Abstract
Purpose
The aim of this randomized clinical trial was to assess whether chemical cleansing using a sulfonic/sulfuric acid gel solution (HBX) as an adjunct to scaling and root planing (SRP) resulted in a decrease in residual plaque and calculus in deep periodontal pockets compared to SRP alone.
Methods
Fifty-six patients with 56 hopeless posterior teeth, scheduled for extraction due to severe periodontitis, were enrolled in this study. Each tooth was randomly assigned to 1 of the 2 experimental procedures. The test teeth were subjected to the irrigation of the subgingival area with HBX for 2 minutes, followed by SRP with hand and ultrasonic instruments for 14 minutes, and then extracted. The control teeth received only mechanical instrumentation before extraction. Residual biofilm was evaluated on photographs and measured as total area and percentage of root surface covered by remaining plaque (RP) or calculus (RC) after treatment.
Results
The initial pocket depth (PD) and total subgingival root surface area were similar between the 2 treatment groups. After treatment, the total subgingival root area covered by RP and RC was statistically significantly larger (P<0.001) in the control group than in the test group. The test teeth showed a lower percentage of RP, but a higher percentage of RC than the control teeth (both P<0.001). Complete calculus removal was achieved in 42% of the control teeth surfaces and in 25% of the test teeth surfaces for a PD of 4 mm.
Conclusions
The additional chemical cleansing with HBX resulted in a statistically significant improvement in bacterial plaque removal during SRP of deep pockets, but it was not effective in reducing calculus deposits.
Keywords: Decontamination, Dental calculus, Periodontitis, Root planing, Sulfates
Graphical Abstract
INTRODUCTION
The accumulation of bacterial plaque, structured as a biofilm, at and below the gingival margin is mandatory for periodontitis to start and progress [1]. Effective biofilm removal and/or control are therefore fundamental in the prevention and treatment of periodontitis. On this basis, scaling and root planing (SRP), performed with ultrasonic or manual instruments, is considered the cornerstone of periodontal treatment [2,3,4]. Although SRP has yielded favorable clinical outcomes when combined with efficient self-performed supragingival plaque control, it may result in both inadequate calculus removal and pocket depth (PD) reduction, mainly in areas of limited access [5]. Deep periodontal defects, furcation lesions, and root irregularities are most likely to exhibit residual calculus following subgingival instrumentation [6,7]. Although dental calculus is not considered to be a primary cause of periodontitis, it seems to play a role in the course of the disease by providing an ideal surface for plaque accumulation [8,9]. Calculus may also amplify the detrimental effects of bacterial plaque by keeping the bacterial deposits in close contact with the tissue interface [10]. A site harboring calculus is more likely to display ongoing inflammation and attachment loss [11,12].
Rabbani et al. [13] demonstrated that a single episode of SRP seldom allowed the complete removal of subgingival calculus and that the percentage of residual calculus increased with increasing PD and size of the scaled root surface. As the pocket deepens, more irregularities are usually observed on the tooth surface and the apical part of the pocket narrows, thus impairing the accessibility and efficacy of SRP. In diseased sites deeper than 5 mm, complete calculus removal was achieved only 11% of the time [14].
In view of these considerations, it is reasonable to hypothesize that additional chemical cleansing that would facilitate the removal of plaque and calculus from the root surface could improve the outcome of periodontitis treatment. A chemical desiccant containing sulfated phenols in a water-based gel solution has been recently introduced and proven effective in root canal decontamination and safe on oral mucosal tissues [15,16,17]. Unlike standard cleansing agents with antimicrobial activity, it dehydrates and dislodges the biofilm extracellular matrix and desiccates the bacteria that live in it [18]. Previous investigations demonstrated the clinical and microbiological efficacy of this desiccant as an adjunct to SRP in treating moderate and severe periodontitis, being safe and harmless for the surrounding healthy periodontal tissues [19,20,21,22]. However, no data are yet available on its capability in vivo to facilitate calculus removal from periodontally diseased root surfaces. Therefore, the aim of this study was to assess whether chemical cleansing using a sulfonic/sulfuric acid gel solution as an adjunct to SRP resulted in a decrease in residual plaque and calculus (RPC) in deep periodontal pockets compared to SRP alone.
