Abstract
Purpose
Periodontitis is considered a local risk factor for medication-related osteonecrosis of the jaws (MRONJ). However, little is known about the progression of periodontitis in the presence of zoledronic acid (ZOL). The aim of this study was to evaluate the effects of the systemic use of ZOL on the progression of experimental periodontitis (EP) in rats, as ZOL could modulate the progression of periodontitis and concomitantly cause MRONJ in individuals with periodontitis.
Methods
Forty-eight male Wistar rats were randomly distributed in 6 groups (n=8 each). To induce EP, ligatures were placed around the right first mandibular molars. Three groups were treated with ZOL (0.15 mg/kg/week, intraperitoneal), and 3 with 0.9% saline solution (controls). In the ZOL/Lig30 and ZOL/Lig 15 groups, after 4 weeks of treatment with ZOL, EP was induced and euthanasia was performed after 30 and 15 days of EP induction, respectively. In both groups, the animals continued to receive ZOL after EP until the end of the experiment. In the Lig/ZOL group, EP was induced first, and 15 days later, ZOL was administered for 8 weeks, with euthanasia 1 week after the last dose. After euthanasia, the mandibles were evaluated using micro-computed microtomography (micro-CT) and histomorphometry. Bone loss was measured, and the presence of osteonecrosis was evaluated histologically. The data were evaluated using the Student t-test and the Mann-Whitney test, with a significance level of 5%.
Results
In the Lig/ZOL group, micro-CT revealed less alveolar bone resorption in the distal root (P<0.01) than in the control group (Lig/Con). Histomorphometric analysis confirmed less alveolar bone resorption in the Lig/ZOL group (P=0.001). Histologically, osteonecrosis was more common in the ZOL groups.
Conclusion
ZOL decreased alveolar bone resorption in rats with EP. However, it presented a higher risk for MRONJ.
Keywords: Histology, Osteonecrosis, Periodontitis, Rats, X-ray microtomography, Zoledronic acid
Graphical Abstract
INTRODUCTION
Bisphosphonates are synthetic analogs of pyrophosphate, in which carbon replaces the central oxygen in the molecule, providing resistance to hydrolysis and to degradation. They present an ability to bind to bivalent metal ions, such as calcium [1]. For this reason, bisphosphonates are rapidly eliminated from the circulation and adhere to bone mineral surfaces at sites of active bone remodeling, which are subject to osteoclastic resorption [2].
Despite the beneficial anti-resorptive effects of bisphosphonates, gnathic bones may be affected by a potentially serious adverse effect after prolonged administration of nitrogenated bisphosphonates [1]. Specifically, excessive suppression of bone turnover, reduced remodeling capacity, and a decreased ability to repair damage can lead to accumulation of micro-damage, causing medication-related osteonecrosis of the jaws (MRONJ) [1,3].
MRONJ is defined as an area of exposed bone, or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region, which does not heal within 8 weeks after identification by a health care provider, in a patient receiving or exposed to an antiangiogenic or antiresorptive agent, such as a bisphosphonate, without a previous history of radiation therapy in the craniofacial region [1,3]. MRONJ has been reported after dental extraction, trauma to oral tissues, and dental implant placement, and is associated with periapical disease and periodontitis [1,3,4,5].
Periodontitis is a common inflammatory disease caused by biofilm accumulation that leads to the onset of destruction of the alveolar bone and the periodontal ligament. In periodontitis, proteolytic enzymes, such as matrix metalloproteinases (MMPs), are released by osteoclasts to promote tissue destruction during bone resorption. In addition to MMPs, other important causative factors in the inflammatory process are involved in alveolar bone loss in periodontitis, such as lipopolysaccharide (LPS), prostaglandin E2, interleukin-1β, and tumor necrosis factor alpha [6]. The cascade of periodontal destruction is initiated by LPS in the outer membrane of Gram-negative bacteria present in the biofilm. Monocytes and macrophages are activated and release proinflammatory cytokines that promote destructive processes. Osteoclasts, in turn, release proteolytic enzymes such as MMPs, which induce bone tissue destruction during resorption [6,7].
Previous studies have investigated the use of alendronate, which antagonizes the action of important factors in inflammation, as a possible adjuvant to the basic periodontal treatment of periodontitis induced in rats [8,9]. Alendronate was also evaluated as a topical treatment for intra-bony defects caused by chronic periodontitis [10] and as a way to avoid bone loss around the implant, thereby improving osseointegration [11]. However, little is known about the progression of periodontitis in the presence of zoledronic acid (ZOL). Meanwhile, periodontitis is considered a local risk factor for MRONJ [5]. While the use of systemic ZOL could modulate the progression of periodontitis, it could cause MRONJ in individuals with periodontitis. Therefore, the objectives of this study were to evaluate the in vivo effects of ZOL on the progression of periodontitis in rats, and to explore the local effects of periodontitis as a probable risk factor for the development of MRONJ.
MATERIALS AND METHODS
Animals and ethical considerations
This study was approved by the Local Animal Ethics Committee (process# 23076.019257/2013-80), and it followed the ethical recommendations of the National Council for Control of Animal Experimentation in Brazil and the National Institute of Health Guide for Care and Use of Laboratory Animals. The entire study was designed in accordance with the ARRIVE guidelines.
