Abstract
Purpose
Periodontitis is associated with a dysbiosis of periodontopathic bacteria, which stimulate the interleukin (IL)-23/IL-17 axis that plays an essential role in the immunopathogenesis of this disease, leading to alveolar bone destruction through receptor activator of nuclear factor κB ligand (RANKL). IL-23 receptor mRNA (IL-23R) has been identified in periodontitis, and IL-17 receptor A mRNA (IL-17RA) and its protein have not yet been evaluated in patients with periodontitis. In this study was measure IL-23R and IL-17RA in gingival tissue (GT) from patients with generalized chronic periodontitis (GCP) and generalized aggressive periodontitis (GAP) and to explore correlations with clinical parameters.
Methods
We included 16 healthy subjects (HS), 18 patients with GCP, and 14 with GAP. GT samples were collected during periodontal surgery. Both IL-23R and IL-17RA were detected by enzyme-linked immunosorbent assay.
Results
The results were analyzed with Mann-Whitney U test and Spearman' rank correlation coefficients using SPSS version 25.0. We found lower IL-23R levels in patients with GCP and GAP than in HS. Contrarily, we observed higher IL-17RA levels in GCP and GAP patients than in HS. Moreover, we found negative correlations between IL-23R in GT and probing depth and clinical attachment loss (CAL). Likewise, a positive correlation of IL-17RA in GT with CAL was found.
Conclusions
The results of these findings suggest that the reverse behavior between IL-23R and IL-17RA in periodontitis patients may also be involved with the activation of RANKL, which promotes alveolar bone loss.
Keywords: Aggressive periodontitis, Chronic periodontitis, Interleukine-17RA, Interleukine-23R
Graphical Abstract
INTRODUCTION
Periodontitis is a multifactorial chronic disease associated with a dysbiotic biofilms and deregulation of the periodontal inflammatory response. The predominant clinical manifestations include loss of periodontal tissue support manifested by clinical attachment loss (CAL), periodontal probing depth (PD), percentage of bleeding on probing (%BoP), and alveolar bone loss. According to the 1999 classification, chronic periodontitis is the most common periodontal disease, which is characterized by moderate progression and differs from aggressive periodontitis, which shows faster destruction of alveolar bone [1].
Periodontopathogenic bacteria such as Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia stimulate dendritic cells to secrete pro-inflammatory cytokines such as interleukin (IL)-23, which in turn, stimulates Th17 cells through its specific receptor (IL-23 receptor mRNA [IL-23R]) [2]. IL-23R is constituted by an IL-23R subunit (maintaining clonal expansion) and beta1 of an IL-12 receptor subunit (IL-12Rβ1); this receptor is expressed mainly in Th17 cells, which produce IL-17, IL-2, and receptor activator of nuclear factor κB ligand (RANKL), among others [3,4]. IL-17 joins its receptor (IL-17R), formed by the heterodimer IL-17 receptor A (IL-17RA) and IL-17RC isoforms, expressed in some cells such as fibroblasts to produce RANKL [5]. Moreover, IL-23R and IL-17RA can be present in soluble form (sIL-23R and sIL-17RA) through a different mechanism [6,7,8].
The IL-23/IL-17 axis and its receptors play a fundamental role in the immunopathogenesis of periodontal disease to stimulate RANKL production, activate pre-osteoclasts, and initiate degradation of the bone matrix [9] (Figure 1). In this sense, a discrepancy in IL-23/IL-17 in different biological samples has been reported in chronic and aggressive periodontitis [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25].
Figure 1. Role of IL-23 and IL-17 and their receptors (IL-23R and IL-17R) in periodontal disease.
LPS and other bacterial components induce the secretion of pro-inflammatory cytokines when they are recognized by APCs found in the gum, such as dendritic cells, and these produce cytokines such as IL-1, IL-6, and IL-23, among other pro-inflammatory cytokines [2]. IL-23 joins to a specific receptor (IL-23R) in Th17 cells. IL-23R consists of a subunit known as IL-23R that forms a complex with the beta-1 subunit of the receptor to IL-12 (IL-12Rβ1) [3]. The binding of IL-23 to its IL-23R receptor in Th17 cells promotes the phosphorylation of JAK2 and tyk2, which activates STAT3, allowing the overregulation of RORγT [4] and subsequently increasing the expression of pro-inflammatory cytokines such as IL-17 and RANKL [32,34]. Moreover, IL-17, by binding to its IL-17RA receptor expressed in fibroblasts, initiates the signaling cascade by ACT1 and TRAF6, giving way to the transduction of various pro-inflammatory cytokines like RANKL [5]. RANKL produced by Th17 or fibroblasts activates preosteoclasts to differentiate into mature osteoclasts, leading to alveolar bone reabsorption [32].