MATERIALS AND METHODS
Experimental design
This study was a parallel, randomized, single-center, clinical trial designed to compare 2 different non-surgical periodontal treatment modalities in deep pockets: SRP using hand and ultrasonic instruments with chemical cleansing (test) versus SRP using hand and ultrasonic instruments alone (control). Study subjects were consecutively recruited from a pool of patients referred to the Section of Periodontology, University of Turin (Italy) between November 2018 and May 2019 for the treatment of periodontitis. Ethical approval to participate was granted by the Ethical Committee of AOU Città della Salute e della Scienza of Turin (approval Nr. 0008908) and written informed consent was obtained from participants. The investigation was performed according to the ethical principles of the Helsinki Declaration and the results were reported according to the CONSORT guidelines.
Patients were recruited if they were diagnosed as having stage III or IV periodontitis [23] and presented at least 1 posterior tooth with a hopeless periodontal prognosis (loss of periodontal support involving at least two-thirds of the total root length and/or mobility of grade III) and at least an interproximal site with PD ≥6 mm. Lactating or pregnant women and patients suffering from systemic diseases or conditions contraindicating tooth extraction or who had received SRP at the study teeth within the last 6 months were excluded. Third molars were also excluded.
Randomization
Each patient had a hopeless tooth. When the patient presented with more than one tooth requiring extraction, the tooth to be included was randomly selected. The treatment regimen was randomly assigned using a computer-generated random list with patients numbered according to the order in which they were enrolled. To conceal allocation, the corresponding forms were put into an opaque envelope with the patient number on the outside. A clinician not involved in the treatment delivery (F.C.) opened the envelope immediately after local anesthesia administration and applied the chemical desiccant on the root surface of the test teeth. SRP and tooth extraction were performed by a second clinician (F.Z.) blinded to the group assignment.
Experimental procedure
Clinical measurements were recorded at 6 sites per experimental tooth prior to the treatment using a 1-mm periodontal probe (PUNC 15, Hu Friedy, Chicago, IL, USA). The highest reading of PD, gingival recession and clinical attachment level (CAL) was recorded. The degree of furcation involvement was recorded using a Nabers Probe (Hu Friedy, Chicago, IL, USA). After local anesthesia, the teeth in the test group were subjected to irrigation of the subgingival area for 2 minutes with a chemical desiccant (HybenX©, EPIEN Medical, Inc., St. Paul, MN, USA) and thoroughly rinsed with sterile saline to remove all the gel. Mechanical debridement was then carried out by means of ultrasonic instrumentation (Cavitron, DENTSPLY, York, PA, USA) for 7 minutes followed by 7 minutes of manual SRP (Gracey Curettes Mini-Five, Hu-Friedy, Chicago, IL, USA). Teeth in the control group received only the mechanical instrumentation (7 minutes of ultrasonic and 7 minutes of manual instrumentation). Before extraction, the position of the gingival margin was marked with a notch (N), using a round diamond bur 1.4 mm in diameter (Komet Dental Italia, Italy, Milano) on a 1:5 red ring contra-angle handpiece (Kavo Italia, Italy, Sesto San Giovanni) under continuous irrigation with tap water.
