Abstract
Background
Evidence show that Matrix metalloproteinases (MMPs) have been associated with neurological complications in the viral infections. Here in the current investigation, we intended to reveal if MMPs are potentially involved in the development of neurological symptoms in the patients with Coronavirus disease 2019 (COVID-19).
Methods
The levels of MMPs, inflammatory cytokines, chemokines, and adhesion molecules were evaluated in the serum and cerebrospinal fluid (CSF) samples from 10 COVID-19 patients with neurological syndrome (NS) and 10 COVID-19 patients lacking NS. Monocytes from the CSF samples were treated with TNF-α and the secreted levels of MMPs were determined.
Results
The frequency of monocytes were increased in the CSF samples of COVID-19 patients with NS compared to patients without NS. Levels of inflammatory cytokines IL-1β, IL-6, and TNF-α, chemokines CCL2, CCL3, CCL4, CCL7, CCL12, CXCL8, and CX3CL1, MMPs MMP-2, MMP-3, MMP-9, and MMP-12, and adhesion molecules ICAM-1, VCAM-1, and E-selectin were significantly increased in the CSF samples of COVID-19 patients with NS compared with patients without NS. Treatment of CSF-derived monocytes obtained from COVID-19 patients with NS caused increased production of MMP-2, MMP-3, MMP-9, and MMP-12.
Conclusions
Higher levels of inflammatory cytokines might promote the expression of adhesion molecules on blood-CSF barrier (BCSFB), resulting in facilitation of monocyte recruitment. Increased levels of CSF chemokines might also help to the trafficking of monocytes to CSF. Inflammatory cytokines might enhance production of MMPs from monocytes, leading to disruption of BCSFB (and therefore further infiltration of inflammatory cells to CSF) in COVID-19 patients with NS.
Keywords: Coronavirus disease 2019, Neurological symptom, Matrix metalloproteinases, Inflammatory cytokine, Chemokine, Adhesion molecule
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection that cause coronavirus disease 2019 (COVID-19) has been associated with a wide range of clinical symptoms that is presented as acute respiratory distress syndrome (ARDS) in the severe forms. The virus targets and attacks various body organs where angiotensin-converting enzyme 2 (ACE2) receptor is exerted as the major receptor for virus S protein [1], [2]. Among the typical clinical manifestations of COVID-19 are fever, fatigue, dry cough, and sore throat [3]. A growing body of evidence reveals the neurological symptoms in COVID-19 subjects such as headache, neuroinflammatory presentations, and cerebrovascular complications [4]. Molecular tests have recognized the SARS-CoV-2 nucleic acid in the cerebrospinal fluid (CSF) of COVID-19 patients [5]. Moreover, virus particles have also been detected in the autopsy samples of brain in a subject [6].
Matrix metalloproteinases (MMPs) are zinc-dependent enzymes that degrade extracellular matrix (ECM) proteins, such as collagen, fibronectin, and laminin as well as basement membrane structures. MMPs have been associated with diverse pathophysiological conditions like inflammation as well as metastasis and angiogenesis in malignancies [6], [7]. These enzymes play a role in promoting the passing of inflammatory immune cells through the blood–brain barrier (BBB) and accumulation in the CSF and Central nervous system (CNS) [8]. Serum MMP-3 concentration was reported to be higher in COVID-19 patients and correlated with increased levels of inflammatory mediators [9]. Increased levels of chemokines as well as MMPs, like MMP-2, MMP-3, MMP-8, and MMP-9 in the CSF of patients with Varicella-zoster virus (VZV) infection were reported [7]. Increased expression levels of MMP-3, MMP-12, and tissue inhibitor of matrix metalloproteinases 1 (TIMP-1) was reported in mice with lethal infection with neurotropic mouse hepatitis virus compared to sublethal infection. Moreover, activity of MMP-9 was associated with neutrophil recruitment to CNS [8]. In the CSF of patients with human immunodeficiency virus (HIV) dementia, increased levels of pro-MMP-2 and pro-MMP-7 were detected. Even though high serum levels of MMPs might be associated with promoted levels of MMPs in the CSF, the brain-derived cells produced MMP-2, MMP-7, and MMP-9, which were stimulated upon induction of these cells with tumor necrosis factor (TNF)-α. Hence, increased levels of MMPs in the CSF mirrored the aberrant/hyper activation of immune system in CNS of patients with HIV dementia [9].
CNS is regarded as an immune-privileged site and, therefore, trafficking of immune cells into the CNS is limited through the blood–brain barrier (BBB) as well as the blood-CSF barrier (BCSFB). That notwithstanding, immune surveillance occurs steadily in the CNS during normal physiological conditions, demonstrating that infiltration of immune cells into CNS happens regardless of inflammation or BBB/BCSFB injury [10], [11]. On the other hand, pathological conditions like inflammation promote the infiltration of immune cells into CNS that is occurred when BBB is injured and is mediated by chemokines. In such condition, immune cells present in the parenchymal basement membrane surrounding the spinal cord and brain enters the CNS [12].
MMPs and adhesion molecules have been hypothesized to be involved in the CNS injury during coronavirus infections. After entry of coronaviruses into lung, they infect epithelial cells and may pass across the epithelium and enter into blood to infect the monocytes. MMPs, especially MMP-9 (induces the permeability of BBB) and inflammatory mediators, like TNF-α (that triggers overexpression of Intercellular adhesion molecule 1 (ICAM-1) on the endothelial cells) contribute the infected immune cells (like monocytes) to pass through the BBB and enter into CNS. These infected immune cells might produce inflammatory mediators in the CNS and cause neuron injury. These infected immune cells also secret several chemokines, such as CCL5, CXCL10, CXCL11, leading to infiltration of inflammatory immune cells (like T cells) into CNS [10].
