Heterogeneity of MTT in CTP after aneurysmal SAH correlates with patient outcomes. Because the findings are in line with the pathophysiologic concept of the capillary transit time heterogeneity, future studies should seek to verify the coefficient of variation for MTT as a potential imaging biomarker for outcome.
Abstract
BACKGROUND AND PURPOSE:
Impairment of tissue oxygenation caused by inhomogeneous microscopic blood flow distribution, the so-called capillary transit time heterogeneity, is thought to contribute to delayed cerebral ischemia after aneurysmal SAH but has so far not been systematically evaluated in patients. We hypothesized that heterogeneity of the MTT, derived from CTP parameters, would give insight into the clinical course of patients with aneurysmal SAH and may identify patients at risk of poor outcome.
MATERIALS AND METHODS:
We retrospectively analyzed the heterogeneity of the MTT using the coefficient of variation in CTP scans from 132 patients. A multivariable logistic regression model was used to model the dichotomized mRS outcome. Linear regression was used to eliminate variables with high linear dependence. T tests were used to compare the means of 2 groups. Furthermore, the time of the maximum coefficient of variation for MTT after bleeding was evaluated for correlation with the mRS after 6 months.
RESULTS:
On average, each patient underwent 5.3 CTP scans during his or her stay. Patients with high coefficient of variation for MTT presented more often with higher modified Fisher (P = .011) and World Federation of Neurosurgical Societies grades (P = .014). A high coefficient of variation for MTT at days 3–21 after aneurysmal SAH correlated significantly with a worse mRS score after 6 months (P = .016). We found no correlation between the time of the maximum coefficient of variation for MTT after bleeding and the patients' outcomes after 6 months (P = .203).
CONCLUSIONS:
Heterogeneity of MTT in CTP after aneurysmal SAH correlates with the patients' outcomes. Because the findings are in line with the pathophysiologic concept of the capillary transit time heterogeneity, future studies should seek to verify the coefficient of variation for MTT as a potential imaging biomarker for outcome.
From the 9 in 100,000 individuals with an aneurysmal SAH (aSAH) per year, up to 40% die within 1 month despite improved intensive care and current treatment strategies.1-4 Subsequent physical impairment, cognitive impairment, and secondary long-term psychosocial deterioration mean that most survivors cannot return to their former work life.5,6
The etiology of the detrimental long-term changes after SAH remains poorly understood. In terms of temporal progression, 2 distinct harmful phases of pathophysiologic changes can be distinguished. The term “early brain injury” describes initial pathophysiologic changes within the first 3 days, whereas “delayed cerebral ischemia” (DCI) represents a complex of reactions that occur later during the course of the disease.7
During DCI, various pathophysiologic reactions and mechanisms result in cerebral ischemia and neuronal energy depletion. DCI likely involves microvascular dysfunction; disturbances in cerebral microcirculation; angiographic vasospasms; thrombosis of cerebral, primarily cortical, vessels; cortical spreading depolarization and ischemia; as well as inflammatory reactions.7-10 Finally, DCI may lead to cerebral infarction and, therefore, irreversible loss of function.
In general, the important factors for brain tissue survival are adequate oxygenation and glucose supply, among others. Historically, it was assumed that oxygenation mainly relies on the CBF, and previously macroscopic, angiographically visible vasospasms were assumed to be the main driver of DCI. However, it turned out that for this simplified assumption, the blood flow in capillaries must be identical throughout the whole capillary bed, which is not the case. In addition to the CBF, the microvascular blood distribution across capillaries, also known as the capillary transit time heterogeneity (CTH), was found to be the a crucial factor for the oxygenation of brain tissue.11,12 Recently, Østergaard et al11 presumed that the CTH also contributes to DCI-related ischemia. According to the group's model, an increased CBF can lead to better tissue oxygenation as long as the CTH has not crossed a critical threshold. If the threshold is exceeded, any further increase in CBF will lead to a reduced oxygen extraction efficacy in brain tissue, due to capillary shunting in hyperemic areas, resulting in hypoxic brain tissue.12 Østergaard et al defined this state as malignant CTH.11,12 In such a case, adequate oxygenation of the brain tissue may be achieved only by reducing the CBF and thereby slowing the flow of erythrocytes through the shunting capillaries. This mechanism could be an explanation for the occurrence of vasospasm in patients with aSAH.11
CTH has been mainly evaluated in simulations, rodent models, and humans predominately with ischemic stroke or neurodegenerative diseases, such as Alzheimer disease. In the patient population, evaluation has so far mainly relied on MR imaging. Moreover, Østergaard et al11 recently established the relative transit time heterogeneity (RTH) as the ratio of CTH to MTT.13 In doing so, the inherent dependency of CTH on the MTT was removed, making the RTH an improved indicator of the capillary transit heterogeneity.13 Furthermore, Østergaard et al have based the calculation of CTH and RTH on elaborated Bayesian approaches, and CTH evaluation is not readily available in clinical software for perfusion evaluation. Hence, to date, there is no routinely available radiologic readout for the CTH or RTH in the clinical setting of aSAH.
So far, the concept of capillary transit time heterogeneity has not been systemically evaluated in patients with aSAH. We hypothesized that the higher heterogeneity of MTT derived from CTP parameters will allow us to gain insight into the clinical course of patients with aSAH and to predict a worse outcome. In the present study, we, therefore, retrospectively analyzed the heterogeneity of MTT in CTP scans obtained within 3 weeks after SAH and assessed a potential association with the initial neurologic status on admission and clinical outcome after 6 months.