IL: interleukin, LPS: lipopolysaccharide, APC: antigen presenting cell, JAK2: Janus kinase 2, tyk2: tyrosine kinase 2, RANKL: receptor activator of nuclear factor κB ligand, TNF: tumor necrosis factor, MMP: matriz metalloproteinase.
In a pilot study, we reported higher sIL-23R levels in plasma from patients with generalized chronic periodontitis (GCP) and generalized aggressive periodontitis (GAP) than from healthy subjects (HS) [26]. Otherwise, we found lower levels of sIL-17RA in serum and plasma from GCP and GAP patients than from HS [21]. Regarding IL-23R in gingival tissue (GT), higher mRNA expression has been reported in moderate and severe chronic periodontitis sites than in healthy sites [27]. Likewise, IL-17RA has been evaluated at the mRNA level [28] and protein level by immunohistochemistry [29], but only in patients with rheumatoid arthritis. The aim of this study was to determine IL-23R and IL-17RA at the protein level in GT samples from patients with GCP and GAP because these receptors are involved in transduction signals that promote the production of RANKL, which plays a role in alveolar bone destruction.
MATERIALS AND METHODS
Study subjects
Fifty-three subjects who attended the periodontal clinic of the University of Guadalajara were recruited to participate in this study from February 15, 2017, to July 30, 2018. Patients who had clinical features typical of GCP and GAP were enrolled consecutively in the study [1]. All subjects were in good general health and had not received previous periodontal therapy or taken any antibiotics, immunomodulatory, or anti-inflammatory drugs months before the study. Pregnant women, smokers, patients who were undergoing antibiotic prophylaxis for dental treatment, patients with any systemic disease, and patients on long-term medications that could affect the expression of gingivitis or periodontitis were excluded from the study.
Medical and dental records were obtained from participants, and the diagnosis and classification of periodontal disease were performed according to the 1999 classification of the American Academy of Periodontology [1].
The calculation of the sample size was performed using a formula for studies that evaluate the difference of independent means, with a significance level of 95% and statistical power of 80%. Mean and standard deviation values provided by 2 studies that evaluated the cytokine levels of the IL-23/IL-17 axis in GT in periodontal disease were used [10,12]; the results of both studies were averaged and a sample size of 12 subjects was obtained per study group (n=12).
Ethical approval of studies and informed consent
The Ethics Committee of the University of Guadalajara approved this study (approval number: CI-00714). The objective was explained to each participant before they agreed to participate. Written informed consent was obtained from all participants according to the 2013 Declaration of Helsinki.
Periodontal clinical measurements
Clinical examinations were performed on all existing teeth of the participants, and the periodontal conditions were assessed based in terms of %BoP, PD, and CAL.
In all participants, the examinations were performed using a periodontal probe (15 mm, probe tip diameter 0.5 mm; University of North Carolina UNC-15 Hu-Friedy® (Hu-Friedy, Chicago, IL, USA) by a single researcher, and the PD and CAL were determined.
Clinical parameters (PD, CAL, and %BoP) were measures at 6 sites per tooth, and periapical radiographs were obtained by the same researcher using the long-cone paralleling technique with the same radiographic equipment, film, exposure, and development conditions for all subjects to obtain the diagnosis [1,30,31].
Study groups
HS group
This group included 16 patients (15 women and 1 man; median age, 40 years; interquartile range [IQR], 12 years) who attended the clinic for dental esthetic surgery (crown lengthening procedures) without clinical inflammation, or BoP, with ≤3 mm PD, and with no evidence of CAL, or radiographic bone loss [1,30,31].
GCP group
This study group included 23 patients (19 and 4 men; median age, 46 years; IQR, 21 years) who presented pockets in more than 30% of the oral cavity, including ≥5 mm CAL and ≥6 mm PD with radiographic evidence of alveolar bone loss [1,30,31].
GAP group
This group comprised 14 GAP patients (10 women and 4 men; median range, 32 years; IQR, 5.75 years) who had a family history of ≥1 family member with a history of severe periodontal problems. Showing CAL in more than 3 teeth further than the incisors and first molars, with a minimum of ≥5 mm CAL, ≥6 mm PD and radiographic evidence of advanced alveolar bone loss [1,30,31].
