Graphical abstract
Keywords: Hematotoxicity, Hepatotoxicity, Micronucleus, Oxidative status
Highlights
-
•
Quercetin reduced the Benzene-induced micronucleus formation.
-
•
Quercetin attenuated the Benzene-induced hematoxicity.
-
•
Quercetin lowered remarkably the Benzene-declined WBC density.
-
•
Quercetin ameliorated the Benzene-induced oxidative stress.
Abstract
The protective effects of Quercetin (QCN) on Benzene (BNZ)-induced hemato- and hepatotoxicy were investigated. To reach this goal, 36 adult male mice were divided into 6 groups (n = 6). The control group was not exposed to BNZ, while animals in BNZ group were exposed to BNZ (30 ppm) and the animals of QCN group were received QCN (50 mg/kg, orally), the fourth, fifth and sixth groups were exposed to 30 ppm BNZ and received 10, 50 and 100 mg/kg QCN one h before the BNZ exposure, for 28 days. The day after the last exposure following anesthesia and the blood collection, the liver and femur tissues were collected. The bone marrow samples were extracted and subjected to micronucleus assay. The blood samples were processed for hematological and biochemical analyses. Histopathological examinations were performed on the liver samples. QCN reduced significantly (p < 0.05) the BNZ-elevated hepatic enzymes and ameliorated the BNZ-induced WBC and RBC reduction. The BNZ-elevated micronucleus percentage both in the bone marrow and peripheral blood was remarkably declined in the QCN-received groups. QCN improved the BNZ-induced histopathological changes and oxidative status in the liver and serum. Our results suggest that QCN could be a protective supplement to reduce the BNZ-induced hemato- and hepatotoxicities.
1. Introduction
Benzene (BNZ) as an aromatic hydrocarbon has been used in the past as a solvent in many industries and currently is used in the synthesis of styrene, phenol, cyclohexane, aniline, plastics, and resins and also in the synthesis of many pharmaceuticals including pesticides and detergents. At the same time, BNZ is a natural constitute of crude oil, which is emitted in large amounts from oil refineries, cigarette smoke and vehicle exhausts and considered one of the major sources of BNZ-exposure [1]. Among the other ways, inhalation rout of exposure accounts for more than 90 % of exposure in the general population [2]. Benzene as a ubiquitous volatile compound is well absorbed and metabolized. Although BNZ is not generally regarded as an acute toxic agent, its chronic exposure however related to neurological impairment, hematotoxicity and the development of aplastic anemia and leukemia [3]. Previously, its hepatotoxicity and nephrotoxicity in rodent models after exposing to low concentration of BNZ has been documented [4].
Early studies showed that the toxicity of BNZ is mainly mediated via various metabolites, which are produced during its biotransformation. The main metabolism of BNZ take place in the liver by Cytochrome p450 2E1 and produces primarily benzene-oxide and then hydroxylated metabolite of phenol, hydroquinone and catechol, which ultimately oxidized to banzoquinone [5]. The produced metabolites and in particular benzene oxide as an electrophilic reactive intermediate is able to adduct with cellular DNA, indicating the benzene’s genotoxicity. The Cytochrome p450-mediated biotransformation of BNZ and the production of phenol and other hydroxylated metabolites occurs in lesser extend in the other tissues including the bone marrow. There are evidence indicating that the bone marrow due to having highly available myeloperoxidase, is able to further metabolize the phenolic metabolites of BNZ. The bone marrow myeloperoxidase activity results in oxidation of phenolic metabolites and production of radical intermediates, which are able to bind covalently to proteins, suggesting the bone marrow toxicity of benzene [6]
Another important factor in the toxicity of BNZ is related to the excessive generation of reactive oxygen species (ROS) along with remarkable reduction of hepatic glutathione (GSH), which plays a key role in the antioxidant system of cells [4]. Therefore, two important pathways are considered in the BNZ toxicity including the production of metabolism-dependent clastogenic metabolites and concomitantly the excessive ROS generation that both lead to the clinical outcomes of BNZ toxicity as hematological disorders such as acute myeloid leukemia and hepatic injuries. Taken in account two aforementioned pathways in the BNZ-induced toxicity, it would be worth to minimize the BNZ biotransformation and equally important to enhance the antioxidant capacity of intoxicated patients.
