Abstract
Aortic insufficiency with recirculation can be difficult to diagnose echocardiographically in patients who have continuous-flow left ventricular assist devices. Transthoracic and transesophageal echocardiography can underestimate its severity; moreover, transesophageal echocardiography necessitates general anesthesia. We report the case of a 58-year-old man with obesity and end-stage nonischemic cardiomyopathy who, after 3 months of support with a continuous-flow left ventricular assist device, underwent intracardiac echocardiography to evaluate complications potentially associated with the device. The findings ruled out aortic insufficiency, preventing an unnecessary valvular intervention.
Keywords: Aortic valve insufficiency; echocardiography/methods; echocardiography, Doppler, color; heart-assist devices/adverse effects; heart failure/diagnostic imaging; obesity
Continuous-flow left ventricular assist devices (LVADs) can cause aortic insufficiency (AI) in the native heart. Untreated, AI caused by continuous-flow LVAD support can lead to recirculation and worsening heart failure, which in turn may necessitate surgical aortic valve replacement or urgent cardiac transplant.1 Intracardiac echocardiography (ICE) is standardly used in interventional and electrophysiologic procedures, but its use in LVAD-supported patients is rarely reported.2,3 We report the case of a 58-year-old man undergoing continuous-flow LVAD support who underwent ICE for evaluation of possible AI and concomitant recirculation.
Case Report
A 58-year-old man with obesity (weight, 171 kg) and end-stage nonischemic cardiomyopathy underwent implant of a HeartWare HVAD (Medtronic) as destination therapy. Postoperative transesophageal echocardiograms (TEEs) and transthoracic echocardiograms (TTEs) showed appropriate device placement, neutral septal position, and no valvular abnormalities. Echocardiographically guided ramp testing was used to optimize device settings, and intermittent aortic valve opening was confirmed. The next day, laboratory tests revealed a lactate dehydrogenase (LDH) level of 389 IU/L (normal range, 122–222 IU/L) and an international normalized ratio (INR) of 2.9 (normal range, 0.9–1.1). Our heart failure team monitored the patient's progress daily for one month, and at hospital discharge, he weighed 159 kg. During the next month, he returned weekly to our outpatient clinic for monitoring. At each visit, he was asymptomatic and euvolemic, and his LVAD was functioning satisfactorily.
Three months after implant, the patient was hospitalized for worsening shortness of breath and peripheral edema. Physical examination revealed a 17-kg weight gain, diffuse inspiratory crackles, and lower extremity pitting edema. Jugular venous pulsations were undetectable because of the patient's body habitus. The LVAD hummed continuously, indicating a functioning pump motor.
A chest radiograph showed bilateral pleural effusions and vascular congestion. Serum laboratory studies revealed an N-terminal pro-brain natriuretic peptide level of 13,613 pg/mL (normal range, <300 pg/mL), an LDH level of 329 IU/L, an INR of 2.1, and no urobilinogen or serum free hemoglobin. The patient's LDH level was monitored daily and remained above normal, as it had been at baseline. Interrogation of the LVAD on the day of admission revealed no alarms, power spikes, or increased pump flow, and consistent speed (2,700 rpm), flow (5.5 L/min), and power (5.1 W).
A ramp-test echocardiogram showed the septum in a neutral position, mitral regurgitation, and an ill-defined aortic regurgitant jet (Fig. 1A–B). When pump speed was increased, the mitral regurgitation and apparent aortic regurgitant jet persisted (Fig. 1C–D). We had expected forward blood flow to increase at the higher pump speed; instead, the aortic regurgitant jet appeared to do so.
Fig. 1.
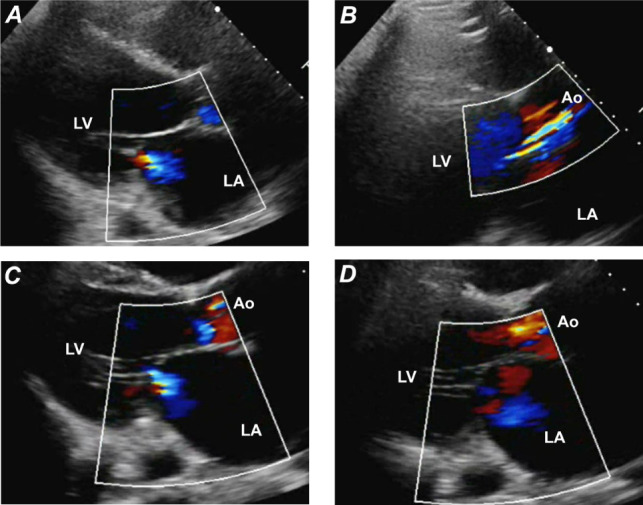
Ramp-test transthoracic echocardiograms (color-flow Doppler mode, parasternal long-axis views) show A) mitral regurgitation and B) a probable aortic regurgitant jet at a pump speed of 2,700 rpm and the same anomalies (C and D) at an increased pump speed of 2,860 rpm.
