Abstract
Aims.
To determine the prevalence of women of childbearing age with schizophrenia and bipolar disorder exposed to antipsychotic (AP) drugs and mood stabilizers (MS) in Lombardy, a European region of 10 million inhabitants and 1 752 285 women of childbearing age.
Methods.
The data concerning psychiatric care, drug treatments and pregnancy outcomes were retrieved from local administrative databases during a 12-month census period.
Results.
During a 12-month census period, 2893 women of childbearing age with schizophrenia (74.8% of all women of childbearing age with schizophrenia) and 918 with bipolar disorder (80.1% of all women of childbearing age with bipolar disorder) were exposed to AP drugs or MS, yielding a prevalence of exposure for women with schizophrenia of 1.65 (95% confidence interval (CI) 1.59–1.71) per 1000 female inhabitants, and for women with bipolar disorder of 0.52 (95% CI 0.49–0.55) per 1000 female inhabitants. Persistent exposure to potentially teratogenic medications accounted for one in every 1000 women of childbearing age. Of the 57 pregnancies in women with schizophrenia, normal delivery was recorded in 23 (40%) cases; of the 26 pregnancies in women with bipolar disorder, normal delivery was recorded in 10 (38%) cases.
Conclusions.
In women of childbearing age with severe mental disorders, exposure to psychotropic drugs is substantial, which suggests that the issue of reproductive health is epidemiologically relevant and a major public health concern.
Key words: bipolar disorder, childbearing age, psychotropic drugs, schizophrenia
Introduction
Schizophrenia and bipolar disorder are severe mental disorders characterized by high prevalence, onset in late childhood or early adolescence, long-term course and similar distribution between men and women (Muller-Oerlinghausen et al. 2002; van Os & Kapur, 2009). Long-term pharmacological treatment with antipsychotics (AP) and/or mood stabilizers (MS) is usually required, as it has been shown to be beneficial during the acute phases and to prevent new illness episodes (Soares-Weiser et al. 2007; Beynon et al. 2009; Leucht et al. 2012).
These epidemiological characteristics have implications for reproductive health, as it is expected that a relevant proportion of women with schizophrenia and bipolar disorder is exposed to AP and MS during childbearing age. Surprisingly, however, no epidemiological data are available on this compelling issue. This potential exposure raises concerns, as these two groups of medicines may increase the risk of congenital abnormalities during the first trimester of pregnancy and may also have metabolic adverse effects that could pose a serious medical challenge for women planning a pregnancy or already pregnant (Gentile, 2004, 2010; Meador et al. 2006; National Institute for Health and Clinical Excellence, 2007; Einarson & Boskovic, 2009; Jentink et al. 2010).
A second line of concern is that, in women with schizophrenia and bipolar disorder, reproductive health, long-term consequences of drug exposure and need for adequate information on contraception options may be very difficult and complex topics to discuss, especially when they are acutely unwell.
In the present epidemiological survey, we sought to determine the prevalence and describe the main epidemiological characteristics of women of childbearing age with schizophrenia and bipolar disorder exposed to AP and MS. Also, we aimed to record the number of documented pregnancies while taking one or more of these drugs and the outcome of these pregnancies, if known.
Methods
Setting
In the Italian system of care, patients with severe mental disorders receive psychiatric care within a specific regional mental health system (Sytema et al. 1997; Tansella et al. 2006; Lora, 2009). Each Italian region set up its own mental health system, and each mental health system is composed of a network of community-based psychiatric services. Each community-based psychiatric service provides care to all residents of a well-defined catchment area, so that individuals living in a specific area can access only the psychiatric facilities of that area. People seeking psychiatric care outside their catchment area are referred to their own catchment area. Therefore, from an epidemiological point of view, each region represents a single evaluation unit that is relatively homogeneous in terms of policies, resources and service delivery (Tansella et al. 1987; Lora et al. 2011).
