Abstract
BACKGROUND AND PURPOSE:
Rescue endovascular and pharmacologic approaches are increasingly being adopted after recanalization failure of acute large-vessel occlusion strokes with mechanical thrombectomy, with encouraging results. The safety and efficacy of glycoprotein IIb/IIIa inhibitors in ischemic stroke have been investigated, though cangrelor, a recent intravenous P2Y12-receptor inhibitor with a rapid onset/offset of action and a short half-life, may be a valuable option. We compared the safety and efficacy of cangrelor with those of glycoprotein IIb/IIIa inhibitors for refractory occlusions.
MATERIALS AND METHODS:
We performed a retrospective analysis of the ongoing prospective, multicenter, observational Endovascular Treatment in Ischemic Stroke Registry in France between May 2012 and February 2020. Refractory intracranial occlusions of the anterior and posterior circulation were included and defined as recanalization failure of large-vessel occlusion stroke, perioperative target artery reocclusion, or high risk of early reocclusion related to an arterial wall lesion. The primary end point was a favorable outcome, defined as a 90-day mRS of 0–2. Secondary end points were reperfusion, intracranial hemorrhage, and procedural complications.
RESULTS:
Among 69 patients, 15 were treated with cangrelor, and 54, with glycoprotein IIb/IIIa inhibitors. The favorable outcome (adjusted OR = 2.22; 95% CI, 0.42–11.75; P = .348) and mortality (adjusted OR = 0.44; 95% CI, 0.06–3.16; P = .411) rates were similar in both groups. There was no difference in the rates of any intracranial hemorrhage (adjusted OR = 0.40; 95% CI, 0.08–2.09; P = .280), symptomatic intracranial hemorrhage (6.7% versus 0.0%, P = .058), or procedural complications (6.7% versus 20.4%, P = .215). Reperfusion rates were higher in the cangrelor group, though the difference did not reach statistical significance (93.3% versus 75.0% for modified TICI 2b–3; adjusted OR =10.88; 95% CI, 0.96–123.84; P = .054).
CONCLUSIONS:
Cangrelor seems to be as safe as glycoprotein IIb/IIIa inhibitors for managing refractory intracranial occlusion and leads to satisfactory brain reperfusion. Cangrelor is a promising agent in this setting, and additional studies are warranted to confirm our findings.
Bridging therapy is the standard approach for acute large-vessel occlusion strokes.1 Successful recanalization is achieved in 70%–80% of cases, and this has been improving continuously in recent years.1-3 However, mechanical thrombectomy failure remains problematic, and the optimal approach in this setting is unknown. In many cases, reperfusion failure may be related to underlying intracranial atherosclerosis with local acute thrombosis.4-7 In this context, adjunct pharmacologic and mechanical strategies with promising results are being reported.8-11 Anti-thrombotic agents such as acute antiplatelet therapy are often considered alone or in combination with intracranial angioplasty or stent placement. Acute antiplatelet therapy has been reported in association with a rescue strategy after standard thrombectomy failure, incomplete recanalization, or lesions considered to have a high risk of early reocclusion.8-10 Such acute antiplatelet therapy is most often deemed essential in the case of rescue intracranial stent placement.
Glycoprotein IIb/IIa (GPIIb/IIIa) inhibitors are used most often, but the optimal antithrombotic strategy is unknown. In this situation, cangrelor (Kengreal), a new P2Y12 inhibitor recently added to the standard pharmacologic arsenal for treating acute myocardial infarction,12 might be used for intracranial reperfusion, given its interesting pharmacologic properties (immediate platelet inhibition, quick platelet function recovery after treatment interruption, and easy transition to oral antiplatelet therapy).13 There are few reports on the use of cangrelor in acute stroke,14-18 and none have compared it with GPIIb/IIIa inhibitors. GPIIb/IIIa inhibitors, which are frequently used in this setting, have a slower onset of antiplatelet action.8,10,11,19,20 Their effect duration is longer, which can be unsafe in the case of intracranial bleeding or any hemorrhagic risk. The role of GPIIb/IIIa inhibitors on intracranial hemorrhage (ICH) risk in the setting of ischemic stroke is controversial.21 Therefore, this preliminary study compared the safety and efficacy of cangrelor with GPIIb/IIIa inhibitors after mechanical thrombectomy failure for intracranial large-vessel occlusion stroke.