A total of 48 male adult rats (Rattus norvegicus albinus, Wistar), weighing between 300 and 350 g and aged 4–6 months on average, were included in the experiment. The animals were fed a standard diet for rodents (Presence®, Paulínia, São Paulo, Brazil), and had free access to food and water. They were maintained in polypropylene cages with zinc-plated wire cover caps with 4 animals in each cage. The animals were kept in a room at a temperature of 23±2ºC and a light/dark cycle of 12 hours.
Induction of periodontitis and administration of ZOL
The animals received atropine to control salivary flow and were anesthetized with 100 mg/kg of ketamine hydrochloride (SESP, Vetbrands Animal Health Division, Jacareí, São Paulo, Brazil) together with 50 mg/kg of xylazine (Bayer do Brasil, Belford Roxo, Rio de Janeiro, Brazil) by the intramuscular route. When the absence of pain sensitivity was confirmed, the animal was positioned in a modified Doku apparatus [12], and periodontal disease was induced by placing a sterile silk 4.0 ligature (Ethicon, Johnson and Johnson, São Paulo, Brazil) around the left first mandibular molar in a submarginal position. After the procedure, the animals were kept alone in individual cages to avoid excessive stress, without restriction of food and water.
The animals treated with ZOL (Zometa, Novartis Pharmaceutical, Basel, Switzerland) received 0.15 mg (once a week) of the drug by intraperitoneal injection. At the end of each experimental period, the animals were euthanized through an anesthetic overdose of ketamine and xylazine.
Study design
The animals were randomly assigned to 6 experimental groups, each containing 8 animals.
• ZOL/Lig30: animals were treated with ZOL for 4 weeks. On the day of the last dose, periodontitis was induced. Thirty days after ligature, the animals were euthanized.
• ZOL/Lig15: animals were treated with ZOL for 4 weeks. On the day of the last dose, periodontitis was induced. Fifteen days later, the animals were euthanized.
In both the ZOL/Lig30 and ZOL/Lig15 groups, the animals continued to receive ZOL after induction of periodontitis until the end of the experiment.
• Con/Lig30: animals were treated with 1 mL of 0.9% saline solution by intraperitoneal injection for 4 weeks (once a week). On the day of the last application, periodontitis was induced. Thirty days after ligature, the animals were euthanized.
• Con/Lig15: animals were treated with 1 mL of 0.9% saline solution by intraperitoneal injection for 4 weeks (once a week). On the day of the last dose, periodontitis was induced and 15 days later, the animals were euthanized.
In both the Con/Lig30 and Con/Lig15 groups, the animals continued to receive the saline solution after the induction of periodontitis until the end of the experiment.
• Lig/ZOL: fifteen days after periodontitis induction, animals were treated with ZOL for 8 weeks. Euthanasia occurred 1 week after the last drug application.
• Lig/Con: fifteen days after periodontitis induction, animals received 1 mL of 0.9% saline solution by intraperitoneal injection for 8 weeks (once a week). Euthanasia occurred 1 week after the last dose.
After euthanasia, the mandibles were removed and fixed in 10% buffered formalin for 72 hours.
Micro-computed tomography (micro-CT) analysis
A third-generation microtomograph (NIKON XT-H 225 ST Microfocus, Nikon, Tokyo, Japan) was used for the exposure of the samples, operating at 80 kV, 222 μA without a filter, and 500 ms, using a protocol with voxel size of 11 μm and an exposure time of 25 minutes.
The images obtained were reconstructed using the CT PRO 3D software version XT3.1.3 (Nikon Metrology NV, Tring, UK) with the .xteck extension, in which the volume of interest was delimited. After delimiting the area of interest, multiplanar (axial, sagittal, and coronal) reconstructions of the selected volume were exported to the DICOM extension using VGStudioMax 2.2 (Volume Graphics Gmbh, Heidelberg, Germany). Next, a Gaussian filter (3×3×3) was applied to smooth over the presence of artifacts on the digital images.
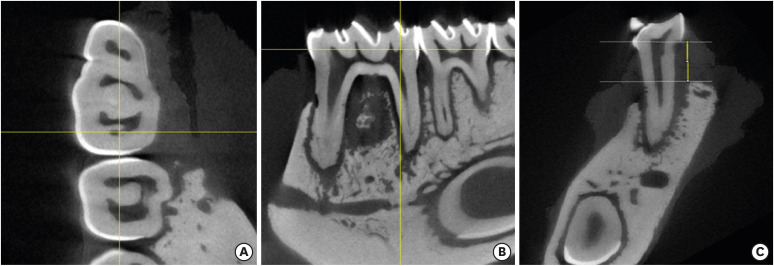
The reconstructed images were analyzed by 1 evaluator (PGCLM), who was previously calibrated, using ImageJ version 14.5 (National Institute of Health, Bethesda, MD, USA). To find the region to be analyzed, the axial section marked the central region of the tooth, and the sagittal section was rotated in order to keep the roots perpendicular to the ground. Measurements were made in the coronal section, where 2 horizontal lines were drawn: 1 at the point of the cementoenamel junction and 1 at the highest part of the alveolar bone crest of the distal root, at the buccal and lingual surfaces. The vertical distance between the 2 horizontal lines was captured in pixels and later converted to millimeters [13]. Bone loss from the furcation region was also analyzed by performing vertical measurements of the furcate dentin to the alveolar bone of the region (Figure 1). All sections referring to the first molar were analyzed and measured on the coronal axis, maintaining the central position obtained in the axial section (Figure 1). Areas of necrotic bone were not included in the measurements of alveolar bone loss [13].