GT collection and protein extraction
GT was obtained during periodontal surgery. The collection included epithelium from the periodontal pocket or sulcus area and underlying connective tissue; the tissue was recollected with a #15c scalpel blade and LaGrange scissors (Hu-Friedy). In total, 23 GCP and 14 GAP patients were treated with flap surgery in sites with ≥5 mm PD and ≥6 mm CAL. Sixteen HS were included who underwent crown lengthening for aesthetic indications and during the surgical phase the tissue was recollected in sites with ≤3 mm PD and without CAL, each tissue sample was placed in a 1.5mL microtube. The microtubes were then immersed in crushed ice (4°C) and immediately transported to the laboratory to be stored in an ultra-freezer at −80°C until analysis.
The frozen GT were solubilized. First, the tissue was blotted, weighed on a microbalance, and then placed in a 1.5 mL microtube with a sufficient volume of RIPA Buffer (Sigma Chemicals, St. Louis, MO, USA) to ensure the following dilution: 10 mg tissue/100 mL RIPA Buffer plus protease inhibitor (cOmplete™ Protease Inhibitor Cocktail, Roche Diagnostic GmbH, Mannheim, Germany). Next, the tissues were macerated with a polypropylene pestle and a vortex. Finally, samples were centrifuged at 3,000 rpm for 15 minutes. The supernatant was frozen at −80°C until the IL-23R and IL-17RA enzyme-linked immunosorbent assay (ELISA) was performed. The procedure was carried out at 4°C [1,30,31].
Protein assay
A standard Bradford micro-method Bradford reagent (Coomassie Brilliant Blue G-250, 95% ethanol, concentrated phosphoric acid, and water) was used to assess the protein concentration in GT samples. The absorbance was read at 570 nm in a microplate spectrophotometer (Poweam Medical Systems Co., Nanjing, China). Protein concentrations were calculated with a bovine serum albumin standard curve (Sigma Chemical) and expressed as milligrams per milliliter [1,30,31].
ELISA
Protein extraction aliquots from GT and standards were added in triplicate to a 96 well microtiter plate to determine IL-23R and IL-17RA concentrations (R&D Systems, Minneapolis, MN, USA). Optical density was measured in a microplate reader set to 450 nm with wavelength correction at 540 nm. The signal was detected using a Microplate Reader WHY101 (Poweam Medical Systems Co.). The IL-23R and IL-17RA concentrations were calculated with the standard curve included in each assay kit. The concentrations of human IL-23R and IL-17RA were expressed as picogram per milligram of GT.
Statistical analyses
IL-23R and IL-17RA levels, age, %BoP, PD, and CAL are expressed as median and IQR, and results according to sex are presented as frequencies. The data showed an abnormal distribution using the Shapiro-Wilk test for a small sample size. To observe the differences in IL-23R, IL-17RA, age, %BoP, PD, and CAL between the study groups, the non-parametric Mann-Whitney U test was used, and χ2 test was used to compare differences by sex and periodontal stage and grade. Spearman' rank correlation coefficients were used to study the relationships between IL-23R and IL-17RA levels in GT and clinical findings. All results were analyzed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). Significance was considered at a P value of ≤0.05.
RESULTS
Demographic characteristics and clinical parameters
The GCP group was older than HS and GAP groups. Women predominated in all 3 study groups. The GCP group showed a higher %BoP than the GAP and HS groups. PD and CAL were higher in the GAP group in the GCP and HS groups (Table 1).
Table 1. Demographic characteristics and clinical parameters.
| Parameters | HS (n=16) | GCP (n=23) | GAP (n=14) | P value |
|---|---|---|---|---|
| Mean age (yr) | 40 (12) | 46 (21) | 32 (5.75)a)b) | 0.001 |
| Female/male | 15/1 | 19/4 | 10/4 | 0.270 |
| %BoP | 0 (0) | 10.87 (46.96)a) | 9.25 (92.33)a) | 0.003 |
| PD (mm) | 2.4 (1) | 5.5 (1)a) | 6.33 (3.05)a)b) | <0.001 |
| CAL (mm) | 0 (1.56) | 5.5 (2.5)a) | 7.75 (3.14)a)b) | <0.001 |
Data are the median and interquartile range or percentages.
HS: healthy subjects, GCP: generalized chronic periodontitis, GAP: generalized aggressive periodontitis, BoP: bleeding on probing, PD: probing depth, CAL: clinical attachment level.
a)Significant differences compared to HS; b)Significant differences compared to GCP.
