Abstract
Background
Extracorporeal membrane oxygenation (ECMO) is a valuable rescue therapy to treat refractory hypoxemia caused by influenza. The present meta-analysis aimed to compare the clinical characteristics and outcomes of ECMO between COVID-19 and influenza.
Methods
We searched the PubMed, Cochrane Library, SCOPUS, and Web of Science databases from inception to May 1, 2021. The included studies compared the clinical characteristics and outcomes of ECMO between adults with COVID-19 and those with influenza.
Results
The study included four retrospective cohorts involving a total of 129 patients with COVID-19 and 140 with influenza who were treated using ECMO. Clinical characteristics were similar between the COVID-19 and influenza groups, including body mass index (BMI), diabetes mellitus, hypertension, and immunocompromised status. A higher proportion of patients with COVID-19 on ECMO were male (75.9% vs. 62.9%; P = 0.04). There was no difference between the groups in terms of illness severity based on sequential organ failure assessment (SOFA) score or serum pH. Patients with COVID-19 had a longer mean duration of mechanical ventilation before ECMO (6.63 vs. 3.38 days; P < 0.01). The pooled mortality rate was 43.8%. The mean ECMO duration (14.13 vs. 12.55 days; P = 0.25) and mortality rate (42.6% vs. 45.0%; P = 0.99) were comparable between the groups.
Conclusion
Clinical characteristics, ECMO duration, and mortality were comparable between patients with COVID-19 and those with influenza who required ECMO to treat refractory hypoxemia. The duration of mechanical ventilation before ECMO did not influence outcomes. Patients with COVID-19 benefit from ECMO salvage therapy similarly to those with influenza.
Keywords: COVID-19, SARS-CoV-2, ECMO, ECLS, Influenza
Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; ECMO, extracorporeal membrane oxygenation; HTN, hypertension; ICU, intensive care unit; MERS, Middle East respiratory syndrome; SOFA, sequential organ failure assessment
1. Introduction
In early December 2019, a novel viral respiratory disease was discovered and termed coronavirus disease 2019 (COVID-19), which has resulted in a worldwide pandemic. COVID-19 can present with a wide variety of clinical manifestations, from a mild flu-like illness to severe respiratory failure due to acute respiratory distress syndrome (ARDS). Advancements in management and therapies to reduce morbidity and mortality among patients with COVID-19 are still being pursued as the disease continues to spread across the globe. At present, there are few therapeutic options for COVID-19, and if lung-protective mechanical ventilation and recruitment maneuvers fail to treat refractory hypoxia or hypercapnia, extracorporeal membrane oxygenation (ECMO) is often required to provide temporary organ support. The benefits of ECMO have been studied among critically ill patients with ARDS during the 2009H1N1 influenza and the 2014 Middle East respiratory syndrome (MERS) outbreak [[1], [2], [3]]. This has resulted in the increasing use of ECMO as a salvage therapy for patients with ARDS suffering from seasonal influenza [4,5]. Due to the similarities shared by COVID-19 and influenza, several studies have compared the clinical characteristics and outcomes between hospitalized patients with COVID-19, including those who were critically ill, and those with seasonal influenza [[6], [7], [8], [9]]. In general, critically ill patients with COVID-19 have more comorbidities and are more likely to develop respiratory and extra-respiratory complications. As a result COVID-19 has a higher mortality rate than influenza, even in patients who are critically ill [[7], [8], [9]]. ECMO is a resource-intensive, highly specialized, demanding, and expensive form of life support that can lead to significant complications; its benefits remains less clear in severe COVID-19 than in influenza. Previous observational studies have demonstrated that critically ill patients with influenza are more likely to be initiated on ECMO than those with COVID-19, although this did not necessarily improve mortality [6,7,10]. Few studies have compared clinical characteristics and outcomes between patients with COVID-19 and those with influenza on ECMO. The present systematic review and meta-analysis used published evidence to compare clinical characteristics and outcomes between patients with COVID-19 and those with influenza who required ECMO.
2. Materials and methods
The present systematic review was conducted and presented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Ethical approval and informed consent were not required as this was a systematic review and meta-analysis of previously published studies. The protocol was registered and published in the International Prospective Register of Systematic Reviews (PROSPERO) under reference number CRD42021249317.