Figure 1. Axial (A), sagittal (B), and coronal (C) reconstructions of the area of interest. The coronal section was used for measurements (C) by drawing 2 horizontal lines at the height of the cementoenamel junction and at the highest alveolar bone crest portion. Measurement of the distance between horizontal lines (vertical line).
A scan was performed on all samples, and all coronal sections of all specimens were measured. The degree of bone resorption was measured throughout the mesial roots, distal roots, and furcation region from the coronal section, maintaining the central position obtained in the axial section. The mean resorption rates of the buccal and lingual surfaces were obtained for both the distal and mesial roots. A general average of resorption on each of the roots (mesial and distal) was calculated from a simple arithmetic mean calculated based on data from the buccal and lingual surfaces of each root alone. Likewise, a general mean of bone resorption was calculated by a simple arithmetic mean based on the general mean of the mesial and distal roots and the mean bone resorption in the furcation area. Eight animals from each group were evaluated, and all microtomographic sections were measured for the 3 regions studied (furcation, distal root, and mesial root). All microtomographic sections were sequentially measured for the 3 regions studied (furcation, distal root, and mesial root). Thus, the entire specimen was assessed.
Histological analysis
The mandibles were decalcified with 5% tetrasodium ethylenediaminetetraacetate (EDTA) for 30 days. Next, they underwent conventional histological processing. Histological sections of 5 μm were obtained from tissues embedded in paraffin and stained with hematoxylin and eosin. Inflammatory reaction intensity was evaluated in the furcation region and scores were assigned according to the degree of inflammation: 1, absent; 2, mild; 3, moderate; and 4, intense. Inflammation was considered to be mild when few inflammatory cells were present, distributed focally or diffusely; moderate when there was a focal inflammatory reaction, and intense when the region evaluated showed many inflammatory cells distributed diffusely [13]. Osteonecrosis was considered present when the bone presented loss of more than 5 contiguous osteocytes with confluent areas of empty gaps [5].
Histomorphometric analysis
Sixteen slides from each group (2 belonging to each animal) were scanned by Panoramic MIDI (3DHISTECH Ltd., Budapest, Hungary). Each slide had 4 serial cuts, which were analyzed at ×2 magnification. To quantify the area of alveolar bone resorption, the Panoramic Viewer software (3DHISTECH Ltd.) was used. The images were rotated in order to leave the distal root and the furcation perpendicular to the ground. For the distal root measurement, a horizontal line was drawn at the height of the cementoenamel junction and the distance between the horizontal line and the alveolar bone crest was measured. The entire length of the furcation region was measured vertically, based on the furcate dentin and the viable alveolar bone crest of the same region [13]. Areas of necrotic bone were not included in the alveolar bone loss measurement.
Statistical analysis
