisher and Mustanski, supra note 37.
- 73.Ybarra ML et al. , “Ethical Considerations in Recruiting Online and Implementing a Text Messaging-based HIV Prevention Program with Gay, Bisexual, and Queer Adolescent Males,” The Journal of Adolescent Health 59, no. 1 (2016): 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]