2.1. Search criteria and selection
A literature search was performed in the PubMed, Cochrane Library, SCOPUS, and Web of Science databases from inception to May 1, 2021, using the keywords and respective Medical Subjects Headings (MeSH) terms: “COVID-19,” “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),” “ECMO,” “extracorporeal life support (ECLS),” and “influenza”. Two authors (W.C. and B.S.) independently reviewed the titles and abstracts of all search results for eligibility. If an article was considered potentially eligible, both authors independently examined the full article for inclusion. Disagreements between the authors were resolved by consensus-based discussion. If no consensus was reached, a third reviewer (B.M.) made the final decision. To detect additional studies, we searched the reference lists of all retrieved articles for more eligible studies.
2.2. Inclusion and exclusion criteria
We included studies that: (1) compared outcomes of clinical characteristics and mortality between critically ill patients with COVID-19 (population) and those with influenza (comparison) who received ECMO (intervention); (2) involved adult patients (aged ≥18 years) with COVID-19 or influenza; (3) had a cross-sectional, case-control, or cohort design; (4) diagnosed COVID-19 or H1N1 infection using real-time reverse transcription-polymerase chain reaction from a nasopharyngeal or oropharyngeal swab. We excluded studies that: (1) were systematic reviews, literature reviews, case reports, case series, editorials, commentaries, or opinion articles, although the references of such articles were screened for articles meeting our inclusion criteria; (2) discussed infectious outbreaks other than the COVID-19 pandemic; (3) were conducted on animals or in vitro; (4) were published in languages other than English if no translated version was available.
2.3. Data collection and synthesis
The extracted data from the full texts of the included studies were compiled into a standardized form. The following information was collected and summarized in Table 1 : study design, period of patient enrollment, clinical characteristics, and associated outcomes. Descriptive statistics were reported as means ± standard deviations (SDs). Any disagreements or discrepancies regarding the extracted data were resolved via discussion between two authors (W.C. and B.S.), or with a third researcher (B.M.).
Table 1.
Summary of clinical characteristics and outcomes of cohort studies.
| ECMO | Charlton et al. [28] | Cousin et al. [29] | Jäckel et al. [30] | Luyt et al. [14] |
|---|---|---|---|---|
| Study design | Single-center, retrospective | Multi-center, retrospective | Single-center, retrospective | Single-center, retrospective |
| Country | UK | France | Germany | France |
| COVID-19 enrollment | April 2020–May 2020 | March 2020–May 2020 | April 2020–May 2020 | March 2020–April 2020 |
| Influenza enrollment | 2018–2019 | January 2014–May 2020 | October 2010–June 2020 | 2017–2020 |
| COVID-19 (N) | 34 | 30 | 15 | 50 |
| Influenza (N) | 26 | 22 | 47 | 45 |
| COVID-19 age (Y) | 46.30 ± 7.50 | 57.00 ± 11.11 | 60.80 ± 9.56 | 48.00 ± 10.37 |
| Influenza age (Y) | 43.10 ± 8.70 | 55.00 ± 8.89 | 52.70 ± 13.93 | 58.00 ± 11.85 |
| COVID-19 male N (%) | 27 (79.4) | 24 (80.0) | 11 (73.3) | 36 (72.0) |
| Influenza male N (%) | 18 (69.2) | 14 (63.6) | 28 (59.6) | 28 (62.2) |
| COVID-19 BMI (kg/m2) | 31.90 ± 6.00 | 33.00 ± 6.67 | 27.80 ± 4.67 | NR |
| Influenza BMI (kg/m2) | 30.60 ± 7.80 | 30.00 ± 5.93 | 27.50 ± 8.15 | NR |
| COVID-19 HTN N (%) | 8 (23.5) | 7 (31.8) | 5 (33.3) | NR |
| Influenza HTN N (%) | 5 (19.2) | 2 (9.1) | 20 (42.8) | NR |
| COVID-19 DM N (%) | 4 (11.8) | 10 (33.3) | 2 (13.3) | NR |