The data obtained were tabulated in Microsoft Office Excel 2013 (Microsoft Corp., Redmond, WA, USA), imported into SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA) and subjected to normality tests (Shapiro–Wilk and Kolmogorov–Smirnov tests), which yielded a P value >0.05 for most variables, except for the histometric analysis in the furcation area of the ZOL/Lig30, Con/Lig30, ZOL/Lig15, and Con/Lig15 groups. Statistical analysis was performed using the Student t-test to analyze microtomography data and inflammation, and the Mann-Whitney test for histometric analysis in the furcation area. Values were expressed as mean and standard deviation, considering a level of significance of 95% (P<0.05).
RESULTS
For data analysis, the groups treated with ZOL were compared with the corresponding control groups. Thus, the ZOL/Lig30 group was compared with the Con/Lig30 group, the ZOL/Lig15 group with the Con/Lig15 group, and the Lig/ZOL group with the Lig/Con group.
Linear measurements using micro-CT
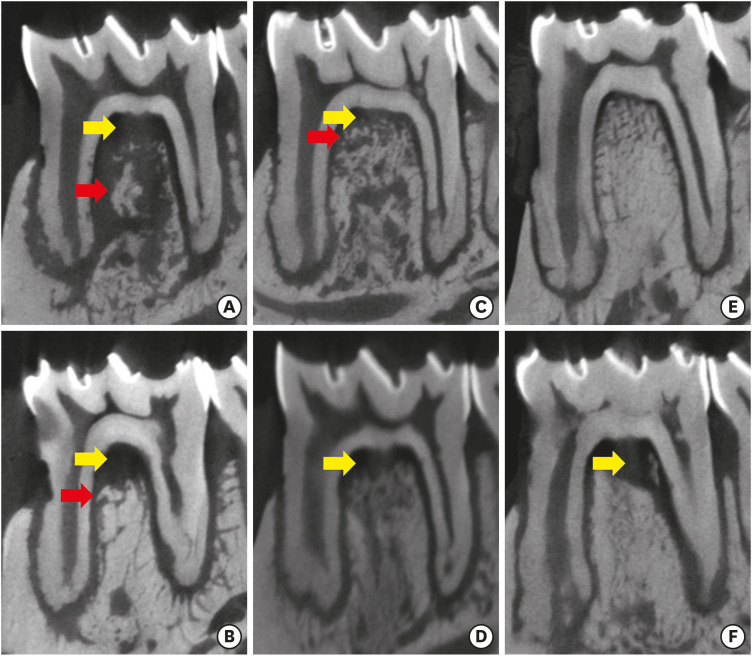
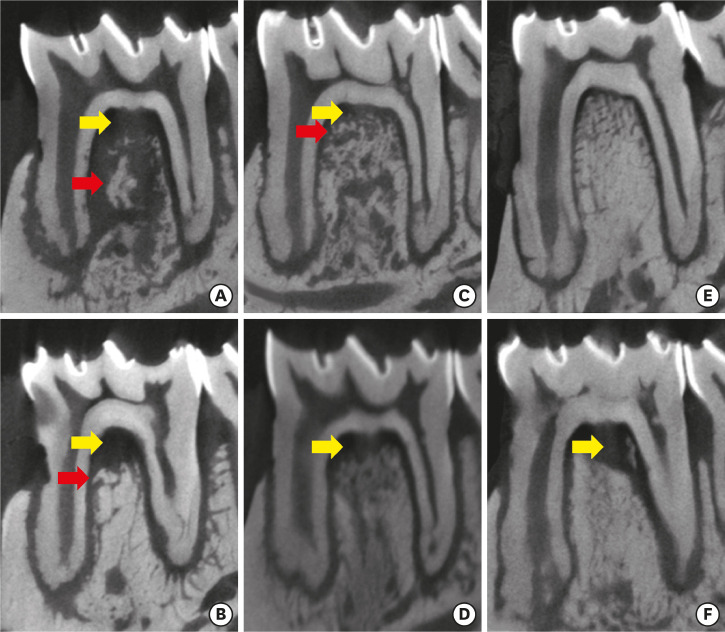
When ZOL was administered before the induction of periodontal disease, there was no significant difference with respect to alveolar bone resorption, irrespective of the time of disease (P>0.05) (Table 1). However, when the drug was administered to animals with the disease already established (Lig/ZOL group), less alveolar bone resorption associated with the distal root (P<0.01) was observed (Table 1). In 3 animals in the ZOL/Lig30 group, and in 1 in the ZOL/Lig15 group, bone fragments with irregular edges and detachment of the alveolar bone were observed in the furcation region, suggesting bone sequestration. This same feature was observed in 1 animal in the Con/Lig 30 group. Figure 2 illustrates the microtomographic findings.
Table 1. Alveolar bone resorption in the distal root and furcation shown by microtomography analysis.
| Groups | Region | Alveolar bone resorption | P valuea) |
|---|---|---|---|
| A | Furca | 0.60±0.20 vs.0.75±0.57 | 0.522 |
| Lingual (DR) | 1.06±0.16 vs. 1.25±0.23 | 0.090 | |
| Vestibular (DR) | 1.14±0.17 vs. 1.19±0.33 | 0.700 | |
| Mean value | 1.10±0.12 vs. 1.22±0.22 | 0.210 | |
| B | Furca | 0.28±0.07 vs. 0.63±0.50 | 0.119 |
| Lingual (DR) | 0.95±0.83 vs. 1.05±0.28 | 0.399 | |
| Vestibular (DR) | 0.90±0.14 vs. 0.82±0.18 | 0.383 | |
| Mean value (DR) | 0.93±0.1 vs. 0.94±0.22 | 0.927 | |
| C | Furca | 0.86±0.77 vs. 0.91±1.06 | 0.933 |
| Lingual (DR) | 0.96±0.12 vs. 1.23±0.64 | 0.001 | |
| Vestibular (DR) | 0.89±0.15 vs. 1.36±0.28 | 0.003 | |
| Mean value (DR) | 0.91±0.12 vs. 1.30±0.6 | <0.001 |
Data are shown as mean±standard deviation. Values in mm.
Groups are represented as below: A, ZOL/Lig30 vs. Con/Lig30; B, ZOL/Lig15 vs. Con/Lig15; C, Lig/ZOL vs. Lig/Con.
DR: distal root, ZOL: zoledronic acid.
a)Student's t-test.
Figure 2. Microtomographic sections (sagittal). ZOL/Lig30 (A), Con/Lig30 (B), ZOL/Lig15 (C), Con/Lig15 (D), Lig/ZOL (E), Lig/Con (F). Alveolar bone resorption in the furcation (yellow arrow) and bone sequestration (red arrow).
ZOL: zoledronic acid.
Histomorphometric analysis
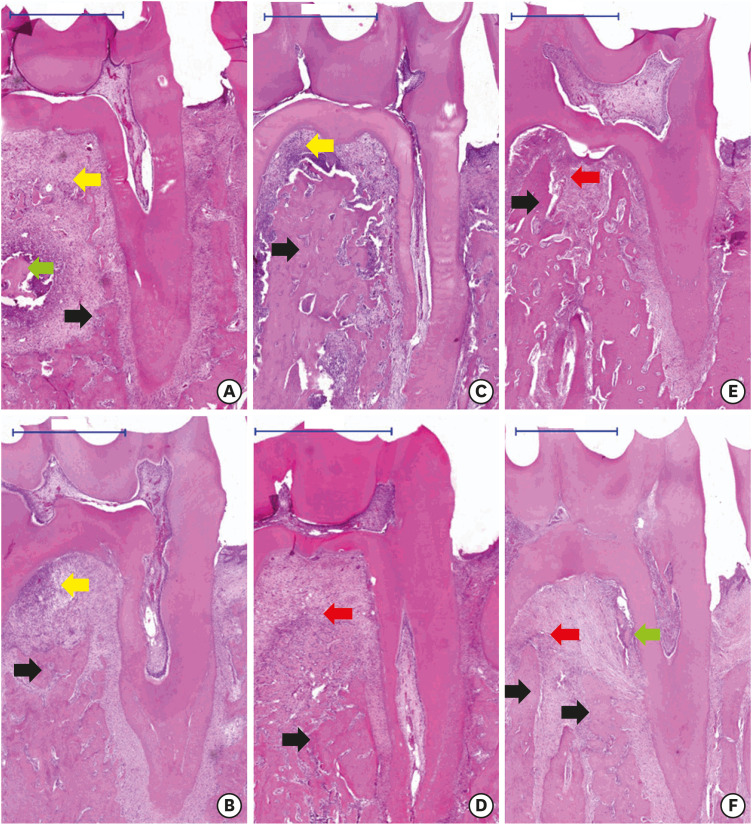
In animals treated with ZOL after induction of periodontitis (Lig/ZOL group), there was less alveolar bone resorption in the distal root (P=0.001), confirming the findings of the micro-CT analysis (Figure 3). The same trend of bone resorption was observed in the furcation region (P<0.001) (Table 2). Similarly, in animals treated with ZOL before the induction of periodontitis (ZOL/Lig15), there was also a decrease in bone resorption in the distal root (P<0.001) (Table 2). However, when the induction of periodontitis lasted up to 30 days, the animals treated with ZOL (ZOL/Lig30) presented increased alveolar bone resorption at distal root sites (P<0.05) (Table 2).