MATERIALS AND METHODS
The data used in this study are available from the corresponding author on reasonable request.
Study Population
We performed a retrospective study of the Endovascular Treatment of Ischemic Stroke (ETIS) Registry from May 2012 to February 2020 (NCT03776877). ETIS is an ongoing prospective, multicenter, observational study that includes all consecutive patients undergoing endovascular treatment for large-vessel occlusion stroke at 13 comprehensive stroke centers in France. The local institutional review boards approved the data collection and analysis. All data in the ETIS registry were collected, stored, and accessed locally following the recommendations of the Comité consultatif sur le traitement de l'information en matière de recherche dans le domaine de santé. This study included patients presenting with refractory occlusion of the anterior or posterior circulation. Refractory occlusion was defined as recanalization failure of a large-vessel occlusion stroke after the optimal standard mechanical thrombectomy approach, perioperative reocclusion of the target artery, or a high risk of early reocclusion due to underlying angiographically identified arterial wall disease after mechanical thrombectomy (mostly underlying intracranial atherosclerotic stenosis but also other possible pathologies such as intracranial dissection or large-artery vasculitis). The exclusion criteria were cangrelor or GPIIb/IIIa inhibitor use for any indication other than those mentioned above, such as cervical carotid artery stent placement, distal embolus, or embolus in a new territory. Tandem occlusions, thrombosis of an underlying previously implanted intracranial stent, and iatrogenic occlusion after a cerebral endovascular procedure (aneurysm or arteriovenous shunt embolization) were also excluded.
Treatments
Mechanical thrombectomy was performed according to the clinical context, timeframe, imaging data, and guidelines. Intravenous thrombolysis was administered within 4.5 hours after stroke onset according to international recommendations, using recombinant tissue plasminogen activator in the absence of contraindications. The endovascular procedure was performed with the patient under conscious sedation or general anesthesia, depending on the patient's condition and local protocol. The initial thrombectomy approach was left to the discretion of the interventionist. Pharmacologic or mechanical adjunctive treatments were used for refractory occlusions according to patient comorbidities, imaging data (potentially completed with additional perioperative flat panel detector CT), and prior or previously administered thrombolysis and antithrombotic treatments. Perioperative adjunctive acute antiplatelet therapy was decided on a case-by-case basis after a multidisciplinary discussion. The cangrelor administration protocol consisted of a 30-µg/kg intravenous bolus continued with a 4-µg/kg/min infusion. The GPIIb/IIIa inhibitors were tirofiban (Agrastat), abciximab (ReoPro), or eptifibatide (Intergrilin) given following their respective standard administration protocols and dose (intravenous bolus first followed by intravenous infusion via a syringe pump). An intravenous bolus of 250 mg of acetylsalicylic acid (ASA; aspirin) was also given at the clinician's discretion. During the endovascular procedure, intracranial angioplasty or stent placement was performed optionally in association with pharmacologic therapy. Mid- and long-term antiplatelet therapy was given depending on the clinical and imaging evolution. Ideally, dual antiplatelet therapy with ASA plus clopidogrel or ticagrelor was initiated during the first 24 hours after endovascular treatment after no evidence of ICH seen on day 1 CT or MR imaging. The dual antiplatelet therapy was continued for 3 months and followed by long-term single antiplatelet therapy with ASA. Illustrative cases are presented in Figs 1 and 2.
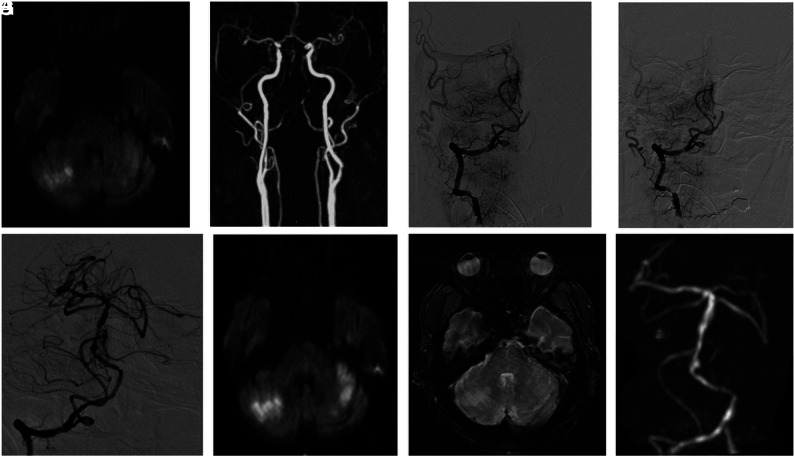
FIG 1.
