Abstract
A series of novel quinolone-based 4-(methoxymethyl)-1,2,3-triazole derivatives were synthesized, and their structures were characterized by 1H, 13C NMR and mass spectroscopy. The compounds (IXa-l) were screened in vitro antibacterial activity against five gram-positive and five gram-negative bacterial strains, viz. M. Tuberculosis, M. Luteus, MRSA, B. Subtilis, B. Cereus, P. Aerginosa, K. Pneumonia, E. Coli, P. Vulgaris and S. Typhi, used and compared with standard gentamycin. The combination of the pharmacologically active moieties in a single scaffold results in their synergistic effect and high antimicrobial activity against several bacterial strains. COVID-19 has spread rapidly around the globe since its first identification in Wuhan, China, in December 2019. Coronavirus Disease 2019 (COVID‐19 Mpro) has become a major health problem causing severe acute respiratory illness in humans. The causative virus is called severe acute respiratory syndrome coronavirus 2, and the World Health Organization named the new epidemic disease Coronavirus Disease (COVID-19). Also, docking studies demonstrated that all derivatives exhibit a good theoretical affinity with Autodock 4.2 software score in between − 9.89 and − 13.4 kCal/mol against the main protease of COVID‐19 Mpro that caused worldwide epidemics. We believe that newly synthesized quinolone-based 4-(methoxymethyl)-1,2,3-triazole derivatives can guide many future studies in organic synthesis, medicine and pharmaceutical applications.
Keywords: 1,2,3-Triazole; Antimicrobial activity; COVID-19; Autodock
Introduction
A series of novel quinolone-based chalcones containing 1,2,3-triazole moiety were synthesized. Several natural products contain quinoline nucleus in (Cinchona Alkaloids) and quinoline analogues displaying a broad range of biological activities [1] such as anticytotoxic [2], antimicrobial [3], anti-tubercular [4] and anti-malarial [5]. Moreover, Chalcones are a class of privileged structures that have a wide range of biological properties such as antimicrobial [6], anti-inflammatory [7] and anticancer [8]. Chalcones are natural biocides and are well-known intermediates used for the synthesis of various heterocyclic compounds. Chalcones constitute an important class of natural products belonging to the flavonoid families [9], which are also key precursors in the synthesis of many biologically important heterocycles such as benzothiazepines [10], pyrazolines [11] and flavones [12]. Furthermore, piperidine derivatives are reported as anti-HIV [13], antitumor [14] and antimicrobial [15] active molecules. Moreover, 1,2,3-triazole derivatives enhanced considerable attention for the past few decades due to their attractive chemotherapeutical importance in antimicrobial [16], anti-inflammatory [17], anticonvulsant [18], anticancer [19], anti-HIV [20] and anti-tubercular [21] activities and help to avert platelet aggregation [22]. Very recently, disubstituted 1,2,3-triazole analogues have been reported as cannabinoid CB1 receptor antagonists [23], antiallergenic [24], antineoplastic [25]. Some of the 1, 2, 3-triazoles are used as deoxyribose nucleic acid (DNA) cleaving agents [26] and potassium channel activators [27], antioxidant [28], antimalarial [29] and antiprotozoal [30]. 1, 2, 3-triazole moieties are attractive connecting units, since they are stable to metabolic degradation and capable of hydrogen bonding which can be favorable in binding of biomolecular targets. In view of pharmacological significance of triazole derivatives, we were planned to synthesize some new triazole; no study on the effect of these compounds on COVID‐19 Mpr° has been conducted so far.
Corona viruses are a large family of viruses which may cause illness in animals and humans. In human beings, several corona viruses are known to cause respiratory infections ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS) and severe acute respiratory syndrome (SARS). The most recently identified corona virus causes lethal SARS. This new virus and disease were unknown before the outbreak began in Wuhan, China, in 2019 [31]. Now the COVID-19 has become a pandemic and has been affecting many countries globally. A novel coronavirus (CoV) is a new strain of coronavirus.
The disease caused by the novel coronavirus first identified in Wuhan, China, has been named Coronavirus Disease 2019 (COVID-19)—‘CO’ stands for corona, ‘VI’ for virus and ‘D’ for disease.
Formerly, this disease was referred to as ‘2019 novel coronavirus’ or ‘2019-nCoV. The COVID-19 virus is a new virus linked to the same family of viruses as severe acute respiratory syndrome (SARS) and some types of common cold.
The treatment regimen identified so far includes prophylactic antibiotics administration to prevent secondary infections and administration of broad spectrum antiviral to prevent viremia. Stopping one transmission chain can prevent many future cases isolation, and quarantine can have a big impact on reducing transmission. But many people in several countries have not been following the instructions of WHO; hence, the disease has been propagating rapidly severe acute respiratory syndrome (SARS) CoV emerged in Guangdong, China, in 2002, Middle East Respiratory Syndrome (MERS) CoV emerged in the Middle East in 2012, SARS-CoV-2 emerged in Wuhan, China, in 2019.
The human-to-the human spreading of the virus occurs due to close contact with an infected person, exposed to coughing, sneezing, respiratory droplets or aerosols. These aerosols can penetrate the human body (lungs) via inhalation through the nose or mouth. The COVID-19 virus is the largest positive-stranded RNA virus consisting of approximately 30,000 nucleotides as a part of their genome, which is 76% similar to the SARS CoV beta corona virus [32]. There are three types of beta corona viruses, which include SARS CoV, MERS and SARS CoV2 [33].
As there is no perfect suitable antiviral drug available for the treatment of COVID-19, the repurposing of existing drugs can be an effective approach for the treatment. Recently, COVID-19-infected patients administered with potential protease inhibitors like lopinavir/ritonavir have shown an improved therapeutic outcome [34] which demonstrates that Mpro or the main protease enzyme can be a promising target for drug discovery against COVID-19. Recently published crystal structure of SARS CoV2 main protease enzyme (Mpro) by Yang et al. [35] is considered as a significant breakthrough in novel coronavirus research. The structure explains that the main protease enzyme has a catalytic domain consisting HIS41 and CYS145 amino acid residues, which regulates the polyprotein to form single polypeptides that are required for replication and interpretation or transcription [36].
In this present study, through virtual screening-based molecular docking analysis, newly synthesized quinolone-based triazole molecules were identified as a potential SARS CoV2 main protease enzyme (Mpro) inhibitor.
Materials and methods
Thin-layer chromatography (TLC) was performed on E.Merk AL Silicagel 60 F254 plates and visualized under UV light. The infrared (IR) spectra were determined in a PerkinElmer Fourier transform (FDIR spectrum). 1H-NMR spectra were recorded on Varian EM-360 (400 MHz mercury plus) spectrometer in DMSO-d6 or CDCl3 and calibrated using solvent signals [7.25(CDCl3) and 2.50(DMSO-d6)]. All chemical shifts are recorded in δ(ppm) using TMS as an internal standard. The mass spectra were recorded on Agilent ion trap MS Spectrometer at energy of ionizing electron equal to 70 eV. All the reagents were purchased from Aldrich, Merck and Acros Organics and used without further purification.