Figure 3. Histological sections of inflammation in induced periodontal disease (scale bar = 1 mm). ZOL/Lig30 (A), ZOL/Lig15 (C), Con/Lig30 (B), Con/Lig15 (D), Lig/ZOL (E) and Lig/Con (F). Mild inflammatory infiltrate (red arrow), moderate inflammatory infiltrate (yellow arrow), osteonecrosis with formation of bone sequestration (green arrow), and viable bone (black arrow).
ZOL: zoledronic acid.
Table 2. Histometric analysis showing mean and SD of bone resorption in the distal root and furcation (A.1, A.2, B.1, B.2, C.1, C.2). Number of animals per group that presented osteonecrosis in the furca and/or distal root (A.3, B.3, C.3).
| Groups | Variables | Bone resorption | P value | |
|---|---|---|---|---|
| A | A.1 | DR | 0.620±0.096 vs. 0.490±0.128 | 0.005a) |
| A.2 | Furca | 1.276±0.689 vs. 0.836±0.376 | 0.046b) | |
| A.3 | Osteonecrosis | 3/6 vs. 1/6 | - | |
| B | B.1 | DR | 0.323±0.143 vs. 0.608±0.301 | <0.001a) |
| B.2 | Furca | 0.429±0.157 vs. 0.492±0.337 | 0.516b) | |
| B.3 | Osteonecrosis | 1/6 vs. 0/6 | - | |
| C | C.1 | DR | 0.408±0.058 vs. 0.589±0.159 | 0.001a) |
| C.2 | Furca | 0.363±0.179 vs. 0.798±0.215 | <0.001a) | |
| C.3 | Osteonecrosis | 0/6 vs. 1/6 | - | |
Data are shown as mean±SD.
Values in mm, except for A.3, B.3, and C.3. In A.3, B.3, and C.3, number of animals with osteonecrosis/total number of animals in group.
Groups are represented as below: A, ZOL/Lig30 vs. Con/Lig30; B, ZOL/Lig15 vs. Con/Lig15; C, Lig/ZOL vs. Lig/Con.
DR: distal root, SD: standard deviation, ZOL: zoledronic acid.
a)Student's t-test; b)Mann-Whitney test.
Histological analysis
The histological analysis revealed moderate inflammation in the furcation of animals treated with ZOL before the induction of periodontitis for 15 days (ZOL/Lig15 group), while the control (Con/Lig15 group) presented mild inflammation (P=0.018) (Table 3). However, in animals with 30 days of periodontal disease and previous treatment with ZOL (ZOL/Lig30) or saline solution (Con/Lig30), the inflammation was moderate (P=0.733) (Table 3). When periodontitis was induced before treatment, the degree of inflammation was mild for both the treated (Lig/ZOL) and control (Lig/Con) groups (P=0.0853) (Table 3).
Table 3. The degree of inflammation in the furcation region shown by histology.
| Groups | Region | Degree of inflammationa) | P valueb) |
|---|---|---|---|
| A | Furca | 3.6±0.89 vs. 3.4±0.89 | 0.733 |
| B | Furca | 3.0±0.81 vs. 2.0±0.01 | 0.018 |
| C | Furca | 2.4±0.55 vs. 2.5±1.0 | 0.853 |
Data are shown as mean±standard deviation.
Groups are represented as below: A, ZOL/Lig30 vs. Con/Lig30; B, ZOL/Lig15 vs. Con/Lig15; C, Lig/ZOL vs. Lig/Con.
ZOL: zoledronic acid.
a)These values refer to the mean scores of the inflammatory reaction intensity; b)Student's t-test.