The present epidemiological survey was carried out in Lombardy, the largest and most affluent region in Italy (in 2011 its population was 9 917 714, 16.4% of Italy's population). Lombardy is located in the northernmost part of the country and includes the metropolitan area of Milan, Italy's second largest city.
Data source
In Lombardy, a regional Psychiatric Information System, a regional database of hospital admissions and a regional database of prescriptions are maintained and routinely updated for administrative and reimbursement reasons.
The data concerning psychiatric care used in this study were retrieved from the regional Psychiatric Information System (Lora et al. 2012). The system gathers information from all public community-based psychiatric services and private day treatment and residential facilities. It collects demographic information and ICD-10 diagnoses (World Health Organization, 1992) of patients in contact with mental health services and records all patient care episodes in any treatment setting (outpatient and home contacts, day treatment attendance and admissions to general hospital or residential facilities).
Information on drug treatment was retrieved from the regional administrative database that stores dispensing records used for community pharmacy reimbursement (Conti et al. 2012). This database includes all community (i.e. outside hospitals) prescriptions reimbursed by the National Health System (NHS) for the population living in this region. Therefore, general practitioner prescriptions, ambulatory prescriptions delivered by specialists (psychiatrists, neurologists and others) and prescriptions delivered in private care are included in the database if reimbursed by the NHS. In Italy, typical and atypical AP and MS are fully reimbursed by the NHS on the basis of a favourable cost-effectiveness profile.
Information on pregnancy outcomes were retrieved from the regional database of hospital admissions, which stores ICD9-CM codes for all admissions to public and private hospitals located in Lombardy.
Data extraction
From the regional Psychiatric Information System we extracted the list of all patients with at least one psychiatric contact between January and December 2007. The first psychiatric contact during 2007 was defined as index contact. On the basis of ICD-10 diagnosis, we selected women aged between 18 and 45 years with a diagnosis of schizophrenia spectrum disorders or bipolar disorder. For schizophrenia spectrum disorders, the following ICD-10 code was included: 2X.XX; for bipolar disorder, the following ICD-10 codes were included: 30.XX, 31.XX, 34.0X and 38.0X.
For the cohorts of women with a diagnosis of schizophrenia and bipolar disorder, we extracted from the regional administrative database storing pharmacy records all prescriptions of AP and MS issued during the 12 months after the index contact. We used an anonymous patient identification code (Corrao et al. 2008).
Drugs were classified following the Anatomical Therapeutic Chemical Classification (ATC) system. AP were defined as medicines in the N05A ATC group (excluding N05AN – lithium); and MS were defined as medicines in the N03AF, N03AG, N03AX, N05AN ATC groups.
For the two cohorts, we additionally extracted from the regional database of hospital admissions all pregnancy-related outcomes for the 12 months after the index contact. Hospital admissions were identified by using ICD9-CM codes 630–677.
According to local regulations, no formal approval of the study protocol was required. However, as previously reported for similar database analyses (Conti et al. 2012), to preserve patient privacy, the identification codes from all the databases were converted to anonymous codes, and the conversion table was then destroyed.
Data analyses
The prevalence of women of childbearing age with schizophrenia or bipolar disorder was calculated by dividing the number of women aged 18–45 years with a recorded diagnosis of schizophrenia or bipolar disorder by the total number of female inhabitants (of the same age group) residents in Lombardy. The prevalence of women of childbearing age exposed to AP and MS was calculated by dividing the number of women aged 18–45 years with a recorded diagnosis of schizophrenia or bipolar disorder who received at least one prescription of AP drugs or MS by the total number of female inhabitants (of the same age group) residents in Lombardy. The prevalence of women of childbearing age with persistent exposure was calculated by dividing the number of women aged 18–45 years with a recorded diagnosis of schizophrenia or bipolar disorder who were persistently prescribed AP drugs or MS over the 12-month study period by the total number of female inhabitants (of the same age group) residents in Lombardy. Rates per 1000 inhabitants were thus calculated, together with the 95% confidence interval (CI).
