Abstract
SARS-CoV-2 infection causes respiratory insufficiency and neurological manifestations, including loss of smell and psychiatric disorders, and can be fatal. Most vaccines are based on the spike antigen alone, and although they have shown efficacy at preventing severe disease and death, they do not always confer sterilizing immunity. Here, we interrogate whether SARS-CoV-2 vaccines could be improved by incorporating nucleocapsid as an antigen. We show that, after 72 h of challenge, a spike-based vaccine confers acute protection in the lung, but not in the brain. However, combining a spike-based vaccine with a nucleocapsid-based vaccine confers acute protection in both the lung and brain. These findings suggest that nucleocapsid-specific immunity can improve the distal control of SARS-CoV-2, warranting the inclusion of nucleocapsid in next-generation COVID-19 vaccines.
Keywords: SARS-CoV-2 vaccines, COVID-19, spike antigen, nucleocapsid antigen
Graphical abstract
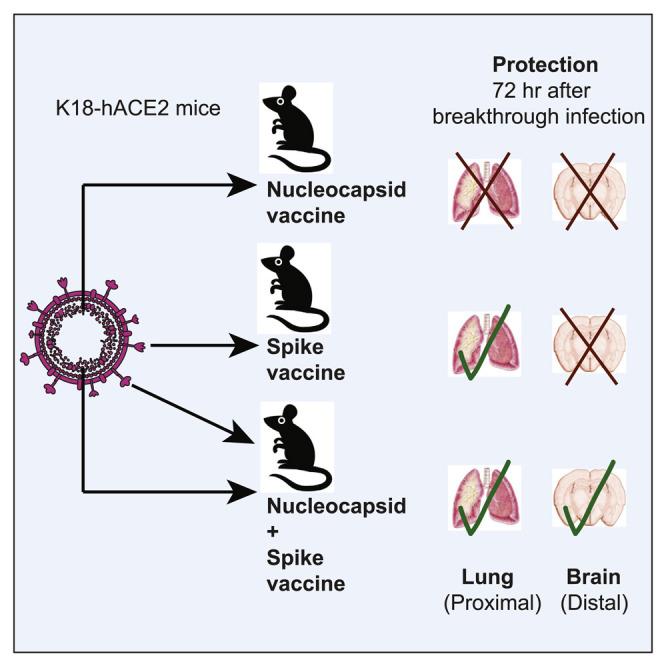
Most SARS-CoV-2 vaccines are based on the spike antigen alone, and it is unknown whether including other viral antigens improves protection. Dangi et al. show that combining a spike vaccine with a nucleocapsid vaccine improves the control of a SARS-CoV-2 infection, warranting the inclusion of nucleocapsid in next-generation SARS-CoV-2 vaccines.
Introduction
Most of the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) vaccines used in humans are based on the spike antigen. These vaccines have shown high efficacy against severe disease and death, but they do not always confer sterilizing immunity (Baden et al., 2021; Logunov et al., 2021; Polack et al., 2020; Sadoff et al., 2021; Voysey et al., 2021; Zhu et al., 2020). In particular, breakthrough infections can be detected in nasopharyngeal swabs of vaccinated individuals (Keehner et al., 2021; Levine-Tiefenbrun et al., 2021; Tande et al., 2021). Nasopharyngeal swabs typically contain viruses derived from the proximal site of challenge (the respiratory system), which may not reflect ongoing virus replication at distal sites of the body. It is unknown whether SARS-CoV-2 vaccines can prevent acute viral dissemination to distal sites of the body, such as the central nervous system, which is considered an immune-privileged site that is relatively “impermeable” to circulating antibodies (Forrester et al., 2018).
The central nervous system has become increasingly important in the management of coronavirus disease 2019 (COVID-19), especially because even mild or asymptomatic infections can trigger neurological manifestations, including “brain fog” and psychiatric conditions (Nalbandian et al., 2021). SARS-CoV-2 is a respiratory virus, but prior reports suggest that it may also disseminate to the brain via the olfactory mucosa (Cantuti-Castelvetri et al., 2020; Meinhardt et al., 2021; Song et al., 2021). Post-mortem analyses of COVID-19 patients have shown the presence of SARS-CoV-2 in the brain (Gasmi et al., 2021; Kumari et al., 2021; Meinhardt et al., 2021; Song et al., 2021). Knowing whether vaccines can block viral dissemination to the central nervous system is important, because this would help elucidate whether vaccinated people who become exposed to the virus could still develop neurological complications. For ethical reasons, it is not feasible to sample the brain of vaccinated individuals to assess breakthrough infections in this distal site.
The SARS-CoV-2 spike protein is a critical antigen in coronavirus vaccines. This antigen alone is the basis for the Pfizer/BioNTek vaccine, Moderna vaccine, Johnson & Johnson’s (J&J) Janssen vaccine, AstraZeneca/Oxford vaccine, CanSino vaccine, Sputnik V vaccine, Novavax vaccine, and others. Vaccines that contain the spike antigen generate robust neutralizing antibodies that prevent the initial entry of SARS-CoV-2 into the respiratory system, but it is unclear whether other viral antigens could provide equally important immune protection. It is also unknown whether the inclusion of multiple viral antigens could provide a synergistic improvement in vaccine-elicited protection. In this study, we compared the efficacy of spike-based versus nucleocapsid-based vaccines after an intranasal SARS-CoV-2 challenge in K18-hACE2 mice, which are highly susceptible to SARS-CoV-2 infection. Surprisingly, we show that a spike-based vaccine does not provide acute protection to the central nervous system; protection against distal viral dissemination to the nervous system was observed only when a spike-based vaccine was co-administered with a nucleocapsid-based vaccine. These findings demonstrate a synergy between spike-specific and nucleocapsid-specific immune responses, and provide a framework for the rational design of next-generation coronavirus vaccines.