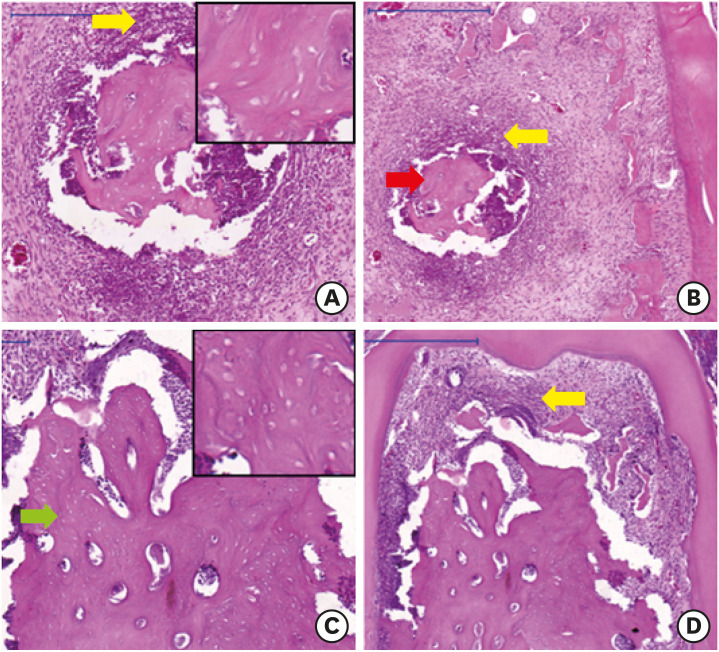
Osteonecrosis was present in the treated groups that received ZOL before the induction of periodontitis (Figures 3 and 4), irrespective of treatment time, in 3 animals in the ZOL/Lig30 group and 1 in the ZOL/Lig15 group. However, only 1 animal with osteonecrosis was present in the Con/Lig30 and Lig/Con groups (Table 2).
Figure 4. Osteonecrosis in the ZOL/Lig30 (A, B) and ZOL/Lig15 (C, D) groups (scale bar = 0.5 mm). Osteonecrosis with formation of bone sequestration (red arrow) surrounded by chronic inflammatory infiltrate (yellow arrow). Necrotic bone (green arrow) with loss of confluent osteocytes.
ZOL: zoledronic acid.
DISCUSSION
Periodontal disease occurs due to changes in the composition of microorganisms in dental biofilm and in the immune response of the host. This initiates the destructive process in the periodontal ligament and resorption of the alveolar bone. The experimental model of periodontal disease induction with a ligature favors the accumulation of biofilm, inducing a local inflammatory process dependent not only on the plaque, but also on the host immune response [9]. Experimental studies have been performed to explain MRONJ. These experiments observed that this side effect occurred in the presence of oral trauma and infection, including oral surgery, periodontal disease, and inflammatory periapical disease [4,5,14,15]. Similar to what has been reported in the literature, an experimental model with ligature was used to evaluate the effects of experimental periodontitis (EP) on bone during treatment with ZOL.
The administration of ZOL before the induction of periodontitis reduced alveolar bone resorption in animals with newly established disease (ZOL/Lig15), although this experimental group presented a higher mean score of inflammatory intensity (3, moderate) than the respective control group Con/Lig15 (mean score: 2, mild). The same findings were observed when the animals began to receive the drug after periodontal disease had already developed (Lig/ZOL), with similar mean scores of inflammatory intensity found in both the experimental and control groups (Lig/Con). The lower bone resorption found in these groups, ZOL/Lig15 and Lig/ZOL, probably occurred due to the antiresorptive activity of the drug, modulating osteoclastic and osteoblastic action [16], irrespective of the degree of inflammation intensity. The inhibition of the enzyme farnesyl diphosphate synthase in the cholesterol synthesis pathway, triggered by ZOL, prevents the recruitment of mature osteoclasts and differentiation of immature osteoclasts [17]. This action results in loss of the resorptive capacity of osteoclasts or even apoptosis [18]. Additionally, a study reported evidence that ZOL and pamidronate also acted on osteoblasts by increasing osteoprotegerin (OPG) secretion, a member of the tumor necrosis factor receptor superfamily. Since OPG inhibits osteoclastogenesis and osteoclast activity by acting as an antagonist of receptor activator of nuclear factor κB ligand, this may be another pathway through which ZOL inhibits bone resorption [19]. In contrast, however, the present study showed the opposite result when the drug was administered before periodontitis induction, and it progressed for 30 days (ZOL/Lig30). The group treated with ZOL had greater alveolar bone resorption at all sites studied. This result probably occurred because osteonecrosis was present in more animals in the ZOL/Lig30 group, and the area of necrotic bone was not measured in the histometric analysis.
Despite its anti-resorptive actions, the current study showed that ZOL did not interfere with the inflammatory reaction, regardless of whether the drug was administered before or after periodontitis induction. Similar findings were reported by other authors who evaluated the action of alendronate in EP [9,20]. Both studies observed less alveolar bone resorption over the course of time in groups treated with bisphosphonate (alendronate). These findings show that, in this experimental model, lower alveolar bone resorption is related to the antiresorptive capacity of the drug.
The highest bone resorption values shown in the control groups were similar to the findings of Aghaloo et al. [5], who used a similar experimental model and drug concentrations to those in this study. However, the time of drug administration was prolonged for 15 weeks, periodontitis induction continued for a longer period (12 weeks), and periodontal disease was not evaluated at another time interval. The authors found greater resorption in the alveolar bone of the distal root of first molars in the control group. In addition, they reported the presence of osteonecrosis with the formation of bone sequestration in the region where the ligature was placed, similar to our study. The current results are also similar to those reported by Moreira et al. [20]. However, they used alendronate sodium when monitoring the progression of EP in rats. Animals treated with alendronate had rare signs of alveolar bone resorption after 14 days of periodontitis induction, in addition to greater bacterial contamination and presence of osteonecrosis with bone exposure at 21 days of ligature use. Likewise, other authors found less resorption in the periapical bone region of the animals that received high doses of ZOL for 8 weeks, by means of imaging and histological analyses [4,21].