The major mechanobiology underlying neurologic syndrome (NS) and neurovirulence by SARS-CoV-2 has not been clarified yet. Here in this study, we tried to explore possible mechanism by investigating the levels of different chemokines, MMPs, as we as adhesion molecules in COVID-19 patients.
2. Materials and methods
2.1. Study participants
In this study, 10 subjects with COVID-19 presenting neurological manifestations and 10 age- and sex-matched COVID-19 patients without neurological presentations were included (Table 1 ). Patients were selected from those who referred to the intensive care unit (ICU) of Shahid Rajaee hospital of Karaj, Iran. Diagnosis of COVID-19 patients was done using Real-time PCR for the infection by SARS-CoV-2 through nasopharyngeal swab sampling. Patients had had respiratory failure and decreased oxygen saturation. A neurologist examined and determined the neurologic symptoms of the patients. CSF samples in excess of the amount required for diagnostic purposes was obtained from all patients by lumbar puncture. Additionally, 5 ml of peripheral blood samples were obtained from all cases. The leukocyte profiling of the samples was determined by routine laboratory hematologic analyzer. The current study was conducted after receiving approval from the local ethical committee of Alborz University of Medical Science (IR.ABZUMS.REC.1399.340). Informed consent forms were signed by all cases prior to sampling.
Table 1.
Demographic characteristics and laboratory findings of the study subjects.
| Item | Cases with NS (n = 10) |
Cases without NS (n = 10) |
P value |
|---|---|---|---|
| Sex; male/female | 5 (50%)/5 (50%) | 5 (50%)/5 (50%) | > 0.05 |
| Age; year | 61.21 ± 12.24 | 59.51 ± 10.50 | > 0.05 |
| WBC; cells/mm3 | 8144.25 ± 2510.38 | 7923.32 ± 2211.32 | > 0.05 |
| Lymphocyte-total leukocyte ratio | 24.66 ± 13.45 | 25.54 ± 13.74 | > 0.05 |
| Neutrophil-lymphocyte ratio | 8.48 ± 11.85 | 7.90 ± 10.74 | > 0.05 |
| CRP (mg/L) | 5.23 ± 3.11 | 4.88 ± 2.41 | > 0.05 |
| AST (IU/L) | 32.28 ± 9.47 | 31.85 ± 9.50 | > 0.05 |
| ALT (IU/L) | 39.11 ± 9.54 | 38.96 ± 9.79 | > 0.05 |
| LDH (IU/L) | 465.52 ± 97.45 | 439.64 ± 98.47 | > 0.05 |
| Fever | 10 (100%) | 8 (80%) | > 0.05 |
| Cough | 9 (90%) | 8 (80%) | > 0.05 |
| Dyspnea | 9 (90%) | 7 (70%) | > 0.05 |
| Sputum | 7 (70%) | 5 (50%) | > 0.05 |
| Vomiting/diarrhea | 7 (70%) | 6 (60%) | > 0.05 |
| Positive PCR of peripheral blood | 4 (40%) | 1 (10%) | > 0.05 |
COVID-19, Coronavirus disease 2019; NS, Neurologic Syndrome; WBC, White blood cell; CRP, C-reactive protein; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; LDH, Lactate dehydrogenase
2.2. Concentrations of MMPs, cytokines, chemokines, and adhesion molecules
CSF samples and serum samples isolated from peripheral blood of all study subjects were evaluated using enzyme linked immunosorbent assay (ELISA) to measure the concentration of MMPs (MMP-2, MMP-3, MMP-8, MMP-9, MMP-12), chemokines (CCL2, CCL3, CCL4, CCL5, CCL7, CCL12, CXCL8, CX3CL1), cytokines (IL-1β, IL-6, and TNF-α), and adhesion molecules (ICAM-1, VCAM-1 and E-selectin). Commercial kits (Invitrogen, Thermo Fisher Scientific, San Diego, CA, USA) and an ELISA reader device (Tecan Spectra, Austria) were used to determine the optical density (OD) of the samples.
2.3. Monocyte isolation incubation by TNF
Total leukocytes were isolated from the CSF samples by centrifugation. Additionally, peripheral blood mononuclear cells (PBMCs) were isolated from blood samples after dilution in Phosphate-buffered saline (PBS) by density-gradient centrifuged using Ficoll/Hypaque 1.077 g/ml (Lymphodex, inno-Train, Kronberg, Germany). Monocyte isolation was conducted by positive selection of CD14 + cells using magnetic-activated cell sorter columns (Miltenyi Biotec, San Diego, CA). The purity of monocytes was determined using flow cytometry (about 92% purity was yielded). The monocytes (55 × 105 cells/well) were cultured in 1 ml of RPMI 1640 media (Gibco, Invitrogen, Germany) supplemented with 10% fetal bovine serum (FBS; Gibco, Invitrogen, Germany), 4 mM L-glutamine, and 10 IU/ml penicillin/streptomycin in 24-well plates and maintained in humidified conditions in 5% CO2 at 37° C for 24 h. Then, cells were incubated with 100 ng/ml Lipopolysaccharide (LPS; Sigma-Aldrich) and in the presence or absence recombinant TNF (5 ng/ml) for 72 h. The supernatant of each well was collected to measure the levels of MMPs.
2.4. Statistical analysis
The Mann-Whitney U test was used to compare the data between the groups. Numeric and nominal data presentation was performed by mean ± standard deviation (SD) and numbers and percentage, respectively. Analysis of data and designing of graphs were conducted by GraphPad PRISM software v.8.00 (GraphPad Software, Inc., San Diego, CA, USA).