Results
Immunogenicity of spike-based and nucleocapsid-based SARS-CoV-2 vaccines
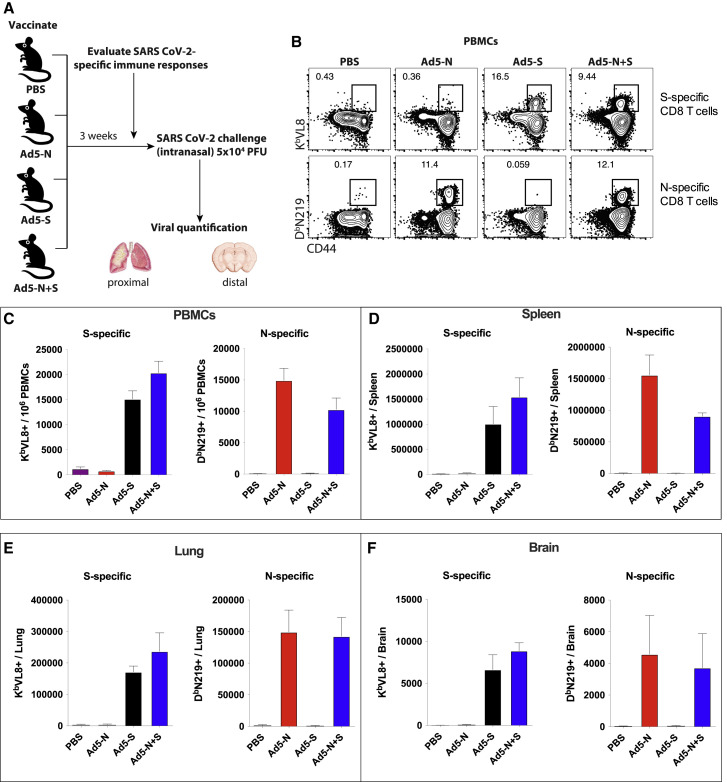
K18-hACE2 mice constitute a robust pre-clinical model that recapitulates salient features of SARS-CoV-2 infection in humans, including respiratory insufficiency, a dysregulated inflammatory response, neurological complications, and death. Therefore, this mouse model has been useful for investigating antiviral therapies, vaccines, and COVID-19 pathogenesis (Bao et al., 2020; Oladunni et al., 2020; Rosenfeld et al., 2021; Winkler et al., 2020; Zheng et al., 2021). We vaccinated K18-hACE2 mice intramuscularly with an adenovirus vector expressing SARS-CoV-2 spike (Ad5-S) or nucleocapsid (Ad5-N), or both (Ad5-S + Ad5-N), at a dose of 109 plaque-forming units (PFUs) per vector per mouse (Figure 1 A).
Figure 1.
Immunogenicity of spike- and nucleocapsid-based vaccines by tetramer binding assays
(A) Experimental approach for evaluating immune responses and immune protection in K18-hACE2 mice.
(B) Representative fluorescence-activated cell sorting (FACS) plots showing the frequencies of SARS-CoV-2-specific CD8 T cells (KbVL8+ and DbN219+) in PBMCs. Gated on total CD8 T cells.
(C) Summary of SARS-CoV-2 spike-specific CD8 T cells in PBMCs.
(D) Summary of SARS-CoV-2 spike-specific CD8 T cells in spleen.
(E) Summary of SARS-CoV-2 spike-specific CD8 T cells in lung.
(F) Summary of SARS-CoV-2 spike-specific CD8 T cells in brain.
Data are from week 3 post-vaccination from an experiment with n = 5 per group. The experiment was performed twice with similar results. Error bars represent SEMs.
