Abstract
D-Allose (D-All), C-3 epimer of D-glucose, is a rare sugar known to suppress reactive oxygen species generation and prevent hypertension. We previously reported that D-allulose, a structural isomer of D-All, prolongs the lifespan of the nematode Caenorhabditis elegans. Thus, D-All was predicted to affect longevity. In this study, we provide the first empirical evidence that D-All extends the lifespan of C. elegans. Lifespan assays revealed that a lifespan extension was induced by 28 mM D-All. In particular, a lifespan extension of 23.8 % was achieved (p < 0.0001). We further revealed that the effects of D-All on lifespan were dependent on the insulin gene daf-16 and the longevity gene sir-2.1, indicating a distinct mechanism from those of other hexoses, such as D-allulose, with previously reported antiaging effects.
Keywords: anti-aging, daf-16, sir-2.1, Caenorhabditis elegans, D-allose, lifespan
Abbreviations
AMP, Adenosine 5´-monophosphate; NAD, nicotinamide adenine dinucleotide; NGM, nematode growth medium; ROS, reactive oxygen species.
D-Allose (D-All), a structural isomer of D-allulose, is a rare sugar that is present in a limited quantity in nature. 1) 2) D-All has been detected at low levels in human cord blood 3) and in Indian seaweed. 4) However, it is challenging to study allose owing to its low abundance. D-All could be produced by the Izumoring strategy, which is a systematic method for the production of all monosaccharide isomers using microbial enzymes. 1) Mass production methods developed by Izumori have provided insight into biological properties. The safety of D-All has been established in rats 5) and in clinical studies, and its utilizable energy value is approximately zero. 6) Studies have demonstrated various functions of D-All, such as anti-tumor activity, 7) anti-hypertension effects, 8) and brain protection from ischemic injury, 9) and these functions are mainly due to the suppression of reactive oxygen species (ROS) generation by competing for D-glucose utilization. 10) 11) Based on the beneficial health effects, D-All is expected to be a potent anti-metabolic syndrome drug and a pharmaceutical precursor.
Caenorhabditis elegans is a powerful animal model for lifespan assays and studies of the mechanisms underlying aging owing to its short lifespan and well-defined genetic pathways. 12) Moreover, most longevity genes and signaling pathways are evolutionarily conserved from nematodes to mammals. 13) A Forkhead box O transcription factor, the primary downstream target of the insulin/IGF-1 signaling pathway, is encoded by daf-16 in C. elegans. It modulates lifespan, metabolism, dauer formation, and stress resistance. 14) Sir-2.1 encodes an NAD +-dependent histone deacetylase (Sirtuin) that regulates lifespan by modulating the transcription of genes involved in the stress response. 15)
We have reported that a low dose of D-allulose, a structural isomer of D-All, prolongs the lifespan of nematodes. 16) The anti-aging effect of D-allulose may be attributed to its ability to mimic the structure of metabolizable D-sugars, thereby modulating carbohydrate metabolism to extend the life span. Therefore, we hypothesized that D-All is also a functional sugar with an effect on longevity, similar to D-allulose. However, the impact of D-All on longevity has not been demonstrated.
We determined the concentration of D-All that did not influence nematode growth and demonstrated its antiaging activity. In addition, to determine the mechanisms underlying the D-All-induced lifespan extension, we evaluated mutant strains deficient for well-established longevity pathway-related genes , daf-16, and sir-2.1.
EXPERIMENTAL
D-All was prepared enzymatically at the International Institute for Rare Sugar Research and Education, Kagawa University. The purity of D-All was higher than 98 %. C. elegans N2 (wild-type), daf-16 two allelic mutant (mgDf50 and mu86), and, sir-2.1 mutant (ok434) strains were obtained from the Caenorhabditis Genetic Center (CGC) at the University of Minnesota. Escherichia coli OP50 was used as a food source for the nematodes.