Measurements
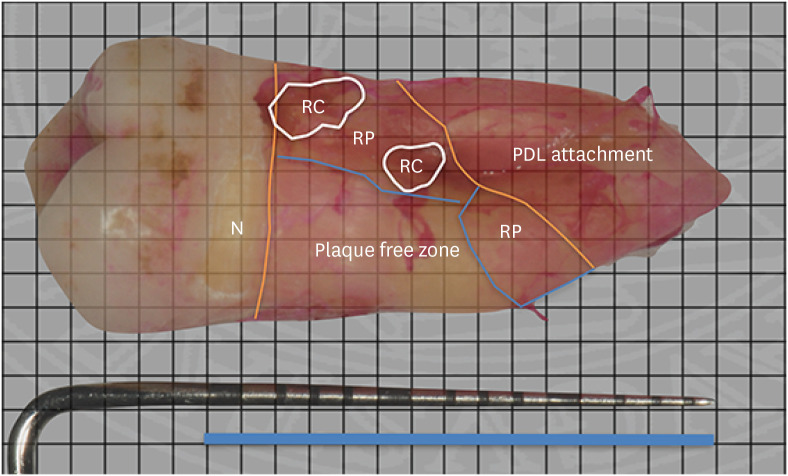
The extracted teeth were immediately rinsed with running water to remove any blood and soft tissue tags. The teeth were transferred to an erythrosine solution for 2 minutes to stain RPC on the root surface, rinsed with running water and stored in individual containers marked with the patient number. Digital images of each tooth were acquired using a digital camera (Nikon D3200, Tokyo, Japan) equipped with a 105-mm macro lens. For each tooth, 4 images of each side were taken along with a graduated periodontal probe, the millimeter markings of which were used as reference points. A blinded examiner (F.F.) measured the extent of RPC using a 1-mm square grid. The total subgingival root surface area that contained residual plaque (RP) and residual calculus (RC) was calculated by counting the boxes spread on the root surface (Figure 1). The total area of RPC was calculated as the sum of RP and RC. The total subgingival root surface was measured using the same method from N to the connective tissue attachment. The percentage of root surface area with RP and RC was then calculated. Only square boxes covering more than 50% of the area were considered positive in the calculations. Finally, the distance between the most coronal plaque and calculus identified on the root surface and N was defined as the cleaning depth, which represented the linear depth to which sub-gingival surfaces were completely clean after treatment. All root surface calculations were repeated 2 times in a random order, and the corresponding mean values were recorded.
Figure 1. Measurements of RC, RP and total subgingival root area in maxillary first molar. Total subgingival root area is calculated as the area between orange lines, RC circled with white line, and RP enclosed in blue lines.
N: notch, RC: residual calculus, RP: residual plaque, PDL: periodontal ligament.
Statistical analysis
The primary outcome of this study was the percentage of subgingival root surface area covered by RP and RC after treatment. Based on the data from a previous study [13], a sample size of 56 teeth (28 teeth in each group) was sufficient to detect a minimum difference of 0.5% between test and control treatment procedures using the 2-tailed t-test with a power of 80% and alpha error of 0.05. Mean values, standard deviations, and cumulative frequencies were calculated as descriptive statistics.
The statistical analysis was performed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). Quantitative data were checked for normality using the Shapiro-Wilk test and for equality of variances using the Levene test. Differences in quantitative parameters were assessed using parametric (the unpaired t-test) and non-parametric tests (Mann-Whitney U-test) as appropriate. Differences in qualitative parameters were analyzed with the chi-square test. A P value <0.05 was considered to indicate statistical significance.
RESULTS
Fifty-six patients with 56 teeth (28 in the test and 28 in the control group) were treated with or without adjunctive delivery of a chemical desiccant. Digital images were captured for all teeth. As each tooth was divided into four surfaces, a total of 224 surfaces were considered in the data analysis. No adverse effects or complications were reported for either treatment modality during the healing phase.
Of the 56 treated teeth, 36 were maxillary teeth, 20 were mandibular teeth, and 47 were multi-rooted teeth. As summarized in Table 1, there were no statistically significant differences between the 2 groups in terms of tooth type and location, the presence of developmental sulci, and furcation involvement.