Persistence of drug treatment with AP and MS was quantified as a medication possession ratio (MPR). The MPR was calculated by dividing the cumulative duration of any AP or MS treatment during follow-up (numerator) by the whole number of days of follow-up (denominator) (Andrade et al. 2006). The cumulative duration of each medication was calculated by dividing the total prescribed amount of AP or MS by each drug's defined daily dose (DDD) (WHO Collaborating Centre for Drug Statistic Methodology, 2003). Drug treatment was considered persistent with MPR ≥0.8 (Andrade et al. 2006).
The cohorts of women of childbearing age with a diagnosis of schizophrenia or bipolar disorder, who were exposed to AP drugs or MS, were described with respect to the following socio-demographic and clinical characteristics: age; completed years of education (1–5, 6–8, 9–13 and ≥14); employment status (employed or unemployed); marital status (married, never married, divorced and widowed); urbanicity (defined according to Eurostat as rural, semi-urban and urban) (Eurostat, 2007); psychiatric hospitalization in the previous 5 years; use of psychiatric services during follow-up; psychological contacts during follow-up: Charlson Comorbidity Index, a score summarizing the overall burden of comorbidity on the basis of hospital admissions during the year before the index contact (categorized as 0 and ≥1) (Charlson et al. 1987).
The top ten most prescribed AP drugs and MS for the two cohorts of women with schizophrenia and bipolar disorder were calculated as the number of prescriptions of each drug during the 12-month period after the index contact out of the total number of prescriptions of AP drugs and MS.
In women of childbearing age with a diagnosis of schizophrenia or bipolar disorder, both exposed to and unexposed to AP drugs or MS, the proportion of pregnancies was calculated. Pregnancy related outcomes were additionally described.
Results
Prevalence rates
During the 12-month census period 3868 women with schizophrenia aged between 18 and 45 years received psychiatric care; of these 2893 (74.8%) were exposed to AP drugs or MS. Similarly, a total of 1146 women with bipolar disorder aged between 18 and 45 years received psychiatric care; of these 918 (80.1%) were exposed to AP drugs or MS. The corresponding prevalence rate of women of childbearing age with schizophrenia was 2.2 per 1000 inhabitants (Table 1), and the prevalence rate of women of childbearing age with schizophrenia and AP and/or MS drug use was 1.65 per 1000 inhabitants (Table 1). Similarly, the prevalence rate of women of childbearing age with bipolar disorder was 0.65 per 1000 inhabitants (Table 1), and the prevalence rate of women of childbearing age with bipolar disorder and AP and/or MS drug use was 0.52 per 1000 inhabitants (Table 1).
Table 1.
Prevalence rates of women of childbearing age with schizophrenia and bipolar disorder exposed to AP and/or MS
| Women aged 18–45 years (5014) | Schizophrenia | Bipolar disorder | ||
|---|---|---|---|---|
| No. | Rate per 1000 inhabitants (95% CI) | No. | Rate per 1000 inhabitants (95% CI) | |
| All | 3868 | 2.20 (2.13–2.27) | 1146 | 0.65 (0.61–0.69) |
| Exposed to AP or MS | 2893 | 1.65 (1.59–1.71) | 918 | 0.52 (0.49–0.55) |
| Persistently exposed to AP or MS | 1196 | 0.68 (0.64–0.72) | 404 | 0.23 (0.20–0.25) |
The prevalence of women of childbearing age persistently exposed to AP drugs or MS was 0.68 per 1000 inhabitants for schizophrenia, and 0.23 per 1000 inhabitants for bipolar disorder (Table 1).