Illustrative case 1. A and B, A 67-year-old patient who presented with a rapidly progressive coma associated with a right hemiplegia. Initial MR imaging demonstrates a basilar artery occlusion with a relatively small infarct core (posteriori circulation posterior circulation-ASPECTS = 8). The patient underwent intravenous thrombolysis combined with endovascular treatment. Considering the absence of recanalization of the basilar artery after 2 mechanical thrombectomy passes with a standard approach (C and D), intracranial angioplasty associated with cangrelor intravenous infusion was performed, allowing complete reperfusion (E). Day 1 MR imaging shows stability of the cerebellar infarction and patency of basilar artery without intracranial hemorrhage (F–H). The patient progressively recovered, and after 3 months, the mRS score was 0.
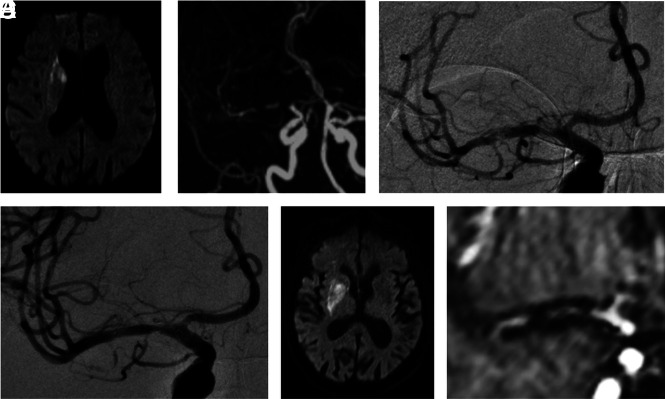
FIG 2.
Illustrative case 2. A and B, This 81-year-old woman had acute left hemiparesis and dysarthria (initial NIHSS score = 8) secondary to an occlusion of the right M1 segment of the MCA, likely due to an underlying intracranial stenosis. Intravenous thrombolysis and endovascular treatment were performed. C, The first angiographic run confirms the pattern of intracranial acute thrombosis associated with an underlying stenosis (intracranial atherosclerosis). D, Considering the poor expected efficacy of a standard mechanical thrombectomy in such occlusion etiology, the limited infarct core, and the absence of intracranial hemorrhage at baseline, intracranial angioplasty and stent placement combined with cangrelor intravenous administration were performed as a first-line strategy. E and F, Clinical evolution and day 1 imaging have favorable results. At 90 days, the mRS score was 2.
Outcomes
Clinical, imaging, timeline, and angiographic data were recorded prospectively. Trained research nurses determined the mRS scores at 90 days during face-to-face interviews or phone conversations with the patients, their relatives, or their general practitioners. Favorable outcome was defined as a 90-day mRS of 0–2. Early neurologic changes (24-hour NIHSS score) were recorded. Successful reperfusion was defined as modified TICI (mTICI) 2b, 2c, or 3. Near-complete reperfusion was defined as a final mTICI 2c or 3, and complete reperfusion, as a final mTICI 3. Intracranial recanalization of the target artery was also assessed with the arterial occlusive lesion score. Favorable reperfusion of the target vessel was defined as a score of 2 or 3. Procedural complications (dissection, embolus in a new territory, perforation, and local puncture-site complication) and 90-day mortality were collected. ICH was assessed according to the European Cooperative Acute Stroke Study (ECASS II) classification. Symptomatic ICH (sICH) was defined as neurologic deterioration (NIHSS worsening of ≥4 points) along with ICH. The ASPECTS and patency of the intracranial artery were evaluated on day 1 of imaging.