Table 1. Distribution and anatomic characteristics of test and control teeth.
| Parameters | Experimental group | P value | |||
|---|---|---|---|---|---|
| Test | Control | ||||
| Tooth level (n=56) | |||||
| Tooth location | 1.000 | ||||
| Maxilla | 18 (64.3) | 18 (64.3) | |||
| Mandible | 10 (35.7) | 10 (35.7) | |||
| Tooth type | 0.716 | ||||
| Premolars | 5 (17.9) | 4 (14.3) | |||
| Molars | 23 (82.1) | 24 (85.7) | |||
| Tooth surface level (n=224) | |||||
| Multi-rooted teeth | 104 (92.9) | 100 (89.3) | 0.340 | ||
| Furcation involvement | 77 (68.8) | 71 (63.4) | 0.370 | ||
| Development sulci | 9 (8.0) | 6 (5.4) | 0.430 | ||
Data are reported as numbers (%).
Table 2 reports data on RPC after treatment. While the initial mean PD and the total subgingival root surface area were similar between the 2 groups (P=0.304 and P=0.197, respectively), the total subgingival root area covered by RP and RC was significantly larger in the control group than in the test group (both P<0.001). In the control group it amounted to 15.96±13.64 mm2 for plaque and 10.90±7.69 mm2 for calculus, while in the test group it was 5.17±6.69 mm2 and 6.67±8.72 mm2, respectively. When comparing the test and control teeth regarding the percentage of root surface harboring either RP or RC, there was a significantly lower percentage of RP, but a higher percentage of RC, at the test versus control sites (both P<0.001). Furthermore, a lower percentage of RC and RP combined was detected on the test (19.53%±18.15%) versus control teeth (40.99%±23.32%, P<0.001).
Table 2. Residual plaque and calculus on subgingival root surfaces of test and control teeth after treatment.
| Parameters | Experimental group | P value | |
|---|---|---|---|
| Test | Control | ||
| Probing depth (mm) | 8.04±2.31 | 8.34±2.71 | 0.369 |
| Cleaning depth (mm) | 2.67±2.69 | 4.41±2.96 | <0.001 |
| Total subgingival root surface area (mm2) | 55.86±24.22 | 60.13±25.09 | 0.197 |
| Residual plaque area (mm2) | 5.17±6.69 | 15.96±13.64 | <0.001 |
| Residual calculus area (mm2) | 6.67±8.72 | 10.90±7.69 | <0.001 |
| Percent of plaque area (%) | 10.63±12.79 | 17.32±10.68 | <0.001 |
| Percent of calculus area (%) | 46.90±33.76 | 23.65±18.27 | <0.001 |
| Percent of plaque and calculus area (%) | 19.53±18.15 | 40.99±233.32 | <0.001 |
Data are reported as mean ± standard deviation.
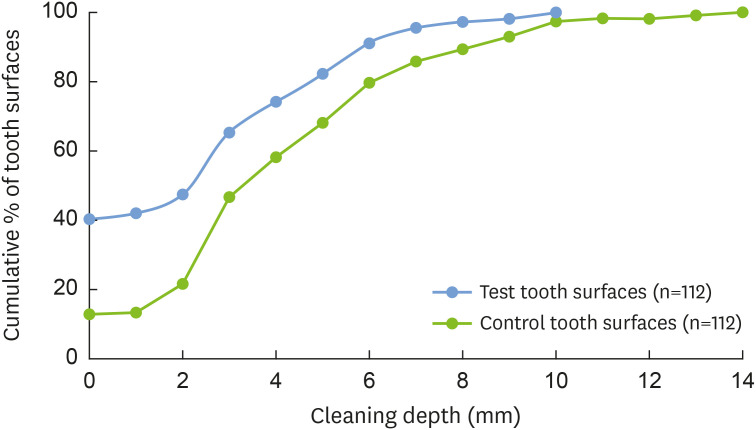
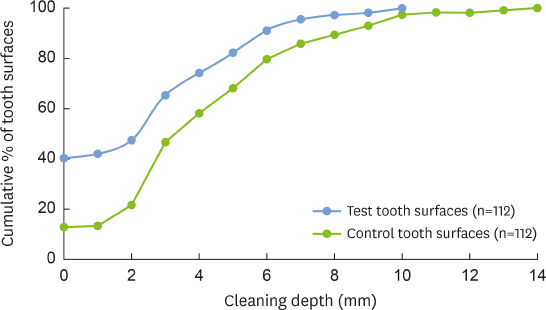
The mean cleaning depth was significantly higher (P<0.001) for the control teeth (4.41±2.96 mm) than the test teeth (2.67±2.70 mm). An analysis of the cumulative percentage distributions of tooth surfaces revealed that 74.1% and 58.0% of the test and control teeth, respectively, achieved a cleaning depth ≤4 mm, having no plaque and calculus in the first 4 mm apical to N (Figure 2).