3. Results
3.1. Baseline characteristics of the study subjects
Demographic data and clinical presentations of study subjects are listed in Table 1. Study subjects were composed of 10 cases with NS and 10 individuals without NS. In both groups, 5 (50%) male cases and 5 (50%) female subjects were included. Among the laboratory tests, both groups had similar levels of white blood cell (WBC), lymphocyte-total leukocyte ratio, neutrophil–lymphocyte ratio, C-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH). It was observed that the SARS-CoV-2 nucleic acid PCR of peripheral blood was positive in 4 (40%) subjects with NS and in 1 (10%) patient without NS. The results of SARS-CoV-2 nucleic acid PCR was negative in all patients. The time from COVID-19 initial diagnosis to lumbar puncture to obtain CSF samples was 6.2 ± 8.7 days in subjects with NS and 5.5 ± 7.9 days in subjects without NS. The neurological presentations of the 10 COVID-19 subjects with NS are shown in Table 2 .
Table 2.
Neurologic manifestation of 10 COVID-19 subjects with NS.
| Patient No. | Sex | Neurologic manifestations | Neurologic diagnosis |
|---|---|---|---|
| 1 | Female | Reduced consciousness level, seizure | Encephalopathy with seizure |
| 2 | Female | Temporary paresis of left arm, changed mental state, higher muscle tone | Encephalopathy, transient ischemic attack |
| 3 | Male | Changed mental state, confusion | Encephalopathy |
| 4 | Female | Cheyne-Stoke breathing, tetraparesis, hyporeflexia | Critical illness polyneuropathy |
| 5 | Male | Headache, changed mental state, increased muscle tone, areflexia, delirium | Encephalopathy |
| 6 | Male | Delirium, hyposmia, hypogeusia, unilateral peripheral vestibular dysfunction | Unilateral vestibular neuritis |
| 7 | Male | Headache, changed mental state, seizure | Seizure |
| 8 | Female | Headache, suspected meningism, areflexia, delirium | Encephalopathy |
| 9 | Male | Headache, confusion, nausea with vomiting | Cerebral venous sinus thrombosis |
| 10 | Female | Headache, changed mental state, declined level of consciousness, areflexia | Critical illness polyneuropathy |
3.2. Leukocyte profiling of CSF
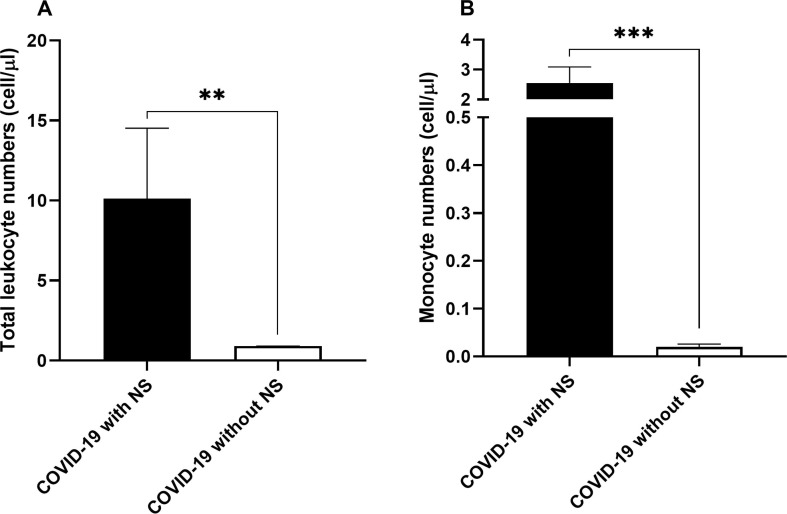
The frequency of leukocytes in the CSF of subjects with NS was 10.12 ± 4.40 cell/μl while it was<1 cell/μl in patients without NS (P < 0.01; Fig. 1 .A). Most importantly, it was observed that the number of monocytes in the CSF samples from COVID-19 cases with NS was significantly higher compared to that of cases without NS (2.55 ± 0.54 cell/μl vs. 0.018 ± 0.006 cell/μl; P < 0.001; Fig. 1.B).
Fig. 1.
Numbers of total leukocytes (A) and monocytes (B) in the CSF samples obtained from 10 COVID-19 patients with NS and 10 COVID-19 patients without NS. The leukocyte profiling of the samples was determined by routine laboratory hematologic analyzer. The Mann-Whitney U test was used to compare the data between two groups. Data are presented by mean ± SD of the results from 10 cases in each group (NS; Neurologic syndrome, ** shows a P < 0.01 and *** shows a P < 0.001).