| Influenza DM N (%) | 2 (7.7) | 2 (9.1) | 8 (17.0) | NR |
| COVID-19 immunocompromised N (%) | NR | 3 (10.0) | 0 (0) | 1 (2.0) |
| Influenza immunocompromised N (%) | NR | 1 (4.5) | 8 (17.0) | 4 (8.9) |
| COVID-19 SOFA score | NR | 10.00 ± 3.70 | 10.00 ± 2.22 | 12.00 ± 2.96 |
| Influenza SOFA score | NR | 11.00 ± 3.70 | 8.00 ± 2.22 | 15.00 ± 5.19 |
| COVID-19 pre-ECMO pH | 7.30 ± 0.10 | 7.37 ± 0.07 | 7.30 ± 0.13 | NR |
| Influenzae pre-ECMO pH | 7.30 ± 0.20 | 7.35 ± 0.12 | 7.28 ± 0.12 | NR |
| COVID-19 ventilated pre-ECMO (D) | 4.90 ± 1.70 | 6.00 ± 3.70 | 4.60 ± 3.41 | 11.00 ± 5.19 |
| Influenzae ventilated pre-ECMO (D) | 2.40 ± 2.50 | 3.00 ± 2.96 | 1.10 ± 1.85 | 7.00 ± 2.96 |
| COVID-19 ECMO duration (D) | 13.20 ± 5.60 | 11.00 ± 5.19 | 11.30 ± 11.85 | 21.00 ± 17.78 |
| Influenzae ECMO duration (D) | 12.30 ± 8.00 | 11.00 ± 9.63 | 8.90 ± 7.63 | 18.00 ± 17.04 |
| COVID-19 mortality N (%) | 16 (47.1) | 16 (53.3) | 6 (40.0) | 17 (34.0) |
| Influenzae mortality N (%) | 8 (30.8) | 10 (45.5) | 27 (57.4) | 18 (40.0) |
Abbreviations: BMI, body mass index; D, days; DM, diabetes mellitus; ECMO, extracorporeal membrane oxygenation; HTN, hypertension; N, numbers; SOFA, sequential organ failure assessment; Y, years.
2.4. Outcomes
The primary outcomes of interest were differences in mortality or ECMO duration between critically ill patients with COVID-19 and those with H1N1 influenza patients requiring ECMO. The secondary outcomes were differences in clinical characteristics between the two populations. The following clinical characteristics were collected: patient demographics (age, sex, and body mass index [BMI]), comorbidities (hypertension [HTN], diabetes mellitus [DM], and immunocompromised status), sequential organ failure assessment (SOFA) score at intensive care unit (ICU) admission, pre-ECMO pH, and duration of mechanical ventilation.
2.5. Quality assessment
The quality of individual observational studies was assessed by two researchers (W.C. and B.S.) using the Newcastle–Ottawa Scale (NOS), which contains nine items. Briefly, the NOS scale assesses three important features of studies: adequacy of the selection of exposed and non-exposed cohorts, comparability of groups, and adequacy of outcome assessment, with a total score ranging from 0 to 9 [11]. The study quality can be divided into three groups: low quality, 0–3; moderate quality, 4–6; high quality, 7–9. During the quality assessment of the included studies, any disagreements were resolved by discussion or by a third researcher (B.M.).
2.6. Statistical analysis
A meta-analysis was performed for the primary and secondary outcomes using the Review Manager (RevMan) software, Version 5.4, The Cochrane Collaboration, 2020. Dichotomous outcomes were assessed using the Mantel–Haenszel statistical method and measured in odds ratios (ORs) with 95% confidence intervals (CIs). Continuous outcomes were evaluated using the inverse variance statistical method and measured in terms of standard mean difference (SMD). The inverse variance method accounts for the differing sample sizes of individual studies by weighting studies based on the variance of their estimates: small studies with large variances have less weighting and large studies with small variances have more weighting. Pooled ORs, SMDs, and 95% CIs were calculated using DerSimonian and Laird's random-effects model, and extracted outcomes were pooled by weighted averages [12]. Statistical heterogeneity among studies was assessed using I2 statistics, with high heterogeneity defined as an I2 value of ≥50% [13]. All P-values < 0.05 were considered statistically significant. Publication bias was examined by visual inspection of the funnel plot.