Quercetin (QCN) as a natural bioflavonoid is found in fruits and vegetables. During the last decades quercetin’s pharmacological effects including anti-inflammatory, anti-proliferative, anti-atherosclerotic and antioxidative effects have been well evidenced [7,8]. Its antioxidant property is related to the presence of a catechol group and a hydroxyl group in quercetin chemical structure, which enables it to act as a hydrogen donor for quenching free radicals [9,10]. On the other hand, it has been recently demonstrated that QCN competitively inhibited CYP 2E1 activity in human recombinant cDNA-expressed CYP 2E1 [11]. To highlight the possible protective effects of QCN on BNZ-induced damages the present study was performed and the results revealed that both hemato- and hepato-toxicity are attenuated by administration of QCN in protective approach.
2. Materials and methods
2.1. Chemicals
Quercetin (Q4951) was purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Ketamine and xylazine were obtained from Alfasan (Woerden, Holland). Benzene, Ethylene diamine tetra acetic acid (EDTA), sulphanilamide, N-(1-naphthyl) ethylene diamine -2HCl, tetramethyl benzidine, 2, 4-dinitrophenylhydrazine (DNPH), thiobarbituric acid, phosphoric acid (85 %), guanidine hydrochloride, and ethyl acetate were purchased from Merck (Darmstadt, Germany). N-Butanol was obtained from Carl Roth, GmbH Co. (Karlsruhe, Germany).
2.2. Experimental design and animal grouping
This experimental study was conducted on male, Swiss albino, healthy, and adult mice with average body weight of 30 ± 4 g. Animals were divided into 6 groups (6 mice/group) as follows:
The first group served as control and did not expose to benzene.
The second group was exposed to 30 ppm of BNZ vapor.
The third group received only QCN (50 mg/kg B.W. orally).
The fourth, fifth and sixth groups were exposed to 30 ppm of BNZ vapor and they received QCN at 10, 50 and 100 mg/Kg B.W. dose levels orally 1 h before the BNZ exposure.
The exposure model was mouse whole-body inhalation exposure and the selected concentration was based on previously published evidence [4]. The experimental protocols were approved by the Ethics Committee of Ardabil University of Medical Sciences, Ardabil, Iran (IR.ARUMS.REC.1398.603).
2.3. Benzene exposure
Mouse whole-body inhalation exposure was performed in a chamber (plastic box; 33 cm long, 23 cm wide and 20 cm deep; volume = 0.015 m3) in an experimental room. Airflow was continuously entered into chamber during the exposure time via a rubber tube connected and adjusted by a flowmeter (Airmax, Japan). Pure BNZ was passed within the airflow at 30 ppm concentration and the mice in different groups were exposed to BNZ for 4 h/day, 5 days/week and for 4 weeks [12].
2.4. Benzene concentration and gas chromatographic analyses
The BNZ concentration in the inhalation chamber was measured by gas chromatography. Samples of chamber’s air, taken at 30-min intervals during 4 h (exposure period) were injected, separated and detected in a 2010 plus gas chromatograph (Shimadzu, Kyoto, Japan), supplemented with a flame ionization detector. An Rtx-5 (30 m ×0.25 mm ×0.25 μm) capillary column was used with nitrogen as a carrier gas and temperature programming from 40 °C (5 min) to 250 °C at 10 min−1. The instrument was checked based on the pattern in retention times and responses of BNZ in the standard calibration injection. The concentrations of BNZ were quantified by an external standard calibration.