Statistical Analysis
Quantitative variables are expressed as the mean (SD) when the distribution is normal or the median (interquartile range) otherwise. Categoric variables are expressed as numbers (percentage). The patients were divided into 2 groups according to the antithrombotic treatment type (GPIIb/IIIa inhibitors and cangrelor). Baseline characteristics were compared between these 2 groups using the Student t test for Gaussian continuous variables, the Mann-Whitney U test for non-Gaussian continuous variables, or the χ2 test (or Fisher exact test when the expected cell frequency was <5) for categoric variables, as appropriate. For outcome parameters, multivariate logistic regression models were adjusted for age, ASA treatment, NIHSS and ASPECTS at presentation, infarct location, and intracranial stent placement using complete case analysis. Statistical testing was conducted at a 2-tailed α level of .05. Data were analyzed using STATA, Version 16.1 (StataCorp).
RESULTS
During the study period, 7527 patients underwent endovascular therapy in the participating centers. Of these, 69 met the inclusion criteria, of whom 15 received cangrelor and 54 received GPIIb/IIIa inhibitors (abciximab, n = 38; tirofiban, n = 11; and eptifibatide, n = 5). The Online Supplemental Data present the patients' baseline characteristics according to the type of acute antiplatelet therapy and global population. The 2 groups were balanced except for the admission diastolic blood pressure (mean, 77.66 versus 100.71 mm Hg; P = .002). Procedural and therapeutic main characteristics were also comparable between groups. ASA administration differed significantly between groups with higher use in the cangrelor group than in the GPIIb/IIIa inhibitors group (93.3% versus 29.6%, P < .001). Table 1 presents the main clinical, angiographic, and radiologic outcomes of the univariate analysis. Favorable outcome was comparable between groups (40.0% versus 31.5%, P = .536). There was also no difference in the mortality rate (20.0% versus 29.6%, P = .460). Successful reperfusion was achieved without a significant difference between groups (93.3% versus 75.0%, P = .124), but the near-complete and complete reperfusion rates significantly favored the cangrelor group (80.0% versus 30.8%, P < .001, and 46.7% versus 21.2%, P = .050, respectively).
Table 1:
Clinical and imaging outcomes
| GPIIb/IIIa Inhibitors (n = 54) | Cangrelor (n = 15) | Total (n = 69) | P Value | |
|---|---|---|---|---|
| Arterial occlusion lesion score 2–3 | 44/54 (81.5%) | 15/15 (100.0%) | 59/69 (85.5%) | .071 |
| Final mTICI 2b-3 | 39/54 (75.0%) | 14/15 (93.3%) | 53/69 (79.1%) | .124 |
| Final mTICI 2c-3 | 16/52 (30.8%) | 12/15 (80.0%) | 28/67 (41.8%) | <.001 |
| Final mTICI 3 | 11/52 (21.2%) | 7/15 (46.7%) | 18/67 (26.9%) | .050 |
| Puncture-to-recanalization delay (median) (IQR) | 80 (55−133) | 107 (82–149) | 87 (58–135) | .573 |
| Perioperative complication | 11/54 (20.4%) | 1/15 (6.7%) | 12/69 (17.4%) | .215 |
| Day 1 NIHSS (median) (IQR)a | 12 (7–16) | 11 (5–16) | 12 (6–16) | .462 |
| Day 1 ASPECTS (median) (IQR) | 6 (4–7) | 7 (6–8) | 6 (4–7) | .089 |
| Intracranial artery patency on day 1 imaging follow-up | 42/54 (79.2%) | 13/15 (86.7%) | 55/69 (80.9%) | .519 |
| Any ICH | 20/54 (37.0%) | 4/15 (26.7%) | 24/69 (34.8%) | .456 |
| sICH | 0/53 (0.0%) | 1/15 (6.7%) | 1 /68 (1.5%) | .058 |
| ECASS hemorrhage subtypes | .798 | |||
| HI-1 | 8/53 (15.1%) | 2/15 (13.3%) | 10/68 (14.7%) | |
| HI-2 | 7/53 (13.2%) | 1/15 (6.7%) | 8/68 (11.8%) | |
| PH-1 | 1/53 (1.9%) | 1/15 (6.7%) | 2/68 (2.9%) | |
| PH-2 | 0/53 (0%) | 0/15 (0%) | 0/69 (0%) | |
| Remote ICH | 1/53 (1.9%) | 0/15 (0.0%) | 1/68 (1.5%) | |
| Subarachnoid hemorrhage | 2/53 (3.8%) | 0/15 (0.0%) | 2/68 (2.9%) | |
| 90-Day mRS 0–2 | 17/54 (31.5%) | 6/15 (40.0%) | 23/69 (33.3%) | .536 |
| 90-Day mortality | 16/54 (29.6%) | 3/15 (20.0%) | 19/69 (27.5%) | .460 |
Note:—IQR indicates interquartile range; HI-1, hemorrhagic infarction type 1; HI-2, hemorrhagic infarction type 2; PH-1, parenchymal hematoma type 1; PH-2, parenchymal hematoma type 2.