Figure 2. Cumulative percentage distribution showing the persistence of plaque and calculus along the root surface at different cleaning depths in test and control tooth surfaces.
DISCUSSION
This randomized clinical trial aimed to assess the additional benefits of a chemical desiccant with subgingival ultrasonic and manual instrumentation of deep periodontal pockets in terms of reduction in RPC. To the best of our knowledge, no previous in vivo studies have measured these treatment outcomes. Although residual calculus in the absence of bacterial plaque can be compatible with gingival health [14], subgingival calculus has the potential to increase the detrimental effect of plaque, acting as a plaque-retaining factor either supragingivally or subgingivally [8,9,10]. It is well known that RPC can perpetuate periodontal infection [24] and are predictive factors for further loss of periodontal support over time [25,26]. Therefore, the removal of calculus with overlying plaque is fundamental in non-surgical periodontal treatment [5]. However, SRP has proven to be less effective in calculus removal with increasing PD [13]. Apart from PD, operator skill and reduced access have been associated with higher amounts of residual calculus [27,28].
To overcome the limitations of SRP, recent studies have proposed the local application of substances with anti-biofilm properties that destroy the biofilm so that bacteria cannot survive [19]. On this basis, we applied in periodontal pockets a new chemical desiccant (HybenX©) that is supposed to reach macroscopic niches inaccessible for proper decontamination, enhancing biofilm removal from root and calculus surfaces [20,21,22]. We selected only posterior teeth with deep pocket sites and, for the most part, multi-rooted teeth, which have more complex anatomy and thus may benefit more of the additional application of such gel solution. The results of the present study showed a statistically significant reduction of the root surface area covered by RPC in the test group. However, the total percentage of residual calculus was significantly greater in the test group than in the control group with high inter-individual variance at the subject and tooth level (46.90%±23.8% vs. 23.7%±18.3%). Most of the residual calculus was present in the furcation area, cemento-enamel junction, and developmental sulci on the root surfaces. These findings are in agreement with those of Jones et al. [29] and Matia et al. [30] reporting an increased prevalence of residual deposits in the irregularities of the root surface. The mean percentage of remaining calculus was in line with that reported in the literature ranging from 12% to 35% [13,29,31]. In PD between 3-5 mm, the chance of obtaining complete calculus removal was reported as high as 39% [14]. In the present study a cleaning depth ≥5 mm was observed in 25.9% and 42.0% of the test and control teeth, respectively.
Interestingly, the test teeth showed lower percentages of both RP and RC covered by bacterial plaque than the control sites. This could be explained by the relevant cleaning potential of the desiccant gel. The bond between sulfated components and water leads to the inactivation and denaturation of the organic components of the biofilm, causing the death of bacterial cells. In addition, the desiccation process of the biofilm matrix endangers its bond to the root surface, thereby favoring the removal of the biofilm by hand or ultrasonic subgingival instrumentation [19 21].
Regarding safety, previous studies reported no harmful effects of desiccants on surrounding healthy periodontal tissues [19,20,21,22]. However, the application of desiccants should be carefully performed in terms of the concentration, amount, and application time to avoid a possible negative effect on adjacent periodontal tissues.