IL-23R and IL-17RA levels in the GT
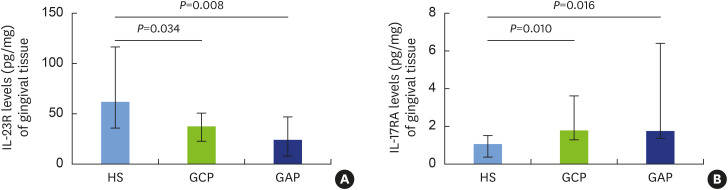
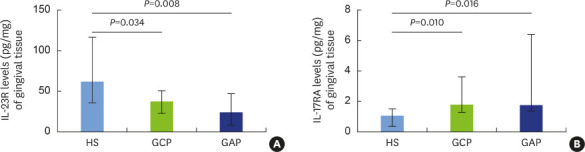
Concentrations of IL-23R were measured in the GT of the study subjects, and significantly lower levels were found in the GCP (median, 37.86; IQR, 27.65) and GAP (median, 24.42; IQR, 39.33) groups in the HS group (median, 62.43; IQR, 81.1) (Figure 2A). Regarding the concentrations of IL-17RA measured in the GT, we found a significantly higher levels in patients with GCP (median, 1.78; IQR, 2.36) and GAP (median, 1.75; IQR, 5.1) than in HS (median, 1.07; IQR, 1.13) (Figure 2B). However, we found no significant differences in IL-23R and IL-17RA levels in GT between the GCP and GAP groups (Figure 2).
Figure 2. IL-23R and IL-17RA levels in gingival tissue from patients with chronic and aggressive periodontitis.
The levels of IL-23 and IL-17 receptors in gingival tissue of HS, patients with GCP, and patients with GAP were measured by enzyme-linked immunosorbent assay and expressed as picograms of IL-23R and IL-17RA per milligram of gingival tissue. The results are shown as median and interquartile range and were analyzed using the Mann-Whitney U test. We found significantly lower IL-23R levels in patients with GCP and GAP than in HS (A). Conversely, we observed significantly higher in IL-17RA levels in patients with GCP and GAP than in HS (B). A P value ≤0.05 was considered to indicate statistical significance.
IL: interleukin, HS: healthy subjects, GCP: generalized chronic periodontitis, GAP: generalized aggressive periodontitis.
Correlations between IL-23R and IL-17RA in the GT clinical parameters
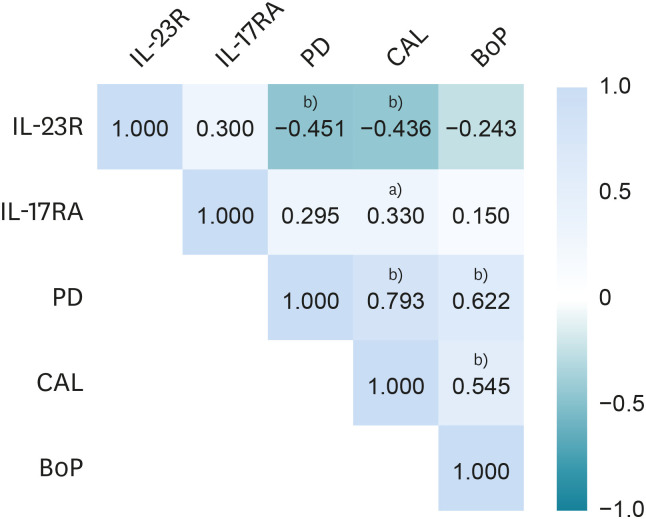
Spearman' rank correlation coefficients were calculated for the clinical parameters and the IL-23R and IL-17RA levels. Since the sample were GT, the clinical parameters used for the correlations correspond to the exact areas where the GT was collected. We found that IL-23R levels showed negative correlation with PD and CAL. Moreover, a positive correlation was found between IL-17RA levels and CAL. Regarding the correlations between the clinical parameters (PD, CAL, and %BoP), we found significant correlations between PD and CAL, between PD and %BoP, and between CAL and %BoP (Figure 3).
Figure 3. Correlations between IL-23R and IL-17RA in the gingival tissue and clinical parameters.
Spearman' rank correlation coefficients were calculated for the values of the evaluated clinical parameters and the concentrations of the receptors.
IL: interleukin, PD: probing depth, CAL: clinical attachment loss, BoP: bleeding on probing.
a)P value ≤0.01; b) P value ≤0.05.