3.3. Concentration of MMPs
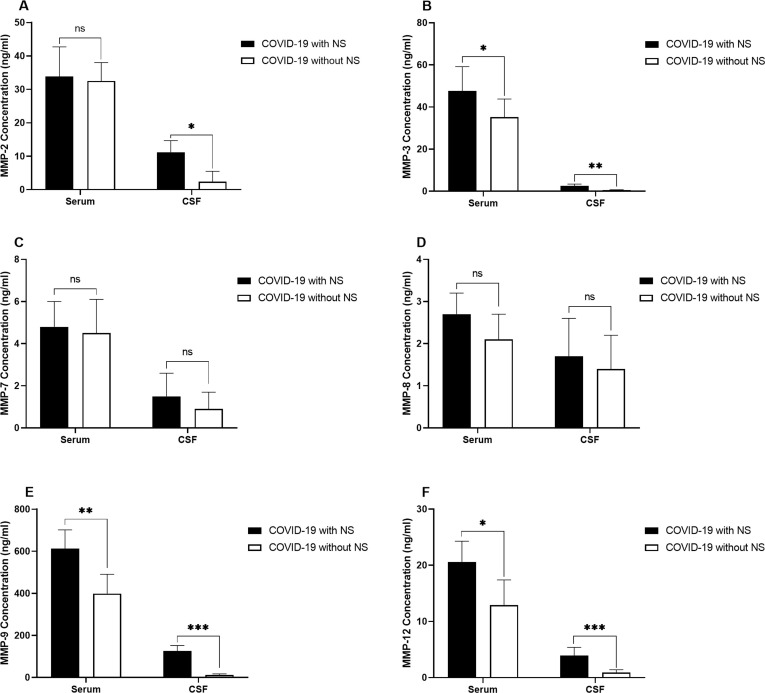
The serum levels of MMP-2 had no significant difference between COVID-19 patients with NS and those without NS, while CSF levels of MMP-2 was significantly higher in COVID-19 patients with NS (P = 0.044; Fig. 2 .A). MMP-3 levels in both serum (P = 0.031) and CSF samples (P = 0.008) were higher in COVID-19 patients with NS and those without NS (Fig. 2.B). No significant differences were observed in serum and CSF levels of both MMP-7 (Fig. 2.C) and MMP-8 (Fig. 2.D) between COVID-19 cases with and without NS. MMP-9 level was significantly increased in both serum (P = 0.004) and CSF (P = 0.0003) samples obtained from the COVID-19 cases with NS (Fig. 2.E). Significantly higher levels of MMP-12 were detected in both serum (P = 0.016) and CSF (P = 0.0009) samples of COVID-19 cases with NS in comparison to cases without NS (Fig. 2.F).
Fig. 2.
Bar graphs show the serum and CSF levels of MMP-2 (A), MMP-3 (B), MMP-7 (C), MMP-8 (D), MMP-9 (E), and MMP-12 (F) in 10 COVID-19 patients with NS and 10 COVID-19 patients without NS. The concentration of MMPs was measured using ELISA method. The Mann-Whitney U test was used to compare the data between two groups. Data are presented by mean ± SD of the results from 10 cases in each group (NS; Neurologic syndrome, ns; non-significant, * shows a P < 0.5 ** shows a P < 0.01, *** shows a P < 0.001).
3.4. Concentration of cytokines
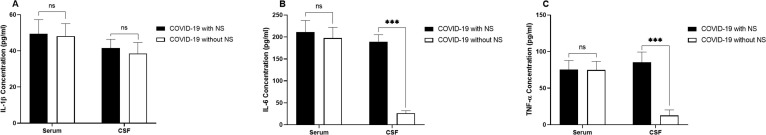
Experiments revealed that the levels of IL-1β had no significant differences in both serum and CSF samples from COVID-19 cases with NS in comparison to COVID-19 cases without NS (Fig. 3 .A). Although IL-6 level had no significant difference in the serum samples of COVID-19 cases with NS compared to patients without NS, it was significantly higher in the CSF samples of COVID-19 patients with NS (P = 0.0004; Fig. 3.B). Additionally, the TNF-α level was significantly higher in the CSF sample, but not serum sample, of COVID-19 patients with NS compare to patients without NS (P = 0.0007; Fig. 3.C).
Fig. 3.
Demonstration of the serum and CSF levels of IL-1β (A), IL-6 (B), and TNF-α (C), in 10 COVID-19 patients with NS and 10 COVID-19 cases without NS. The concentration of cytokines was measured using ELISA method. The Mann-Whitney U test was used to compare the data between two groups. Data are presented by mean ± SD of the results from 10 cases in each group (NS; Neurologic syndrome, ns; non-significant, *** shows a P < 0.001).
3.5. Concentration of chemokines
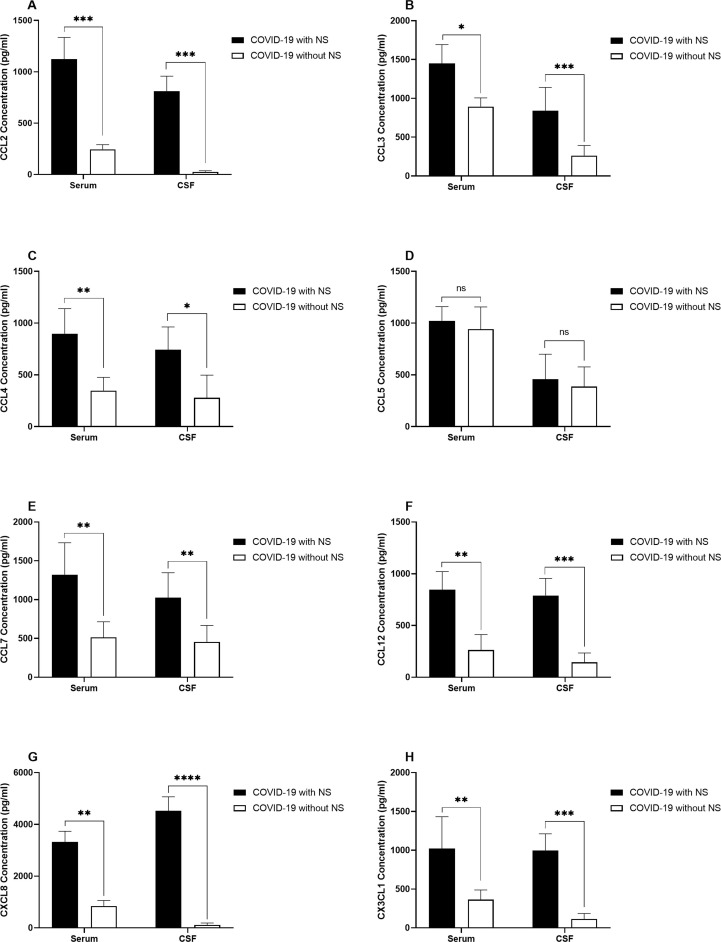
The levels of CCL2, CCL3, CCL4, CCL7, CCL12, CXCL8, and CX3CL1 was higher in both serum and CSF samples from COVID-19 cases with NS compared to patients without NS. Nonetheless, no significant differences were detected in serum and CSF CCL-5 levels between COVID-19 patients with and without NS (Fig. 4 ).