C. elegans was maintained at 20 °C on nematode growth medium (NGM) 17) with E. coli OP50. As a departure from previous studies, peptone-free NGM medium was used. Eggs of C. elegans were collected by treating egg-bearing adults with alkaline hypochlorite solution and were shaken in S basal medium at 20 °C for 20–24 h to prepare synchronized first-stage larvae (L1). 17)
Lifespan assays were performed by conventional methods. 16) One hundred synchronized animals (10 animals/dish) were placed in 2-mL S liquid medium dishes containing D-All with E. coli OP50. Control worms were incubated in medium without sugars. 5-Fluoro-2′-deoxyuridine was added (40 µM, final concentration) to prevent progeny growth. In each assay, 10 dishes (10 animals/dish) were used. The dishes were incubated without shaking at 20 °C. The numbers of live and dead animals were counted every other day under a microscope based on their movement, and the survivors were transferred to the new medium. The survival curves were determined using the Kaplan–Meier method, and survival differences were tested for significance using the log-rank test. Data are expressed as means ± standard error (SE).
D-All was used at a concentration of 28 mM (0.5 %) for the lifespan assay, based on a previous study; 16) this was also in the range of concentrations added to feed in the mixed feed administration method for rodents. 5) Furthermore, we previously showed that 168 mM (3 %) D-All has a weak effect on nematode growth, 18) and our preliminary data showed that 0–56 mM (0–1 %) D-All does not affect nematode growth (data not shown); accordingly, 28 mM is sufficiently low concerning growth inhibition.
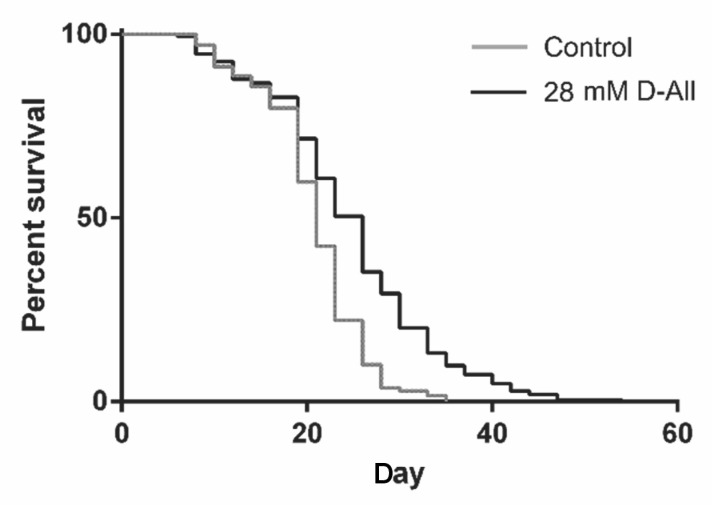
We found that the mean lifespan was significantly extended (23.8 %) in the presence of 28 mM D-All as compared to the control ( p < 0.0001) ( Fig. 1, Table 1). Under almost identical conditions on an OP50 diet for nematodes, the lifetime extension rates of D-allulose were 7.0 and 12.0 % at a sugar concentration of 25 and 10 mM, respectively. Therefore, we can infer that D-All may exert stronger anti-aging activity than D-allulose.
Fig. 1. Survival curves of wild type C. elegans N2 cultured with D-All.
Table 1.
Summary and statistical analysis of C. elegans lifespan assays.
| Strain | Sugar | Mean lifespan ± SE (days) |
Extension (%) |
Number of worms |
p-value ( vs. None) |
|---|---|---|---|---|---|
| N2 | None | 20.6±0.6 | 239 | ||
| D-All | 25.5±0.7 | +23.8 | 212 | < 0.0001 | |
| daf-16( mgDf50) | None | 16.8±0.5 | 101 | ||
| D-All | 17.5±0.4 | +4.2 | 103 | 0.3424, NS | |
| daf-16 (mu86) | None | 14.2±0.6 | 101 | ||
| D-All | 13.9±0.9 | -2.1 | 100 | 0.3626, NS | |
| sir-2.1( ok434) | None | 19.9±0.8 | 108 | ||
| D-All | 19.0±0.4 | -4.5 | 104 | 0.7138, NS |
The p-value ( vs. control) was calculated by the log-rank test. NS, not significant.