2.5. Peripheral blood analysis
A day after the last exposure the mice were anesthetized using a cocktail of ketamine and xylazine (ketamine: 90 mg/kg and xylazine: 8 mg/kg, IP). The blood samples were collected from the heart and analyzed in an automatic hematology analyzer (ADVIA SIEMENS 2120i, Germany). In hematology analyses the number of red blood cells, white blood cells, platelets, lymphocytes, neutrophils and monocytes along with the concentration of hemoglobin and the percentage of hematocrit were determined. At the same time, serum samples were isolated from whole blood by centrifugation at 1500 ×g for 10 min at room temperature and stored at −80 °C for further analyses.
2.6. Blood biochemical analyses
The collected blood samples both subjected to hematological analyses and/or were centrifuged at 1500 ×g for 10 min to separate blood serum. To evaluate the impact of BNZ-exposure and QCN protective effects on the BNZ-induced hepatoxicity, serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP), as hepatic functional enzymes were determined using commercially available Kits (BYERPAUL, Tehran, IRAN) and an automatic biochemical analyzer (MINDRAY BS-480, China).
2.7. Blood and bone marrow collection and micronucleus assay
The day after the last exposure, the mice were anaesthetized by a cocktail of ketamine and xylazine (ketamine: 90 mg/kg and xylazine: 8 mg/kg, IP) and after collecting the blood samples were euthanized using decapitation method. The femur tissues were removed and the bone marrow was flushed using Hanks’ buffered salt solution, 1% (w/v) bovine serum albumin, and 0.15 % (w/v) EDTA (pH 7.2). The bone marrow smears were prepared and fixed in methanol for 10 min. The fixed bone marrow smears were then stained by using the May-Grunwald and Giemsa protocols. After staining a minimum 2000 erythrocytes were counted and the percentage of micronucleus (MN) in polychromatic/normochomatic erythrocytes was determined for each single group [13]. At the same time, the peripheral blood samples from each single animal were smeared on slides and after drying at room temperature were fixed with 100 % methanol for 10 min and stained with Giemsa solution (5%) for 20 min. Following air drying optical microscope was used to estimate the micronucleus frequencies in the study groups [14].
2.8. Oxidative stress biomarkers assessment
2.8.1. Total antioxidant capacity (TAC)
To evaluate the total antioxidant capacity of serum samples, the FRAP method was performed [15]. To provide an acidic environment (pH 3.6) acetate buffer was used and the produced blue color due to the reduction of Fe3+ ions from the Fe3+- 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) complex and converting them into ferrous (Fe2+) ions is assessed at 593 nm by a spectrophotometer. This is a non-specific reaction, and any molecule that is capable to reduce ferric ions in acidic condition participates in this reaction.
2.8.2. Total thiol molecules assessment in the liver
The total thiol molecules (TTM) level was assessed in the liver according to the previously described method [16]. Briefly, 0.1 g of the liver samples were homogenized in 1 mL KCl (150 mM), and then centrifuged at 3000 × g for 10 min. A 0.5 mL of the supernatant was added to 0.6 mL Tris-EDTA buffer (Tris base 0.25 M, EDTA 20 mM, pH 8.2) followed by the addition of 40 μl 5,5′ - Dithiobis (2-nitrobenzoic acid) (DTNB, 10 mM in pure methanol) in a 10 mL glass test tube. The final volume of mixture was expanded up to 4.0 mL by addition of pure methanol. After incubation for 15 min at room temperature, the samples were centrifuged at 3000 × g for 10 min, and the absorbance of the supernatant was assessed at 412 nm. TTM were expressed as nmoles per mg of protein. The protein content of the samples was measured by the Lorry method [17].
2.8.3. Malondialdehyde (MDA) levels in the liver
The lipid peroxidation rate was determined by Thiobarbituric acid (TBA) reaction and by measuring the produced amount of malondialdehyde in the liver samples. Each sample was individually homogenized in a chilled potassium chloride solution (150 mM) and the resulting mixture was centrifuged at 3000 rpm for 10 min. A 500 μl of supernatant was mixed with 3 mL phosphoric acid (1% v/v), vortexed and then 1 mL of TBA (6/7 g/l) was added. The samples were hold at 100 °C for 45 min, and then chilled in ice. The absorbance of supernatant after adding a 3 mL n-butanol and centrifugation at 3000 rpm for 10 min was measured at 532 nm by a spectrophotometer and calculated according to the standard calibration curve of malondialdehyde as nanomole of malondialdehyde equivalents per ml of sample homogenate [18].