Missing values in 26 patients.
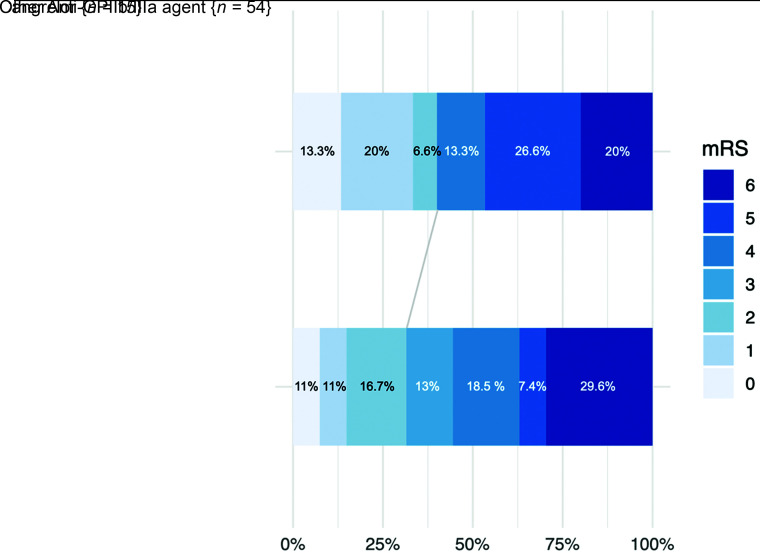
The difference in the rate of target occlusion recanalization (arterial occlusion lesion score of 2 or 3) did not reach significance (100% versus 81.5%, P = .071). Safety criteria did not differ significantly between the 2 groups. Day 1 imaging outcomes were also balanced because no differences were observed in day 1 ASPECTS (median, 7 versus 6; P = .089) or patency of the target artery (86.7% versus 79.2%, P = .519). The multivariate analysis adjusting for major confounding factors revealed improved reperfusion rates in the cangrelor group, but the difference did not reach significance (final mTICI 2b–3, 93.3% versus 75.0%; adjusted OR = 10.88; 95% CI, 0.96–123.84; P = .054) (Table 2). Similarly, we found improved functional outcomes and mortality rates and less ICH in the cangrelor group, without significant differences. Figure 3 presents the distribution of mRS scores at 90 days.
Table 2:
Multivariate analysis of main outcomes
| GPIIb/IIIa Inhibitor | Cangrelor | Adjusted ORsa | 95% CI | P Value | |
|---|---|---|---|---|---|
| 90-Day mRS 0–2 | 31.5% | 40.0% | 2.22 | 0.42–11.75 | .348 |
| 90-Day mortality | 29.6% | 20.0% | 0.44 | 0.06–3.16 | .411 |
| Any ICH | 37.0% | 26.7% | 0.40 | 0.08–2.09 | .280 |
| Final mTICI 2b–3 | 75.0% | 93.3% | 10.88 | 0.96–123.84 | .054 |
| Final mTICI 2c, 3 | 30.8% | 80.0% | 5.33 | 0.97–29.38 | .055 |
| Final mTICI 3 | 21.2% | 46.7% | 2.77 | 0.59– 13.02 | .198 |
Adjusted for age, initial NIHSS score, initial ASPECTS, infarct topography (anterior versus posterior circulation), aspirin administration, and rescue intracranial stent placement.
FIG 3.
Distribution of mRS scores at 90 days according to the antiplatelet therapy type.
In the cangrelor group, 1 patient presented with intraventricular hemorrhage, considered sICH in our study. He initially presented with basilar artery occlusion secondary to underlying stenosis (initial posterior circulation-ASPECTS = 6), treated with stent placement and cangrelor infusion). Day 1 imaging revealed posterior fossa extensive infarction and intraventricular hemorrhage. The patient died at day 3 after stroke onset.