Pregnancies and pregnancy related outcomes
During the study period, the proportion of pregnancies in women with schizophrenia was 0.8% in those exposed to AP drugs and/or MS, and 3.6% in those unexposed (Table 2). Similarly, the proportion of pregnancies in women with bipolar disorder was 1.2% in those exposed to AP and/or MS, and 6.4% in those unexposed (Table 2). Of the 57 pregnancies in women with schizophrenia, normal delivery was recorded in 23 (40%) cases and abortion in 26 (46%) cases (legally induced abortion in 15 cases); outcome was missing for the remaining eight cases. Of the 26 pregnancies in women with bipolar disorder, normal delivery was recorded in 10 (38%) cases and abortion in 10 (38%) cases (legally induced abortion in six cases); outcome was missing for the remaining six cases.
Table 2.
Proportion of pregnancies in women with schizophrenia and bipolar disorder exposed to AP and/or MS
| Women aged 18–45 years | Schizophrenia | Bipolar disorder | ||
|---|---|---|---|---|
| No. of pregnancies | % (95% CI) | No. of pregnancies | % (95% CI) | |
| Exposed to AP or MS | 22 | 0.76 (0.47–1.15) | 11 | 1.20 (0.60–2.14) |
| Not exposed to AP or MS | 35 | 3.56 (2.49–4.91) | 15 | 6.41 (3.63–10.35) |
Characteristics of women of childbearing age with schizophrenia and bipolar disorder exposed to AP and/or MS
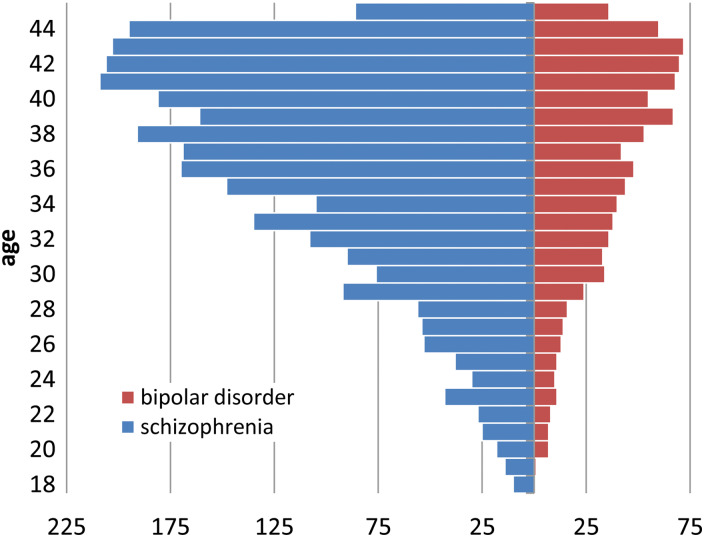
The main epidemiological and clinical characteristics of the two cohorts are presented in Table 3, and the age distribution is shown in Fig. 1. Only a few women received high education and most were unemployed, with a higher rate of unemployment in women with schizophrenia. Most women were unmarried, with a higher rate in those with schizophrenia. Over 85% of women were 30 years or above (Fig. 1). Over 90% of women had at least one outpatient contact with a psychiatrist during follow-up, and around half were hospitalized at least once in the previous 5 years. Charlson comorbidity index failed to reveal substantial medical comorbidities (Table 3).
Table 3.