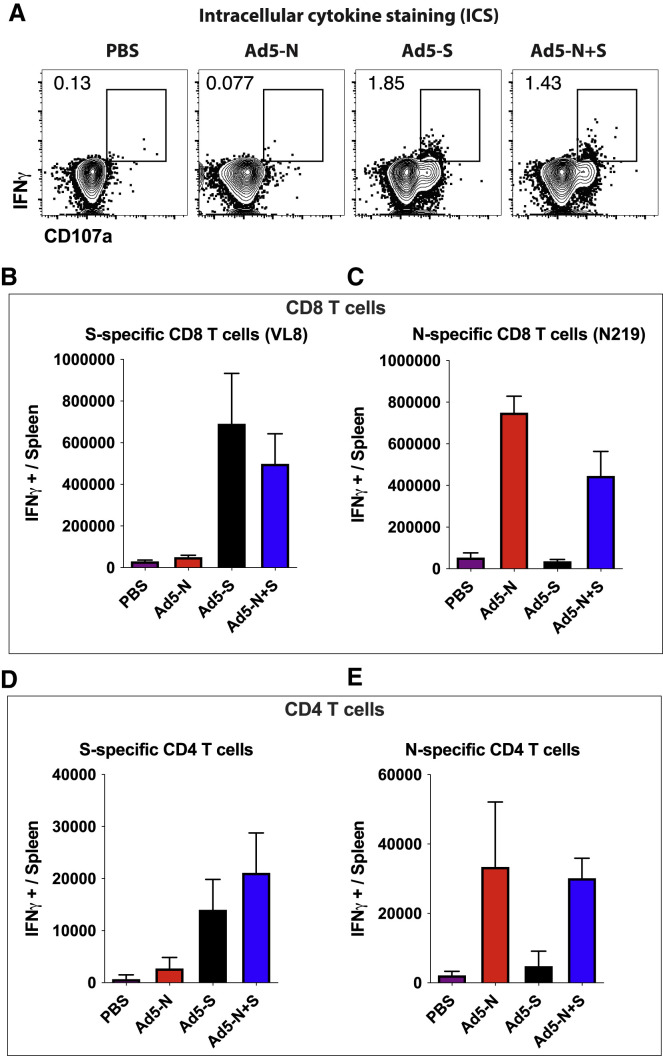
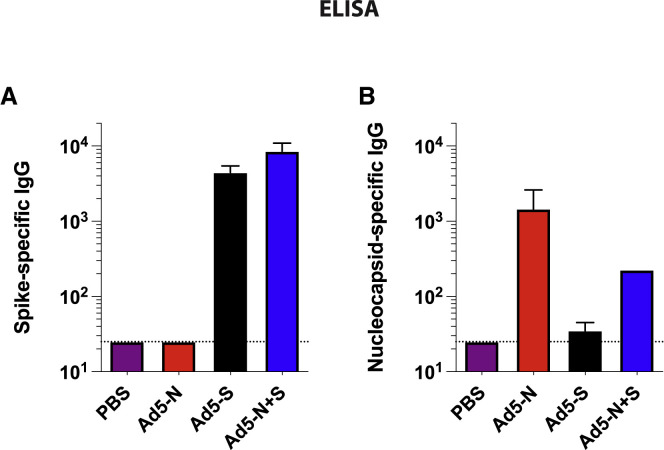
After 3 weeks, we evaluated SARS-CoV-2-specific CD8 T cell responses in peripheral blood mononuclear cells (PBMCs) and tissues by tetramer binding assays (KbVL8 to detect spike-specific CD8 T cells; DbN219 to detect nucleocapsid-specific CD8 T cells). Ad5-S and Ad5-N vaccination elicited detectable CD8 T cell responses in PBMCs and tissues (Figures 1B–1F). We also confirmed the immunogenicity of the Ad5-S and Ad5-N vaccines by intracellular cytokine staining (ICS) assays, which showed interferon-γ (IFN-γ)-expressing CD8 T cells (Figures 2 A–2C), and IFN-γ-expressing CD4 T cells (Figures 2D and 2E). Similar to prior studies with other Ad5-vectored vaccines (Larocca et al., 2016; Penaloza-MacMaster et al., 2013; Provine et al., 2016), we observed modest IFN-γ expression on virus-specific CD8 and CD4 T cells following Ad5 vaccination. The Ad5-S and Ad5-N vaccines also elicited their respective antibody responses by enzyme-linked immunosorbent assay (ELISA) (Figure 3 ). These results show that the spike-based and nucleocapsid-based vaccines elicit CD8 T cells, CD4 T cells, and antibody responses.
Figure 2.
Immunogenicity of spike- and nucleocapsid-based vaccines by intracellular cytokine staining (ICS)
(A) Representative FACS plots showing the frequencies of SARS-CoV-2 spike-specific CD8 T cells (IFN-γ+). Gated on total CD8 T cells.
(B) Summary of SARS-CoV-2 spike-specific CD8 T cells.
(C) Summary of SARS-CoV-2 nucleocapsid-specific CD8 T cells.
(D) Summary of SARS-CoV-2 spike-specific CD4 T cells.
(E) Summary of SARS-CoV-2 nucleocapsid-specific CD4 T cells.
Data are from spleen (5 h peptide stimulation). In (A) and (B), cells were stimulated with VNFNFNGL peptide (VL8). In (C), cells were stimulated with LALLLLDRL peptide (N219). In (D) and (E), cells were stimulated with the respective overlapping peptide pools. Data are from week 3 post-vaccination from an experiment with n = 5 per group. Experiment was performed twice with similar results. Error bars represent SEMs.
Figure 3.
Immunogenicity of spike- and nucleocapsid-based vaccines by ELISA
(A) Summary of SARS-CoV-2 spike-specific antibody responses in sera.
(B) Summary of SARS-CoV-2 nucleocapsid-specific antibody responses in sera.
Data are from week 3 post-vaccination from an experiment with n = 5 per group. The experiment was performed twice with similar results. Error bars represent SEMs.