3. Results
3.1. Study selection
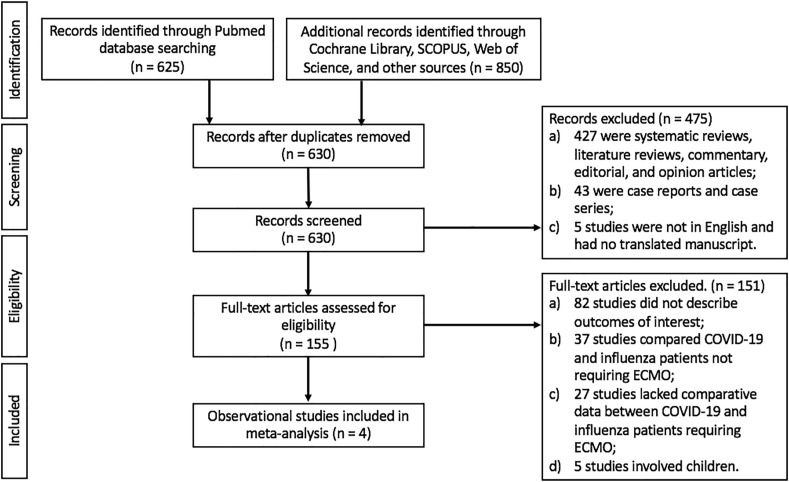
A total of 625 studies were identified in the initial search of the PubMed database, and 850 studies were identified from other databases and sources. After removing duplicates and those not meeting the inclusion criteria (by title, abstract, and full text), four eligible observational studies were included in the present review (Fig. 1 ).
Fig. 1.
Flow diagram of study selection.
3.2. Study characteristics
The study characteristics of the four included studies are described in Table 1. All of them retrospective cohort studies. Two of the studies were from France, one was from Germany, and the other was from the UK. A total of 269 patients requiring ECMO were described—129 with COVID-19 and 140 with influenza. The patients with COVID-19 were recruited between March and May 2020, while those with influenza were recruited between 2010 and 2020. The quality assessment of the studies is shown in Table 2 : three of the studies had the maximum score of nine, and one by Luyt et al. had a score of eight [14].
Table 2.
Results of the Newcastle–Ottawa Scale [11] in the four cohort studies.
1. Representatives of the exposed cohorts
2. Selection of the non-exposed cohorts
3. Ascertainment of exposure
4. The outcome of interest was not present at the start of the study
Comparability: Study controls were compared for the most important factor and additional factors
a) Assessment of the outcome
b) Enough follow-up for the outcome
c) Adequacy of follow-up
| Author(s) | Cohort Studies | Selection |
Comparability |
Outcome |
Total Of 9 Scores | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | (∗∗) | a | b | c | |||
| Charlton et al. [28] | Single-center, retrospective | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Cousin et al. [29] | Multi-center, retrospective | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Jackel et al. [30] | Single-center, retrospective | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Luyt et al. [14] | Single-center, retrospective | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 |
Abbreviations: NR, non-representative; ∗ one positive variable; ∗∗ two positive variable.
3.3. Demographics and comorbidities
Mean age was comparable between patients with COVID-19 and those with influenza on ECMO (53.03 vs. 52.20 years; P = 0.57; Table 1 and Fig. 2 ). A higher proportion of patients with COVID-19 were male (75.9% vs. 62.9%; P = 0.04; Table 1 and Fig. 3 ). The mean BMI was similar between the groups (30.90 vs. 29.37 kg/m2; P = 0.15).
Fig. 2.
Forrest plot of patients with COVID-19 and those with influenza requiring ECMO support. Clinical characteristics of age, BMI, SOFA score, days from mechanical ventilation to ECMO initiation, and outcome of ECMO duration were assessed. Standard mean differences were calculated by inverse variance statistical method with a random-effects model. Abbreviations: BMI: body mass index; CI, confidence intervals; df, degree of freedom; ECMO, extracorporeal membrane oxygenation; IV, inverse variance; SD, standard deviation; SOFA, sequential organ failure assessment.
Fig. 3.
Forrest plot of patients with COVID-19 or influenza requiring ECMO support. Clinical characteristics of male sex, diabetes mellitus, hypertension, immunocompromised status, and outcome of mortality were assessed. The odds ratio was calculated using the Mantel–Haenszel method with a random-effects model. Abbreviations: CI, confidence intervals; df, degree of freedom; DM, diabetes mellitus; ECMO, extracorporeal membrane oxygenation; HTN, hypertension; M − H, Mantel–Haenszel.