DISCUSSION
Despite growing reports in the literature, our study presents substantial results of cangrelor use in the setting of intracranial thrombectomy. We found that cangrelor is safe, with no increased risk of mortality or ICH whether symptomatic or not compared with GPIIb/IIIa inhibitors. Reperfusion rates were higher with cangrelor than with GPIIb/IIIa inhibitors, though this difference did not reach statistical significance.
With the expansion of thrombectomy indications, complex angiographic presentations remain improvement targets. In particular, more complementary treatments for failed intracranial recanalization are being proposed. There are 2 possible scenarios: distal fragmentation impairing reperfusion or proximal nonrecanalization. In the second scenario, thrombectomy failure is frequently related to underlying arterial wall disease, mostly atherosclerotic, albeit not exclusively.4-6 In this setting, aggressive management, including acute antiplatelet therapy and intracranial angioplasty or stent placement, has been reported.8-10,19,22-25 These pharmacologic and mechanical approaches are most often combined with promising results, considering the specific large-vessel occlusion stroke subtype. To date, the optimal pharmacologic adjunctive therapy for this recently described rescue approach for refractory intracranial occlusion is yet to be determined.
GPIIb/IIIa inhibitors are usually administered for refractory intracranial occlusion. In this setting, the reported ICH risk seems comparable with the observed rates in thrombectomy for embolic causes.8,10,11,19,20 The neurologic outcome, intracranial reperfusion, and midterm artery patency are also promising. However, in the setting of large-vessel occlusion stroke, antithrombotic management, especially if intracranial stent placement has been performed, always involves a compromise between hemorrhagic risk and arterial patency. In this sense, reports with GPIIb/IIIa inhibitors are encouraging. However, the specific features of cangrelor might be advantageous in cerebral ischemia.13,17 Its immediate antiplatelet effect and quicker restoration of platelet function after treatment interruption allow safer management in the context of ischemic stroke, as observed here. Indeed, sICH or emergent craniotomy is not unusual and requires rapid restoration of platelet function. This might be much easier with a rapidly active, reversible drug such as cangrelor than with a GPIIb/IIIa inhibitor. The pharmacodynamics of GPIIb/IIIa inhibitors differ substantially, with a more prolonged onset of action time (10–30 minutes) and a substantially longer duration of the antiplatelet effect (4–12 hours).16 Improving the pharmacologic characteristics of antiplatelet therapy without impairing the antithrombotic effect and increasing ICH risk are the ideal goals. Cangrelor has been previously reported in several case series, including acute-phase endovascular treatments (cervical or intracranial stent placement) with an encouraging hemorrhagic risk and arterial patency results.16,18
We found a trend toward a higher risk of sICH in the cangrelor group with univariate analysis (P = .058). However, in our population, ASA use was more frequent in the cangrelor group than in the GPIIb/IIIa inhibitor group. This more frequent ASA use in the cangrelor group might be related to the lack of confidence in its indications. ASA might have been a confounding factor. With the multivariate analysis, after adjusting for ASA use, the ICH risk remained nonsignificant. However, this may indicate that cangrelor should not be systematically associated with ASA in the acute phase. This hypothesis has to be explored with more extensive studies.
Our study revealed that cangrelor might have safety and efficacy profiles similar to those of GPIIb/IIIa inhibitors. Reperfusion rates, including proximal (arterial occlusive lesion score) and global (mTICI score) reperfusion evaluations, favored the cangrelor group, even if statistical significance was not reached. This trend also has to be confirmed.
Our study had several limitations. First, although we presented the largest population of refractory intracranial occlusions treated with rescue therapy and cangrelor, the study sample remains small. The lack of power of the statistical analysis probably explains the absence of a significant difference on multivariate analysis. Still, given the lack of studies on cangrelor for this indication, we believe that our data provide reassurance regarding the potential safety and efficacy of this pharmacologic management. Moreover, considering the specific subtype of large-vessel occlusion stroke, the lack of validated guidelines in its management, and the retrospective nature of our work, the endovascular procedure and perioperative management in our study were not completely standardized. Aggressive rescue management for refractory intracranial occlusions is still not widely applied by operators of the participating centers to date, partly explaining the small sample size. Biologic data regarding aspirin and the P2Y12 response were not available. The study period was also quite extended with potential evolutions in endovascular approaches with time. Our small study sample also reflects the recent introduction of cangrelor; therefore, our results are only preliminary. More extensive studies are necessary to evaluate the value of cangrelor in the treatment of refractory intracranial occlusions.