A spike-based vaccine confers proximal protection in the lung, but not distal protection in the brain
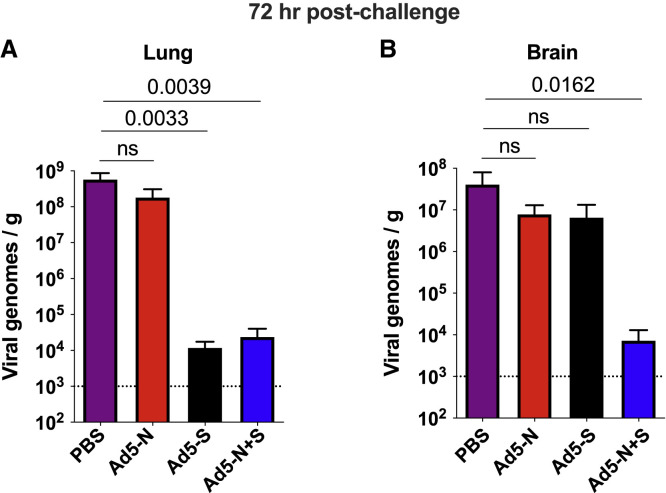
We performed intranasal challenges with 5 × 104 PFU of SARS-CoV-2 (isolate USA-WA1/2020), followed by euthanasia 3 days post-challenge to evaluate acute viral loads in tissues. We also challenged unvaccinated K18-hACE2 mice as controls. We compared viral loads in the lung, which represents a proximal site of challenge. Intranasal challenge of unvaccinated K18-hACE2 mice with SARS-CoV-2 normally results in high viral replication in the lung, followed by distal dissemination to the brain by day 2 post-challenge (Winkler et al., 2020). Consistent with prior studies (Oladunni et al., 2020; Rosenfeld et al., 2021; Winkler et al., 2020; Zheng et al., 2021), the unvaccinated mice showed high viral loads in the lung (Figure 4 A). In contrast, mice vaccinated with the spike-based vaccine alone (but not the nucleocapsid-based vaccine alone) exhibited significant antiviral protection in the lung at day 3 post-challenge (Figure 4A).
Figure 4.
Immune protection of spike- and nucleocapsid-based vaccines
RNA was harvested from the lungs (A) and brains (B) at 72 h post-infection, and viral RNA was quantified by qRT-PCR.
Challenges were performed with a total of 5 mice per group in biosafety level 3 (BSL-3) facilities. Error bars represent SEMs. Indicated p values were calculated using Kruskal-Wallis test (Dunn’s multiple comparisons). NS, not significant (p > 0.05).
Importantly, co-immunization of mice with the spike-based vaccine and the nucleocapsid-based vaccine did not confer any synergistic protective advantage in the lung at day 3 post-challenge, relative to spike-based vaccine alone (Figure 4A). These data show that a spike-based vaccine alone is sufficient to protect the site of initial viral entry, the respiratory system.
Effect of nucleocapsid-specific immunity in the distal control of a SARS-CoV-2 infection
At first glance, the data above suggested that nucleocapsid-specific immunity plays a dispensable role during a SARS-CoV-2 infection. Nucleocapsid-specific immunity has recently garnered attention as a main target for T cell responses (Lineburg et al., 2021; Nguyen et al., 2021; Rydyznski Moderbacher et al., 2020). Antibody responses can block the initial entry of the virus at proximal sites of challenge, but if the virus escapes the antibody response, T cell responses are critical for controlling second-round infections and subsequent viral dissemination to distal sites. SARS-CoV-2 is thought to disseminate to the nervous system after second-round infections that progress from the olfactory mucosa to the brain (Meinhardt et al., 2021). Although current spike-based SARS-CoV-2 vaccines show high efficacy in protecting the respiratory system via their induction of neutralizing antibodies, it is not clear whether they can also provide acute protection at distal sites, outside the respiratory system.
We show that mice immunized with a spike-based vaccine (or a nucleocapsid-based vaccine) did not exhibit a statistically significant improvement in antiviral protection in the brain (Figure 4B). However, combining a spike-based vaccine with a nucleocapsid-based vaccine resulted in significantly improved protection in the brain (Figure 4B). These findings show that spike-specific immunity confers acute protection at the proximal site of viral entry (the respiratory system), but acute protection at distal sites may critically depend on also having nucleocapsid-specific immunity.
Discussion
Several SARS-CoV-2 vaccines have shown efficacy against SARS-CoV-2, including the Pfizer/BioNTek vaccine, Moderna vaccine, J&J Janssen vaccine, AstraZeneca/Oxford vaccine, CanSino vaccine, Sputnik V vaccine, and Novavax vaccine (Baden et al., 2021; Logunov et al., 2021; Polack et al., 2020; Sadoff et al., 2021; Voysey et al., 2021; Zhu et al., 2020). All of these vaccines are based solely on the spike protein of SARS-CoV-2, and have demonstrated high efficacy against severe COVID-19. Although these vaccines prevent severe disease and death, symptomatic infections can occur in vaccinated individuals.
Insidious neurological manifestations are common after SARS-CoV-2 infection. It is unknown whether vaccines can prevent long-term neurological manifestations of SARS CoV-2. An unanticipated result from our studies is that a spike-based vaccine does not fully protect the central nervous system early following infection. This observation could help explain symptomatic infections that are reported in a fraction of vaccinated individuals, which can be associated with the loss of smell and other neurological manifestations.
Spike-specific antibodies are critical to prevent initial infection because they sterically block the binding of the spike protein to the host angiotensin-converting enzyme 2 (ACE2) receptor. However, in the context of a breakthrough infection, infected cells are eliminated by cytotoxic T cells, which do not need to be spike specific to recognize infected cells. The nucleocapsid protein of SARS-CoV-2 has been suggested as an important target for T cell responses (Joag et al., 2021; Lineburg et al., 2021; Nguyen et al., 2021; Rydyznski Moderbacher et al., 2020). First, this protein contains conserved cross-reactive T cell epitopes that are present among different coronaviruses, suggesting that it could be an ideal target for universal coronavirus vaccines (Dutta et al., 2020). Second, the nucleocapsid protein is among the most abundant structural proteins in the coronavirus life cycle (Chang et al., 2014; de Breyne et al., 2020; Weiss and Leibowitz, 2011), which may facilitate early antigen presentation and recognition by T cells.