MATERIALS AND METHODS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments. For this type of study formal consent is not required. The study was approved by the local ethics committee (study ID: 5760R). Data will be made available on reasonable request. We prepared the manuscript according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Inclusion Criteria
We retrospectively included all patients with SAH admitted to our hospital between January 2008 and December 2015 who met the following inclusion criteria: 1) SAH present on initial nonenhanced CT, 2) admission within 24 hours after ictus, 3) subsequent CTP scans with at least 2 CTP scans in addition to the initial CTP imaging after aneurysm occlusion, and 4) documented clinical follow-up examination 6 months after discharge to assess the functional outcome. Patients were excluded from the study on the basis of following criteria: 1) nonaneurysmal SAHs, 2) unknown onset of aSAH, 3) patients with unevaluable CTP (eg, due to severe motion), or 4) patients with a history of cranial neurosurgical or endovascular intervention.
SAH Management
We managed patients according to the applicable aSAH guidelines.14 SAH was graded according to the modified Fisher scale based on initial noncontrast CT imaging.15 In all patients, systolic arterial blood pressure was adjusted between 100 and 140 mm Hg, and the partial pressure of carbon dioxide (pCO2) was maintained in the range of 35 to 40 mm Hg for all intubated patients. External ventricular drainage (EVD) was established in intubated patients and patients with poor World Federation of Neurosurgical Societies (WFNS) grades (Glasgow Coma Scale ≤12 according to our internal guidelines), as well as patients with exacerbated intracranial pressure after initial imaging (CT + CTA) and before subsequent angiography and aneurysm treatment. If a patient showed secondary-progressive deterioration of awareness before or after aneurysm treatment, even leading to insufficient neurologic assessability (Glasgow Coma Scale ≤12 according to our internal guidelines), an increase in intracranial pressure was suspected after exclusion of other causes and an EVD was placed. General care followed a standardized protocol, including surgical or endovascular occlusion of the aneurysm within 24 hours after admission. A CTP scan was performed routinely 6 hours after aneurysm treatment and on days 4, 7, and 10 after bleeding or in case of clinical suspicion of DCI (defined as previously described16). CTP was not performed only when CT was not possible due to critical situations such as a cardiopulmonary instability of a patient or if there was a treatment-resistant absolute contraindication for the administration of the contrast agent (allergy, high-grade renal insufficiency).
Perfusion CT Analysis
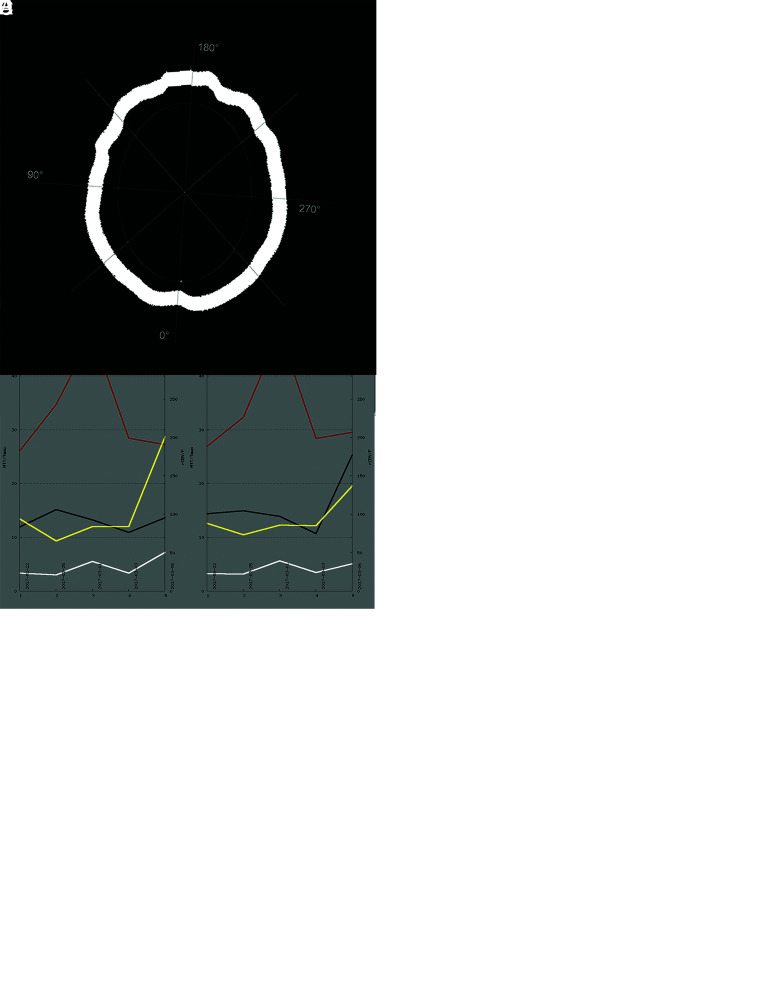
CTP data were acquired with a multisection CT scanner (Somatom Volume Zoom, Definition Flash or AS; Siemens) (80 kV, 120 mAs, 2 adjacent slices, 10-mm section thickness, 1 image/s for 35 seconds).17-19 Three seconds after starting the CT, contrast-enhancing medium (30 mL, 400 mg iodine/mL) followed by a saline chaser (30 mL) was injected with a flow rate of 5 mL/s. Intravenous access of ≤18 gauge in a cubital vein or a high-flow central venous catheter was required for contrast administration. Slices were positioned at the level of the central parts of lateral ventricles, parallel to a plane through the orbital floor and the external auditory meatus, thereby sampling the anterior, middle, and posterior cerebral artery territories as well as the anterior and posterior borderzones.20 Calculation of the parameter images (MTT, CBF, CBV, and time-to-maximum [Tmax]) was performed using STROKETOOL-CT (Digital Image Solutions), which processes data using singular value decomposition.21 We used the software Angiotux CT 2D (ECCET 2006, Beck A. Aurich V., http://www.eccet.de/) for standardized parameter value extraction from the different cortical brain regions.18 A 10-mm-wide band was automatically delineated along the cortex, omitting outer CSF spaces, the rostral falx cerebri, and the superior sagittal sinus. The automated definition of the ROI was reviewed and potential deviations were corrected as needed. A running average spanning 10° of the ROI was computed in 2° steps for each perfusion parameter, yielding 180 measurements per parameter per CTP scan (Fig 1). We assessed the heterogeneity of cerebral perfusion by the coefficient of variation (cv), also called the relative SD, of the MTT (cvMTT) for each individual scan. The cv is a measure of the dispersion of a probability distribution independent of the mean of the examined variable:
FIG 1.