It is difficult to know whether the observed difference between treatment procedures would result in significant differences in clinical outcomes and disease progression at the treated teeth. All teeth included in this trial had a hopeless periodontal prognosis and were extracted upon completion of the treatment procedure. An improvement in clinical parameters was obtained in a case series that reported a decrease of 0.87±1.3 mm in PD and a reduction of the bacterial load at 3 months in patients with moderate to severe chronic periodontitis treated with SRP and chemical cleansing [20]. A randomized controlled trial also showed better results with SRP in combination with the chemical desiccant in terms of PD reduction and CAL gain and microbiological parameters in hospitalized patients who also had chronic periodontitis [22]. Data from histological and clinical studies showed that pocket closure could be achieved even in the presence of calculus treated with chlorhexidine [9]. Furthermore, it has been demonstrated that sterilized calculus may be encapsulated in connective tissue without inducing marked inflammation or tissue reaction [8]. A study by Mombelli et al. [32] clearly demonstrated that if complete removal of sub-gingival plaque on the calculus was achieved, complete healing was observed.
This study has some limitations. The accuracy of the grid square method to assess the RPC on the root surface has been questioned. This method overestimated surface area covered by residual deposits by 2- to 8-fold [33]. To address this problem, only squares more than 50% stained or occupied by residual calculus were counted as positive. In addition, residual debris and fibrin colored by the staining solution could be misidentified as bacterial accretion [34]. Finally, data were not stratified by root surface and tooth type. However, previous studies did not observe any difference in the percentage of remaining calculus in different types of teeth [13,27]. Furthermore, we included only posterior teeth and no differences were found between the test and control groups according to tooth type. Based on the findings of the present study, future research perspectives include analyzing the clinical performance of HBX in the treatment of furcation-involved teeth, especially with difficult clinical access, or infrabony defects.
In conclusion, the use of chemical cleansing as an adjunct to traditional SRP may facilitate the removal of microbial plaque from the root surface, but would provide no significant improvement in calculus removal in deep pockets located at posterior teeth sites.
Footnotes
Funding: This paper was supported by the research funds of Jeonbuk National University in 2017 (M.C.).
- Conceptualization: Fahad Zafar, Federica Romano, Filippo Citterio, Mario Aimetti.
- Formal analysis: Claudia Dellavia, Moontaek Chang.
- Investigation: Fahad Zafar, Filippo Citterio, Francesco Ferrarotti.
- Methodology: Federica Romano, Filippo Citterio, Moontaek Chang, Mario Aimetti.
- Project administration: Mario Aimetti.
- Writing - original draft: Fahad Zafar.
- Writing - review & editing: Federica Romano, Filippo Citterio, Francesco Ferrarotti, Moontaek Chang, Mario Aimetti.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1:821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 2.Cobb CM. Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. J Clin Periodontol. 2002;29(Suppl 2):6–16. [PubMed] [Google Scholar]