Characteristics of women of childbearing age with schizophrenia and bipolar disorder exposed to AP and/or MS
| Type of disorder | |||||
|---|---|---|---|---|---|
| All | Schizophrenia | Bipolar disorder | |||
| Characteristics | No. | % | No. | % | |
| Overall | 3811 | 2893 | 100 | 918 | 100 |
| Education (years) | |||||
| Missing | 180 | 122 | 4.2 | 58 | 6.3 |
| 1–5 | 220 | 179 | 6.2 | 41 | 4.5 |
| 6–8 | 1921 | 1533 | 53.0 | 388 | 42.3 |
| 9–11 | 1253 | 893 | 30.9 | 360 | 39.2 |
| ≥14 | 237 | 166 | 5.7 | 71 | 7.7 |
| Employment | |||||
| Missing | 183 | 126 | 4.4 | 57 | 6.2 |
| Unemployed | 2285 | 1842 | 63.7 | 443 | 48.3 |
| Employed | 1343 | 925 | 32.0 | 418 | 45.5 |
| Marital status | |||||
| Missing | 101 | 67 | 2.3 | 34 | 3.7 |
| Never married | 2363 | 1924 | 66.5 | 439 | 47.8 |
| Married | 1052 | 702 | 24.3 | 350 | 38.1 |
| Divorced or separated | 278 | 188 | 6.5 | 90 | 9.8 |
| Widowed | 17 | 12 | 0.4 | 5 | 0.5 |
| Urbanicity | |||||
| Rural | 289 | 211 | 7.3 | 78 | 8.5 |
| Semiurban | 1056 | 744 | 25.7 | 312 | 34.0 |
| Urban | 2466 | 1938 | 67.0 | 528 | 57.5 |
| Psychiatric hospitalization in last 5 years | |||||
| No | 1998 | 1558 | 53.9 | 440 | 47.9 |
| Yes | 1813 | 1335 | 46.1 | 478 | 52.1 |
| Used psychiatrist services during follow-up | |||||
| No | 307 | 218 | 7.5 | 89 | 9.7 |
| Yes | 3504 | 2675 | 92.5 | 829 | 90.3 |
| Psychological contacts during follow-up | |||||
| No | 2692 | 2034 | 70.3 | 658 | 71.7 |
| Yes | 1119 | 859 | 29.7 | 260 | 28.3 |
| Charlson comorbidity score | |||||
| 0 | 3778 | 2869 | 99.2 | 909 | 99.0 |
| ≥1 | 33 | 24 | 0.8 | 9 | 1.0 |
Fig. 1.
Age distribution of women of childbearing age with schizophrenia and bipolar disorder exposed to AP and/or MS.
Top ten drugs in women of childbearing age with schizophrenia and bipolar disorder
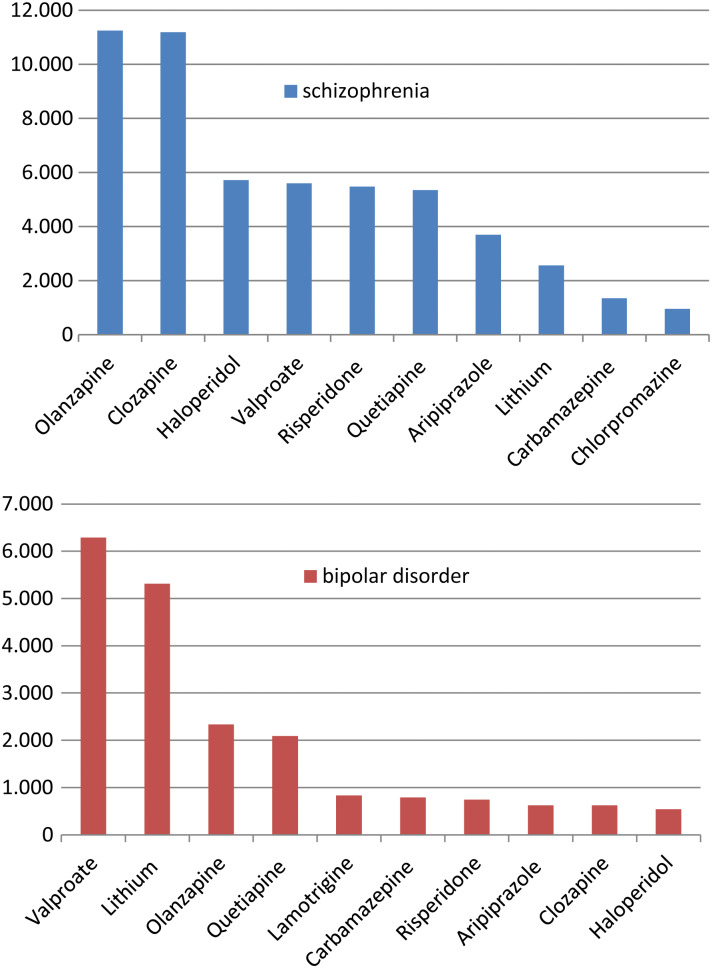
The distribution of women with schizophrenia and bipolar disorder receiving the top ten agents, which represented over 90% of all prescriptions in both cohorts, is presented in Fig. 2. Interestingly, the MS valproate, lithium and carbamazepine were among the top ten agents in both groups and, similarly, the AP haloperidol, clozapine, olanzapine, aripiprazole, risperidone and quetiapine were among the top ten agents in both groups.