Exemplary illustration of CTP imaging in patients with SAH. A, A series of axial CTP scans at the level of the cella media of the lateral ventricles, parallel to the orbitomeatal plane, thereby representing 6 supratentorial vessel territories. B, Calculated perfusion parameter maps in clockwise order from top left: MTT, Tmax, and semi-quantitative relative CBF (rCBF) as well as CBV (rCBV). C, Depiction of the ROI automatically defined along the cortex. Readout starts in the territory of the right posterior cerebral artery and is carried out in a clockwise manner using a sliding-window approach (mean across 10° in 2° steps). D, Display of 180 measurements across 360° (red, MTT; black, Tmax; yellow, rCBF; white, rCBV). E, Mean perfusion parameters over 5 CTP scans for the left hemisphere on the left and the right hemisphere on the right side. Note the marked pathologic elevation of MTT (red) in the third scan.
The statistical analysis of this study included all late CTP scans gathered in the nonacute phase of a patient, thus all CTP scans between days 3 and 21 postbleeding. To further evaluate the impact of very large heterogeneity, we determined the point in time of the maximum cvMTT across all CTP scans from an individual patient during the entire clinical course, including the CTP scans in the first 3 days after ictus.
Definition of Grading/Outcome Measures
We dichotomized the initial neurologic condition using the WFNS grading system:22 A WFNS score of I–III represents a good neurologic grade on admission, whereas a WFNS score of IV–V is considered a poor grade. The functional outcome was assessed using the mRS 6 months after ictus.23 An mRS score of 0–2 was considered a favorable outcome, and a score of 3–6, an unfavorable outcome. The Fisher score was used without dichotomization.24,25
Statistical Analysis
We analyzed presenting variables for correlation with cvMTT as well as Rankin outcomes. A multivariable logistic regression model would be used to model the dichotomized patient outcome according to the mRS (good versus poor). In a first step, to avoid multicollinearities in predictor variables, we used pair-wise linear regression among numeric variables to eliminate variables with high linear dependence. The remaining predictors, including sex (binary indicator, male or female), age (numeric), WFNS score (undichotomized, treated as a continuous numeric variable), Fisher score (undichotomized, treated as continuous numeric variable), and the logarithm of the cvMTT (numeric), were later used in the logistic regression. We used the logarithm of cvMTT because cvMTT is, by definition, always positive and thus non-normally distributed. Logistic regression, by definition, assumes log-odds to linearly depend on predictor variables. This condition is automatically satisfied when the 2 outcome groups are normally distributed with the same variance. In our case, the variances of cvMTT between the 2 groups, mRS ≤ 2 and mRS ≥ 3, differed strongly (0.0044 versus 0.0072). Using the logarithm achieved a more similar distribution (0.11 and 0.16), thus leading to more reliable predictions. For the outcome variable, we dichotomized patient mRS scores to 2 values: 0, representing mRS ≤ 2, and 1 representing mRS ≥ 3. We applied both forward and backward selection of predictor variables. The fitted logistic regression model was used to assess the effect of predictor variables on the patient outcome. To examine the difference in the means of a numeric variable between the 2 groups, we used the Student t test, and to compare multiple groups, ANOVA.
RESULTS
Patients
From the initial 476 patients with SAH, a total of 344 were excluded due to various factors, including those with a nonaneurysmal SAH, <2 follow-up CTP scans being performed, the onset of SAH being unknown, aneurysm occlusion not performed within 24 hours after SAH, mRS after 6 months not recorded, or the patient already having a history of cranial neurosurgical intervention. One hundred thirty-two patients were included in the analysis. The mean age was 54 (SD, 11) years, and 69% were women (Table 1).