DISCUSSION
The IL-23/IL-17 axis plays an essential role in the immunopathogenesis of periodontitis [2]. The binding of IL-23 to its IL-23R receptor in Th17 cells promotes the expression of pro-inflammatory cytokines such as IL-17 and RANKL [32]. IL-17, by binding to its IL-17RA receptor expressed in fibroblasts, induces the transduction of various pro-inflammatory cytokines such as RANKL. RANKL produced by Th17 or fibroblasts activates preosteoclasts differentiate into mature osteoclasts, leading to alveolar bone reabsorption [2].
Several studies have shown differences in the levels of the IL-23/IL-17 axis in biological samples from patients with periodontal disease [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. However, only 1 study has evaluated the expression of IL-23R mRNA in the GT of periodontal diseases [27], and IL-17RA has only been evaluated in patients with rheumatoid arthritis at the mRNA level [28] and protein level [29].
In pilot studies, we determined the levels of IL-23R and IL-17RA in serum and plasma from patients with GCP and GAP [21,26]. However, IL-23R and IL-17RA levels at the protein level in GT samples from patients with chronic and aggressive periodontitis have not been reported.
IL-23R can also be present in a soluble form (sIL-23R), which is generated by 2 mechanisms: alternative splicing [6] and cleavage of cell membranes by proteases, such as ADAM10 and ADAM17 [7]. In this sense, Bostanci et al. [33] did not observe a difference between ADAM17 concentrations in gingival crevicular fluid from healthy HS and GCP patients. In GT higher mRNA IL-23R expression has been reported in sites with periodontitis than in HS [27]. Our results suggest the possibility that once the mRNA of IL-23R is translated into protein, others adamalysins such ADAM10 could cleave IL-23R [7], which probably leads to reduced levels of this receptor in the GT of patients with GCP and GAP.
In addition to these results, we also found a negative correlation between IL-23R in the GT and clinical parameters (PD and CAL). Therefore, we can infer that the expression pattern of this receptor might depend on disease progression since, with higher PD and CAL, the concentration of IL-23R in the GT decreased. IL-23R may be overexpressed in the early stages of periodontal disease since IL-23 stimulates IL-23R overexpression in Th17 cells to activate clonal expansion [34].
Concerning IL-17RA in the GT, we found higher concentrations in GCP and GAP patients than in HS. Contrary to these results, in a previous study, we reported lower levels of sIL-17RA in plasma from GAP and GCP patients than in HS [21]. In this context, it has been demonstrated that several isoforms of IL-17RA, including the sIL-17RA soluble isoform, lack the intramembrane region and are generated by alternative splicing [6]. This isoform can capture IL-17A, which functions as an antagonist to IL-17RA, and by this mechanism it may inhibit signaling pathways [8,35]. It is possible that the IL-17RA expression in the GT is higher in GCP and GAP patients, and this membrane-bound receptor is not being converted to sIL-17RA by splicing, this possible explanation is consistent with the low levels of sIL-17RA in the plasma of patients with GCP and GAP that we previously demonstrated [21].
Furthermore, it is essential to note that the IL-17RA levels in the GT of patients with GCP and GAP are similar to the IL-17 levels reported in various types of samples from patients with periodontitis [10,12,13,14,15,16,17,18,19,20,21,22,23,25].
In contrast, we found a positive correlation between IL-17RA in the GT with CAL. In the GT, we evaluated the total amount of IL-17RA and found support for the idea that increased levels of this receptor in tissue are strongly related to a more severe loss of gingival insertion and, consequently, the progression of periodontitis. IL-17A is known to play a fundamental role in alveolar bone destruction; most likely, in the GT, IL-17RA plays a pivotal role in RANKL production, mainly by fibroblast [36].
It is important to point out that analysis of IL-23R and IL-17RA protein levels in GT from GAP and GCP patients initiates with the knowledge of the function of these receptors in situ. These molecules will also be evaluated in periodontitis, according to the new classification of periodontal disease [37,38]. A previous report described IL-23R mRNA expression in periodontitis [27]; however, no studies have investigated IL-17RA mRNA expression in the GT of these patients. Moreover, it is unknown whether several adamalysins can cleave IL-17RA from the membrane as ADAM17 does with IL-23R in periodontitis.
However, there is strong evidence that chronic periodontitis is linked to both the initiation and progression of systemic diseases such as rheumatoid arthritis or neuroinflammatory/neurodegenerative disorders such as Alzheimer's disease and multiple sclerosis [39,40].