Fig. 4.
Bar graphs show the serum and CSF levels of CCL2 (A), CCL3 (B), CCL4 (C), CCL5 (D), CCL7 (E), CCL12 (F), CXCL8 (G), and CX3CL1 (H) in COVID-19 patients with NS and COVID-19 patients without NS. The concentration of chemokines was measured using ELISA method. The Mann-Whitney U test was used to compare the data between two groups. Data are presented by mean ± SD of the results from 10 cases in each group (NS; Neurologic syndrome, ns; non-significant, * shows a P < 0.5 ** shows a P < 0.01, *** shows a P < 0.001, **** shows a P < 0.0001).
3.6. Concentration of adhesion molecules
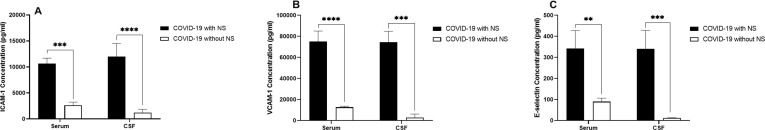
It was detected that ICAM-1 level was significantly higher in the serum (P = 0.0005) and CSF (P = 0.00009) samples obtained from COVID-19 patients with and without NS (Fig. 5 .A). In addition, it was observed that the serum (P = 0.00006) and CSF (P = 0.0008) levels of VCAM-1 were significantly higher in COVID-19 patients with NS compared with patients without NS (Fig. 5.B). Experiments indicated that level of E-selectin in both serum (P = 0.0018) and CSF (P = 0.0007) samples was significantly higher in COVID-19 cases with NS in comparison to patients without NS (Fig. 5.C).
Fig. 5.
Illustration of the serum and CSF levels of ICAM-1 (A), VCAM-1 (B), and E-selectin (C), in 1 0COVID-19 patients with NS and 10 COVID-19 cases without NS. The concentration of adhesion molecules was measured using ELISA method. The Mann-Whitney U test was used to compare the data between two groups. Data are presented by mean ± SD of the results from 10 cases in each group (NS; Neurologic syndrome, ns; non-significant, *** shows a P < 0.001).
3.7. MMP levels after monocyte stimulation
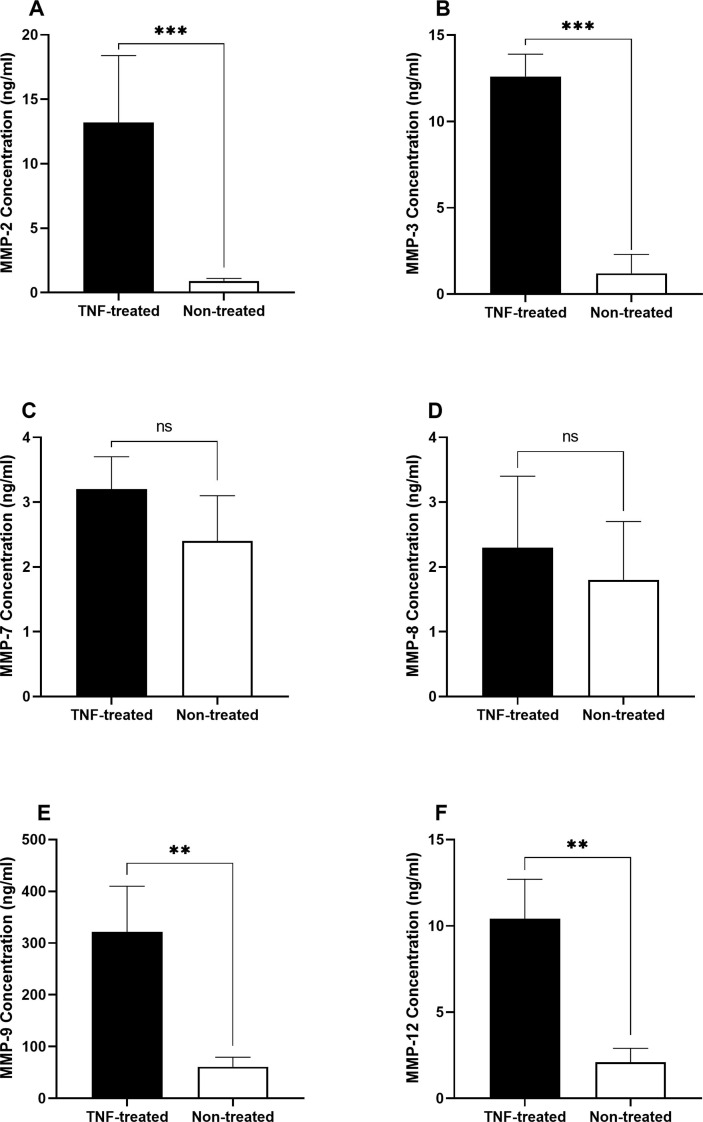
In the supernatant of TNF-α treated monocytes derived from CSF samples of COVID-19 patients with NS, levels of MMP-2 (P = 0.0002; Fig. 6 .A), MMP-3 (P = 0.0007; Fig. 6.B), MMP-9 (P = 0.0074; Fig. 6.E), and MMP-12 (P = 0.0056; Fig. 6.F) was significantly higher in comparison to untreated monocytes obtained from CSF samples of COVID-19 patients with NS. However, no significant differences were observed in secreted levels of MMP-7 (Fig. 6.C) and MMP-8 (Fig. 6.D).