Ao = aorta; LA = left atrium; LV = left ventricle
Because this heightened the suspicion of recirculation due to AI,4 invasive diagnostic testing was performed. At the baseline pump speed (2,700 rpm), right-sided heart catheterization revealed a mean right atrial pressure (mRAP) of 16 mmHg and a mean pulmonary capillary wedge pressure (mPCWP) of 21 mmHg (cardiac index, 2.6 L/min/m2). At 2,800 rpm, the mRAP increased to 18 mmHg, and the mPCWP, to 23 mmHg (cardiac index, 2.3 L/min/m2). These increases indicated that blood was recirculating into the heart instead of flowing forward into the systemic circulation.
Because of the mixed hemodynamic and echocardiographic findings, we performed ICE. An ultrasound-tipped catheter was advanced through the right femoral vein into the right atrium, where the probe was rotated to obtain a long-axis view of the left ventricular outflow tract. The resulting color-flow Doppler echocardiogram showed flow turbulence within the aortic annulus, but no evidence of AI (Fig. 2).
Fig. 2.
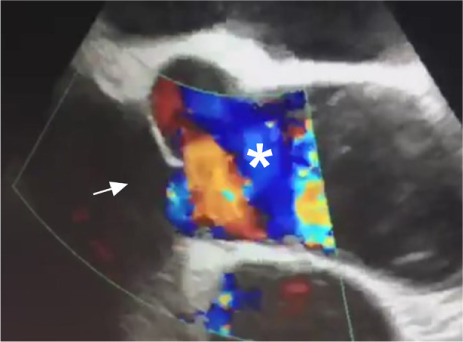
Intracardiac echocardiogram (color-flow Doppler mode, long-axis view) with the probe in the right atrium, shows turbulence within the aortic annulus (asterisk) but no evidence of aortic insufficiency (arrow).
Supplemental motion image is available for Figure 2.
On ruling out AI, we determined that the patient's symptoms were caused by acute fluid overload secondary to dietary noncompliance and inadequate diuresis. Aggressive intravenous diuresis was initiated, and pump speed was increased to 2,800 rpm. One week later, after euvolemia was achieved, the patient was prescribed bumetanide 5 mg twice daily and was encouraged to follow his medication and dietary regimens. Serum laboratory studies revealed an LDH level of 349 IU/L, an INR of 2.6, and no urobilinogen. The patient was discharged from the hospital on postadmission day 9, weighing 162 kg. At regular follow-up visits, the patient's weight remained stable between 161 and 164 kg, and he continued following his prescribed regimens.
Discussion
The incidence of new-onset AI increases with duration of LVAD use. In one retrospective study, approximately 10% of patients had at least moderate AI within 6 months after implant, and 25% to 30% of patients had it within the first year.5 Persistent aortic valve closure is a risk factor for de novo AI after LVAD implant.5,6 Patients who have de novo AI one year after implant are at significantly higher risk of death than those without AI.7 Therefore, prompt detection of AI in LVAD-supported patients is vital.
The American Society of Echocardiography has published recommendations for echocardiography in the management of patients supported with LVADs.8 However, standard echocardiographic methods are not adequate for grading AI. For example, one group of investigators9,10 found that TTE underestimated AI in 33% of cases, especially when regurgitation levels were low. Moreover, the guidelines have not been validated for patients supported with continuous-flow LVADs. As a result, the clinical importance of AI in this population can be missed, ultimately leading to a poor long-term prognosis.
In addition, patient body habitus must be considered in obtaining adequate TTEs. Imaging is difficult in patients who are obese, have chest wall deformities, or have obstructive lung disease. Obesity also increases the need for left-sided heart intravenous contrast.11
Transesophageal echocardiography remains crucial during the perioperative and immediate postoperative periods of LVAD implant.12 However, the associated anesthesia requirements can be prohibitive. General anesthesia lowers systemic vascular resistance so that the reduced afterload increases forward flow through the LVAD and reduces AI, thus leading to underestimates of AI severity. Because of the potential adverse effects of anesthesia on cardiac hemodynamics, we did not use TEE during our patient's ramp test 3 months after LVAD implant.