In this regard, IL-1 and tumor necrosis alpha-1 produced in response to lipopolysaccharides (LPS) derived from periodontopathogenic bacteria in periodontitis have been described as trigger neuroinflammatory/neurodegenerative diseases [39,40]. Ballerini et al. [40] proposed a model of anti-inflammatory effects derived from stem cells from relapsing-remitting multiple sclerosis patients as a new perspective on the therapeutic use of autologous periodontal stem cells in neuro-inflammatory/neurodegenerative diseases.
This advancement is of paramount importance because the IL-23/IL-17 axis is also triggered by the LPS stimulus from periodontopathogenic bacteria [2], so the model proposed by Ballerini et al. [40] could be applied as a new therapeutic approach to periodontitis and its associated systemic diseases.
In conclusion, the inverse behavior of IL-23R and IL-17RA in GT from patients with GCP and GAP showed the role of these molecules in situ regarding the severity of periodontitis and the involvement of these molecules in the production of RANKL, which promotes alveolar bone loss in periodontitis. Lower levels of IL-23R in GCP and GAP, were associated with a higher severity of clinical parameters such as PD, CAL, and %BoP. Conversely, higher levels of IL-17RA were present in GCP and GAP patients, and were associated whit more severe CAL.
Finally, molecular and functional tests of IL-23R and IL-17RA would provide knowledge about the immunopathogenesis of periodontal disease, including the mechanisms involved in the destruction of periodontal supporting tissues. In the near future, it may be possible to implement immunological therapies that regulate these mechanisms to decrease the destruction of tooth supporting tissues.
Footnotes
Funding: This study was carried out with financial support from the University of Guadalajara, and the Mexican Association of Periodontology.
- Conceptualization: Alondra del Carmen Ruíz-Gutiérrez, Ruth Rodríguez-Montaño, Celia Guerrero-Velázquez.
- Data curation: Alondra del Carmen Ruíz-Gutiérrez, Ruth Rodríguez-Montaño, Celia Guerrero-Velázquez.
- Formal analysis: Alondra del Carmen Ruíz-Gutiérrez, Ruth Rodríguez-Montaño, Celia Guerrero-Velázquez.
- Investigation: Alondra del Carmen Ruíz-Gutiérrez, Ruth Rodríguez-Montaño, Ana Lourdes Zamora-Perez.
- Methodology: Alondra del Carmen Ruíz-Gutiérrez, Ruth Rodríguez-Montaño, María Luisa Pita-López.
- Project administration: Ana Lourdes Zamora-Perez, María Luisa Pita-López.
- Writing - original draft: Alondra del Carmen Ruíz-Gutiérrez, Ruth Rodríguez-Montaño, Celia Guerrero-Velázquez.
- Writing - review & editing: Ana Lourdes Zamora-Perez, Celia Guerrero-Velázquez.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Weighardt H, Jusek G, Mages J, Lang R, Hoebe K, Beutler B, et al. Identification of a TLR4- and TRIF-dependent activation program of dendritic cells. Eur J Immunol. 2004;34:558–564. doi: 10.1002/eji.200324714. [DOI] [PubMed] [Google Scholar]
- 3.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 4.Floss DM, Klöcker T, Schröder J, Lamertz L, Mrotzek S, Strobl B, et al. Defining the functional binding sites of interleukin 12 receptor β1 and interleukin 23 receptor to Janus kinases. Mol Biol Cell. 2016;27:2301–2316. doi: 10.1091/mbc.E14-12-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song X, Qian Y. IL-17 family cytokines mediated signaling in the pathogenesis of inflammatory diseases. Cell Signal. 2013;25:2335–2347. doi: 10.1016/j.cellsig.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Kan SH, Mancini G, Gallagher G. Identification and characterization of multiple splice forms of the human interleukin-23 receptor alpha chain in mitogen-activated leukocytes. Genes Immun. 2008;9:631–639. doi: 10.1038/gene.2008.64. [DOI] [PubMed] [Google Scholar]