Fig. 6.
Levels of MMPs in the supernatant of TNF-α treated and non-treated monocytes derived from CSF samples of COVID-19 patients with NS. Monocytes were isolated from the total leukocytes in the CSF samples by positive selection of CD14 + cells using magnetic-activated cell sorter columns. Afterwards, monocytes were incubated with 100 ng/ml Lipopolysaccharides and 5 ng/ml recombinant TNF for 72 h. The supernatant of each well was collected to measure the levels of MMPs. The concentration of MMPs was measured using ELISA method. The Mann-Whitney U test was used to compare the data between two groups. Data are presented by mean ± SD of the results from 10 cases in each group. After treating monocytes with TNF, level of MMP-2 (A), MMP-3 (B), MMP-9 (E), and MMP-12 (F) was significantly higher in comparison to untreated monocytes obtained from CSF samples of COVID-19 patients with NS. There were no significant differences in the secreted levels of MMP-7 (C) and MMP-8 (D) (TNF; Tumor necrosis factor, ns; non-significant, ** shows a P < 0.01 and *** shows a P < 0.001).
Additionally, we performed the same experiment in the monocytes obtained from CSF of COVID-19 cases without NS, but there were no statistically significant differences between levels of MMPs before and after treatment of monocytes with TNF-α (Data not shown).
4. Discussion
Research show that numerous viruses are able to invade the CNS and infect the immune cells as well as neurons. In spite of little understandings with respect to invasion of viruses into CNS, such events are not rare and are progressively identified through advancements in sophisticated diagnostic tools. Viruses exert various routs to enter CNS and infect glial cells as well as neurons and contribute to the development of neurological disorders [13]. Evidence shows that patients with COVID-19 suffer from several forms of neurological complications [14]. Approximately 20% of subjects with COVID-19 who need ICU admission have shown neurological manifestations that are probably at increased susceptibility to mortality [15]. Infection by SARS-CoV-2 was associated with postmortem neuropathological modifications. In addition, the virus was identified in the brain of COVID-19 cases with preexisting microvascular disorders that exhibited encephalopathy. As a result, microvascular comorbidities might confer an increased susceptibility towards neurological disorders in patients with COVID-19 [16]. ACE2 are expressed on the endothelium of various tissues, including brain [17]. It was reported that SARS-CoV-1 were able to bind directly to the ACE2 expressed on the endothelial cells of the cerebral microvasculature. Therefore, it seems that SARS-CoV-2 might also be able to bind the BBB endothelial cells, resulting in accesses of virus to CNS and infect neurons and glial cells [18], [19].
Several viruses might enter the CNS through epithelial cells of the BCSFB in the choroid plexus of the brain ventricles [20], [21]. The gene expression profile of choroid plexus might be modulated through the inflammation caused by coronaviruses, leading to disrupted integrity of BCSFB [22]. Additionally, such inflammation might activate nuclear factor (NF)-κB pathway, leading to overproduction of MMP-9 and increased permeability of BCSFB [23]. These events might also upmodulate the expression of CCL-2, IL-1, IL-6, TNF-α, MMP-8, and ICAM-1 that further promote the permeability of BCSFB as well as infiltration of inflammatory immune cells [24], [25], [26]. Moreover, higher levels of pro-MMP-2 and pro-MMP-7 were detected in the CSF of patients with HIV dementia. The brain-derived cells produced MMP-2, MMP-7, and MMP-9, which were triggered after induction of these cells with TNF-α. Therefore, increased levels of MMPs in the CSF demonstrate the aberrant/hyper activation of immune system in CNS of patients with HIV dementia [9]. In the Japanese encephalitis virus infected mice, increased expression and activity of MMP-2 and MMP-9 in the serum samples [27]. The human coronavirus OC43 strain was observed to modulated MMP-2 and MMP-9 activity produced by the astrocytic and microglial cell lines [28]. Therefore, viruses might develop neuropathology through promoting neuroinflammation and modulation of diverse MMPs. We observed increased levels of MMP-2, MMP-3, MMP-9, and MMP-12 in the CSF samples from COVID-19 patients with NS in comparison to COVID-19 cases without NS. Higher serum levels of MMP-3, MMP-9, and MMP-12 was also detected in the COVID-19 patients with NS.
Our experiments indicated that the number of monocytes were higher in the CSF samples of the COVID-19 patients with neurological symptoms. Additionally, the serum and CSF levels of inflammatory cytokines (IL-1β, IL-6, and TNF-α) as well as chemokines involved in the infiltration of monocytes (CCL2, CCL3, CCL4, CCL7, CCL12, CXCL8, and CX3CL1) was higher in the COVID-19 patients with NS. The levels of MMP-2, MMP-3, MMP-9, and MMP-12 was higher in the CSF samples from COVID-19 patients with NS. Although we did not find the infection of CSF samples in COVID-19 patients with NS, the SARS-CoV-2 nucleic acid PCR was positive for the peripheral blood samples of 40% of patients. Nonetheless, we suggest that monocytes in the CSF might produce higher levels of MMPs that might disrupt the integrity of BCSFB. Additionally, increased production of inflammatory cytokines in the CSF might promote the expression of adhesion molecules (as we detected increased levels of ICAM-1, VCAM-1, and E-selectin in both serum and CSF samples), which in turn promote the infiltration of inflammatory immune cells (particularly monocytes) through a disrupted BCSFB.