Table 1:
Descriptive statistics
| All Patients | mRS <3 | mRS ≥3 | |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Age | |||
| Younger than 50 yr | 41 (31.06) | 34 (42.5) | 7 (13.5) |
| 50 Years or older | 91 (68.94) | 46 (57.5) | 45 (86.5) |
| Sex | |||
| Female | 91 (68.9) | 57 (71.2) | 34 (65.4) |
| Male | 41 (31.1) | 23 (28.8) | 18 (34.6) |
| WFNS grade | |||
| I–III | 64 (48.48) | 50 (62.5) | 14 (26.9) |
| IV–V | 68 (51.52) | 30 (37.5) | 38 (73.1) |
| Fisher grade | |||
| 0–2 | 23 (17.42) | 20 (25) | 3 (5.8) |
| 3–4 | 109 (82.58) | 60 (75) | 49 (92.2) |
| Aneurysm location | |||
| ACA | 1 (0.76) | 0 (0) | 1 (1.9) |
| AcomA | 53 (40.15) | 31 (38.8) | 22 (42.3) |
| BA | 13 (9.85) | 8 (10) | 5 (9.6) |
| ICA | 6 (4.55) | 3 (3.75) | 3 (5.8) |
| MCA | 21 (15.91) | 12 (15) | 9 (17.3) |
| PcaA | 4 (3.03) | 1 (1.25) | 3 (5.8) |
| PcomA | 18 (13.64) | 15 (18.8) | 3 (5.8) |
| PICA | 9 (6.82) | 5 (6.25) | 4 (7.7) |
| SCA | 2 (1.52) | 2 (2.5) | 0 (0) |
| VA | 3 (2.27) | 1 (1.25) | 2 (3.8) |
| Other | 2 (1.52) | 2 (2.5) | 0 (0) |
Note:—ACA indicates anterior cerebral artery; AcomA, anterior communicating artery; BA, basilar artery; PcaA, pericallosal artery; PcomA, posterior communicating artery; VA, vertebral artery; SCA, superior cerebellar artery.
On admission, 64 patients (48%) were of good neurologic grade (WFNS IIII), and 68 patients (52%) presented with a poor grade (WFNS IV–V). Twenty-three patients (17%) had minor SAH (modified Fisher grade 1–2), and 109 (83%) had severe SAH (modified Fisher grade 3–4) (Table 1). Eighty-seven patients (66%) received an EVD during their treatment, and 45 patients (34%) did not need an EVD implantation. Sixty-six patients (50%) received endovascular aneurysm treatment, 60 patients (45%) were treated surgically, 2 patients (2%) received neither treatment, and 4 patients (3%) received both endovascular and surgical treatment. On average, patients were discharged at day 22 (SD), and each patient included in this study underwent 5.3 (SD, 2.1) CTP scans (Table 2).
Table 2:
Treatment modalities and outcome after 6 months
| No. (%) | |
|---|---|
| Extraventricular drainage | |
| Yes | 87 (65.9) |
| No | 45 (34.1) |
| Endovascular treatment | 66 (50) |
| Surgical treatment | 60 (45.5) |
| Combined/no treatment | 6 (4.5) |
| mRS 6 mo | |
| 0–2 | 80 (60.6) |
| 3–5 | 27 (20.5) |
| 6 | 25 (18.9) |
Six months after ictus, 25 patients (19%) had died (mRS 6), a further 27 patients (20%) had an unfavorable outcome (mRS 3–5), and 80 patients (61%) were in a favorable clinical condition (mRS 0–2) (Table 2).
CTP Data
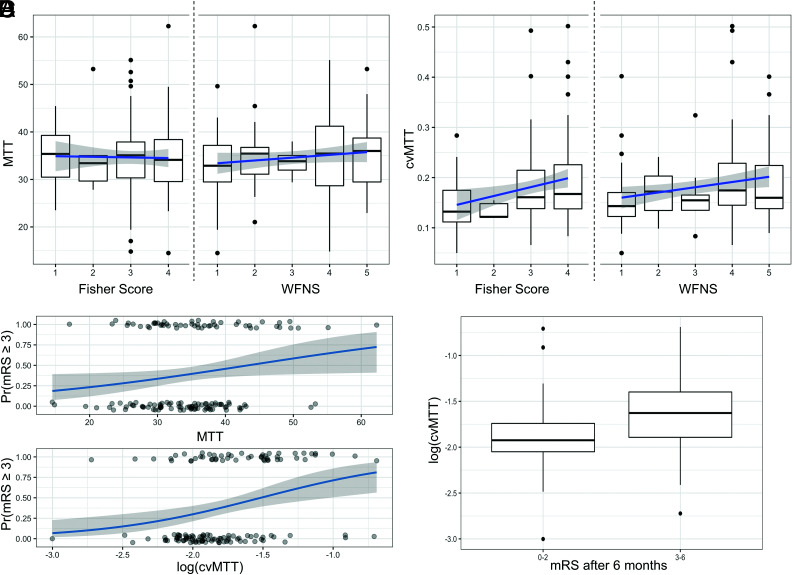
The late perfusion data showed that the MTT was 3.5 (SD, 0.79) seconds. The MTT did not correlate with either the initial WFNS grade (β = 0.59; 95% CI, −0.26−1.43; P = .18) or the Fisher grade (β = −0.13; 94% CI, −1.53−1.27; P = .86) (Fig 2A). Besides age (β = 0.077; 95% CI, 0.043−0.111; P ≤ .001) and Fisher grade (β = 0.98; 95% CI, 0.61−1.35; P ≤ .001), the dichotomized mRS after 6 months also positively correlated with the MTT (logistic regression: OR =1.05; 95% CI, 1.004−1.103; P = .034) (Fig 2C, upper graph).
FIG 2.