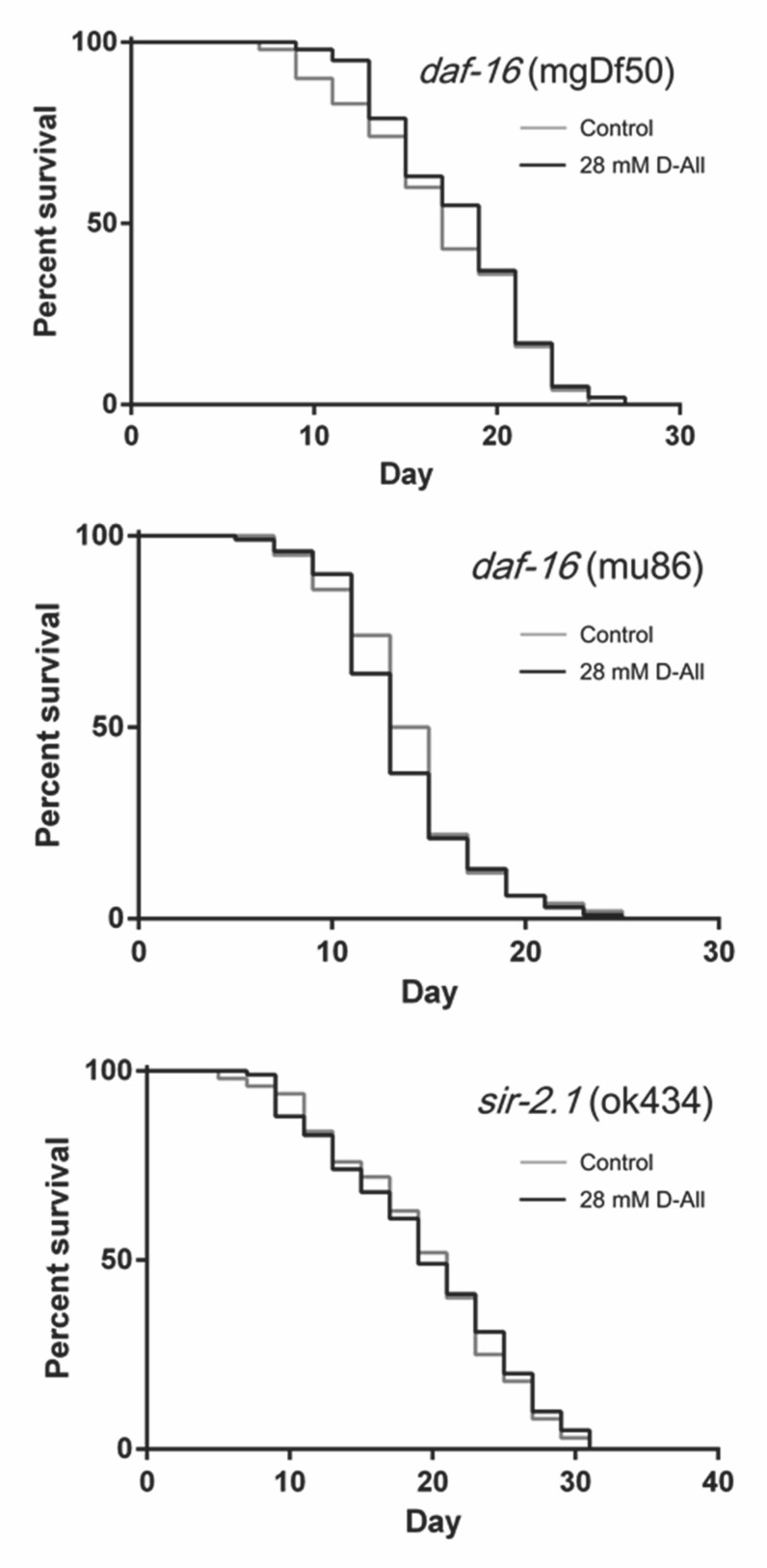
Several molecules involved in longevity, such as DAF-16 and SIR-2.1, have been identified in C. elegans. 15) 16) To determine whether the extension of lifespan by D-All is mediated by pathways involving these molecules, we used daf-16 and sir-2.1 mutants for lifespan assays using D-All. The lifespans of both daf-16 and sir-2.1 mutants did not increase in the presence of D-All ( Fig. 2, Table 1). These results indicated that the D-All-induced lifespan extension was dependent on both DAF-16 and SIR-2.1.