Fig. 2.
Top ten drugs prescribed to women of childbearing age with schizophrenia and bipolar disorder (number of prescriptions).
Discussion
To our knowledge, this is the first report describing the prevalence and characteristics of women of childbearing age with schizophrenia and bipolar disorder exposed to AP and MS in a large catchment area. We found that slightly more than two every 1000 women aged 18–45 years are exposed to psychotropic drugs, and that persistent exposure accounts for one in every 1000 women of childbearing age. We do not know how these figures were compared with those from other catchment areas, as existing surveys have either enrolled psychiatric populations of women all receiving or starting treatment, or already pregnant, or non-psychiatric populations. In the U.S., for example, Schwarz et al., who described dispensing of potentially teratogenic medications to female Veterans treated by the Veterans Affairs Healthcare System, showed that prescriptions for potentially teratogenic medications were filled by 48.8% of female Veterans who received medications (Schwarz et al. 2010). In our sample, the finding that 74.8% and 80.1% of women of childbearing age with schizophrenia and bipolar disorders were exposed to AP and/or MS, respectively, suggests that in this patient population the issue of reproductive health is epidemiologically relevant and a major public health concern.
The relevance is further corroborated by the finding that in the sample of women aged 18–45 years with schizophrenia and bipolar disorder who were exposed to psychotropic drugs the proportion of pregnancies was substantially lower compared with those not exposed to psychotropic drugs. We did not attempt to compare these frequencies statistically, as the two populations may not be comparable, in the sense that psychotropic drugs may have been systematically more often prescribed to women less likely to become pregnant. If this systematic selection had occurred, it would further reinforce that in this patient population prescribing psychotropic drugs poses a serious challenge to doctors, women and their families. The finding that normal delivery accounted only for 40–50% of pregnancies must be seen cautiously, as only few pregnancies were recorded and pregnancy outcome was unknown in more than 10% of cases.
Another important clinical finding is that a significant proportion of women with schizophrenia and bipolar disorder (about 25% and 20%, respectively) were not prescribed AP and/or MS. This is in contrast with the evidence that it is best that people with schizophrenia and bipolar disorder receive long-term treatment to prevent relapse (Soares-Weiser et al. 2007; Beynon et al. 2009; Leucht et al. 2012). It is, however, common for women who are planning a pregnancy or do not employ adequate contraception, either to use drugs intermittently or to stop treatments that may be perceived as potentially dangerous.
This analysis has limitations. First, the lack of data on whether women eventually took the prescribed drugs should be highlighted, since a relevant proportion of the medicines prescribed for people with chronic conditions are not taken (Jones, 2003). A second limitation is that under reporting cannot be excluded because the database collects outside-the-hospital prescriptions only, while prescriptions issued in general hospitals, psychiatric residential facilities and in all other in-patient services were not considered. In addition, it may be possible that women with stable symptomatology have been followed by primary care doctors with no psychiatric contacts during the census period. This would represent another source of underreporting, although in the Italian system of community psychiatric care it is very unlikely that women with schizophrenia and bipolar disorder are not seen for such a long period of time. Another potential limitation is that some prescriptions might theoretically have been missed by the database. It is possible, for example, that retail pharmacies failed to provide all data to the regional Health Authority or, in the case of long-acting AP drugs, it is possible that some injections might have been directly dispensed by mental health services without providing the requested information to the Regional Health Authority. Although these possibilities cannot be completely ruled out, retail pharmacies and mental health services are reimbursed only for those prescriptions provided to the Regional Health Authority. In this system, therefore, under reporting would have produced negative economic consequences.