Correlation of MTT and the cvMTT with the dichotomized outcome after 6 months. A, Mean MTT distribution of the Fisher grade (left) and the WFNS grades (right). The MTT did not correlate with the initial WFNS grade (P = .18) or Fisher grade (P = .86) at admission. B, cvMTT distribution for the Fisher grade (left) and the WFNS grades (right). The cvMTT correlates significantly with the WFNS (P = .014) and the Fisher grade (P = .011) at admission. C, Upper graph: MTT as a predictor of the dichotomized mRS after 6 months (logistic regression: OR =1.05, 95% CI, 1.004–1.103; P = .034); lower graph: cvMTT as a predictor of the dichotomized mRS after 6 months (logistic regression: OR = 5.09; 95% CI, 1.46–17.77; P = .011). D, cvMTT for favorable and unfavorable outcomes (mRS after 6 months ≤2 and ≥3, respectively). Pr(mRS ≥ 3) indicates probability (mRS >= 3).
CTP Heterogeneity Correlates with 6-Month mRS
The cvMTT correlated significantly with the WFNS (β = 0.010; 95% CI, 0.002−0.019; P = .014) and the Fisher grade (β = 0.018; 95% CI, 0.004−0.031; P = .011) at admission (Fig 2B). The cvMTT significantly correlated with the mRS after 6 months (logistic regression: OR =5.09; 95% CI, 1.46−17.77; P = .011) (Fig 2C, lower graph). A higher cvMTT was associated with a worse mRS after 6 months (Fig 2D). Further statistical tests revealed a positive association of the cvMTT with the presence of an EVD (t test; 95% CI, −0.054−0.007; P = .012). cvMTT also remained a significant predictor for the outcome when only patients with an EVD were considered (logistic regression: OR = 7.15; 95% CI, 1.51−33.9; P = .013). No significant correlation was found between the aneurysm location and the cvMTT (ANOVA, F10,121 = 1.315, P = .23).
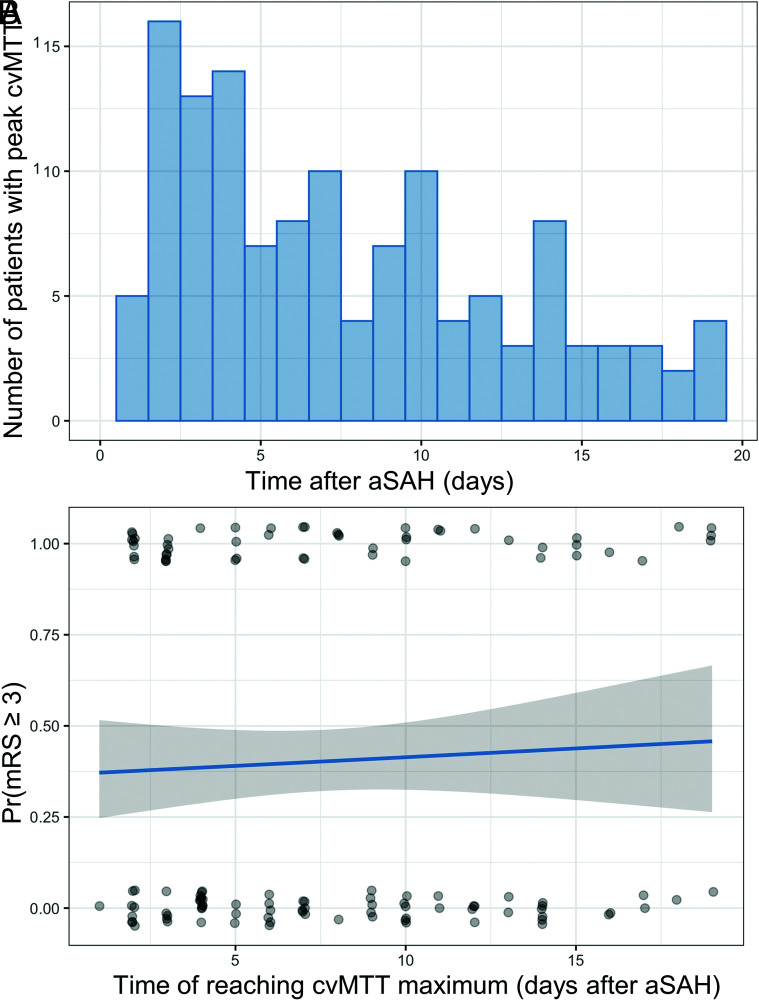
CTP Heterogeneity Peak
The peak of the MTT heterogeneity occurred most frequently at 7.3 (SD, 5.1) days after bleeding (Fig 3A). We found no significant correlation between the maximum cvMTT after bleeding (cvMTT-peak) and the dichotomized patient outcomes after 6 months (logistic regression: OR = 1.03; 95% CI, 0.55–1.93; P = .4) (Fig 3B). There was no correlation between the outcome after 6 months and the number of CTP scans obtained in an individual patient (OR =1.01; 95% CI, 0.85–1.20; P = .89).
FIG 3.
The time of cvMTT-peak does not correlate with the outcome after 6 months. A, Histogram shows the number of patients with the cvMTT-peak with respect to the days after SAH. B, No significant correlation between the time of reaching the cvMTT-peak and the dichotomized patient outcomes after 6 months could be observed (logistic regression: OR =1.03; 95% CI, 0.55–1.93; P = .4). Pr(mRS ≥ 3) indicates probability (mRS >= 3).
DISCUSSION
This retrospective study in patients with SAH has 3 main findings:
The heterogeneity of MTT, as defined by cvMTT in late CTP imaging, correlates significantly with the initial WFNS or Fisher grade
The heterogeneity of MTT, as defined by cvMTT in late CTP imaging, correlates significantly with the dichotomized outcome after 6 months
The point in time of the MTT heterogeneity peak does not correlate with the outcome after 6 months.