Comorbidities of DM, HTN, and immunocompromised status were common in both groups. The prevalences of DM (20.3% vs. 12.6%; P = 0.28) and HTN (25.3% vs. 28.4%; P = 0.66) were comparable. The proportion of immunocompromised patients was similar between groups (4.2% vs. 11.4%; P = 0.37; Table 1 and Fig. 3). Luyt et al. identified immunocompromised status in patients who had undergone solid-organ transplant or those with underlying vasculitis requiring treatment using chronic immunosuppressant, such as corticosteroids [14].
3.4. Pre-ECMO variables
Upon ICU admission, illness severity was similar between patients with COVID-19 and those with influenza (mean SOFA score: 10.67 vs. 11.33; P = 0.14). The serum pH levels before ECMO initiation were comparable between groups, ranging from 7.30 to 7.37 in the patients with COVID-19 and from 7.28 to 7.35 in those with influenza (Table 1). Patients with COVID-19 had a longer mean duration of mechanical ventilation prior to ECMO initiation (6.63 vs. 3.38 days; P < 0.01; Table 1 and Fig. 2).
3.5. Outcomes
The mean ECMO duration was similar between patients with COVID-19 and those with influenza (14.13 vs. 12.55 days; P = 0.25). The pooled mortality rate among all patients was 43.8% (118/269). The mortality rate did not differ between the groups (42.6% vs. 45.0%; P = 0.99).
4. Discussion
We reviewed four studies with comparative data describing 129 patients with COVID-19 and 140 with influenza requiring ECMO, with a pooled mortality rate of 43.8%. The demographics and comorbidities were similar between the groups. A higher proportion of patients with COVID-19 on ECMO were male. There was no difference between the two groups in terms of illness severity according to serum pH levels or SOFA score upon ICU admission. Patients with COVID-19 receiving ECMO had a longer duration of mechanical ventilation prior to ECMO initiation. Lastly, the ECMO duration and mortality rate were comparable.
It is likely that clinical characteristics were similar between patients with COVID-19 and those with influenza on ECMO because clinicians applied similar selection criteria. Advanced age, morbid obesity, and multiple comorbidities, especially those that are disabling, incurable, or life-threatening, are associated with lower survival rates and are listed as contraindications to ECMO in the Extracorporeal Life Support Organization 2017 and updated 2020 guidelines [[15], [16], [17], [18]]. The comparable outcomes indicate that refractory respiratory failure can be treated in a similar manner in both viral syndromes. Neither mortality nor ECMO duration differed significantly between the two groups. Sex difference has not been shown to affect survivability among ECMO patients [19,20]. The higher rate of men among patients with COVID-19 is unsurprising because men with this disease are known to have a greater likelihood of severe illness and hospitalization [7,8,20].
Correctly timing ECMO is challenging in practice. Initiating the treatment too soon may unnecessarily subject the patient to a risky and costly procedure, but clinicians who wait too long risk missing the window in which the therapy could make a difference. The current guidelines caution against ECMO in patients intubated for longer than 7–10 days [15,16]. However, many centers are reluctant to withhold this potentially life-saving salvage therapy in critically ill patients [18,19], especially those with COVID-19, which follows a more indolent course initially and takes longer to reach a point of clinical decompensation [[21], [22], [23]]. For this reason, it is unsurprising that patients with COVID-19 showed a longer duration of mechanical ventilation before ECMO initiation. In addition, COVID-related ARDS often demonstrates a unique atypical phenotype of significant gas exchange derangements with relatively preserved lung compliance [[23], [24], [25]]. The work of breathing is, therefore, less taxing to patients, with less resultant dyspnea and distress. In contrast, the fulminant nature and low lung compliance of influenza-related ARDS can result in ventilation challenges and may necessitate earlier ECMO initiation. Moreover, concerns about depleting valuable healthcare resources in the catastrophic COVID-19 pandemic may have led to more cautious and delayed use of ECMO in patients with COVID-19 than in those with influenza [15].
The strength of our study was that it was the only meta-analysis to objectively compare clinical characteristics and outcomes between patients with COVID-19 and those with influenza on ECMO therapy. The rapid COVID-19 pandemic did not allow for more structured and methodical criteria for initiating salvage ECMO. To further improve COVID-19 management, researchers must ascertain the parallels and differences between severe COVID-19 and the better-studied influenza.