The results of the present study would call for the development of psychosocial and psychoeducational interventions aimed at systematically taking into consideration the short- and long-term consequences of psychotropic drug exposure, as well as the need for adequate information on contraception options, in women of childbearing age (Barbui & Cipriani, 2011b). Most pregnancies are unplanned in this group, so prescribing needs to bear in mind both the risks of not being treated and the risks of treatment. Women should be given information on contraception and on the importance of planning pregnancy care – including medication options – before trying to conceive. These intervention packages should be pragmatically tailored to the main epidemiological characteristics of women with schizophrenia and bipolar disorder: according to the present survey, only a few received high education and most were unemployed and unmarried. Age should additionally be carefully considered, as teenagers with schizophrenia and bipolar disorder might need a different approach in comparison with older women. The episodic nature of schizophrenia and bipolar disorder would require the development of information strategies that allow to reach an agreement between the woman, her family and the treating medical and psychiatric staff on what should be done in case of a new acute illness episode in childbearing age. Women may this way have regular discussions around their plans so that they can make informed choices on the risks and benefits of different drug options.
In addition to the epidemiological characteristics of women, the therapeutic characteristics of AP and MS drugs, and their specific risks during childbearing age, should also be covered. It is of interest to note that valproate is still the most popular MS, as the evidence of its teratogenic properties are particularly alarming and also has adverse impact on cognitive development. According to NICE recommendations, for example, valproate should not be prescribed other than when no other medication is effective. The finding that, among the top ten drugs, nine were in common for the two groups of women with schizophrenia and bipolar disorder, suggests that information packages for schizophrenia may have substantial overlap with those for bipolar disorder.
Ideally, pragmatic cluster trials (Barbui & Cipriani, 2011a) should be developed to investigate whether different psychosocial and psychoeducational intervention strategies, administered under real-world circumstances, may have a beneficial and sustained effect on women reproductive health outcomes.
Declaration of interest
All authors declare that they have no conflicts of interest.
References
- Andrade SE, Kahler KH, Frech F, Chan KA (2006). Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiology and Drug Safety 15, 565–574. [DOI] [PubMed] [Google Scholar]
- Barbui C, Cipriani A (2011a). Cluster randomized trials. Epidemiology and Psychiatric Sciences 20, 307–309. [DOI] [PubMed] [Google Scholar]
- Barbui C, Cipriani A (2011b). What are evidence-based treatment recommendations? Epidemiology and Psychiatric Sciences 20, 29–31. [DOI] [PubMed] [Google Scholar]
- Beynon S, Soares-Weiser K, Woolacott N, Duffy S, Geddes JR (2009). Pharmacological interventions for the prevention of relapse in bipolar disorder: a systematic review of controlled trials. Journal of Psychopharmacology 23, 574–591. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987). A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases 40, 373–383. [DOI] [PubMed] [Google Scholar]
- Conti V, Lora A, Cipriani A, Fortino I, Merlino L, Barbui C (2012). Persistence with pharmacological treatment in the specialist mental healthcare of patients with severe mental disorders. European Journal of Clinical Pharmacology 68, 1647–1655. [DOI] [PubMed] [Google Scholar]