Why does the nucleocapsid-based vaccine fail to confer acute protection at the proximal site of challenge (the lung)? It is possible that acute viral loads in the lung represent primarily viruses derived from the primary foci of infection. During the hyperacute phase, nucleocapsid-specific immune responses may not prevent breakthrough infection in the respiratory system because they target an internal protein that is not involved in viral entry. However, during subsequent second-round infections that progress from proximal sites of challenge to distal sites, nucleocapsid-specific T cells may provide a synergistic antiviral effect by killing virally infected cells, curtailing further the dissemination of the virus to distal sites. Co-immunization with a spike-based vaccine and a nucleocapsid-based vaccine accelerates the acute control of SARS-CoV-2, suggesting an advantage of including other viral antigens besides the spike protein in future COVID-19 vaccines.
A limitation of our study is that we only measured viral loads at a very early time point. We did not compare viral loads at later time points, because unvaccinated animals succumb (or must be euthanized) after day 5 post-challenge (Oladunni et al., 2020), and because it is already known that spike-based vaccines elicit immune responses that ultimately clear SARS-CoV-2 within 1 week of challenge (Feng et al., 2020; Wu et al., 2020). Our study was focused on evaluating hyperacute viral control to assess breakthrough infection. Future studies will assess the contribution of nucleocapsid-specific antibodies versus T cells, and whether vaccines that encode other viral antigens (e.g., envelope, membrane) can improve further distal protection after a SARS-CoV-2 challenge. Although the K18-hACE2 model is considered useful to evaluate vaccines and antivirals, it is possible that the extensive viral dissemination in the brain may be a peculiarity of this mouse model. Nevertheless, it is still reasonable to conclude that nucleocapsid-specific immunity improves the acute distal control of SARS-CoV-2. In conclusion, we show a substantial benefit of including both spike and nucleocapsid antigens in COVID-19 vaccines. These results warrant the incorporation of nucleocapsid in future coronavirus vaccines.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| αCD8, clone 53-6.7 in PerCP-Cy5.5 | BD PharMingen | 551162 |
| αCD44, clone IM7 in Pacific Blue | Biolegend | 103020 |
| αIFNy, clone XMG1.2 in APC | BD PharMingen | 554413 |
| αCD107a, clone 1D4B in FITC | BD PharMingen | 553793 |
| αCD4, clone RM4-5 FITC | eBioscience | 11-0042-82 |
| Bacterial and virus strains | ||
| Ad5-SARS-CoV-2 N | A gift from Dr. David Masopust at University of Minnesota | N/A |
| Ad5-SARS-CoV-2 S | University of Iowa Vector Core, custom made | N/A |
| SARS-Related Coronavirus 2, Isolate USA-WA1/2020, | BEI resources | NR-52281 |
| Chemicals, peptides, and recombinant proteins | ||
| SARS-CoV-2 S protein overlapping peptide pools (181 peptides) | BEI resources | NR-52402 |
| SARS-CoV-2 N protein overlapping peptide pools (59 peptides) | BEI resources | NR-52404 |
| VL8 (VNFNFNGL) | GenScript | N/A |
| N219 (LALLLLDRL) | GenScript | N/A |
| SARS CoV-2 S protein for ELISA | Made by the Northwestern University recombinant protein production core using a SARS CoV-2 S protein gene provided by BEI (NR-52394, see above) | N/A |
| SARS CoV-2 N protein for ELISA | BEI resources | NR-53797 |
| Critical commercial assays | ||
| Quick RNA 96 kit (RNA extraction) | Zymo Research | R1052 |
| Taqman RNA-to-CT step kit | ThermoFisher | 4392653 |
| Experimental models: Cell lines | ||
| HEK293 | ATCC | CRL-1573 |
| Vero-E6 | ATCC | CRL-1586 |
| Experimental models: Organisms/strains | ||
| K18-hACE2 mice | Jackson Laboratories | 034060 |
| Recombinant DNA | ||
| Plasmid containing SARS CoV-2 spike (S) gene | BEI resources | NR-52394 |
| Plasmid containing SARS CoV-2 nucleocapsid (N) gene | BEI resources | NR-53507 |
| Software and algorithms | ||
| GraphPad Prism software version 9.1.0 | https://www.graphpad.com/scientific-software/prism/ | N/A |
| FlowJo version 10 | https://www.flowjo.com | N/A |
| Other | ||
| KbVL8 tetramer (APC) | Provided by the NIH tetramer core at Emory University | N/A |
| KbN219 tetramer (PE) | Provided by the NIH tetramer core at Emory University | N/A |
| Forward primer, 5′ GACCCCAAAATCAGCGAAAT 3′ | Integrated DNA Technologies | 10006713 |
| Reverse primer, 5′ TCTGTTTACTGCCAGTTGAATCTG 3′ | Integrated DNA Technologies | 10006713 |
| Probe, 5′ TCTGGTTACTGCCAGTTGAATCTG 3′ | Integrated DNA Technologies | 10006713 |
| SARS-CoV-2 copy number control | BEI resources | NR-52358 |
| Live dead dye for flow cytometry | Invitrogen | L34976 |
| Cytofix/cytoperm (fixing agent for flow cytometry) | BD Biosciences | 554722 |
| Phosphate buffer saline (PBS) | GIBCO | 14190-144 |
| Golgi Plug protein transport inhibitor | BD Biosciences | 555029 |
| Golgi Stop (Monensin) | BD Biosciences | 554724 |
| DMEM | GIBCO | 11965-092 |
| Fetal bovine serum | Sigma | F0926-500 |
Resource availability
Lead contacts
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact Pablo Penaloza-MacMaster (ppm@northwestern.edu) and/or Justin Richner (richner@uic.edu).