Antibacterial activity
The synthesized compounds (IXa-l) were screened in vitro antibacterial activity against five gram-positive and five gram-negative bacterial strains such as M. Tuberculosis, M. Luteus, MRSA, B. Subtilis, B. Cereus, P. Aerginosa, K. Pneumonia, E. Coli, P. Vulgaris and S. Typhi compared with standard gentamycin drug. The MIC assay was performed to test the sensitivity of microorganisms to an antimicrobial agent. The tubes were incubated for 37 °C for 24 h, and later, antibacterial activity was recorded the bacterial growth at concentrations of 75 and 100 µg/mL. Among all synthesized compounds, the compounds IXe and IXg showed good activity against all the bacteria strains, compounds IXb and IXl showed more activity against K. Pneumonia and E. Coli, and this may be due to presence of electron withdrawing nature of nitro and chloro substituents on phenyl ring. The compounds IXa and IXi exhibited excellent potent activity against gram-negative bacteria K. pneumonia and E. coli. The compounds IXa and IXf showed more activity against gram-positive bacteria B. Subtilis and B. Cereus (Table 1).
Table 1.
Antibacterial activity of newly synthesized compounds
| Concentration µg/mL | Gram-positive Zone of inhibition |
Gram-negative Zone of inhibition |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M. tuberculosis | M. luteus | MRSA | B. subtilis | B. cereus | P. aeruginosa | K. pneumoniae | E. coli | P. vulgaris | S. typhi | ||
| IXa | 75 | 26 | 23 | 18 | 23 | 26 | 20 | 22 | 25 | 27 | 20 |
| 100 | 28 | 31 | 32 | 28 | 32 | 24 | 25 | 28 | 28 | 23 | |
| IXb | 75 | 17 | 21 | 16 | 20 | 23 | 22 | 20 | 18 | 19 | 17 |
| 100 | 20 | 26 | 22 | 26 | 27 | 27 | 26 | 25 | 24 | 17 | |
| IXc | 75 | 16 | 18 | 12 | 15 | 14 | 13 | 17 | 18 | 16 | 13 |
| 100 | 17 | 20 | 14 | 17 | 16 | 15 | 20 | 20 | 17 | 15 | |
| IXd | 75 | 17 | 21 | 19 | 25 | 26 | 19 | 26 | 29 | 22 | 18 |
| 100 | 25 | 25 | 24 | 28 | 28 | 23 | 23 | 22 | 28 | 23 | |
| IXe | 75 | 29 | 31 | 19 | 24 | 28 | 21 | 23 | 24 | 31 | 21 |
| 100 | 31 | 35 | 34 | 29 | 36 | 28 | 26 | 27 | 35 | 23 | |
| IXf | 75 | 25 | 24 | 19 | 23 | 24 | 20 | 19 | 26 | 27 | 18 |
| 100 | 27 | 31 | 32 | 29 | 32 | 25 | 22 | 27 | 29 | 23 | |
| IXg | 75 | 23 | 22 | 18 | 21 | 24 | 19 | 20 | 23 | 27 | 20 |
| 100 | 28 | 28 | 30 | 28 | 29 | 26 | 25 | 28 | 29 | 25 | |
| IXh | 75 | 18 | 19 | 19 | 24 | 23 | 18 | 19 | 20 | 21 | 18 |
| 100 | 20 | 22 | 24 | 27 | 26 | 22 | 24 | 26 | 24 | 23 | |
| IXi | 75 | 19 | 17 | 22 | 18 | 20 | 18 | 18 | 22 | 19 | 17 |
| 100 | 20 | 23 | 25 | 22 | 23 | 21 | 22 | 24 | 22 | 23 | |
| IXj | 75 | 16 | 20 | 18 | 22 | 22 | 16 | 17 | 21 | 17 | 16 |
| 100 | 19 | 23 | 20 | 26 | 26 | 19 | 19 | 23 | 18 | 18 | |
| IXk | 75 | 24 | 28 | 21 | 22 | 26 | 20 | 21 | 25 | 30 | 20 |
| 100 | 31 | 32 | 30 | 25 | 31 | 26 | 26 | 26 | 35 | 24 | |
| IXl | 75 | 19 | 22 | 19 | 22 | 25 | 16 | 22 | 24 | 27 | 17 |
| 100 | 25 | 29 | 30 | 26 | 29 | 20 | 26 | 28 | 29 | 23 | |
| Standard | 75 | 32 | 36 | 39 | 33 | 37 | 28 | 30 | 29 | 35 | 32 |
| 100 | 38 | 42 | 45 | 38 | 43 | 35 | 33 | 31 | 38 | 36 | |
The MIC experiments were performed for all the tested compounds against antibacterial strains, viz. M. Tuberculosis, M. Luteus, MRSA, B. Subtilis, B. Cereus. The detailed MIC results are presented in Table 2.
Table 2.
MIC values of newly synthesized compounds
| Comp. code | Gram-positive bacteria | Gram-negative bacteria | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M. tuberculosis | M. luteus | MRSA | B. subtilis | B. cereus | P. aeruginosa | K. pneumonia | E. coli | P. vulgaris | S. typhi | |
| IXa | > 150 | 104.6 | > 100 | > 50 | 93.2 | > 120 | < 100 | < 50 | < 100 | > 120 |
| IXb | < 100 | > 70 | > 100 | < 50 | > 100 | 93.0 | < 100 | < 50 | < 100 | > 100 |
| IXc | > 150 | > 250 | > 250 | < 150 | 161.4 | 169.3 | > 150 | > 150 | > 160 | > 200 |
| IXd | < 100 | > 100 | > 120 | < 50 | 110.1 | 109.8 | < 100 | 39.8 | > 100 | > 120 |
| IXe | > 250 | > 150 | > 200 | > 200 | > 150 | > 175 | 125.7 | < 100 | > 135 | > 160 |
| IXf | > 100 | > 70 | > 100 | < 50 | 104.6 | 104.6 | < 100 | < 50 | > 50 | > 150 |
| IXg | > 150 | 104.6 | > 100 | > 50 | 93.2 | > 120 | < 150 | < 50 | > 70 | > 120 |
| IXh | < 100 | > 130 | > 150 | > 100 | < 100 | > 130 | > 125 | < 100 | > 130 | > 125 |
| IXi | > 120 | 138.1 | 166.9 | < 100 | 109.8 | > 145 | > 130 | < 100 | > 135 | > 130 |
| IXj | > 120 | > 100 | > 70 | < 120 | > 120 | > 100 | > 75 | > 50 | > 135 | > 140 |
| IXk | > 250 | > 175 | > 200 | > 100 | > 150 | > 135 | 125.7 | < 100 | > 135 | > 160 |
| IXl | > 150 | > 120 | > 100 | < 50 | 93.2 | 104.6 | < 100 | < 50 | < 100 | > 100 |
Results and discussions
A Vilsmeier–Haack adduct 2-chloroquinoline-3-carbaldehyde was synthesized from phosphorusoxytrichloride (6.5 ml, 70 mmol) and N,N-dimethylformamide (2.3 ml, 30 mmol) at 0 °C. N-phenylacetamide (1.35 gm, 10 mmol) was added to the formed Vilsmeier–Haack adduct, and it was stirred at room temperature for overnight. To a solution of acetanilide (I) in dry DMF, POCl3 was added dropwise by maintaining 0–5 °C and the mixture was stirred at room temperature for 12 h to yield 2-chloroquinoline-3-carbaldehyde (II). Progress of the reaction was monitored by TLC, after completion of the reaction, the reaction mixture was poured in ice cold water, and the white product was filtered and dried. The compound was purified by recrystallization from petroleum ether: ethyl acetate mixture to yield pure 2-chloroquinoline-3-carbaldehyde.