CONCLUSIONS
In this preliminary study, cangrelor use in the management of intracranial refractory occlusions was associated with encouraging safety and efficacy profiles in comparison with GPIIb/IIIa inhibitors. Due to the limited sample size, no significant influence on 90-day clinical outcome was observed. Given its ease of handling and favorable pharmacodynamics, cangrelor may be a promising alternative to other intravenous acute antiplatelet therapies. Larger studies are mandatory to determine the optimal acute antiplatelet therapy strategy for refractory intracranial large-vessel occlusion stroke.
Supplementary Material
ABBREVIATIONS:
- ASA
acetylsalicylic acid
- GPIIb/IIIa
glycoprotein IIb/IIIa
- ICH
intracranial hemorrhage
- mTICI
modified TICI
- sICH
symptomatic intracranial hemorrhage
Footnotes
Disclosures: Gaultier Marnat—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Medtronic, MicroVention. Bertrand Lapergue—UNRELATED: Grants/Grants Pending: MicroVention, Phenox.* Mikael Mazighi—UNRELATED: Consultancy: Air Liquide, Amgen, Boerhinger Ingelheim, Acticor Biotech; Grants/Grants Pending: RHU BOOSTER, Agence Nationale pour la Recherche, funding from French Ministry of Health.* Frédéric Clarençon—UNRELATED: Board Membership: ArteDrone; Consultancy: Medtronic; Payment for Lectures Including Service on Speakers Bureaus: Balt, Penumbra, Stryker. Sébastien Richard—UNRELATED: Grants/Grants Pending: French Ministry, Comments: grants for 2 academic studies*; Payment for Development of Educational Presentations: Boehringer Ingelheim. *Money paid to the institution.
References
- 1.Goyal M, Menon BK, Van Zwam WH, et al. HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Lapergue B, Blanc R, Gory B, et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large-vessel occlusion: the ASTER randomized clinical trial. JAMA 2017;318:443–52 10.1001/jama.2017.9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turk AS, Siddiqui A, Fifi JT, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large-vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet 2019;393:998–1008 10.1016/S0140-6736(19)30297-1 [DOI] [PubMed] [Google Scholar]
- 4.Yeo LLL, Bhogal P, Gopinathan A, et al. Why does mechanical thrombectomy in large-vessel occlusion sometimes fail?: a review of the literature. Clin Neuroradiol 2019;29:401–14 10.1007/s00062-019-00777-1 [DOI] [PubMed] [Google Scholar]
- 5.Li H, Zhang Y, Zhang L, et al. Endovascular treatment of acute ischemic stroke due to intracranial atherosclerotic large-vessel occlusion: a systematic review. Clin Neuroradiol 2020;30:777–87 10.1007/s00062-019-00839-4 [DOI] [PubMed] [Google Scholar]
- 6.Kim BM. Causes and solutions of endovascular treatment failure. J Stroke 2017;19:131–42 10.5853/jos.2017.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaesmacher J, Gralla J, Mosimann PJ, et al. Reasons for reperfusion failures in stent-retriever-based thrombectomy: registry analysis and proposal of a classification system. AJNR Am J Neuroradiol 2018;39:1848–53 10.3174/ajnr.A5759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Premat K, Dechartres A, Lenck S, et al. Rescue stenting versus medical care alone in refractory large-vessel occlusions: a systematic review and meta-analysis. Neuroradiology 2020;62:629–37 10.1007/s00234-020-02360-9 [DOI] [PubMed] [Google Scholar]
- 9.Baek JH, Kim BM, Kim DJ, et al. Stenting as a rescue treatment after failure of mechanical thrombectomy for anterior circulation large artery occlusion. Stroke 2016;47:2360–67 10.1161/STROKEAHA.116.014073 [DOI] [PubMed] [Google Scholar]
- 10.Chang Y, Kim BM, Bang OY, et al. Rescue stenting for failed mechanical thrombectomy in acute ischemic stroke a multicenter experience. Stroke 2018;49:958–64 10.1161/STROKEAHA.117.020072 [DOI] [PubMed] [Google Scholar]