Previously, it was seen that MMP-3 concentration was higher in serum of COVID-19 patients that correlated with increased levels of inflammatory cytokines [9]. In addition, inflammatory cytokines have been shown to promote the production of MMPs from monocytes [29]. We observed that TNF treatment of CSF-derived monocytes obtained from COVID-19 patients with NS caused increased production of MMP-2, MMP-3, MMP-9, and MMP-12. However, the TNF treatment of monocytes from COVID-19 patients without NS did not affect on the levels of MMPs. As a result, it is suggested that increased inflammatory cytokines in the CSF of COVID-19 patients with NS might induce production of MMPs from monocytes. However, it is not clear why a number of patients with COVID-19 develop CNS inflammation. It seems that genetic background, life style, even the variant of virus might increase susceptibility of individuals to promote higher levels of inflammatory cytokines and chemokines in the CNS, increased leukocyte infiltration into CNS, and finally development of neurological complications. Probably, genetic factors as well as unexpected interactions of immune system with virus might develop neuropathology in the COVID-19 cases through the mechanism by inflammatory cytokines, chemokines, adhesion molecules, and MMPs.
It should be noted that levels of MMP-7 and MMP-8 in the serum and CSF samples from COVID-19 patients with NS were comparable to those from the COVID-19 patients without NS. Evidence shows that almost all MMPs are ubiquitously expressed in mammalian organisms. The expression of MMPs are generally occurs and their levels are low and only upregulated during pathological conditions [30]. Therefore, the presence of MMP-2, MMP-3, MMP-9, and MMP-12 in the COVID-19 cases without neurological involvement and similar levels of MMP-7 and MMP-8 in the serum and CSF samples from COVID-19 patients with NS compared to those from the COVID-19 patients without NS are not considered an inconsistent. Basically, the baseline levels of MMPs are present even in the physiological settings and specific pathological conditions promote expression of specific MMPs.
Among the 25 different MMPs identified, MMP-2, MMP-3, MMP-7, MMP-9, and MMP-12 have mostly been implicated in the viral infections, especially in those with neuropathogenesis [31]. More specifically, in the murine coronavirus-induced viral encephalitis, higher levels of MMP-3 and MMP-9 have been reported [32]. This issue might stem from the specific function of MMPs, as MMP-1, MMP-8 and MMP-13 are collagenases, MMP-2 and MMP-9 are gelatinases, MMP-3, MMP-10, MMP-11 are stromelysins, MMP-7 and MMP-12 are elastases, and MMP-14, MMP-15, MMP-16 and MMP-17 are membrane-type MMPs that target gelatin, fibronectin, laminin, and collagen [33]. Our experiments revealed that levels of MMP-2, MMP-3, MMP-9, and MMP-12 in the CSF samples from COVID-19 patients with NS were increased in comparison to COVID-19 cases without NS. Additionally, higher serum levels of MMP-3, MMP-9, and MMP-12 was also detected in the COVID-19 patients with NS compared to COVID-19 patients without NS.
Considering all the facts, this study aimed to explore the possible involvement of MMPs in the development of neurological symptoms in COVID-19 patients. Our experiments suggest that increased levels of inflammatory cytokines might promote the expression of adhesion molecules (probably on the BCSFB) which in turn might result in facilitation of monocyte trafficking. Increased levels of CSF chemokines might also contribute to the infiltration of monocytes to CSF in COVID-19 patients with NS. High level of inflammatory cytokines, particularly TNF-α, might help to increased production of MMPs from monocytes, leading to disruption of BCSFB. Although the conclusions were raised mostly from the previous experiences from other viral infections, still further experiments are needed to disclose several aspects of the involvement of MMPs in the etiopathogenesis of neurological complications in the COVID-19 subjects.
CRediT authorship contribution statement
Mina Mohammadhosayni: Methodology, Writing – original draft, Writing – review & editing. Fatemeh Sadat Mohammadi: Methodology, Writing – original draft, Writing – review & editing. Fatemeh Ezzatifar: Methodology, Writing – original draft, Writing – review & editing. Armita Mahdavi Gorabi: Methodology, Writing – original draft, Writing – review & editing. Arezou Khosrojerdi: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Saeed Aslani: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Maryam Hemmatzadeh: Methodology, Writing – original draft, Writing – review & editing. Shahrooz Yazdani: Formal analysis, Writing – original draft, Writing – review & editing. Mohsen Arabi: Formal analysis, Writing – original draft, Writing – review & editing. Faroogh Marofi: Conceptualization, Writing – original draft, Writing – review & editing. Farhad Jadidi-Niaragh: Conceptualization, Writing – original draft, Writing – review & editing. Navid Shomali: Visualization, Writing – original draft, Writing – review & editing. Hamed Mohammadi: Writing – original draft, Writing – review & editing, Conceptualization, Supervision, Funding acquisition, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors are grateful of patients for their participation in the study.
Funding
This study was supported by a grant from Alborz University of Medical Sciences (Grant No. 99-4209).