In contrast, our patient's case shows that ICE enables superior imaging of the aortic annulus, without the need for anesthesia. Furthermore, it can supplement traditional TTE methods in evaluating suspected AI. We were able to rule out AI, thus avoiding an unnecessary procedure such as valvular intervention in an LVAD-supported patient.
Meeting presentation: Presented as a moderated poster at the ACC.20 Scientific Session Together With World Congress of Cardiology virtual meeting, Chicago, 28–30 March 2020.
Supplementary Material
References
- 1.Truby LK, Garan AR, Givens RC, Wayda B, Takeda K, Yuzefpolskaya M et al. Aortic insufficiency during contemporary left ventricular assist device support: analysis of the INTERMACS registry. JACC Heart Fail. 2018;6(11):951–60. doi: 10.1016/j.jchf.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassi M, Rosenbaum AN, Wiley BM, Behfar A. Novel use for intracardiac echocardiography: evaluation of patients with continuous flow left ventricular assist devices. JACC Cardiovasc Imaging. 2019;12(2):363–6. doi: 10.1016/j.jcmg.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 3.El Banayosy A, Koerner MM, Brehm C, Stevenson ER, Pae WE, Jr, Clemson B et al. Intracardiac echocardiography for diagnosis and management of left ventricular assist device inlet obstruction. ASAIO J. 2014;60(6):e1–2. doi: 10.1097/MAT.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 4.Iizuka K, Nishinaka T, Takewa Y, Yamazaki K, Tatsumi E. The influence of pump rotation speed on hemodynamics and myocardial oxygen metabolism in left ventricular assist device support with aortic valve regurgitation. J Artif Organs. 2017;20(3):194–9. doi: 10.1007/s10047-017-0960-y. [DOI] [PubMed] [Google Scholar]
- 5.Cowger J, Pagani FD, Haft JW, Romano MA, Aaronson KD, Kolias TJ. The development of aortic insufficiency in left ventricular assist device-supported patients. Circ Heart Fail. 2010;3(6):668–74. doi: 10.1161/CIRCHEARTFAILURE.109.917765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorde UP, Uriel N, Nahumi N, Bejar D, Gonzalez-Costello J, Thomas SS et al. Prevalence, significance, and management of aortic insufficiency in continuous flow left ventricular assist device recipients. Circ Heart Fail. 2014;7(2):310–9. doi: 10.1161/CIRCHEARTFAILURE.113.000878. [DOI] [PubMed] [Google Scholar]
- 7.Toda K, Fujita T, Domae K, Shimahara Y, Kobayashi J, Nakatani T. Late aortic insufficiency related to poor prognosis during left ventricular assist device support. Ann Thorac Surg. 2011;92(3):929–34. doi: 10.1016/j.athoracsur.2011.04.115. [DOI] [PubMed] [Google Scholar]
- 8.Stainback RF, Estep JD, Agler DA, Birks EJ, Bremer M, Hung J et al. Echocardiography in the management of patients with left ventricular assist devices: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015;28(8):853–909. doi: 10.1016/j.echo.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Grinstein J, Kruse E, Sayer G, Fedson S, Kim GH, Sarswat N et al. Novel echocardiographic parameters of aortic insufficiency in continuous-flow left ventricular assist devices and clinical outcome. J Heart Lung Transplant. 2016;35(8):976–85. doi: 10.1016/j.healun.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grinstein J, Kruse E, Sayer G, Fedson S, Kim GH, Jorde UP et al. Accurate quantification methods for aortic insufficiency severity in patients with LVAD: role of diastolic flow acceleration and systolic-to-diastolic peak velocity ratio of outflow cannula. JACC Cardiovasc Imaging. 2016;9(6):641–51. doi: 10.1016/j.jcmg.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Finkelhor RS, Moallem M, Bahler RC. Characteristics and impact of obesity on the outpatient echocardiography laboratory. Am J Cardiol. 2006;97(7):1082–4. doi: 10.1016/j.amjcard.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 12.Flores AS, Essandoh M, Yerington GC, Bhatt AM, Iyer MH, Perez W et al. Echocardiographic assessment for ventricular assist device placement. J Thorac Dis. 2015;7(12):2139–50. doi: 10.3978/j.issn.2072-1439.2015.10.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