CTP is an essential diagnostic tool in the detection of hypoperfused areas of the brain in acute stroke and is gaining increasing relevance in aSAH management as a diagnostic tool for delayed cerebral ischemia.18,26,27 A prolonged MTT and TTP in an early CTP after aSAH is known to correlate with the initial neurologic status and clinical outcome.17,19,26-28 Furthermore, these might serve together with additional imaging markers, such as a large intracerebral blood clot in the early noncontrast cranial CT scan, as a surrogate for DCI and as a predictive feature of neurologic outcome.19 While the aforementioned studies evaluated early CTP scans, with various definitions of “early” ranging from ≤6 hours to ≤72 hours after ictus, our study focused on late CTP scans in the phase when a patient is more susceptible to DCI (>72 hours after ictus).17,19,27,28
Increased CTH is considered an important feature of DCI.7,11,12 Elevated CTH during DCI results in a decreased net oxygen supply to cerebral tissue and, therefore, in tissue hypoxia, resulting in a poor clinical outcome.11
Recently, the RTH was established for MR imaging in stroke, representing the CTH without the inherent dependency of CTH on MTT.13 In our opinion, this further developed parameter is even better for displaying changes in the microvascular blood flow than the CTH itself, and we followed the approach by determining the heterogeneity of the MTT using the coefficient of variation, resembling the approach of the previously discussed RTH. Mathematically, the cvMTT represents the dispersion of MTT values around the brain cortex of individual patients. Falsification of the normal SD by extraordinarily high or low MTT values is thereby diminished, further underlining the potential use of the cvMTT as an imaging biomarker. While previously discussed,11,29 the current study is the first to systematically assess CTP heterogeneity in patients with SAH. While Østergaard et al11 applied an elaborated Bayesian statistical model to derive CTH and RTH, our approach is based on the widely clinically available singular value decomposition.
From the various values measured in CTP imaging, the MTT has been established as the surrogate parameter for changes in the microvasculature perfusion. Furthermore, the MTT has the greatest practical relevance in the context of SAH because the indication for an endovascular intervention (spasmolysis) or, in general, intensified DCI-protective therapy is often based on MTT values.19,30,31
Overall, in our opinion, the MTT of CTP imaging is the most likely correlation to detect changes in the microvascular blood flow in the clinical setting of aSAH. As previously mentioned, we did not simply use the SD of MTT as a correlate of CTH but went 1 step further. To exclude the influence of MTT on the measured heterogeneity, we calculated the cvMTT. The coefficient of variation, also known as the relative SD, depicts the heterogeneity of MTT independent of the absolute numeric value of MTT.
The main result of this study is the correlation between the heterogeneity of MTT, as measured by the cvMTT, and the outcome of the patient. We have shown that a higher cvMTT, ie, a more nonuniform cerebral capillary blood flow, correlates with an unfavorable mRS after 6 months. We can set this result in the context of the numerous previous works by Østergaard et al.11 As previously mentioned, the group hypothesized that the presence of malignant CTH results in decreased oxygenation of the brain, resulting in a worse outcome.12,29 This hypothesis fits well with patients with an increased cvMTT in this study also more likely having a poor outcome. It is conceivable that the increased cvMTT is a surrogate of decreased oxygenation of the brain, resulting in brain tissue damage and thus leading to a worse outcome.
Most interesting, this study also reveals a correlation between the cvMTT and the WFNS and the Fisher grades. Like the cvMTT, both of these parameters are related to the outcome after SAH. The Fisher score, in particular, predicts “symptomatic vasospasms.” Therefore, it is understandable that there is a mutual relationship, though it is unclear whether and which physiopathologic mechanisms are the basis for this. Yet, even the combination of both WFNS and Fisher score is not sufficient to predict the outcome very well, 32 in contrast to the cvMTT.
A further result of this study is the correlation of the cvMTT with the presence of an EVD. Taking into consideration that the patients with an EVD are predominantly those with a worse initial WFNS and/or Fisher grade associated with a more severe clinical course and worse outcome, a possible reason for this correlation becomes appreciable. We consider it unlikely that the EVD itself influences the cvMTT. At the scanning level, the EVD component may cross the imaging plane, though the ribbonlike ROI generally does not include the EVD; therefore, an artifact due to the EVD is almost impossible. For the patients without an EVD, a minimal Glasgow Coma Scale of 13 and normal wakefulness were required. Therefore, in these patients, a physiologic intracranial pressure was assumed. In patients with secondary-progressive deterioration of awareness, even leading to insufficient neurologic assessability (Glasgow Coma Scale ≤12 according to our internal guidelines), an increase of intracranial pressure was suspected and an EVD was subsequently placed. For patients with an EVD, the intracranial pressure was adjusted to a physiologic level of <18 mm Hg by cerebrospinal fluid drainage. As a result, a significant effect of intracranial pressure on our results seems unlikely. The finding that cvMTT also remained a significant predictor for the outcome when only patients with an EVD were considered supports the assumption.
Caspers et al33 demonstrated, in 2017, that the point in time of maximum mean MTT after aSAH is of importance for the outcome of a patient. An earlier peak of mean MTT was associated with an unfavorable outcome. We, therefore, examined a possible connection between the point in time of the greatest MTT heterogeneity (cvMTT-peak) and the outcome. In contrast to the earlier findings, however, no evidence for a correlation was detected for cvMTT.