The groups treated with ZOL before the induction of periodontitis (ZOL/Lig30 and ZOL/Lig15) presented bone portions detached of the alveolar ridge, which showed irregular borders, in the furcation region, suggesting bone sequestration shown in the microtomography images. Histological analysis confirmed necrotic bone in both groups. Similar findings were observed in another study, which administered alendronate in rats with periodontitis [5]. Imaging findings such as sequestration, periosteal bone formation, and alveolar crest expansion are common in patients with MRONJ [1,3]. The pathophysiology of MRONJ is believed to be linked to inhibition of bone remodeling by osteoclast apoptosis, presence of local inflammation or infection, as observed in periodontal disease, inhibition of angiogenesis, soft tissue toxicity, and dysfunction of native or acquired immunity [2]. However, Bonnet et al. [22] found no areas of MRONJ in rats with periodontitis induced by periostin deficiency and treated with high doses of ZOL. The divergent results may be attributed to variation in the experimental models used to investigate the association between osteonecrosis and periodontal disease [16]. Additionally, ZOL increases the prevalence of MRONJ in rats with periodontitis is a dose-dependent manner [23]. In contrast, although the animals in the LIG/ZOL group also received the same dose of ZOL for 8 weeks as those in the ZOL/LIG30 group, none of them presented osteonecrosis, both in histological and microtomographic analyses. Thus, it has been suggested that there is apparently an increased risk of MRONJ in those individuals who are being treated with ZOL and develop periodontitis. Clinically, this reinforces the need for rigorous dental care for these patients.
Osteonecrosis also occurred in animals treated with vehicle, similar to other studies [5,24], affecting only 1 animal from each control group (Con/Lig30 and Lig/Con). In the control groups, osteonecrosis may have occurred due to bone denudation in the region caused by ligature trauma, with consequent infection and cell death of osteocytes [5]. In the same way, Yamashita et al. [24], studying repair of bone wounds in the palate of animals treated with ZOL, reported that necrosis may occur in animals treated with ZOL and with vehicle (control). In the control group, the necrosis was considered to be trauma-induced, with a subsequent infection that impaired tissue vascularization.
Despite the interesting findings, it is important to emphasize that this is an in vivo study and its results cannot be directly applied to humans. Further studies should be carried out to confirm these findings and improve the experimental model. In addition, molecular and immunohistochemical analyses can be important to understand the mechanisms involved in the development of MRONJ associated with periodontal disease.
In the present study, ZOL modulated the extension of alveolar bone resorption in periodontitis induced in rats. Although this finding indicates a probable adjuvant effect in the treatment of periodontitis, some animals treated with ZOL nonetheless presented osteonecrosis. Thus, since ZOL is widely used to treat systemic bone diseases, and periodontal disease is a common oral disease, dental and medical practitioners should pay attention to the risk of osteonecrosis in these patients. Moreover, according to the current findings, the use of local or systemic ZOL as an adjuvant treatment of periodontitis is inadvisable.
Footnotes
Funding: This study was supported in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Finance code 001, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. The authors also thank the Computed Tomography Laboratory (LTC) of the Nucleus in Non-Destructive Testing of X-Ray Applications (NENDARX) of the Department of Nuclear Energy, Universidade Federal de Pernambuco, for technical support in obtaining the microtomographic images.
- Conceptualization: Danyel Elias da Cruz Perez, Flávia Maria de Moraes Ramos-Perez.
- Formal analysis: Priscylla Gonçalves Correia Leite de Marcelos, Flávia Maria de Moraes Ramos-Perez.
- Investigation: Priscylla Gonçalves Correia Leite de Marcelos, Diego Moura Soares, Samuel Silva de Araújo.
- Methodology: Priscylla Gonçalves Correia Leite de Marcelos, Maria Luiza dos Anjos Pontual, Diego Moura Soares, Liriane Baratella Evêncio, Danyel Elias da Cruz Perez.
- Project administration: Flávia Maria de Moraes Ramos-Perez.
- Writing - original draft: Priscylla Gonçalves Correia Leite de Marcelos.
- Writing - review & editing: Danyel Elias da Cruz Perez, Maria Luiza dos Anjos Pontual, Flávia Maria de Moraes Ramos-Perez.