A mixture of 2-Chloroquinoline-3-carbaldehyde (0.191 gm, 1 mmol), piperidine (0.102 m, 1.2 mmol) and K2CO3 (0.552 gm, 4 mmol) in DMF (20 ml) was heated to 80–90 °C for 10–12 h to yield corresponding 2-(piperidin-1-yl) quinoline-3-carbaldehyde (III). Progress of the reaction was monitored by TLC; after completion of the reaction, the reaction mixture was poured in ice cold water. The solid separated was filtered, washed with water, dried and purified by column chromatography using silica gel (hexane:ethyl acetate, 10: 1, v/v) to yield the pure 2-(piperidin-1-yl)quinoline-3-carbaldehyde. 1-(2/3/4-(prop-2-yn-1-yloxy)phenyl)ethanones were synthesized from the mixture of 1-(2/3/4-hydroxyphenyl) ethanone (Iva–c) (10 mmol), propargyl bromide (V) (1.40 gm, 1.2 mmol) and anhydrous K2CO3 (2 gm, 14 mmol) in 15 ml of dry acetone was taken in round-bottom flask and refluxed for 8 h. After completion of the reaction, acetone was distilled under vacuum and it was diluted with cold water and the precipitate formed was filtered, washed with water and crystallized from methanol to give 1-(2/3/4-(prop-2-yn-1-yloxy)phenyl)ethanones (Via–c).
M.P.: 136 °C
A mixture of 2-(piperidin-1-yl)quinoline-3-carbaldehyde, 1-(2/3/4-(prop-2-yn-1-yloxy)phenyl)ethanones (Via–c) and potassium hydroxide in ethanol was stirred at room temperature for 5–6 h to afford (E)-1-(2/3/4-(prop-2-yn-1-yloxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-ones (VIIa–c).
Synthetic procedure for (E)-3-(2-(piperidin-1-yl)quinoline-3-yl)-1-(2/3/4-(prop-2-yn-1-yloxy)phenyl)prop-2-en-1-ones (VIIa–c):
A methanolic solution of 2-(Piperidin-1-yl)quinoline-3-carbaldehyde (III) (1 mmol), propargylated acetophenones (Via–c) (1 mmol) and potassium hydroxide was subjected to microwave irradiation at 180 W for 5–7 min. Progress of the reaction was monitored by TLC, after completion of the reaction. The reaction mixture was poured into ice cold water, slowly the solid separates out, it filtered, washed with water, dried and purified by using column chromatography using n-hexane:ethyl acetate (9:1) to afford pure (E)-3-(2-(piperidin-1-yl)quinolin-3-yl)-1-(2/3/4-(prop-2-yn-1-yloxy)phenyl)prop-2-en-1-ones (VIIa–c).
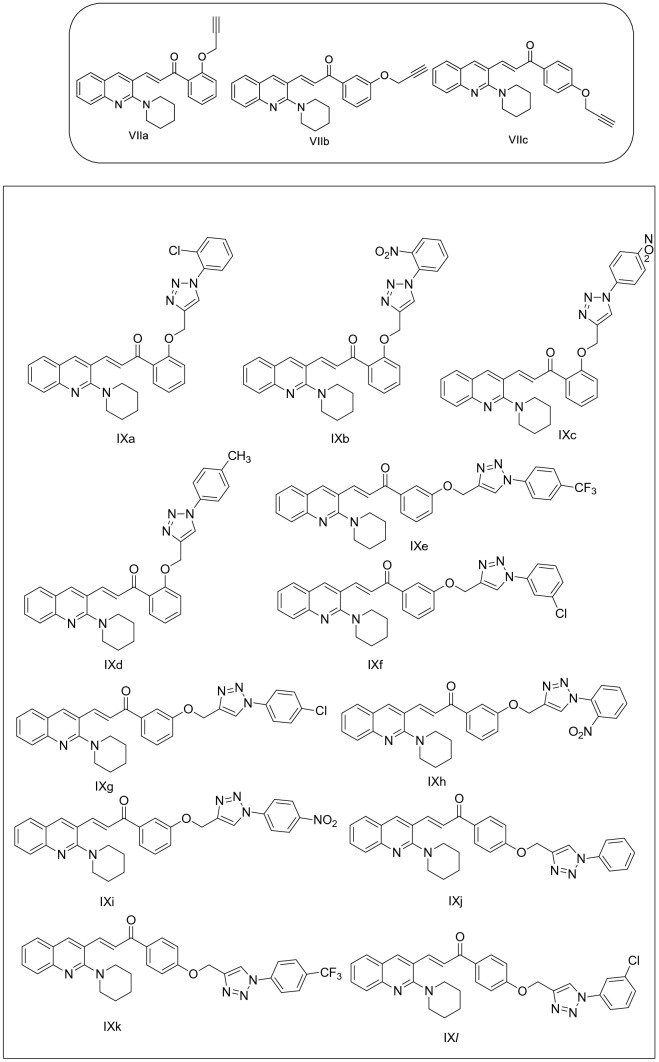
The target compounds were synthesized starting from acetanilide, Vilsmeier–Haack Reaction on acetanilide to yield 2-chloroquinoline-3-carbaldehyde; the 2-(Piperidin-1-yl)quinoline-3-carbaldehyde was prepared by substitution of chloro to piperidine of compound. The compound on Claisen–Schmidt condensed with propargylated acetophenones under reflux to yield propargylated chalcones. The triazole derivatives were prepared by Huisgen 1,3-dipolar cycloaddition of propargylated chalcones with aromatic azides in presence of copper sulfate pentahydrate catalyst and sodium ascorbate in DMF:water solvent medium, with excellent yield.