- 7.Franke M, Schröder J, Monhasery N, Ackfeld T, Hummel TM, Rabe B, et al. Human and murine interleukin 23 receptors are novel substrates for a disintegrin and metalloproteases ADAM10 and ADAM17. J Biol Chem. 2016;291:10551–10561. doi: 10.1074/jbc.M115.710541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohda M, Misumi Y, Tashiro K, Yamazaki M, Saku T, Oda K. Identification of a soluble isoform of human IL-17RA generated by alternative splicing. Cytokine. 2013;64:642–645. doi: 10.1016/j.cyto.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Hienz SA, Paliwal S, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res. 2015;2015:615486. doi: 10.1155/2015/615486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson RB, Wood N, Serio FG. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J Periodontol. 2004;75:37–43. doi: 10.1902/jop.2004.75.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Azuma T, Motohira H, Kinane DF, Kitetsu S. The potential role of interleukin-17 in the immunopathology of periodontal disease. J Clin Periodontol. 2005;32:369–374. doi: 10.1111/j.1600-051X.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 12.Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005;32:383–389. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 13.Duarte PM, da Rocha M, Sampaio E, Mestnik MJ, Feres M, Figueiredo LC, et al. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: a pilot study. J Periodontol. 2010;81:1056–1063. doi: 10.1902/jop.2010.090732. [DOI] [PubMed] [Google Scholar]
- 14.Ozçaka O, Nalbantsoy A, Buduneli N. Interleukin-17 and interleukin-18 levels in saliva and plasma of patients with chronic periodontitis. J Periodontal Res. 2011;46:592–598. doi: 10.1111/j.1600-0765.2011.01377.x. [DOI] [PubMed] [Google Scholar]
- 15.Himani GS, Prabhuji ML, Karthikeyan BV. Gingival crevicular fluid and interleukin-23 concentration in systemically healthy subjects: their relationship in periodontal health and disease. J Periodontal Res. 2014;49:237–245. doi: 10.1111/jre.12100. [DOI] [PubMed] [Google Scholar]
- 16.Fu QY, Zhang L, Duan L, Qian SY, Pang HX. Correlation of chronic periodontitis in tropical area and IFN-γ, IL-10, IL-17 levels. Asian Pac J Trop Med. 2013;6:489–492. doi: 10.1016/S1995-7645(13)60080-2. [DOI] [PubMed] [Google Scholar]
- 17.Ruíz-Gutiérrez AC, Herrera-Mora MC, Zamora-Pérez AL, Meléndez-Ruíz JL, Martínez-Rodríguez VC, Guerrero-Velázquez C. Determinación de los niveles de IL-17 en el líquido crevicular gingival de pacientes con periodontitis crónica y agresiva. Rev Mex Periodontol. 2014;5:46–50. [Google Scholar]
- 18.Mitani A, Niedbala W, Fujimura T, Mogi M, Miyamae S, Higuchi N, et al. Increased expression of interleukin (IL)-35 and IL-17, but not IL-27, in gingival tissues with chronic periodontitis. J Periodontol. 2015;86:301–309. doi: 10.1902/jop.2014.140293. [DOI] [PubMed] [Google Scholar]
- 19.Cifcibasi E, Koyuncuoglu C, Ciblak M, Badur S, Kasali K, Firatli E, et al. Evaluation of local and systemic levels of interleukin-17, interleukin-23, and myeloperoxidase in response to periodontal therapy in patients with generalized aggressive periodontitis. Inflammation. 2015;38:1959–1968. doi: 10.1007/s10753-015-0176-3. [DOI] [PubMed] [Google Scholar]
- 20.Liukkonen J, Gürsoy UK, Pussinen PJ, Suominen AL, Könönen E. Salivary concentrations of interleukin (IL)-1β, IL-17A, and IL-23 vary in relation to periodontal status. J Periodontol. 2016;87:1484–1491. doi: 10.1902/jop.2016.160146. [DOI] [PubMed] [Google Scholar]
- 21.Peña-Echeverría PA, Rodríguez-Montaño R, Ruiz-Gutiérrez AC, Martínez-Rodríguez VMC, Gómez-Meda BC, Cervantes-Cabrera JJ, et al. Determinación de la concentración de IL-23 y el receptor soluble a IL-17 (IL-17RA) en suero y plasma de pacientes con periodontitis crónica y agresiva: un estudio piloto. Rev Mex Periodontol. 2017;8:46–53. [Google Scholar]