- Corrao G, Cesana G, Merlino L (2008). Pharmacoepidemiological research and the linking of electronic healthcare databases available in the Italian region of Lombardy. Biomedical Statistics and Clinical Epidemiology 2, 117–125. [Google Scholar]
- Einarson A, Boskovic R (2009). Use and safety of antipsychotic drugs during pregnancy. Journal of Psychiatric Practice 15, 183–192. [DOI] [PubMed] [Google Scholar]
- Eurostat (2007). European Regional and Urban Statistics Reference Guide. European Communities: Luxemburg. [Google Scholar]
- Gentile S (2004). Clinical utilization of atypical antipsychotics in pregnancy and lactation. Annals of Pharmacotherapy 38, 1265–1271. [DOI] [PubMed] [Google Scholar]
- Gentile S (2010). Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophrenia Bulletin 36, 518–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentink J, Loane M, Dolk H, Barisic I, Garne E, Morris J, de Jong-van der Berg L (2010). Valproic acid monotherapy in pregnancy and major congenital malformations. New England Journal of Medicine 362, 2185–2193. [DOI] [PubMed] [Google Scholar]
- Jones G (2003). Prescribing and taking medicines. British Medical Journal 327, 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Salanti G, Davis JM (2012). Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 379, 2063–2071. [DOI] [PubMed] [Google Scholar]
- Lora A (2009). An overview of the mental health system in Italy. Annali dell'Istituto Superiore di Sanita 45, 5–16. [PubMed] [Google Scholar]
- Lora A, Barbato A, Cerati G, Erlicher A, Percudani M (2012). The mental health system in Lombardy, Italy: access to services and patterns of care. Social Psychiatry and Psychiatric Epidemiology 47, 447–454. [DOI] [PubMed] [Google Scholar]
- Lora A, Conti V, Leoni O, Rivolta AL (2011). Adequacy of treatment for patients with schizophrenia spectrum disorders and affective disorders in Lombardy, Italy. Psychiatric Services 62, 1079–1084. [DOI] [PubMed] [Google Scholar]
- Meador K, Baker G, Finnell R (2006). In utero antiepileptic drug exposure: fetal death and malformations. Neurology 67, 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oerlinghausen B, Berghofer A, Bauer M (2002). Bipolar disorder. Lancet 359, 241–247. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (2007). Antenatal and Postnatal Mental Health. NICE: Great Britain. [PubMed] [Google Scholar]
- Schwarz EB, Longo LS, Zhao X, Stone RA, Cunningham F, Good CB (2010). Provision of potentially teratogenic medications to female veterans of childbearing age. Medical Care 48, 834–842. [DOI] [PubMed] [Google Scholar]
- Soares-Weiser K, Bravo VY, Beynon S, Dunn G, Barbieri M, Duffy S, Geddes J, Gilbody S, Palmer S, Woolacott N (2007). A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder. Health Technology Assessment 11, iii–206. [DOI] [PubMed] [Google Scholar]
- Sytema S, Micciolo R, Tansella M (1997). Continuity of care for patients with schizophrenia and related disorders: a comparative south-Verona and Groningen case-register study. Psychological Medicine 27, 1355–1362. [DOI] [PubMed] [Google Scholar]
- Tansella M, De Salvia D, Williams P (1987). The Italian psychiatric reform: some quantitative evidence. Social Psychiatry 22, 37–48. [DOI] [PubMed] [Google Scholar]
- Tansella M, Amaddeo F, Burti L, Lasalvia A, Ruggeri M (2006). Evaluating a community-based mental health service focusing on severe mental illness. The Verona experience. Acta Psychiatrica Scandinavica, Supplement 429, 90–94. [DOI] [PubMed] [Google Scholar]
- van Os J, Kapur S (2009). Schizophrenia. Lancet 374, 635–645. [DOI] [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistic Methodology (2003). Guidelines for ATC Classification and DDD Assignment. WHO: Oslo. [Google Scholar]
- World Health Organization (1992). Manual of the International Classification of Diseases, Injuries, and Causes of Death. World Health Organization: Geneva. [Google Scholar]