Our study has several limitations:
Data were retrospectively collected, and to obtain a study group with a comparable timeline of therapeutic and diagnostic procedures in the pathophysiologic sequence occurring after SAH,7 very strict inclusion and exclusion criteria were necessary, which meant that many patients could not be included in this study. Nevertheless, the study population is relatively large for a single-center study and is overall well-balanced, for example, regarding patients with good- and poor-grade SAH according to their WFNS score. We believe it is unlikely that our inclusion/exclusion criteria introduced a relevant bias. Larger prospective, preferably multicentric, and more inclusive future studies would need to be conducted to validate our results.
Some of the results might be affected by alteration of the intracranial pressure (ICP). However, the MTT is primarily a correlate for microvascular perfusion, whereas Tmax (as a modification of the TTP) is thought to best reflect the intracranial pressure—ie, studies hinting at a Tmax decrease after EVD placement or decompressive hemicraniectomy.34,35 Therefore, we consider a decisive influence of the ICP on our results unlikely. Additionally, at the time of imaging, the ICP was controlled and maintained at physiologic values by means of an EVD in most patients. Patients without an EVD in place had a Glasgow Coma Scale score of at least 13 and were, therefore, likely to have a physiologic ICP. The influence of ICP should, therefore, be limited.
We correlated heterogeneity of cerebral perfusion with the midterm outcome 6 months after discharge.
Other factors such as comorbidities, intracerebral hemorrhage, secondary brain edema, or seizures might additionally influence the outcome. These potential confounders were not taken into account in the present analysis. Furthermore, even larger studies are required to confirm the heterogeneity of the MTT in CTP scans after aSAH as an independent risk factor for poor outcome.
This is one of the first studies addressing the value of cortical perfusion heterogeneity in CTP imaging. Standard values for normal brain tissue have, therefore, not yet been established. Due to the high variation in the CTP protocols and postprocessing, our results may not be translatable one-to-one to other setups.
The influence of confounding factors on the maximum heterogeneity of MTT, such as diagnosed or undiagnosed diseases affecting the microvasculature such as diabetes, atherosclerosis, or hypertension, are unknown. However, this study is the first to investigate the heterogeneity of MTT as a predictor of outcome in SAH. Future prospective studies should, therefore, conduct subgroup analyses with a greater number of patients.
CONCLUSIONS
In the present cohort of 132 patients with aSAH, heterogeneity of the late cerebral perfusion (cvMTT) correlated with the initial WFNS and Fisher grades. The cvMTT has prognostic value because it correlated significantly with the dichotomized outcome after 6 months. A high degree of heterogeneity was associated with an unfavorable outcome, making the cvMTT an attractive candidate as a robust imaging biomarker for further studies.
ABBREVIATIONS:
- aSAH
aneurysmal SAH
- CTH
capillary transit time heterogeneity
- cvMTT
coefficient of variation for MTT
- cvMTT-peak
maximum cvMTT after bleeding
- DCI
delayed cerebral ischemia
- EVD
external ventricular drainage
- ICP
intracranial pressure
- RTH
relative transit time heterogeneity
- Tmax
time-to-maximum
- WFNS
World Federation of Neurosurgical Societies
References
- 1.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;369:306–18 10.1016/S0140-6736(07)60153-6 [DOI] [PubMed] [Google Scholar]
- 2.de Rooij NK, Rinkel GJ, Dankbaar JW, et al. Delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review of clinical, laboratory, and radiological predictors. Stroke 2013;44:43–54 10.1161/STROKEAHA.112.674291 [DOI] [PubMed] [Google Scholar]
- 3.Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology 2010;74:1494–1501 10.1212/WNL.0b013e3181dd42b3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamp MA, Lieshout JHV, Dibue-Adjei M, et al. A systematic and meta-analysis of mortality in experimental mouse models analyzing delayed cerebral ischemia after subarachnoid hemorrhage. Transl Stroke Res 2017;8:206–19 10.1007/s12975-016-0513-3 [DOI] [PubMed] [Google Scholar]
- 5.Kreiter KT, Copeland D, Bernardini GL, et al. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke 2002;33:200–08 10.1161/hs0102.101080 [DOI] [PubMed] [Google Scholar]
- 6.Hutter BO, Kreitschmann-Andermahr I, Mayfrank L, et al. Functional outcome after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl 1999;72:157–74 10.1007/978-3-7091-6377-1_13 [DOI] [PubMed] [Google Scholar]
- 7.van Lieshout JH, Dibue-Adjei M, Cornelius JF, et al. An introduction to the pathophysiology of aneurysmal subarachnoid hemorrhage. Neurosurg Rev 2017;41:917–30 10.1007/s10143-017-0827-y [DOI] [PubMed] [Google Scholar]
- 8.Friedrich B, Muller F, Feiler S, et al. Experimental subarachnoid hemorrhage causes early and long-lasting microarterial constriction and microthrombosis: an in-vivo microscopy study. J Cereb Blood Flow Metab 2012;32:447–55 10.1038/jcbfm.2011.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 2011;17:439–47 10.1038/nm.2333 [DOI] [PubMed] [Google Scholar]