Materials availability
For Ad5-SARS CoV-2 spike viral vector access, contact the University of Iowa vector core at vector@uiowa.edu. For Ad5-SARS CoV-2 nucleocapsid viral vector access, contact David Masopust at masopust@umn.edu. For VNFNFNGL and LALLLLDRL peptides, contact GenScript at sales.US@genscript.com.
Experimental models and subject details
Animals and ethics statement
Mice were purchased from Jackson laboratories and were housed at Northwestern University or University of Illinois at Chicago (UIC) animal facility. All procedures were performed with the approval of the center for comparative medicine at Northwestern University and the UIC IACUC. Adult mice, approximately half females and half males were used for the immunogenicity experiments included in this study. For the challenge studies, female mice were used.
Method details
Cell lines
Adenoviral vectors were propagated using HEK293 cells purchased from ATCC (cat # CRL-1573). Vero E6 cells were used to propagate SARS CoV-2 isolate USA-WA1/2020 (BEI resources, NR-52281). Cells were not authenticated as they were purchased from a reputable vendor and certificate of analysis was obtained.
Mice and vaccinations
6-8-week-old K18-hACE2 mice were used. These mice express the human ACE2 protein behind the keratin 18 promoter, directing expression in epithelial cells. Mice were purchased from Jackson laboratories (Stock No: 034860). Mice were immunized intramuscularly (50 μL per quadriceps) with an Ad5 vector expressing SARS-CoV-2 spike protein (Ad5-S), or nucleocapsid protein (Ad5-N), or both vectors combined; diluted in sterile PBS, at 109 PFU per mouse. Ad5-N was a kind gift of the Masopust/Vezys laboratory (Joag et al., 2021). These are non-replicating Ad5 vectors (E1/E3 deleted). The vectors contain a CMV (Cytomegalovirus) promoter driving the expression of the respective proteins. The Ad5 vectors were propagated on trans-complementing HEK293 cells (ATCC), purified by cesium chloride density gradient centrifugation, titrated, and then frozen at −80 °C.
SARS-CoV-2 virus and infections
SARS-Related Coronavirus 2, Isolate USA-WA1/2020, NR-52281 was deposited by the Centers for Disease Control and Prevention and obtained through BEI Resources, NIAID, NIH. Virus was propagated and tittered on Vero-E6 cells (ATCC). In brief, Vero cells were passaged in DMEM with 10% Fetal bovine serum (FBS) and Glutamax. Cells less than 20 passages were used for all studies. Virus stocks were expanded in Vero-E6 cells following a low MOI (0.01) inoculation and harvested after 4 days. Viral titers were determined by plaque assay on Vero-E6 cells. Viral stocks were used after a single expansion (passage = 1) to prevent genetic drift.
K18-hACE2 mice were anesthetized with isoflurane and challenged with 5x104 PFU of virus intranasally. Mouse infectious were performed at the University of Illinois at Chicago (UIC) following BL3 guidelines with approval by the UIC Institutional Animal Care and Use Committee (IACUC).
SARS-CoV-2 quantification in tissues
Tissues were isolated from infected mice and homogenized in sterile PBS. RNA was isolated with the Zymo 96-well RNA isolation kit (Catalog #: R1052) following the manufacturer’s protocol. SARS-CoV-2 viral burden was measured by RT-qPCR using Taqman primer and probe sets from IDT with the following sequences: Forward 5′ GAC CCC AAA ATC AGC GAA AT 3′, Reverse 5′ TCT GGT TAC TGC CAG TTG AAT CTG 3′, Probe 5′ ACC CCG CAT TAC GTT TGG TGG ACC 3′. A SARS-CoV-2 copy number control was obtained from BEI (NR-52358) and used to quantify SARS-CoV-2 genomes.
Reagents, flow cytometry and equipment
Single cell suspensions were obtained from PBMCs or tissues. Dead cells were gated out using Live/Dead fixable dead cell stain (Invitrogen). SARS-CoV-2 nucleocapsid or spike peptides were used for intracellular cytokine staining (ICS) to detect nucleocapsid- and spike-specific CD8 T cells. The peptide stimulation was performed in the presence of GolgiPlug and GolgiStop (BD Biosciences) for 5 hr at 37°C in a 5% CO2 incubator. MHC class I monomers (KbVL8, VNFNFNGL and Kb219, LALLLLDRL) were used for detecting virus-specific CD8 T cells, and were obtained from the NIH tetramer facility located at Emory University. MHC monomers were tetramerized in-house. The VNFNFNGL epitope is located in position 539-546 of the SARS-CoV-2 spike protein. Cells were stained with fluorescently-labeled antibodies against CD8α (53-6.7 on PerCP-Cy5.5), CD44 (IM7 on Pacific Blue), KbVL8 (APC), KbN219 (PE), CD107a (1D4B on FITC), and IFNγ (XMG1.2 on APC). Fluorescently-labeled antibodies were purchased from BD PharMingen, except for anti-CD44 (which was from Biolegend). Flow cytometry samples were acquired with a Becton Dickinson Canto II or an LSRII and analyzed using FlowJo v10 (Treestar).