Conflict of Interest: No potential conflict of interest relevant to this article was reported
References
- 1.Yarom N, Shapiro CL, Peterson DE, Van Poznak CH, Bohlke K, Ruggiero SL, et al. Medication-related osteonecrosis of the jaw: MASCC/ISOO/ASCO clinical practice guideline. J Clin Oncol. 2019;37:2270–2290. doi: 10.1200/JCO.19.01186. [DOI] [PubMed] [Google Scholar]
- 2.Aghaloo T, Hazboun R, Tetradis S. Pathophysiology of osteonecrosis of the jaws. Oral Maxillofac Surg Clin North Am. 2015;27:489–496. doi: 10.1016/j.coms.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Kang B, Cheong S, Chaichanasakul T, Bezouglaia O, Atti E, Dry SM, et al. Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice. J Bone Miner Res. 2013;28:1631–1640. doi: 10.1002/jbmr.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, et al. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res. 2011;26:1871–1882. doi: 10.1002/jbmr.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannobile WV. Host-response therapeutics for periodontal diseases. J Periodontol. 2008;79:1592–1600. doi: 10.1902/jop.2008.080174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akram Z, Abduljabbar T, Kellesarian SV, Abu Hassan MI, Javed F, Vohra F. Efficacy of bisphosphonate as an adjunct to nonsurgical periodontal therapy in the management of periodontal disease: a systematic review. Br J Clin Pharmacol. 2017;83:444–454. doi: 10.1111/bcp.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Almeida J, Ervolino E, Bonfietti LH, Novaes VC, Theodoro LH, Fernandes LA, et al. Adjuvant therapy with sodium alendronate for the treatment of experimental periodontitis in rats. J Periodontol. 2015;86:1166–1175. doi: 10.1902/jop.2015.150166. [DOI] [PubMed] [Google Scholar]
- 10.Kanoriya D, Pradeep AR, Singhal S, Garg V, Guruprasad CN. Synergistic approach using platelet-rich fibrin and 1% alendronate for intrabony defect treatment in chronic periodontitis: a randomized clinical trial. J Periodontol. 2016;87:1427–1435. doi: 10.1902/jop.2016.150698. [DOI] [PubMed] [Google Scholar]
- 11.Duarte PM, de Vasconcelos Gurgel BC, Sallum AW, Filho GR, Sallum EA, Nociti FH., Jr Alendronate therapy may be effective in the prevention of bone loss around titanium implants inserted in estrogen-deficient rats. J Periodontol. 2005;76:107–114. doi: 10.1902/jop.2005.76.1.107. [DOI] [PubMed] [Google Scholar]
- 12.Doku HC, Shklar G, Bugbee B. The effect of epsilon aminocaproic acid on the healing of extraction wounds in hamsters. Oral Surg Oral Med Oral Pathol. 1966;22:569–577. doi: 10.1016/0030-4220(66)90159-9. [DOI] [PubMed] [Google Scholar]
- 13.Soares DM, Ramos-Perez F, Araújo SS, Correia Leite de Marcelos PG, Pontual AA, Perez D. Sildenafil citrate on experimental periodontitis in rats: microtomographic and histological analyses. Oral Dis. 2018;24:1073–1082. doi: 10.1111/odi.12846. [DOI] [PubMed] [Google Scholar]
- 14.Silva PG, Ferreira Junior AE, Teófilo CR, Barbosa MC, Lima Júnior RC, Sousa FB, et al. Effect of different doses of zoledronic acid in establishing of bisphosphonate-related osteonecrosis. Arch Oral Biol. 2015;60:1237–1245. doi: 10.1016/j.archoralbio.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto-Silva FP, Bradaschia-Correa V, Lima LA, Arana-Chavez VE. Ultrastructural and immunohistochemical study of early repair of alveolar sockets after the extraction of molars from alendronate-treated rats. Microsc Res Tech. 2013;76:633–640. doi: 10.1002/jemt.22210. [DOI] [PubMed] [Google Scholar]
- 16.Li CL, Lu WW, Seneviratne CJ, Leung WK, Zwahlen RA, Zheng LW. Role of periodontal disease in bisphosphonate-related osteonecrosis of the jaws in ovariectomized rats. Clin Oral Implants Res. 2016;27:1–6. doi: 10.1111/clr.12502. [DOI] [PubMed] [Google Scholar]
- 17.McClung MR. Bisphosphonate therapy: how long is long enough? Osteoporos Int. 2015;26:1455–1457. doi: 10.1007/s00198-014-3019-4. [DOI] [PubMed] [Google Scholar]
- 18.Dannemann C, Zwahlen R, Grätz KW. Clinical experiences with bisphopsphonate induced osteochemonecrosis of the jaws. Swiss Med Wkly. 2006;136:504–509. doi: 10.4414/smw.2006.11431. [DOI] [PubMed] [Google Scholar]
- 19.Viereck V, Emons G, Lauck V, Frosch KH, Blaschke S, Gründker C, et al. Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. Biochem Biophys Res Commun. 2002;291:680–686. doi: 10.1006/bbrc.2002.6510. [DOI] [PubMed] [Google Scholar]
- 20.Moreira MM, Bradaschia-Correa V, Marques ND, Ferreira LB, Arana-Chavez VE. Ultrastructural and immunohistochemical study of the effect of sodium alendronate in the progression of experimental periodontitis in rats. Microsc Res Tech. 2014;77:902–909. doi: 10.1002/jemt.22413. [DOI] [PubMed] [Google Scholar]
- 21.França TRT, Ramos-Perez FMM, Pontual ADA, Castro JFL, Bonan PRF, Perez DEDC. Effects of zoledronic acid in experimental periapical lesions in rats: an imaging and histological analysis. Braz Dent J. 2017;28:566–572. doi: 10.1590/0103-6440201601558. [DOI] [PubMed] [Google Scholar]
- 22.Bonnet N, Lesclous P, Saffar JL, Ferrari S. Zoledronate effects on systemic and jaw osteopenias in ovariectomized periostin-deficient mice. PLoS One. 2013;8:e58726. doi: 10.1371/journal.pone.0058726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messer JG, Mendieta Calle JL, Jiron JM, Castillo EJ, Van Poznak C, Bhattacharyya N, et al. Zoledronic acid increases the prevalence of medication-related osteonecrosis of the jaw in a dose dependent manner in rice rats (Oryzomys palustris) with localized periodontitis. Bone. 2018;108:79–88. doi: 10.1016/j.bone.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita J, Koi K, Yang DY, McCauley LK. Effect of zoledronate on oral wound healing in rats. Clin Cancer Res. 2011;17:1405–1414. doi: 10.1158/1078-0432.CCR-10-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]