- 3.Smiley CJ, Tracy SL, Abt E, Michalowicz BS, John MT, Gunsolley J, et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 2015;146:508–24.e5. doi: 10.1016/j.adaj.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 4.John MT, Michalowicz BS, Kotsakis GA, Chu H. Network meta-analysis of studies included in the Clinical Practice Guideline on the nonsurgical treatment of chronic periodontitis. J Clin Periodontol. 2017;44:603–611. doi: 10.1111/jcpe.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aimetti M. Nonsurgical periodontal treatment. Int J Esthet Dent. 2014;9:251–267. [PubMed] [Google Scholar]
- 6.Buchanan SA, Robertson PB. Calculus removal by scaling/root planing with and without surgical access. J Periodontol. 1987;58:159–163. doi: 10.1902/jop.1987.58.3.159. [DOI] [PubMed] [Google Scholar]
- 7.Tomasi C, Leyland AH, Wennström JL. Factors influencing the outcome of non-surgical periodontal treatment: a multilevel approach. J Clin Periodontol. 2007;34:682–690. doi: 10.1111/j.1600-051X.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 8.Allen DL, Kerr DA. Tissue response in the guinea pig to sterile and non-sterile calculus. J Periodontol. 1965;36:121–126. doi: 10.1902/jop.1965.36.2.121. [DOI] [PubMed] [Google Scholar]
- 9.Listgarten MA, Ellegaard B. Electron microscopic evidence of a cellular attachment between junctional epithelium and dental calculus. J Periodontal Res. 1973;8:143–150. doi: 10.1111/j.1600-0765.1973.tb01752.x. [DOI] [PubMed] [Google Scholar]
- 10.Friskopp J, Hammarström L. A comparative, scanning electron microscopic study of supragingival and subgingival calculus. J Periodontol. 1980;51:553–562. doi: 10.1902/jop.1980.51.10.553. [DOI] [PubMed] [Google Scholar]
- 11.Sherman PR, Hutchens LH, Jr, Jewson LG Clinical Responses Related to Residual Calculus. The effectiveness of subgingival scaling and root planing. II. Clinical responses related to residual calculus. J Periodontol. 1990;61:9–15. doi: 10.1902/jop.1990.61.1.9. [DOI] [PubMed] [Google Scholar]
- 12.Anerud A, Löe H, Boysen H. The natural history and clinical course of calculus formation in man. J Clin Periodontol. 1991;18:160–170. doi: 10.1111/j.1600-051x.1991.tb01128.x. [DOI] [PubMed] [Google Scholar]
- 13.Rabbani GM, Ash MM, Jr, Caffesse RG. The effectiveness of subgingival scaling and root planing in calculus removal. J Periodontol. 1981;52:119–123. doi: 10.1902/jop.1981.52.3.119. [DOI] [PubMed] [Google Scholar]
- 14.Waerhaug J. Healing of the dento-epithelial junction following subgingival plaque control. II: As observed on extracted teeth. J Periodontol. 1978;49:119–134. doi: 10.1902/jop.1978.49.3.119. [DOI] [PubMed] [Google Scholar]
- 15.Lopez MA, Andreasi Bassi M, Confalone L, Silvestre F, Arcuri C. The treatment of peri-implant diseases: a new approach using hybenx® as a decontaminant for implant surface and oral tissues. Oral Implantol (Rome) 2016;9:106–114. doi: 10.11138/orl/2016.9.3.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye WH, Fan B, Purcell W, Meghil MM, Cutler CW, Bergeron BE, et al. Anti-biofilm efficacy of root canal irrigants against in-situ Enterococcus faecalis biofilms in root canals, isthmuses and dentinal tubules. J Dent. 2018;79:68–76. doi: 10.1016/j.jdent.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Porter SR, Al-Johani K, Fedele S, Moles DR. Randomised controlled trial of the efficacy of HybenX in the symptomatic treatment of recurrent aphthous stomatitis. Oral Dis. 2009;15:155–161. doi: 10.1111/j.1601-0825.2008.01503.x. [DOI] [PubMed] [Google Scholar]
- 18.Antonelli A, Giovannini L, Baccani I, Giuliani V, Pace R, Rossolini GM. In vitro antimicrobial activity of the decontaminant HybenX® compared to chlorhexidine and sodium hypochlorite against common bacterial and yeast pathogens. Antibiotics (Basel) 2019;8:188. doi: 10.3390/antibiotics8040188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pini-Prato G, Magnani C, Rotundo R. Treatment of acute periodontal abscesses using biofilm decontamination approach: a case report study. Int J Periodontics Restorative Dent. 2016;36:55–63. doi: 10.11607/prd.2557. [DOI] [PubMed] [Google Scholar]