Structures of the compounds were established based on spectral analysis. As a representative case, the spectral analysis of (E)-3-(2-(piperidin-1-yl)quinolin-3-yl)-1-(2-(prop-2-yn-1-yloxy)phenyl)prop-2-en-1-one was discussed in IR spectrum (KBr, cm−1) of the characteristic peaks observed at 1649 and 2105 cm−1 assigned to C=O and acetyleneic group, respectively. In the 1H-NMR spectrum (CDCl3, 400 MHz, Fig. 1) of the newly formed two protons of O–CH2 groups resonated at δ 4.79–4.80 ppm as doublet and proton of acetylenic group appeared at δ 2.50–2.51 ppm as a triplet. A multiplet appeared at δ 1.63–1.70 ppm integrating for six protons was assigned to three methylene group protons of piperidine ring, and a triplet at δ 3.31–3.33 ppm integrating for four protons was assigned to two N-methylene group protons of piperidine ring. All the remaining aromatic protons appeared between δ7.10 and 7.82 ppm.
Fig. 1.
Structures of newly synthesized propoxylated intermediates
Synthesis of (E)-1-(2/3/4-((1-aryl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-ones
A mixture of (E)-1-(2/3/4-(prop-2-yn-1-yloxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-ones, arylazide, sodium ascorbate and CuSO4.5H2O in t-butanol:water (2:1) medium were stirred to afford (E)-1-(2/3/4-((1-aryl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-ones.
Structures of the products were established based on spectral analysis. As a representative case, the spectral analysis of (E)-1-(2-((1-(substituted)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one was discussed in IR spectrum (KBr, cm−1) of the characteristic peaks observed at 1649 and 1590 assigned to C=O and C=N, respectively. In the 1H-NMR spectrum (CDCl3, 400 MHz) of the two protons of O-CH2 group resonated at δ 5.31 ppm as singlet. A multiplet appeared at δ 1.68–1.78 integrating for six protons was assigned to three methylene group protons of piperidine ring, and a triplet at δ 3.33–3.36 integrating for four protons was assigned to two N-methylene group protons of piperidine ring.
Various substituted anilines were allowed to react with sodium nitrate and dil. Hydrochloric acid in presence of sodium azide for 1 h at room temperature to obtain aryl azides.
Synthesis of (E)-1-(2/3/4-((1-aryl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-ones
A mixture of (E)-3-(2-(Piperidin-1-yl)quinolin-3-yl)-1-(2/3/4-(prop-2-yn-1-yloxy)phenyl)prop-2-en-1-ones (VIIa-c) (1 mmol), aryl azides (VIIIa-d) (1.1 mmol) and catalytic amount of Copper iodide in DMF:water (3 ml) was stirred for 8–10 h at room temperature. After completion of the reaction, the organic compound was extracted with ethyl acetate (2 × 20 ml) and the combined organic solvent was washed with water, brine solution and dried over sodium sulfate. The dried solvent was concentrated under reduced pressure and purified with column chromatography by using n-hexane:ethyl acetate (7:3) to afford pure 1,2,3-triazole derivatives (IXa-l). All the remaining aromatic protons appeared between δ7.09 and 8.29.
Docking experimental
Initially, all the molecules were sketched in chemsketch and saved it in.mol format. The molecules were converted into.pdb format by using PyMOL tool. The molecules were docked by using Autodock 4.2. The protein was loaded; Chain A was saved in.PDBQT after removal of water molecules.
All the ligands were docked individually. Each and every molecule was loaded and saved it in.PDBQT format. Coordinates were assigned and generated grid to binding the molecule in that particular receptor area to form a grid using AutoGrid. Coordinates of X, Y and Z (– 11.824, 14.735 and 74.152) were selected. Autodock uses Lamarkian Genetic Algorithm to dock the molecules.
Docking methodology
Molecular docking study was performed by using AUTODOCK 4.2 which was a suite of automated docking tools and was used to predict the affinity, activity and binding orientation of ligand with the target protein and to analyze best conformations. The proteins with all the compounds were loaded individually into ADT and evaluated ten finest conformations. In the present investigation, we focused mainly on the binding energy values of the title compounds which use genetic algorithm (GA). All proteins were loaded separately into AutoDock Tools (ADT ver.1.5.6) (http://autodock.scripps.edu/resources).
Three-dimensional coordinates of COVID-19 main protease (PDB ID: 6LU7) [37] were obtained in.pdb format from the Protein Data Bank (https:/www.rcsb.org/) where 6LU7 is the protein sequence of 2019-nCoV. The.pdb file was entered into AutodockTools (ADT ver.1.5.6) for preparation of a.pdbqt file and grid box creation. Water molecules and other atoms were excluded, and ADT measured the Gasteiger charges for protein atoms; Auto-Grid was used with a grid box to create the grid map. The size of grid was determined at 60 × 60 × 60 xyz points with a grid spacing of 0.375 A, and a grid center at dimensions (x, y and z, respectively): – 11.824, 14.735 and 74.152 was designated. The PDB file of the enzyme (PDB ID: 6LU7) was downloaded from the Protein Data Bank (PDB) [38].
Docking results
The structural interactions between PDB with inhibitors were docked separately. Docking studies are commonly performed for predicting binding modes to proteins and their binding energies of ligands. X, Y, Z coordinates of PDB were selected by using SPDBV. Molecular docking studies was carried out for three propoxylated intermediates and all corresponding newly synthesized triazole derivatives against crystal structure of SARS CoV19 main protease enzyme (Mpro) (PDB ID: 6LU7). The binding energy, inhibition constant, hydrogen bond forming residues and interacting residues of all the synthesized derivatives when docked with SARS CoV19 main protease enzyme are presented in Table 3. The binding energy for all the molecules ranges from − 9.89 to − 13.4 kCal/mol. Propargylated compounds (VIIa, VIIb and VIIc) were shown lower binding energy compared to triazole derivatives (IXa to IXl). Compound IXf, a chloro-substituted derivative, had shown highest binding energy of -13.4 kCal/mol with two interactions (GLY143, CYS145). Compounds have IXg not shown interactions. All the derivatives have good interactions with GLY143, ARG188, GLN192, GLU166, THR26 and CYS145 shown in Fig. 2.
Table 3.