Fig. 2. Survival curves of C. elegans longevity gene mutants cultured with D-All.
D-Allulose and D-glucosamine, that are D-glucose analog as same as D-All, have been reported to extend the lifespan of nematodes not via sirtuin and insulin-dependent signaling pathways but nutrient signaling. Similar to D-glucosamine, 19) D-allulose enters cells via glucose transporters and inhibits glycolysis, inducing the metabolism of fat and mitochondrial respiration via AMP-activated protein kinase (AMPK). Increased breathing can induce the temporary upregulation of ROS, leading to increased anti-oxidative enzyme activity and survival rates. 16) 20) Orally administrated D-All also reduces body weight in rodents, 5) which might affect carbohydrate or lipid metabolism. However, the mechanism underlying the antiaging or antimetabolic effect of D-All is not precise, and the role of the nutrient sensor AMPK should be evaluated in the future. Taken together, the anti-aging effect of D-All may be similar to those of D-allulose and D-glucosamine, with differences in the underlying mechanisms and precise outcomes.
In conclusion, we revealed that D-All extends the nematode lifespan and that this effect is dependent on the insulin gene daf-16 and the longevity gene sir-2.1. Further studies are needed to confirm whether D-All extends the lifespan under other experimental conditions, for practical antiaging applications.
CONFLICTS OF INTEREST
Tomoya Shintani is an employee of Matsutani Chemical Industry Co., Ltd.
ACKNOWLEDGMENTS
The strains of C. elegans and E. coli were provided by the Caenorhabditis Genetic Center in the University of Minnesota. We thank the late Dr. Kazuhiro Okuma (Matsutani Chemical Industry Co., Ltd., Hyogo, Japan) for valuable advice. This research was partially supported by JSPS KAKENHI Grant number 25450156. This research was also partly supported by the Sasakawa Scientific Research Grant from The Japan Science Society (to T.S.).
REFERENCES
- 1).Izumori K.: Izumoring: a strategy for bioproduction of all hexoses. J. Biotechnol., 124, 717–722 (2006). [DOI] [PubMed] [Google Scholar]
- 2).Mooradian A.D., Smith M., and Tokuda M.: The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin. Nutr. ESPEN, 18, 1–8 (2017). [DOI] [PubMed] [Google Scholar]
- 3).Hashimoto F., Nishiumi S., Miyake O., Takeichi H., Chitose M., Ohtsubo H., Ishimori S., Ninchoji T., Hashimura Y., Kaito H., Morisada N., Morioka I., Fukuoka H., Yoshida M., and Iijima K.: Metabolomics analysis of umbilical cord blood clarifies changes in saccharides associated with delivery method. Early Hum. Dev., 89, 315–320 (2013). [DOI] [PubMed] [Google Scholar]
- 4).Ragupathi R.K.R., Arumugam R., and Anantharaman P.: Chemical composition and antibacterial activity of Indian seagrasses against urinary tract pathogens. Food Chem., 135, 2470–2473 (2012). [DOI] [PubMed] [Google Scholar]
- 5).Iga Y., Nakamichi K., Shirai Y., and Matsuo T.: Acute and sub-chronic toxicity of D-allose in rats. Biosci Biotechnol Biochem., 74, 1476–1478 (2010). [DOI] [PubMed] [Google Scholar]
- 6).Kitagawa M., Tanaka M., Yoshikawa Y., Iida T., and Kishimoto Y.: Evaluation of Absorption and Fermentability of D-Mannose, D-Sorbose, and D-Allose in humans. Luminacoids Res., 22, 75–82 (2018). [Google Scholar]