SARS-CoV-2 spike and nucleocapsid specific ELISA
Binding antibody titers were quantified using ELISA as described previously (Dangi et al., 2020; Palacio et al., 2020), using spike protein and nucleocapsid protein as coating antigens. 96-well flat bottom plates MaxiSorp (Thermo Scientific) were coated with 0.1 μg/well of SARS-CoV-2 spike protein or nucleocapsid protein, for 48 hr at 4°C. Plates were washed with PBS + 0.05% Tween-20. Blocking was performed for 4 hr at room temperature with 200 μL of PBS + 0.05% Tween-20 + bovine serum albumin. 6 μL of sera were added to 144 μL of blocking solution in first column of plate, 1:3 serial dilutions were performed until row 12 for each sample and plates were incubated for 60 minutes at room temperature. Plates were washed three times followed by addition of goat anti-mouse horseradish peroxidase-conjugated IgG (Southern Biotech) diluted in blocking solution (1:5000), at 100 μL/well and incubated for 60 minutes at room temperature. Plates were washed three times and 100 μL /well of Sure Blue substrate (Sera Care) was added for approximately 8 minutes. Reaction was stopped using 100 μL/well of KPL TMB stop solution (Sera Care). Absorbance was measured at 450 nm using a Spectramax Plus 384 (Molecular Devices). SARS-CoV-2 spike protein was made at the Northwestern Recombinant Protein Production Core by Dr. Sergii Pshenychnyi using plasmid that was produced under HHSN272201400008C and obtained through BEI Resources, NIAID, NIH: Vector pCAGGS Containing the SARS-Related Coronavirus 2, Wuhan-Hu-1 Spike Glycoprotein Gene (soluble, stabilized), NR-52394. SARS-CoV-2 nucleocapsid protein was obtained through BEI Resources, NIAID, NIH: Nucleocapsid Protein from SARS-Related Coronavirus 2, Wuhan-Hu-1 with C-Terminal Histidine Tag, Recombinant from Baculovirus, NR-53797.
Quantification and statistical analysis
Statistical analyses are indicated on the figure legend. Dashed lines in data figures represent limit of detection. Statistical significance was established at p ≤ 0.05 and was generally assessed by two tailed unpaired Student’s t tests, unless indicated otherwise in figure legends. Data were analyzed using Prism (Graphpad).
Acknowledgments
We thank Drs. Susan Weiss, Stanley Perlman, Thomas Gallagher, David Masopust, and Vaiva Vezys for discussions and reagents. This work was possible with startup funds to J.M.R., a grant from the Emerging and Re-Emerging Pathogens Program (EREPP) at Northwestern University to P.P.M., and a grant from the National Institute on Drug Abuse (NIDA, DP2DA051912) to P.P.M.
Author contributions
T.D. and N.P. performed the immunogenicity experiments. J.C. performed the challenge experiments. P.P.M. and J.M.R. designed the experiments and secured the funding. P.P.M. and J.M.R. wrote the paper, with feedback from all of the authors.
Declaration of interests
P.P.M. reports being Task Force Advisor to the Illinois Department of Public Health (IDPH) on SARS-CoV-2 vaccines. P.P.M. is also a member/advisor of the COVID-19 Vaccine Regulatory Science Consortium (CoVAXCEN) at Northwestern University’s Institute for Global Health.
Published: August 17, 2021
Data and code availability
This article does not report original code. The article includes all the datasets and analyses generated for this study. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. COVE Study Group Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F., et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.K., Hou M.H., Chang C.F., Hsiao C.D., Huang T.H. The SARS coronavirus nucleocapsid protein--forms and functions. Antiviral Res. 2014;103:39–50. doi: 10.1016/j.antiviral.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangi T., Chung Y.R., Palacio N., Penaloza-MacMaster P. Interrogating Adaptive Immunity Using LCMV. Curr. Protoc. Immunol. 2020;130:e99. doi: 10.1002/cpim.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Breyne S., Vindry C., Guillin O., Condé L., Mure F., Gruffat H., Chavatte L., Ohlmann T. Translational control of coronaviruses. Nucleic Acids Res. 2020;48:12502–12522. doi: 10.1093/nar/gkaa1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta N.K., Mazumdar K., Gordy J.T. The Nucleocapsid Protein of SARS-CoV-2: A Target for Vaccine Development. J. Virol. 2020;94 doi: 10.1128/JVI.00647-20. e00647-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Wang Q., Shan C., Yang C., Feng Y., Wu J., Liu X., Zhou Y., Jiang R., Hu P., et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020;11:4207. doi: 10.1038/s41467-020-18077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester J.V., McMenamin P.G., Dando S.J. CNS infection and immune privilege. Nat. Rev. Neurosci. 2018;19:655–671. doi: 10.1038/s41583-018-0070-8. [DOI] [PubMed] [Google Scholar]