There were several limitations to the present meta-analysis. Few studies have described the outcomes and characteristics of both critically ill patients with COVID-19 and those with influenza, and the total number of subjects was therefore small and the results were susceptible to heterogeneity, selection, and publication bias. Predictors associated with clinical outcomes could have been missed. Moreover, existing case series reviewing COVID-19 and influenza separately were highly variable in the type of data collected, so meta-analytic pooling of data became nearly impossible. For this reason, we limited our analysis to studies that provided comparative data on both viral syndromes. As further experience is gained in the unfolding COVID-19 pandemic, we believe more data will become available. Additionally, the dissimilarities between the two study populations, with enrollments period of several months for patients with COVID-19 but several years for influenza patients, likely also predisposed the study to selection and publication bias. Although we only included four studies, the funnel plot was symmetrical, with all studies within the threshold indicating a low probability of publication bias (Fig. 4 ).
Fig. 4.
Funnel plot for primary outcome of mortality.
Despite similarities in clinical characteristics and outcomes, critically ill patients with COVID-19 are a unique population. Their further delineation and characterization in the context of ECMO should continue as more data are collected. For instance, thromboembolism during ECMO occurs more frequently in patients with COVID-19 than in those without (63.6% vs. 18.2%; P < 0.05) because the viral infection causes a prothrombotic state in the context of extracorporeal circulation [26]. The risk of bleeding is also increased in COVID-19 because there is a higher prevalence of disseminated intravascular coagulation and sepsis-induced thrombocytopenia [27].
5. Conclusion
In the midst of the ongoing pandemic, the challenges in patient selection and timing of ECMO initiation are significant barriers preventing the escalation of care in critically ill patients with COVID-19. Because few comparative data were available, it was initially unclear whether the outcomes of patients with COVID-19 would be similar to those of influenza patients on ECMO. In our meta-analysis of four cohort studies involving 129 patients with COVID-19 and 140 with influenza, critically ill patients requiring ECMO support had comparable clinical characteristics, ECMO duration, and mortality. Patients with COVID-19 had a longer duration of mechanical ventilation prior to ECMO. Until more data are available, patients with COVID-19 in refractory respiratory failure can be treated similarly to those with influenza.
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, or publication of this article.
References
- 1.Alshahrani M.S., Sindi A., Alshamsi F., Al-Omari A., El Tahan M., Alahmadi B., et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018 Dec;8(1):3. doi: 10.1186/s13613-017-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A., Jones D., Bailey M., Beca J., Bellomo R., et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. J Am Med Assoc. 2009 Nov 4;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 3.Patroniti N., Zangrillo A., Pappalardo F., Peris A., Cianchi G., Braschi A., et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011 Sep;37(9):1447–1457. doi: 10.1007/s00134-011-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pham T., Combes A., Rozé H., Chevret S., Mercat A., Roch A., et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)–induced acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013 Feb 1;187(3):276–285. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 5.Munshi L., Walkey A., Goligher E., Pham T., Uleryk E.M., Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019 Feb;7(2):163–172. doi: 10.1016/S2213-2600(18)30452-1. [DOI] [PubMed] [Google Scholar]
- 6.Tang X., Du R.-H., Wang R., Cao T.-Z., Guan L.-L., Yang C.-Q., et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020 Jul;158(1):195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludwig M., Jacob J., Basedow F., Andersohn F., Walker J. Clinical outcomes and characteristics of patients hospitalized for Influenza or COVID-19 in Germany. Int J Infect Dis. 2021 Feb;103:316–322. doi: 10.1016/j.ijid.2020.11.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piroth L., Cottenet J., Mariet A.-S., Bonniaud P., Blot M., Tubert-Bitter P., et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021 Mar;9(3):251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Y., Bowe B., Maddukuri G., Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ. 