Binding energy and predicted contacting residues of newly synthesized derivatives that interact with protein 6LU7
| S. nos | Code | Name of the compound | Binding Score (Kcal/mol) | Interacting amino acid |
|---|---|---|---|---|
| 1 | VIIa | (E)-1-(2-(prop-2-yn-1-yloxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one | − 9.89 | GLY143 |
| 2 | VIIb | (E)-3-(2-(piperidin-1-yl)quinolin-3-yl)-1-(3-(prop-2-yn-1-yloxy)phenyl)prop-2-en-1-one | − 10.44 | GLU166:HN1 |
| 3 | VIIc | (E)-3-(2-(piperidin-1-yl)quinolin-3-yl)-1-(4-(prop-2-yn-1-yloxy)phenyl)prop-2-en-1-one | − 10.06 | ARG188:O1 |
| 4 | IXa | (E)-1-(2-((1-(2-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one | − 12.06 | GLY143:HN1 |
| 5 | IXb | (E)-1-(2-((1-(2-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one | − 11.96 | GLY143:HN1 |
| 6 | IXc | (E)-1-(2-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one | − 11.53 |
GLU166:HN1 GLN192:HN1 |
| 7 | IXd | (E)-3-(2-(piperidin-1-yl)quinolin-3-yl)-1-(2-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)prop-2-en-1-one | − 11.81 | GLU166:HN1 |
| 8 | IXe | (E)-3-(2-(piperidin-1-yl)quinolin-3-yl)-1-(3-((1-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)prop-2-en-1-one | − 11.77 | THR26 |
| 9 | IXf | (E)-1-(3-((1-(3-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one | − 13.4 |
GLY143:HN1 CYS145:HN1 |
| 10 | IXg | (E)-1-(3-((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one | − 12.4 | – |
| 11 | IXh | (E)-1-(3-((1-(2-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one | − 13.38 | GLY143:HN1 |
| 12 | IXi | (E)-1-(3-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one | − 11.28 | GLU166:HN1 |
| 13 | IXj | (E)-1-(4-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one | − 12.49 |
GLY143:HN1 CYS145:HN1 |
| 14 | IXk | (E)-3-(2-(piperidin-1-yl)quinolin-3-yl)-1-(4-((1-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)prop-2-en-1-one | − 11.95 | THR26 |
| 15 | IXl | (E)-1-(4-((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one | − 12.66 | CYS145:HN1 |
Fig. 2.
Docking mode of the compounds IXa–IXl in active site of 6LU7
The comparison of free energies corresponding to binding of title compounds with target protein reveals that maximum of the compounds has shown good interactions. Almost all molecules exhibited lower free energy values, indicating more thermodynamically favored interactions.
VIIa) (E)-1-(2-(prop-2-yn-1-yloxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1428, 1592, 1649 and 2105; 1H NMR spectrum, δ, ppm: 1.63–1.70 (m, 2H, 3 X CH2), 2.51–2.52 (t, 1H, acetylene proton), 3.31–3.33 (t, 4H, 2 X N-CH2-), 4.79–4.80 (d, 2H, -CH2-), 7.10–7.14 (m, 2H, Ar–H), 7.31–7.35 (m, 1H, Ar–H), 7.46–7.52 (m, 2H, Ar–H), 7.57–7.61 (m, 1H, Ar–H), 7.65–7.70 (m, 2H, Ar–H), 7.74–7.82 (m, 2H, Ar–H), 8.24 (s, 1H, quinoline-H); 13C NMR spectrum, δC, ppm: 24.6, 25.9, 51.7, 56.6, 76.1, 78.0, 113.3, 121.9, 123.2, 124.1, 124.7, 127.4, 127.7, 127.8, 129.9, 130.2, 130.4, 132.6, 136.6, 141.3, 147.8, 155.9, 160.7, 192.9; MS 397 (M + H)+.
VIIb) (E)-3-(2-(piperidin-1-yl)quinolin-3-yl)-1-(3-(prop-2-yn-1-yloxy)phenyl)prop-2-en-1-one:
Yield: 83%; m.p. 82–85 °C; IR spectrum, v, cm−1: 1428, 1594, 1657 and 2110; 1H NMR spectrum, δ, ppm: 1.66–1.77 (m, 6H, 3 X CH2), 2.55–2.26 (t, 1H, acetylene proton), 3.55 (t, 4H, 2 X N-CH2-), 4.79 (d, 2H, CH2), 7.22–7.24 (m, 1H, Ar–H), 7.34–7.37 (m, 1H, Ar–H), 7.45–7.49 (m, 1H, Ar–H), 7.60–7.73 (m, 5H, Ar–H), 7.83–7.84 (m, 1H, Ar–H), 7.99–8.03 (m, 1H, Ar) 8.24 (s, 1H, quinoline-H); 13C NMR spectrum, δC, ppm: 24.6, 26.0, 51.8, 56.0, 75.9, 77.8, 114.2, 120.0, 121.8, 122.5, 123.0, 124.3, 124.6, 127.4, 127.8, 129.7, 130.4, 136.9, 139.4, 142.8, 157.8, 189.9; MS 397 (M + H)+.
VIIc) (E)-3-(2-(piperidin-1-yl)quinolin-3-yl)-1-(4-(prop-2-yn-1-yloxy)phenyl)prop-2-en-1-one:
Yield: 85%; m.p. 82–85 °C; IR spectrum, v, cm−1: 1425, 1595, 1653 and 2108; 1H NMR spectrum, δ, ppm: 1.66–1.81 (m, 4H, 3 X CH2), 2.57–2.58 (t, 1H, acetylene proton), 3.34–3.36 (t, 4H, 2 X N-CH2-), 4.79–4.80 (d, 2H, -CH2), 7.08–7.11 (m, 2H, Ar–H), 7.33–7.37 (t, 1H, Ar–H), 7.59–7.73 (m, 3H, Ar–H), 7.82–7.84 (d, 1H, Ar–H), 7.98–8.02 (d, 1H, Ar–H), 8.09–8.11 (d, 2H, Ar–H), 8.23 (s, 1H, quinoline-H); 13C NMR spectrum, δC, ppm: 24.6, 26.0, 51.7, 55.9, 76.1, 77.7, 114.8, 122.3, 123.3, 124.2, 124.7, 130.3, 130.7, 131.7, 136.8, 142.0, 147.8, 160.7, 162.2, 188.7; MS 397 (M + H)+.
IXa) (E)-1-(2-((1-(2-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1420, 1590 and 1649; 1H NMR spectrum, δ, ppm: 1.66–1.78 (m, 6H, 3 X CH2), 3.36 (t, 4H, 2 X N-CH2-), 5.41 (s, 2H, -OCH2), 7.09–7.10 (m, 2H, Ar–H), 7.36–7.47 (m, 5H, Ar–H), 7.60–7.75 (m, 4H, Ar–H), 7.84–7.86 (m, 2H, Ar–H), 8.00–8.04 (m, 1H, ArH), 8.13 (d, 1H, ArH), 8.29 (m, 1H, quinoline-H); 13C NMR spectrum, δC, ppm: 24.5, 26.0, 29.7, 51.8, 62.1, 114.3, 119.9, 121.7, 121.8, 122.5, 123.1, 124.3, 124.7, 125.4, 127.4, 127.6, 127.8, 127.9, 128.0, 129.7, 129.8, 130.1, 130.4, 130.8, 130.9, 137.0, 142.6, 146.6, 158.5, 189.9; MS 551 (M + H)+.