- Gasmi A., Tippairote T., Mujawdiya P.K., Gasmi Benahmed A., Menzel A., Dadar M., Bjørklund G. Neurological Involvements of SARS-CoV2 Infection. Mol. Neurobiol. 2021;58:944–949. doi: 10.1007/s12035-020-02070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joag V., Wijeyesinghe S., Stolley J.M., Quarnstrom C.F., Dileepan T., Soerens A.G., Sangala J.A., O’Flanagan S.D., Gavil N.V., Hong S.W., et al. Cutting Edge: Mouse SARS-CoV-2 Epitope Reveals Infection and Vaccine-Elicited CD8 T Cell Responses. J. Immunol. 2021;206:931–935. doi: 10.4049/jimmunol.2001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehner J., Horton L.E., Pfeffer M.A., Longhurst C.A., Schooley R.T., Currier J.S., Abeles S.R., Torriani F.J. SARS-CoV-2 Infection after Vaccination in Health Care Workers in California. N. Engl. J. Med. 2021;384:1774–1775. doi: 10.1056/NEJMc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari P., Rothan H.A., Natekar J.P., Stone S., Pathak H., Strate P.G., Arora K., Brinton M.A., Kumar M. Neuroinvasion and Encephalitis Following Intranasal Inoculation of SARS-CoV-2 in K18-hACE2 Mice. Viruses. 2021;13:132. doi: 10.3390/v13010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca R.A., Provine N.M., Aid M., Iampietro M.J., Borducchi E.N., Badamchi-Zadeh A., Abbink P., Ng’ang’a D., Bricault C.A., Blass E., et al. Adenovirus serotype 5 vaccine vectors trigger IL-27-dependent inhibitory CD4+ T cell responses that impair CD8+ T cell function. Sci. Immunol. 2016;1:eaaf7643. doi: 10.1126/sciimmunol.aaf7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine-Tiefenbrun M., Yelin I., Katz R., Herzel E., Golan Z., Schreiber L., Wolf T., Nadler V., Ben-Tov A., Kuint J., et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat. Med. 2021;27:790–792. doi: 10.1038/s41591-021-01316-7. [DOI] [PubMed] [Google Scholar]
- Lineburg K.E., Grant E.J., Swaminathan S., Chatzileontiadou D.S.M., Szeto C., Sloane H., Panikkar A., Raju J., Crooks P., Rehan S., et al. CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses. Immunity. 2021;54:1055–1065.e5. doi: 10.1016/j.immuni.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., et al. Gam-COVID-Vac Vaccine Trial Group Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brünink S., Greuel S., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.H.O., Rowntree L.C., Petersen J., Chua B.Y., Hensen L., Kedzierski L., van de Sandt C.E., Chaurasia P., Tan H.-X., Habel J.R., et al. CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope display high naive precursor frequency and TCR promiscuity. Immunity. 2021;54:1066–1082.e5. doi: 10.1016/j.immuni.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladunni F.S., Park J.G., Pino P.A., Gonzalez O., Akhter A., Allué-Guardia A., Olmo-Fontánez A., Gautam S., Garcia-Vilanova A., Ye C., et al. Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat. Commun. 2020;11:6122. doi: 10.1038/s41467-020-19891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio N., Dangi T., Chung Y.R., Wang Y., Loredo-Varela J.L., Zhang Z., Penaloza-MacMaster P. Early type I IFN blockade improves the efficacy of viral vaccines. J. Exp. Med. 2020;217:e20191220. doi: 10.1084/jem.20191220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaloza-MacMaster P., Provine N.M., Ra J., Borducchi E.N., McNally A., Simmons N.L., Iampietro M.J., Barouch D.H. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J. Virol. 2013;87:1373–1384. doi: 10.1128/JVI.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. C4591001 Clinical Trial Group Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provine N.M., Larocca R.A., Aid M., Penaloza-MacMaster P., Badamchi-Zadeh A., Borducchi E.N., Yates K.B., Abbink P., Kirilova M., Ng’ang’a D., et al. Immediate Dysfunction of Vaccine-Elicited CD8+ T Cells Primed in the Absence of CD4+ T Cells. J. Immunol. 2016;197:1809–1822. doi: 10.4049/jimmunol.1600591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld R., Noy-Porat T., Mechaly A., Makdasi E., Levy Y., Alcalay R., Falach R., Aftalion M., Epstein E., Gur D., et al. Post-exposure protection of SARS-CoV-2 lethal infected K18-hACE2 transgenic mice by neutralizing human monoclonal antibody. Nat. Commun. 2021;12:944. doi: 10.1038/s41467-021-21239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., Belanger S., Abbott R.K., Kim C., Choi J., et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., et al. ENSEMBLE Study Group Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., Lu P., Weizman O.E., Liu F., Dai Y., et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;218:e20202135. doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tande A.J., Pollock B.D., Shah N.D., Farrugia G., Virk A., Swift M., Breeher L., Binnicker M., Berbari E.F. Impact of the COVID-19 Vaccine on Asymptomatic Infection Among Patients Undergoing Pre-Procedural COVID-19 Molecular Screening. Clin. Infect. Dis. 2021:ciab229. doi: 10.1093/cid/ciab229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Oxford COVID Vaccine Trial Group Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv. Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler E.S., Bailey A.L., Kafai N.M., Nair S., McCune B.T., Yu J., Fox J.M., Chen R.E., Earnest J.T., Keeler S.P., et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020;21:1327–1335. doi: 10.1038/s41590-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Zhong G., Zhang J., Shuai L., Zhang Z., Wen Z., Wang B., Zhao Z., Song X., Chen Y., et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020;11:4081. doi: 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Wong L.R., Li K., Verma A.K., Ortiz M.E., Wohlford-Lenane C., Leidinger M.R., Knudson C.M., Meyerholz D.K., McCray P.B., Jr., Perlman S. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature. 2021;589:603–607. doi: 10.1038/s41586-020-2943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not report original code. The article includes all the datasets and analyses generated for this study. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.