- 11.Maingard J, Phan K, Lamanna A, et al. Rescue intracranial stenting after failed mechanical thrombectomy for acute ischemic stroke: a systematic review and meta-analysis. World Neurosurg 2019;132:e235–45 10.1016/j.wneu.2019.08.192 [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med 2013;368:1303–13 10.1056/NEJMoa1300815 [DOI] [PubMed] [Google Scholar]
- 13.Sible AM, Nawarskas JJ. Cangrelor: a new route for P2Y12 inhibition. Cardiol Rev 2017;25:133–39 10.1097/CRD.0000000000000142 [DOI] [PubMed] [Google Scholar]
- 14.Elhorany M, Lenck S, Degos V, et al. Cangrelor and stenting in acute ischemic stroke: monocentric case series. Clin Neuroradiol 2020May[Epub ahead of print] 10.1007/s00062-020-00907-0 [DOI] [PubMed] [Google Scholar]
- 15.Abdennour L, Sourour N, Drir M, et al. Preliminary experience with cangrelor for endovascular treatment of challenging intracranial aneurysms. Clin Neuroradiol 2020;30:453–61 10.1007/s00062-019-00811-2 [DOI] [PubMed] [Google Scholar]
- 16.Aguilar-Salinas P, Agnoletto GJ, Brasiliense LBC, et al. Safety and efficacy of cangrelor in acute stenting for the treatment of cerebrovascular pathology: preliminary experience in a single-center pilot study. J NeuroInterv Surg 2019;11:347–51 10.1136/neurintsurg-2018-014396 [DOI] [PubMed] [Google Scholar]
- 17.Godier A, Mesnil M, De Mesmay M, et al. Bridging antiplatelet therapy with cangrelor in patients with recent intracranial stenting undergoing invasive procedures: a prospective case series. Br J Anaesth 2019;123:e2–5 10.1016/j.bja.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 18.Cervo A, Ferrari F, Barchetti G, et al. Use of cangrelor in cervical and intracranial stenting for the treatment of acute ischemic stroke: a “real life” single-center experience. AJNR Am J Neuroradiol 2020;41:2094–99 10.3174/ajnr.A6785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng F, Wan J, Liu W, et al. Efficacy and safety of rescue stenting following failed mechanical thrombectomy for anterior circulation large-vessel occlusion: propensity score analysis. J Neurointerv Surg 2020;12:271–73 10.1136/neurintsurg-2019-015154 [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Wu Y, Gao X, et al. Intraarterial versus intravenous tirofiban as an adjunct to endovascular thrombectomy for acute ischemic stroke. Stroke 2020;51:2925–33 10.1161/STROKEAHA.120.029994 [DOI] [PubMed] [Google Scholar]
- 21.Ciccone A, Motto C, Abraha I, et al. Glycoprotein IIb-IIIa inhibitors for acute ischaemic stroke. Cochrane Database Syst Rev 2014;CD005208. 10.1002/14651858.CD005208.pub3] [DOI] [PubMed] [Google Scholar]
- 22.Park H, Baek JH, Kim BM. Endovascular treatment of acute stroke due to intracranial atherosclerotic stenosis–related large-vessel occlusion. Front Neurol 2019;10:308. 10.3389/fneur.2019.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek JH, Kim BM, Heo JH, et al. Outcomes of endovascular treatment for acute intracranial atherosclerosis-related large-vessel occlusion. Stroke 2018;49:2699–705 10.1161/STROKEAHA.118.022327 [DOI] [PubMed] [Google Scholar]
- 24.Zhang G, Ling Y, Zhu S, et al. Direct angioplasty for acute ischemic stroke due intracranial atherosclerotic stenosis-related large-vessel occlusion. Interv Neuroradiol 2020;26:602–06 10.1177/1591019920949674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsang ACO, Orru E, Klostranec JM, et al. Thrombectomy outcomes of intracranial atherosclerosis-related occlusions: a systematic review and meta-analysis. Stroke 2019;50:1460–66 10.1161/STROKEAHA.119.024889 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.