2020 Dec 15:m4677. doi: 10.1136/bmj.m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gjurašin B., Santini M., Krajinović V., Papić N., Atelj A., Kotarski V., et al. A retrospective comparison between influenza and COVID-19-associated ARDS in a Croatian tertiary care center. Wien Klin Wochenschr. 2020 Nov 20 doi: 10.1007/s00508-020-01759-x. http://link.springer.com/10.1007/s00508-020-01759-x [Internet]. [cited 2021 Mar 6]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010 Sep;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luyt C.-E., Sahnoun T., Gautier M., Vidal P., Burrel S., Pineton de Chambrun M., et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: a retrospective cohort study. Ann Intensive Care. 2020 Dec;10(1):158. doi: 10.1186/s13613-020-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shekar K., Badulak J., Peek G., Boeken U., Dalton H.J., Arora L., et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. Am Soc Artif Intern Organs J. 2020 Jul;66(7):707–721. doi: 10.1097/MAT.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.USA Extracorporeal Life Support Organization - ECMO and ECLS ELSO guidelines for cardiopulmonary extracorporeal life support extracorporeal life support organization. Resour Guidel. 2017 Aug 1 https://www.elso.org/Resources/Guidelines.aspx [Internet]. Available from: [Google Scholar]
- 17.Posluszny J., Rycus P.T., Bartlett R.H., Engoren M., Haft J.W., Lynch W.R., et al. Outcome of adult respiratory failure patients receiving prolonged (≥14 Days) ECMO. Ann Surg. 2016 Mar;263(3):573–581. doi: 10.1097/SLA.0000000000001176. [DOI] [PubMed] [Google Scholar]
- 18.Chang C.-H., Chen H.-C., Caffrey J.L., Hsu J., Lin J.-W., Lai M.-S., et al. Survival analysis after extracorporeal membrane oxygenation in critically ill adults: a nationwide cohort study. Circulation. 2016 Jun 14;133(24):2423–2433. doi: 10.1161/CIRCULATIONAHA.115.019143. [DOI] [PubMed] [Google Scholar]
- 19.Barbaro R.P., Odetola F.O., Kidwell K.M., Paden M.L., Bartlett R.H., Davis M.M., et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015 Apr 15;191(8):894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbaro R.P., MacLaren G., Boonstra P.S., Iwashyna T.J., Slutsky A.S., Fan E., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020 Oct;396(10257):1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 Jun;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020 May 15;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jounieaux V., Rodenstein D.O., Mahjoub Y. On happy hypoxia and on sadly ignored “acute vascular distress syndrome” in patients with COVID-19. Am J Respir Crit Care Med. 2020 Dec 1;202(11):1598–1599. doi: 10.1164/rccm.202006-2521LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan C., Chen L., Lu C., Zhang W., Xia J.-A., Sklar M.C., et al. Lung recruitability in COVID-19–associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med. 2020 May 15;201(10):1294–1297. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bemtgen X., Zotzmann V., Benk C., Rilinger J., Steiner K., Asmussen A., et al. Thrombotic circuit complications during venovenous extracorporeal membrane oxygenation in COVID-19. J Thromb Thrombolysis. 2021 Feb;51(2):301–307. doi: 10.1007/s11239-020-02217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yusuff H., Zochios V., Brodie D. Thrombosis and coagulopathy in COVID-19 patients requiring extracorporeal membrane oxygenation. ASAIO J. 2020 Aug;66(8):844–846. doi: 10.1097/MAT.0000000000001208. Am Soc Artif Intern Organs 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlton M., Dashey S., Stubbs A., Lai F.Y., Bird P.W., Badhwar V., et al. Comparing SARS-CoV-2 and influenza A(H1N1)pdm09-infected patients requiring ECMO – a single-centre, retrospective observational cohort experience. J Infect. 2021;82(4):84–123. doi: 10.1016/j.jinf.2020.11.003. S0163445320306988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cousin N., Bourel C., Carpentier D., Goutay J., Mugnier A., Labreuche J., et al. SARS-CoV-2 versus influenza associated acute respiratory distress syndrome requiring veno-venous extracorporeal membrane oxygenation support. ASAIO J. 2020 Oct 13 doi: 10.1097/MAT.0000000000001325. https://journals.lww.com/10.1097/MAT.0000000000001325 [Internet]. [cited 2021 Feb 27];Publish Ahead of Print. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jäckel M., Rilinger J., Lang C.N., Zotzmann V., Kaier K., Stachon P., et al. Outcome of acute respiratory distress syndrome requiring extracorporeal membrane oxygenation in Covid-19 or influenza: a single-center registry study. Artif Organs. 2020 Dec 18 doi: 10.1111/aor.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]