- 20.Lombardo G, Signoretto C, Corrocher G, Pardo A, Pighi J, Rovera A, et al. A topical desiccant agent in association with ultrasonic debridement in the initial treatment of chronic periodontitis: a clinical and microbiological study. New Microbiol. 2015;38:393–407. [PubMed] [Google Scholar]
- 21.Bracke J, Basara M, Savord E, Dunaway A, Watkins M. Pilot evaluation of a simple adjunctive method for improved removal of oral biofilm during conventional scaling and root planing therapy. J Biol Regul Homeost Agents. 2015;29(Suppl 1):6–9. [PubMed] [Google Scholar]
- 22.Isola G, Matarese G, Williams RC, Siciliano VI, Alibrandi A, Cordasco G, et al. The effects of a desiccant agent in the treatment of chronic periodontitis: a randomized, controlled clinical trial. Clin Oral Investig. 2018;22:791–800. doi: 10.1007/s00784-017-2154-7. [DOI] [PubMed] [Google Scholar]
- 23.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Clin Periodontol. 2018;45(Suppl 20):S149–61. doi: 10.1111/jcpe.12945. [DOI] [PubMed] [Google Scholar]
- 24.Wilson TG, Jr, Harrel SK, Nunn ME, Francis B, Webb K. The relationship between the presence of tooth-borne subgingival deposits and inflammation found with a dental endoscope. J Periodontol. 2008;79:2029–2035. doi: 10.1902/jop.2008.080189. [DOI] [PubMed] [Google Scholar]
- 25.Badersten A, Nilveus R, Egelberg J. Effect of nonsurgical periodontal therapy. II. Severely advanced periodontitis. J Clin Periodontol. 1984;11:63–76. doi: 10.1111/j.1600-051x.1984.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaldahl WB, Kalkwarf KL, Patil KD, Molvar MP, Dyer JK. Long-term evaluation of periodontal therapy: I. Response to 4 therapeutic modalities. J Periodontol. 1996;67:93–102. doi: 10.1902/jop.1996.67.2.93. [DOI] [PubMed] [Google Scholar]
- 27.Caffesse RG, Sweeney PL, Smith BA. Scaling and root planing with and without periodontal flap surgery. J Clin Periodontol. 1986;13:205–210. doi: 10.1111/j.1600-051x.1986.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 28.Fleischer HC, Mellonig JT, Brayer WK, Gray JL, Barnett JD. Scaling and root planing efficacy in multirooted teeth. J Periodontol. 1989;60:402–409. doi: 10.1902/jop.1989.60.7.402. [DOI] [PubMed] [Google Scholar]
- 29.Jones WA, O'Leary TJ. The effectiveness of in vivo root planing in removing bacterial endotoxin from the roots of periodontally involved teeth. J Periodontol. 1978;49:337–342. doi: 10.1902/jop.1978.49.7.337. [DOI] [PubMed] [Google Scholar]
- 30.Matia JI, Bissada NF, Maybury JE, Ricchetti P. Efficiency of scaling of the molar furcation area with and without surgical access. Int J Periodontics Restorative Dent. 1986;6:24–35. [PubMed] [Google Scholar]
- 31.Nishimine D, O'Leary TJ. Hand instrumentation versus ultrasonics in the removal of endotoxins from root surfaces. J Periodontol. 1979;50:345–349. doi: 10.1902/jop.1979.50.7.345. [DOI] [PubMed] [Google Scholar]
- 32.Mombelli A, Nyman S, Brägger U, Wennström J, Lang NP. Clinical and microbiological changes associated with an altered subgingival environment induced by periodontal pocket reduction. J Clin Periodontol. 1995;22:780–787. doi: 10.1111/j.1600-051x.1995.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 33.Eschler B, Moskowicz DG, Alvares O. Measurements of residual stained material on treated periodontally involved roots. J Dent Res. 1987;66:149. [Google Scholar]
- 34.Breininger DR, O'Leary TJ, Blumenshine RV. Comparative effectiveness of ultrasonic and hand scaling for the removal of subgingival plaque and calculus. J Periodontol. 1987;58:9–18. doi: 10.1902/jop.1987.58.1.9. [DOI] [PubMed] [Google Scholar]