2.8.4. Protein Carbonylation (CO) in the liver
The rate of protein oxidation in the liver samples were assessed by measuring the amount of carbonyl content. The reaction of 2, 4-dinitrophenylhydrazine (DNPH) and carbonyl groups of proteins was measured based on Levine et al. technique [19]. A 0.1 g of the liver samples was homogenized in a chilled phosphate buffer (50 mM, pH 7.6, containing EDTA 1 mM), and the resulting mixture was centrifuged at 10,000 × g and at 4 °C for 10 min. A 200 μl of supernatant from each sample was used as test (T) and the same volume of supernatant was used as control (C). Thereafter 800 μl of DNPH and hydrochloric acid solution (2 M) was added to the test and control samples, respectively. Samples were hold in dark place at room temperature for one hour and vortexed every 15 min. Then, 0.5 mL of trichloroacetic acid (30 %) was added to each sample and vortexed for 30 s. The samples were centrifuged for 10 min at 10,000 ×g for 3 min, the supernatant was removed and remaining pellet re-suspended in 1 mL ethanol/ethyl acetate solution (1:1) for 15 min. Following centrifugation at 10,000 ×g for 3 min and dispersing the supernatant, the above step was repeated. The precipitate was dissolved in 0.6 mL of guanidine hydrochloride solution (6 M) at 37°C for 15 min and then the samples were centrifuged for 10 min at 10,000 ×g for isolating and depositing any residues. For each sample, optical density (OD) of control and test was measured against a solution of guanidine hydrochloride (6 M) at wavelength of 370 nm. The carbonyl content was determined as follows:
| Carbonyl (nmol/mL) = [(CA)/ (0.011 mM−1)] (600 μl/200 μl); |
Where CA is the corrected absorbance and estimated as the average OD for each control sample was subtracted from average OD of test sample at 370 nm. The extinction coefficient for DNPH at 370 nm is 22,000 M−1 cm−1.
2.8.5. Histopathological examination of the liver tissue
Previously fixed liver samples in formalin solution (10 %) were subjected to histopathological examinations. The samples embedded in paraffin, sectioned into 5−6 μm sections and were stained with Hematoxylin and Eosin (H&E) and then analyzed by a light microscope.
2.8.6. Statistical analyses
Statistical comparisons were performed using the GraphPad Prism (Version 7.0; GraphPad software Inc., San Diego, USA). The comparisons between groups were made by analysis of variance (ANOVA) followed by Bonferroni post hoc test. A p value <0.05 was considered significant.
3. Results
3.1. Quercetin protected from the BNZ-induced hematological changes
Benzene exposure for 4 weeks resulted in a remarkable and significant reduction in WBC number and a significant and slight decrease in RBC number in mice. A significant (p < 0.05) reduction of lymphocyte and neutrophil numbers also was recorded in the BNZ-exposed mice. While QCN alone enhanced significantly the WBC number, we found no major changes in the RBC numbers. Quercetin treatment at medium and high dose levels resulted in a significant (p < 0.05) elevation in the BNZ-reduced numbers of both WBC and RBC (Table 1). There was no statistically significant difference between the control and other groups in terms of platelets density. Benzene exposure resulted in a significant reduction of HCT and a non-significant reduction of Hb concentration in mice and QCN only at the highest given dose level (100 mg/kg) was able to recover the BNZ-reduced HCT (%) and Hb concentration. Although QCN increased the BNZ-reduced lymphocyte number but this increase was not statistically significant (p > 0.05).
Table 1.