IXb) (E)-1-(2-((1-(2-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1428, 1595 and 1655; 1H NMR spectrum, δ, ppm: 1.61–1.67 (m, 6H, 3 X CH2), 3.24 (t, 4H, 2 X N-CH2-), 5.42 (s, 2H, -OCH2), 7.00–7.02 (d, 1H, Ar–H), 7.12–7.20 (m, 2H, Ar–H), 7.27–7.29 (m, 1H, Ar–H), 7.39–7.43 (m, 1H, Ar–H), 7.51–7.58 (m, 4H, Ar–H), 7.64–7.79 (m, 5H, Ar–H), 7.89–7.91 (d, 1H, Ar–H), 8.14 (m, 1H, quinoline-H); 13C NMR spectrum, δC, ppm: 24.5, 25.9, 51.8, 62.8, 113.0, 121.8, 122.7, 124.2, 124.6, 125.4, 127.3, 127.8, 128.0, 129.5, 129.7, 130.2, 130.5, 130.6, 133.1, 133.6, 136.4, 140.5, 147.7, 156.6, 160.7, 192.7; MS 562 (M + H)+.
IXc) (E)-1-(2-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1425, 1595 and 1651; 1H NMR spectrum, δ, ppm: 1.59–1.68 (m, 6H, 3 X -CH2), 3.21–3.22 (t, 4H, 2 X N-CH2-), 5.43 (s, 2H, -OCH2), 7.14–7.20 (m, 2H, Ar–H), 7.27–7.29 (m, 1H, Ar–H), 7.45–7.47 (d, 2H, Ar–H), 7.52–7.59 (m, 3H, Ar–H), 7.69–7.79 (m, 4H, Ar–H), 7.95 (s, 1H, Ar–H), 8.02–8.04 (d, 2H, Ar–H), 8.09 (s, 1H, quinoline-H); 13C NMR spectrum, δC, ppm: 25.9, 29.7, 51.0, 119.8, 124.5, 125.2, 127.4, 127.7, 127.9, 130.6, 132.6, 133.2, 134.9, 135.3, 136.0, 146.8, 147.7, 148.1, 148.9, 149.5, 150.1, 150.3, 151.9, 152.0, 168.2, 174.2, 174.7, 174.8, 198.3; MS 562 (M + H)+.
IXd) (E)-3-(2-(piperidin-1-yl)quinoline-3-yl)-1-(2-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1424, 1594 and 1653; 1H NMR spectrum, δ, ppm: 1.67–1.78 (m, 6H, 3 X -CH2), 2.60 (s, 3H, CH3), 3.44 (t, 4H, 2 X N-CH2), 5.38 (s, 2H, -OCH2), 7.04–7.13 (m, 2H, Ar–H), 7.34–7.47 (m, 4H, Ar–H), 7.53–7.57 (m, 4H, Ar–H), 7.63–7.93 (m, 5H, Ar–H), 8.09 (s, 1H, quinoline-H); 13C NMR spectrum, δC, ppm: 24.5, 25.9, 52.5, 118.5, 117.8, 120.4, 120.8, 121.4, 121.7, 122.3, 123.9, 124.2, 124.3, 127.3, 127.4, 127.7, 127.9, 128.7, 128.9, 129.0, 129.3, 130.3, 130.0.9, 132.2, 133.0, 133.6, 135.6, 136.4, 140.6, 141.1, 157.1, 160.1, 190.6; MS 547 (M + H)+.
IXe) (E)-3-(2-(piperidin-1-yl)quinoline-3-yl)-1-(3-((1-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1427, 1596 and 1655; 1H NMR spectrum, δ, ppm: 1.66–1.77 (m, 6H, 3 XCH2), 3.34 (t, 4H, 2 X N-CH2-), 5.38 (s, 2H, -CH2), 7.26–7.33 (m, 3H, Ar–H), 7.46–7.82 (m, 10H, Ar–H), 8.00–8.04 (d, 1H, Ar–H), 8.13 (s, 1H, Ar–H), 8.27 (s, 1H, quinoline-H); 13C NMR spectrum, δC, ppm: 24.6, 26.0, 51.8, 62.0, 113.5, 114.1, 118.5, 119.8, 121.0, 121.7, 122.4, 123.0, 124.3, 124.7, 127.4, 127.8, 130.4, 131.2, 136.9, 139.6, 142.8, 147.9, 149.9, 158.4, 160.7, 189.9; MS 585 (M + H)+.
IXf) (E)-1-(3-((1-(3-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1426, 1593 and 1652; 1H NMR spectrum, δ, ppm: 1.66–1.78 (m, 6H, 3 X CH2), 3.34–3.36 (t, 4H, 2 X N-CH2-), 5.39 (s, 2H, -CH2), 7.27–7.49 (m, 5H, Ar–H), 7.57–7.84 (m, 7H, Ar–H), 8.00–8.10 (m, 3H, Ar–H), 8.27 (s, 1H, quinoline-H); 13C NMR spectrum, δC, ppm: 25.7, 507, 62.8, 113.0, 117.8, 118.5, 120.6, 120.7, 120.9, 121.4, 121.7, 122.6, 123.9, 126.4, 127.3, 127.8, 128.8, 129.1, 129.7, 130.4, 130.6, 133.1, 133.6, 136.6, 142.0, 144.6, 148.2, 156.6, 192.2; MS 551(M + H)+.
IXg) (E)-1-(3-((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1422, 1590 and 1651; 1H NMR spectrum, δ, ppm: 1.66–1.76 (m, 6H, 3 X CH2), 3.33 (t, 4H, 2 X N-CH2-), 5.36 (s, 2H, -CH2), 7.19–7.47 (m, 5H, Ar–H), 7.55–7.83 (m, 7H, Ar–H), 7.98–8.02 (d, 1H, Ar–H), 8.08–8.12(d, 1H, Ar–H), 8.26 (s, 1H, quinoline-H); 13C NMR spectrum, δC, ppm: 24.6, 26.0, 51.8, 61.9, 113.6, 114.1, 118.4, 119.8, 120.7, 121.0, 122.3, 123.0, 124.0.3, 127.4, 127.8, 129.8, 130.4, 132.2, 135.6, 136.9, 137.7, 139.5, 141.1, 142.7, 147.9, 158.4, 160.7, 189.9; MS 547 (M + H)+.
IXh) (E)-1-(3-((1-(2-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1425, 1590 and 1650; 1H NMR spectrum, δ, ppm: 1.67–1.78 (m, 6H, 3 X CH2), 3.35 (t, 4H, 2 X N-CH2-), 5.41 (s, 2H, -CH2), 7.26–7.48 (m, 3H, Ar–H), 7.66–7.81 (m, 9H, Ar–H), 7.98–8.10 (d, 3H, Ar–H), 8.28 (s, 1H, quinoline-H)); 13C NMR spectrum, δC, ppm: 24.6, 26.0, 51.8, 62.0, 114.3, 119.8, 121.7, 122.4, 123.0, 124.3, 124.5, 124.7, 125.6, 127.4, 127.8, 128.0, 129.9, 130.1, 130.4, 130.9, 133.8, 136.9, 139.6, 142.7, 144.4, 147.9, 153.8, 158.4, 189.9; MS 547 (M + H)+.