References
- 1.N. Ebrahimi, S. Aslani, F. Babaie, M. Hemmatzadeh, R. Hosseinzadeh, Z. Joneidi, Z.M. Tourzani, N. Pakravan, H. Mohammadi, Recent findings on the Coronavirus disease 2019 (COVID-19); immunopathogenesis and immunotherapeutics, International immunopharmacology (2020) 107082. [DOI] [PMC free article] [PubMed]
- 2.Tabrizi Z.A., Khosrojerdi A., Aslani S., Hemmatzadeh M., Babaie F., Bairami A., Shomali N., Hosseinzadeh R., Safari R., Mohammadi H. Multi-facets of neutrophil extracellular trap in infectious diseases: Moving beyond immunity. Microb. Pathog. 2021;158 doi: 10.1016/j.micpath.2021.105066. [DOI] [PubMed] [Google Scholar]
- 3.Abobaker A., Raba A.A., Alzwi A. Extrapulmonary and atypical clinical presentations of COVID-19. J. Med. Virol. 2020;92(11):2458–2464. doi: 10.1002/jmv.26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. International Journal of Infectious Diseases. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lind L., Eriksson K., Grahn A. Chemokines and matrix metalloproteinases in cerebrospinal fluid of patients with central nervous system complications caused by varicella-zoster virus. Journal of neuroinflammation. 2019;16(1):1–11. doi: 10.1186/s12974-019-1428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J., Stohlman S.A., Atkinson R., Hinton D.R., Marten N.W. Matrix metalloproteinase expression correlates with virulence following neurotropic mouse hepatitis virus infection. J. Virol. 2002;76(15):7374–7384. doi: 10.1128/JVI.76.15.7374-7384.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conant K., McArthur J.C., Griffin D.E., Sjulson L., Wahl L.M., Irani D.N. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1999;46(3):391–398. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Engelhardt B., Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids and Barriers of the CNS. 2011;8(1):1–9. doi: 10.1186/2045-8118-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ransohoff R.M., Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012;12(9):623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt B., Ransohoff R.M. Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol. 2012;33(12):579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Giraudon P., Bernard A. Inflammation in neuroviral diseases. J. Neural Transm. 2010;117(8):899–906. doi: 10.1007/s00702-010-0402-y. [DOI] [PubMed] [Google Scholar]
- 14.Fotuhi M., Mian A., Meysami S., Raji C.A. Neurobiology of COVID-19. Journal of Alzheimer's disease : JAD. 2020;76(1):3–19. doi: 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phua J., Weng L., Ling L., Egi M., Lim C.M., Divatia J.V., Shrestha B.R., Arabi Y.M., Ng J., Gomersall C.D., Nishimura M., Koh Y., Du B. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. The Lancet. Respiratory medicine. 2020;8(5):506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., Adams G., Hornick J.L., Padera R.F., Jr, Sabeti P. Neuropathological features of Covid-19. N. Engl. J. Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infectious diseases of poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desforges M., Le Coupanec A., Stodola J.K., Meessen-Pinard M., Talbot P.J. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.. Hamming, W. Timens, M. Bulthuis, A. Lely, G.v. Navis, H. van Goor, Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis, The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland 203(2) (2004) 631-637. [DOI] [PMC free article] [PubMed]
- 20.Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., Talbot P.J. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2020;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solár P., Zamani A., Kubíčková L., Dubový P., Joukal M. Choroid plexus and the blood–cerebrospinal fluid barrier in disease. Fluids and Barriers of the CNS. 2020;17:1–29. doi: 10.1186/s12987-020-00196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marques F., Sousa J.C., Coppola G., Falcao A.M., Rodrigues A.J., Geschwind D.H., Sousa N., Correia-Neves M., Palha J.A. Kinetic profile of the transcriptome changes induced in the choroid plexus by peripheral inflammation. J. Cereb. Blood Flow Metab. 2009;29(5):921–932. doi: 10.1038/jcbfm.2009.15. [DOI] [PubMed] [Google Scholar]
- 23.Chiu P.-S., Lai S.-C. Matrix metalloproteinase-9 leads to claudin-5 degradation via the NF-κB pathway in BALB/c mice with eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0053370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dando S.J., Mackay-Sim A., Norton R., Currie B.J., John J.A.S., Ekberg J.A., Batzloff M., Ulett G.C., Beacham I.R. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev. 2014;27(4):691–726. doi: 10.1128/CMR.00118-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meeker R.B., Williams K., Killebrew D.A., Hudson L.C. Cell trafficking through the choroid plexus. Cell adhesion & migration. 2012;6(5):390–396. doi: 10.4161/cam.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gram M., Sveinsdottir S., Cinthio M., Sveinsdottir K., Hansson S.R., Mörgelin M., Åkerström B., Ley D. Extracellular hemoglobin-mediator of inflammation and cell death in the choroid plexus following preterm intraventricular hemorrhage. Journal of neuroinflammation. 2014;11(1):1–15. doi: 10.1186/s12974-014-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shukla V., Shakya A.K., Shukla M., Kumari N., Krishnani N., Dhole T., Misra U.K. Circulating levels of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases during Japanese encephalitis virus infection. Virusdisease. 2016;27(1):63–76. doi: 10.1007/s13337-015-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards J.A., Denis F., Talbot P.J. Activation of glial cells by human coronavirus OC43 infection. J. Neuroimmunol. 2000;108(1–2):73–81. doi: 10.1016/S0165-5728(00)00266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., McCluskey K., Fujii K., Wahl L.M. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-α, granulocyte-macrophage CSF, and IL-1β through prostaglandin-dependent and-independent mechanisms. J. Immunol. 1998;161(6):3071–3076. [PubMed] [Google Scholar]
- 30.Rempe R.G., Hartz A.M.S., Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36(9):1481–1507. doi: 10.1177/0271678X16655551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muri L., Leppert D., Grandgirard D., Leib S.L. MMPs and ADAMs in neurological infectious diseases and multiple sclerosis. Cell. Mol. Life Sci. 2019;76(16):3097–3116. doi: 10.1007/s00018-019-03174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J., Marten N.W., Bergmann C.C., Macklin W.B., Hinton D.R., Stohlman S.A. Expression of matrix metalloproteinases and their tissue inhibitor during viral encephalitis. J. Virol. 2005;79(8):4764–4773. doi: 10.1128/JVI.79.8.4764-4773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laronha H., Caldeira J. Structure and function of human matrix metalloproteinases. Cells. 2020;9(5):1076. doi: 10.3390/cells9051076. [DOI] [PMC free article] [PubMed] [Google Scholar]