- 22.Chitrapriya MN, Rao SR, Lavu V. Interleukin-17 and interleukin-18 levels in different stages of inflammatory periodontal disease. J Indian Soc Periodontol. 2015;19:14–17. doi: 10.4103/0972-124X.145798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batool H, Nadeem A, Kashif M, Shahzad F, Tahir R, Afzal N. Salivary levels of IL-6 and IL-17 could be an indicator of disease severity in patients with calculus associated chronic periodontitis. Biomed Res Int. 2018;2018:8531961. doi: 10.1155/2018/8531961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadeghi R, Sattari M, Dehghan F, Akbari S. Interleukin-17 and interleukin-23 levels in gingival crevicular fluid of patients with chronic and aggressive periodontitis. Cent Eur J Immunol. 2018;43:76–80. doi: 10.5114/ceji.2018.74876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorbert‐Mros S, Larsson L, Kalm J, Berglundh T. Interleukin‐17 producing T cells and interleukin‐17 mRNA expression in periodontitis and longstanding gingivitis lesions. J Periodontol. 2019;90:516–521. doi: 10.1002/JPER.18-0326. [DOI] [PubMed] [Google Scholar]
- 26.Rivadeneyra-Burgos C, Rodríguez-Montaño R, Ruíz-Gutiérrez AC, Martínez-Rodríguez VC, Meléndez-Ruiz JL, Pita-López ML, et al. Determinación de los niveles del receptor soluble de IL-23 en suero y plasma de pacientes con periodontitis crónica y agresiva. Rev Mex Periodontol. 2017;8:5–10. [Google Scholar]
- 27.Ohyama H, Kato-Kogoe N, Kuhara A, Nishimura F, Nakasho K, Yamanegi K, et al. The involvement of IL-23 and the Th17 pathway in periodontitis. J Dent Res. 2009;88:633–638. doi: 10.1177/0022034509339889. [DOI] [PubMed] [Google Scholar]
- 28.Hillyer P, Larché MJ, Bowman EP, McClanahan TK, de Waal Malefyt R, Schewitz LP, et al. Investigating the role of the interleukin-23/-17A axis in rheumatoid arthritis. Rheumatology (Oxford) 2009;48:1581–1589. doi: 10.1093/rheumatology/kep293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Baarsen LG, Lebre MC, van der Coelen D, Aarrass S, Tang MW, Ramwadhdoebe TH, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi: 10.1186/s13075-014-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sánchez-Hernández PE, Zamora-Perez AL, Fuentes-Lerma M, Robles-Gómez C, Mariaud-Schmidt RP, Guerrero-Velázquez C. IL-12 and IL-18 levels in serum and gingival tissue in aggressive and chronic periodontitis. Oral Dis. 2011;17:522–529. doi: 10.1111/j.1601-0825.2011.01798.x. [DOI] [PubMed] [Google Scholar]
- 31.Franco-Topete R, Zepeda-Nuño JS, Zamora-Perez AL, Fuentes-Lerma MG, Gómez-Meda BC, Guerrero-Velázquez C. IFN-γR2 is strongly expressed on endothelial cells of gingival tissues from patients with chronic periodontitis. J Appl Oral Sci. 2018;26:e20170291. doi: 10.1590/1678-7757-2017-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bostanci N, Emingil G, Afacan B, Han B, Ilgenli T, Atilla G, et al. Tumor necrosis factor-alpha-converting enzyme (TACE) levels in periodontal diseases. J Dent Res. 2008;87:273–277. doi: 10.1177/154405910808700311. [DOI] [PubMed] [Google Scholar]
- 34.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Desharnais J, Sahasrabudhe PV, Jin P, Li W, Oates BD, et al. Inhibiting complex IL-17A and IL-17RA interactions with a linear peptide. Sci Rep. 2016;6:26071. doi: 10.1038/srep26071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin D, Li L, Sun Y, Wang W, Wang X, Ye Y, et al. IL-17 regulates the expressions of RANKL and OPG in human periodontal ligament cells via TRAF6/TBK1-JNK/NF-κB pathways. Immunology. 2014;144:472–485. doi: 10.1111/imm.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caton JG, Armitage G, Berglundh T, Chapple IL, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45(Suppl 20):S1–8. doi: 10.1111/jcpe.12935. [DOI] [PubMed] [Google Scholar]
- 38.Zaki AA. The new classification of periodontal diseases. BDJ Team. 2020;7:32–33. [Google Scholar]
- 39.Gaur S, Agnihotri R. Alzheimer's disease and chronic periodontitis: Is there an association? Geriatr Gerontol Int. 2015;15:391–404. doi: 10.1111/ggi.12425. [DOI] [PubMed] [Google Scholar]
- 40.Ballerini P, Diomede F, Petragnani N, Cicchitti S, Merciaro I, Cavalcanti MF, et al. Conditioned medium from relapsing-remitting multiple sclerosis patients reduces the expression and release of inflammatory cytokines induced by LPS-gingivalis in THP-1 and MO3.13 cell lines. Cytokine. 2017;96:261–272. doi: 10.1016/j.cyto.2017.04.022. [DOI] [PubMed] [Google Scholar]