IXi) (E)-1-(3-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1427, 1592 and 1652; 1H NMR spectrum, δ, ppm: 1.66–1.77 (m, 6H, 3 X CH2), 3.35 (t, 4H, 2 X N-CH2-), 5.41 (s, 2H, -CH2), 7.26–7.27 (m, 1H, Ar–H), 7.34–7.37 (m, 1H, Ar–H), 7.46–7.50 (m, 1H, Ar–H), 7.60–7.74 (m, 5H, Ar–H), 7.82–7.84 (d, 1H, Ar–H), 7.99–8.04 (m, 3H, Ar–H) 8.23–8.27 (d, 2H, Ar–H), 8.41–8.43 (d, 2H, Ar–H); 13C NMR spectrum, δC, ppm: 24.6, 26.0, 51.8, 61.9, 114.0, 119.8, 120.5, 120.9, 121.8, 122.3, 123.0, 124.3, 124.6, 125.6, 127.4, 127.8, 129.9, 130.5, 137.0, 139.6, 141.0, 142.9, 147.9, 158.3, 160.7, 189.9; MS 502 (M + H)+.
IXj) (E)-1-(4-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1419, 1588 and 1648; 1H NMR spectrum, δ, ppm: 1.66–1.77 (m, 6H, 3 X CH2), 3.35 (t, 4H, 2 X N-CH2-), 5.58 (s, 2H, -CH2), 7.17–7.35 (m, 4H, Ar–H), 7.40–7.84 (d, 5H, Ar–H), 8.00–8.24 (m, 7H, Ar–H), 8.44 (m, 1H, quinoline-H); 13C NMR spectrum, δC, ppm: 24.6, 26.0, 51.7, 62.0, 114.7, 120.9, 122.3, 123.3, 123.4, 124.2, 127.4, 127.7, 129.2, 129.9, 130.3, 130.9, 136.8, 141.9, 147.7, 160.7, 162.0, 188.5; MS 536 (M + H)+.
IXk) (E)-3-(2-(piperidin-1-yl)quinoline-3-yl)-1-(4-((1-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1429, 1595 and 1652; 1H NMR spectrum, δ, ppm: 1.67–1.78 (m, 6H, 3 X CH2), 3.35 (t, 4H, 2 X N-CH2-), 5.40 (s, 2H, -CH2), 7.15–7.34 (m, 5H, Ar–H), 7.60–7.88 (m, 7H, Ar–H), 7.99–8.23 (m, 4H, Ar–H & quinoline-H); 13C NMR spectrum, δC, ppm: 24.6, 26.0, 51.7, 62.0, 113.5, 114.6, 114.7, 118.5, 121.0, 121.1, 122.2, 123.2, 124.2, 124.7, 127.4, 127.7, 130.3, 130.9, 131.2, 131.6, 136.8, 142.0, 147.8, 150.0, 160.7, 161.8, 188.6; MS 547 (M + H)+.
IXl) (E)-1-(4-((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-(piperidin-1-yl)quinolin-3-yl)prop-2-en-1-one:
IR spectrum, v, cm−1: 1421, 1589 and 1649; 1H NMR spectrum, δ, ppm: 1.66–1.77 (m, 6H, 3 X N–C-CH2), 3.35 (t, 4H, 2 X N-CH2-), 5.40 (s, 2H, -CH2), 7.13–7.14 (d, 1H, Ar–H), 7.35–7.36 (t, 1H, Ar–H), 7.45–7.48 (m, 2H, Ar–H), 7.59–7.72 (m, 4H, Ar–H), 7.80–7.84 (m, 2H, Ar–H), 7.98–8.02 (d, 1H, Ar–H), 8.09–8.11 (m, 2H, Ar–H), 8.23 (s, 1H, quinoline-H); 13C NMR spectrum, δC, ppm: 24.6, 26.0, 51.7, 62.0, 114.7, 118.5, 120.8, 122.2, 123.2, 124.2, 124.7, 127.4, 127.7, 129.1, 130.3, 130.9, 131.6, 135.7, 136.8, 137.7, 141.9, 147.8, 160.7, 161.8, 188.6; MS 547 (M + H)+.
Conclusion
Novel series of 1,2,3-Triazolyl-piperidinyl-quinolinolines have successfully synthesized starting from acetanilide by transformation of different chemical reaction. The formation of triazole derivative was synthesized by the reaction terminal alkyne with different aryl azide in the presence of copper sulfate pentahydrate with sodium ascorbate in DMF:water medium, this method is proved to be an easy and efficient new synthetic protocol for preparation triazole derivatives, and this protocol gave in excellent yields with shorter reaction. Molecular docking studies were also performed for all compounds into the binding cavity of protein 6LU7. All the compounds have shown interactions with good binding energies except IXg. Compound IXf, a chloro-substituted derivative, showed highest binding energy of − 13.4 kCal/mol with three interactions (GLY143, CYS145). Hence, we conclude that all these newly synthesized molecules would be the potential lead molecules.