- 7).Kimura S., Zhang G.X., Nishiyama A., Nagai Y., Nakagawa T., Miyanaka H., Fujisawa Y., Miyatake A., Nagai T., Tokuda M., and Abe Y.: D-allose, an all-cis aldo-hexose, suppresses development of salt-induced hypertension in Dahl rats. J. Hypertens., 23, 1887–1894 (2005). [DOI] [PubMed] [Google Scholar]
- 8).Jia J.J., Geng W.S., Wang Z.Q., Chen L., and Zeng X.S.: The role of thioredoxin system in cancer: strategy for cancer therapy. Cancer Chemother Pharmacol., 84, 453–470 (2019). [DOI] [PubMed] [Google Scholar]
- 9).Shinohara N., Nakamura T., Abe Y., Hifumi T., Kawakita K., Shinomiya A., Tamiya T., Tokuda M., Keep R.F., Yamamoto T., and Kuroda Y.: D-Allose attenuates overexpression of inflammatory cytokines after cerebral ischemia/reperfusion injury in gerbil. J. Stroke Cerebrovasc. Dis., 25, 2184–2188 (2016). [DOI] [PubMed] [Google Scholar]
- 10).Ishihara Y., Katayama K., Sakabe M., Kitamura M., Aizawa M., Takara M., and Itoh K.: Antioxidant properties of rare sugar D-allose: Effects on mitochondrial reactive oxygen species production in Neuro2A cells. J. Biosci. Bioeng., 112, 638–642 (2011). [DOI] [PubMed] [Google Scholar]
- 11).Chen Z., Chen J., Zhang W., Zhang T., Guang C., and Mu W.: Recent research on the physiological functions, applications, and biotechnological production of D-allose. Appl. Microbiol. Biotechnol., 102, 4269–4278 (2018). [DOI] [PubMed] [Google Scholar]
- 12).Lapierre L.R., and Hansen M.: Lessons from C. elegans: Signaling pathways for longevity. Trends Endocrinol. Metab., 23, 637–644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Fontana L., Partridge L., and Longo V.D.: Extending healthy life span—From yeast to humans. Science., 328, 321–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Kenyon C., Chang J., Gensch E., Rudner A., and Tabtiang R.: AC. elegans mutant that lives twice as long as wild type. Nature., 366, 461–464 (1993). [DOI] [PubMed] [Google Scholar]
- 15).Tissenbaum H.A., and Guarente L.: Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature., 410, 227–230 (2001). [DOI] [PubMed] [Google Scholar]
- 16).Shintani T., Sakoguchi H., Yoshihara A., Izumori K., and Sato M.: D-Allulose, a stereoisomer of D-fructose, extends Caenorhabditis elegans lifespan through a dietary restriction mechanism: a new candidate dietary restriction mimetic. Biochem. Biophys. Res. Commun., 493, 1528–1533 (2017). [DOI] [PubMed] [Google Scholar]
- 17).Lewis J.A., and Fleming J.T.: Basic culture methods. Methods Cell Biol., 48, 3–29 (1995). [PubMed] [Google Scholar]
- 18).Sakoguchi H., Yoshihara A., Izumori K., and Sato M.: Screening of biologically active monosaccharides: growth inhibitory effects of D-allose, D-talose, and L-idose against the nematode Caenorhabditis elegans. Biosci Biotechnol Biochem., 80, 1058–1061 (2016). [DOI] [PubMed] [Google Scholar]
- 19).Shintani T., Kosuge Y., and Ashida H.: Glucosamine extends the lifespan of Caenorhabditis elegans via autophagy induction glucosamine extends nematode lifespan via autophagy induction. J. Appl. Glycosci., 65, 37–43 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Shintani H., Shintani T., Ashida H., and Sato M.: Calorie restriction mimetics: upstream-type compounds for modulating glucose metabolism. Nutrients., 10, 1821–1821 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]