- 10.Schneider UC, Davids AM, Brandenburg S, et al. Microglia inflict delayed brain injury after subarachnoid hemorrhage. Acta Neuropathol 2015;130:215–31 10.1007/s00401-015-1440-1 [DOI] [PubMed] [Google Scholar]
- 11.Østergaard L, Aamand R, Karabegovic S, et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2013;33:1825–37 10.1038/jcbfm.2013.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jespersen SN, Ostergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab 2012;32:264–77 10.1038/jcbfm.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engedal TS, Hjort N, Hougaard KD, et al. Transit time homogenization in ischemic stroke: a novel biomarker of penumbral microvascular failure? J Cereb Blood Flow Metab 2018;38:2006–20 10.1177/0271678X17721666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012;43:1711–37 10.1161/STR.0b013e3182587839 [DOI] [PubMed] [Google Scholar]
- 15.Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery 2006;59:21–27; discussion 21–27 10.1227/01.NEU.0000218821.34014.1B [DOI] [PubMed] [Google Scholar]
- 16.Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010;41:2391–95 10.1161/STROKEAHA.110.589275 [DOI] [PubMed] [Google Scholar]
- 17.Kamp MA, Heiroth HJ, Beseoglu K, et al. Early CT perfusion measurement after aneurysmal subarachnoid hemorrhage: a screening method to predict outcome? Acta Neurochir Suppl 2012;114:329–32 10.1007/978-3-7091-0956-4_63 [DOI] [PubMed] [Google Scholar]
- 18.Turowski B, Haenggi D, Wittsack J, et al. Cerebral perfusion computerized tomography in vasospasm after subarachnoid hemorrhage: diagnostic value of MTT [in German]. Rofo: 2007;179:847–54 10.1055/s-2007-963197 [DOI] [PubMed] [Google Scholar]
- 19.Etminan N, Beseoglu K, Heiroth HJ, et al. Early perfusion computerized tomography imaging as a radiographic surrogate for delayed cerebral ischemia and functional outcome after subarachnoid hemorrhage. Stroke 2013;44:1260–66 10.1161/STROKEAHA.111.675975 [DOI] [PubMed] [Google Scholar]
- 20.Turowski B, Haenggi D, Wittsack HJ, et al. Computerized analysis of brain perfusion parameter images [in German]. Rofo 2007;179:525–29 10.1055/s-2007-962853 [DOI] [PubMed] [Google Scholar]
- 21.Wittsack HJ, Wohlschlager AM, Ritzl EK, et al. CT-perfusion imaging of the human brain: advanced deconvolution analysis using circulant singular value decomposition. Comput Med Imaging Graph 2008;32:67–77 10.1016/j.compmedimag.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 22.Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 1988;51:1457. 10.1136/jnnp.51.11.1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rankin J. Cerebral vascular accidents in patients over the age of 60. Scott Med J 1957;2:200–15 10.1177/003693305700200504 [DOI] [PubMed] [Google Scholar]
- 24.Claassen J, Bernardini GL, Kreiter K, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke 2001;32:2012–20 10.1161/hs0901.095677 [DOI] [PubMed] [Google Scholar]
- 25.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980;6:1–9 10.1227/00006123-198001000-00001 [DOI] [PubMed] [Google Scholar]
- 26.Mathys C, Martens D, Reichelt DC, et al. Long-term impact of perfusion CT data after subarachnoid hemorrhage. Neuroradiology 2013;55:1323–31 10.1007/s00234-013-1278-y [DOI] [PubMed] [Google Scholar]
- 27.Beseoglu K, Etminan N, Hanggi D. The value of perfusion computed tomography (PCT) imaging after aneurysmal subarachnoid hemorrhage: a review of the current data. Acta Neurochir Suppl 2015;120:35–38 10.1007/978-3-319-04981-6_6 [DOI] [PubMed] [Google Scholar]
- 28.Murphy A, Manoel AL, Burgers K, et al. Early CT perfusion changes and blood-brain barrier permeability after aneurysmal subarachnoid hemorrhage. Neuroradiology 2015;57:767–73 10.1007/s00234-015-1529-1 [DOI] [PubMed] [Google Scholar]
- 29.Anzabi M, Angleys H, Aamand R, et al. Capillary flow disturbances after experimental subarachnoid hemorrhage: a contributor to delayed cerebral ischemia? Microcirculation 2019;26:e12516. 10.1111/micc.12516 [DOI] [PubMed] [Google Scholar]
- 30.Wintermark M, Ko NU, Smith WS, et al. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. AJNR Am J Neuroradiol 2006;27:26–34 [PMC free article] [PubMed] [Google Scholar]
- 31.Mir DI, Gupta A, Dunning A, et al. CT perfusion for detection of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2014;35:866–71 10.3174/ajnr.A3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen DS, Macdonald RL. Subarachnoid hemorrhage grading scales: a systematic review. NCC 2005;2:110–18 10.1385/NCC:2:2:110 [DOI] [PubMed] [Google Scholar]
- 33.Caspers J, Rubbert C, Turowski B, et al. Timing of mean transit time maximization is associated with neurological outcome after subarachnoid hemorrhage. Clin Neuroradiol 2017;27:15–22 10.1007/s00062-015-0399-6 [DOI] [PubMed] [Google Scholar]
- 34.Fragata I, Alves M, Papoila AL, et al. Temporal evolution of cerebral computed tomography perfusion after acute subarachnoid hemorrhage: a prospective cohort study. Acta Radiol 2020;61:376–85 10.1177/0284185119858701 [DOI] [PubMed] [Google Scholar]
- 35.Philipp JS, Marcel AK, Thomas B, et al. The influence of decompressive craniectomy for major stroke on early cerebral perfusion. J Neurosurg 2015;123:59–64 10.3171/2014.12.JNS141250 [DOI] [PubMed] [Google Scholar]