References
- 1.Marella A, Tanwar OP, Saha R, Rahmat Ali M, Srivastava S, Akhter M, Shaquiquzzaman M, Mumtaz Alam M. Saudi Pharm. J. 2013;21(1):1–12. doi: 10.1016/j.jsps.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang B, Li Q, Shi W, Chen Li, Sun J. Chem. Biol. Drug. Des. 2018;91(4):957. doi: 10.1111/cbdd.13154. [DOI] [PubMed] [Google Scholar]
- 3.Ashok D, Ganesh A, Vijaya Lakshmi B, Ravi S. Russ. J. Gen. Chem. 2014;84:1237. doi: 10.1134/S1070363214060309. [DOI] [Google Scholar]
- 4.Bodke YD, Shankerrao S, Kenchappa R, Telkar S. Russ. J. Gen. Chem. 2017;87(8):1843. doi: 10.1134/S1070363217080321. [DOI] [Google Scholar]
- 5.Jain S, Kumar A, Saini D. Exp. Parasitol. 2018;185:107. doi: 10.1016/j.exppara.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Patel D, Kumari P, Navin Patel B. Med. Chem. Res. 2013;22(2):726. doi: 10.1007/s00044-012-0073-3. [DOI] [Google Scholar]
- 7.Rojas J, Paya M, Dominguez JN, Ferrandiz ML. Bioorg. Med. Chem. Lett. 2002;12:1951. doi: 10.1016/S0960-894X(02)00317-7. [DOI] [PubMed] [Google Scholar]
- 8.Echeverria C, Santibanez JS, Donoso-Tauda O, Escobar CA, Ramirez-Tagle R. Int. J. Mol. Sci. 2009;10:221. doi: 10.3390/ijms10010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopinathan A, Moidu M, Mukundan M, Ellickal Narayanan S, Narayanan H, Adhikari N. Drug Dev. Res. 2020 doi: 10.1002/ddr.21727. [DOI] [PubMed] [Google Scholar]
- 10.Prakash O, Kumar A, Sadana A, Prakash R, Singh SP, Claramunt RM, Sanz D, Alkorta I, Elguero J. Tetrahedron. 2005;61:6642. doi: 10.1016/j.tet.2005.03.035. [DOI] [Google Scholar]
- 11.Prasad RY, Rao LA, Prasoona L, Murali K, Kumar RP. Bioorg. Med. Chem. Lett. 2005;15:5030. doi: 10.1016/j.bmcl.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 12.Bohn BA. Introduction to Flavonoids. Amsterdam: Harwood Academic; 1998. [Google Scholar]
- 13.Hwang DJ, Kim SN, Choi JH, Lee YS. Bioorg. Med. Chem. 2001;9(6):1429. doi: 10.1016/S0968-0896(01)00013-X. [DOI] [PubMed] [Google Scholar]
- 14.Arun Y, Bhaskar G, Balachandran C, Ignacimuthu S, Perumal PT. Bioorg. Med. Chem. Lett. 2013;23(6):1839. doi: 10.1016/j.bmcl.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Mukovoz PP, Slepukhin PA, Eltsov OS, Ganebnykh IN, Gorbunova AV, Sizentsov AN, Rusyaev ML. Russ. J. Gen. Chem. 2017;87(10):2291. doi: 10.1134/S1070363217100085. [DOI] [Google Scholar]
- 16.Abdel-Wahab BF, Abdel-Latif E, Mohamed HA, Awad GEA. Eur. J. Med. Chem. 2012;52:263. doi: 10.1016/j.ejmech.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Cheng C-Y, Haque A, Hsieh M-F, Hassan SI, Faizi MSH, Dege N, Khan MS. Int. J. Mol. Sci. 2020;21(11):3823. doi: 10.3390/ijms21113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Khokra SL, Yadav A. Futur. J. Pharm. Sci. 2021;7(1):106. doi: 10.1186/s43094-021-00241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashok Kumar B, Sathish Kumar E, SreenivasSubbaiah T. Russ. J. Gen. Chem. 2018;88(3):587. doi: 10.1134/S1070363218030313. [DOI] [Google Scholar]
- 20.Lazrek HB, Taourirte M, Oulih T, Barascut JL, Imbach JL, Pannecouque C, Witrouw M, De Clercq E. Nucleosides Nucleotides Nucl. Acids. 2001;20(12):1949. doi: 10.1081/NCN-100108325. [DOI] [PubMed] [Google Scholar]
- 21.Angajala KK, Vianala S, Macha R, Raghavender M, Krishna Thupurani M, Pathi PJ. Springerplus. 2016;5:423. doi: 10.1186/s40064-016-2052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunha AC, Figueiredo JM, Tributino JLM, Miranda ALP, Castro HC, Zingali RB, Fraga CAM, de Souza MCBV, Ferreirac VF, Barreiroa EJ. Bioorg. Med. Chem. 2003;11:2051. doi: 10.1016/S0968-0896(03)00055-5. [DOI] [PubMed] [Google Scholar]
- 23.Hou DR, Alam S, Kuan TC, Ramanathan M, Lin TP, Hung MS. Bioorg. Med. Chem. Lett. 2009;19:1022. doi: 10.1016/j.bmcl.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Buckle DR, Rockell CJM, Smith H, Spicer BA. J. Med. Chem. 1986;29:2262. doi: 10.1021/jm00161a022. [DOI] [PubMed] [Google Scholar]
- 25.Danoun S, Baziard-Mouysset G, Stigliani JL, Payard M, Selkti M, Viossat B, Tomas A. Heterocycl. Commun. 1998;4:45–51. doi: 10.1515/HC.1998.4.1.45. [DOI] [Google Scholar]
- 26.Stefano M, Chiara Beatrice V, Maurizio M, Nicoletta B, Cristina R, Carlo M, Roberto G. Pyrazolo-triazoles as light achievable DNA cleaving agents. Bioorg. Med. Chem. 2000;8:2343–2346. doi: 10.1016/S0968-0896(00)00160-7. [DOI] [PubMed] [Google Scholar]
- 27.Biagi IG, Calderone V, Giorgi I, Livi O, Scartoni V, Baragatti B, Martinotti E. Eur. J. Med. Chem. 2000;35(7–8):715. doi: 10.1016/S0223-5234(00)00180-X. [DOI] [PubMed] [Google Scholar]
- 28.Khan I, Ali S, Hameed S, Rama NH, Hussain MT, Wadood A, Ul-Haq Z, Khan A, Ali S, Choudhary MI. Eur. J. Med. Chem. 2010;45:5200. doi: 10.1016/j.ejmech.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 29.Boechat N, Ferreira Mde L, Pinheiro LC, Jesus AM, Leite MM, Júnior CC, Aguiar AC, de Andrade IM, Krettli AU. Chem. Biol. Drug. Des. 2014;84:325. doi: 10.1111/cbdd.12321. [DOI] [PubMed] [Google Scholar]
- 30.Bakunov AS, Bakunova SM, Wenzler T, et al. J. Med. Chem. 2010;53:254. doi: 10.1021/jm901178d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bach AU, Barrila J, Velazquez-Campoy A, Leavitt SA, Freire E. Biochemistry. 2004;43(17):4906. doi: 10.1021/bi0361766. [DOI] [PubMed] [Google Scholar]
- 32.Indwiani Astuti*, Ysrafil (2020) Diabetes & Metabolic Syndrome: Clinical Research & Reviews., 14, 407. [DOI] [PMC free article] [PubMed]
- 33.Letko M, Marzi A, Munster V. Nat. Microbiol. 2020;5:562. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.T-T Yao, J-D Qian, W-Y Zhu, Y Wang, G-Q Wang First published, J. Med. Virol. (2020)
- 35.Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, Zhang B, Li X, Zhang L, Peng C, Duan Y, Yu J, Wang L, Yang K, Liu F, Jiang R, Yang X, You T, Liu X, Yang X, Bai F, Liu H, Liu X, Guddat LW, Xu W, Xiao G, Qin C, Shi Z, Jiang H, Rao Z, Yang H. Nature. 2020;582:289. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 36.Chang G-G. Molecular Biology of the SARS-Coronavirus. Berlin, Heidelberg: Springer; 2009. p. 115. [Google Scholar]
- 37.Peele KA, Durthi CP, Srihansa T, Krupanidhi S, Ayyagari VS, John Babu D, Indira M, Ranganadha Reddy A, Venkateswarulu TC. Inf. Med. Unlocked. 2020;19:100345. doi: 10.1016/j.imu.2020.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berman HM. The protein data bank. Nucl. Acids Res. 2000;28(1):235. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]