Effects of Quercetin on the Benzene-induced alterations in hematological factors.
| Experimental Groups | Control | BNZ | QCN | BNZ+QCN 10 | BNZ+QCN 50 | BNZ+QCN 100 |
|---|---|---|---|---|---|---|
| RBC (×106)/μl | 9.8 ± 0.7 | 8.2 ± 0.3 * | 9.1 ± 0.7 | 8.6 ± 0.4 | 9.3 ± 0.6 # | 9.2 ± 0.5 # |
| WBC (×103)/μl | 5.7 ± 881 | 2.6 ± 407 * | 7.8 ± 1005* | 2.3 ± 5.9 | 3.8 ± 613 # | 4.3 ± 804 # |
| Lymphocyte (%) | 56.8 ± 2.6 | 43 ± 4.3 * | 53.3 ± 5.5 | 44 ± 6.1 | 49.2 ± 5.4 | 47.3 ± 3.9 |
| Neutrophil (%) | 5.4 ± 0.7 | 3.4 ± 0.8 * | 4.5 ± 0.2 | 3.6 ± 0.3 | 4.3 ± 0.2 | 4.1 ± 0.2 |
| Monocyte (%) | 36.8 ± 3.2 | 32.6 ± 6.9 | 40.1 ± 3.8 | 36.9 ± 0.6 | 34.2 ± 4.3 | 37.3 ± 2.3 |
| Hb (g/dl) | 13.6 ± 0.9 | 12.6 ± 0.5 | 13.2 ± 0.2 | 12.5 ± 0.5 | 13.5 ± 0.6 | 13.9 ± 0.4 # |
| HCT (%) | 57.8 ± 2.4 | 52.7 ± 1.2 * | 57.6 ± 4.7 | 52.7 ± 2.1 | 54.1 ± 4.5 | 54.4 ± 0.7 # |
| Platelets (×103)/μl | 1170 ± 101 | 1242 ± 94.7 | 1031 ± 74.1 | 1152 ± 203 | 1140 ± 100.5 | 1157 ± 72.9 |
Asterisks are representing significant differences (p < 0.05) between the control and Benzene-exposed groups and #s are demonstrating significant differences between the Benzene-exposed group and those groups, which concurrently both were exposed to benzene and treated with various dose levels of quercetin. BNZ: Benzene; QCN: Quercetin; RBC: Red blood cells; WBC: White blood cells; Hb: Hemoglobin and HCT: Hematocrit.
3.2. Quercetin lowered the BNZ-induced biochemical changes in the blood
After 28 days exposure to BNZ and the administration of QCN at different dose levels, only those animals, which received QCN alone, showed a significant (p < 0.05) reduction in body weight gain (BWG) when was compared with the control group. The serum level of hepatic functional enzymes determination revealed that both ALT and AST significantly and ALP were insignificantly elevated in the BNZ-exposed group. The BNZ-induced elevation of hepatic enzymes was lowered in the animals that were treated with QCN. Although we failed to find any statistically significant differences between the highest given dose and medium dose of QCN, QCN hepatoprotective effect however was found to be dose-dependent (Table 2).
Table 2.
Effects of Quercetin on body-weight gain (g) and serum level of the hepatic functional enzymes concentration in the Benzene-exposed animals.
| Groups | BWG (g) | ALT (U/L) | AST (U/L) | ALP (IU/L) |
|---|---|---|---|---|
| Control | 6 ± 0.21 | 100 ± 9.6 | 41 ± 3.6 | 268 ± 50.9 |
| BNZ | 5.6 ± 0.28 | 135 ± 12.6 * | 56.2 ± 5.2 * | 295 ± 31.4 |
| QCN | 4.3 ± 0.18 * | 86.6 ± 5.0 | 42 ± 3.1 | 170 ± 12.5 * |
| BNZ + QCN 10 | 5.8 ± 0.21 | 128 ± 10.4 | 39.7 ± 3 # | 257 ± 34 |
| BNZ + QCN 50 | 6.3 ± 0.35 | 97.6 ± 2.6 # | 36.6 ± 6.5 # | 232 ± 14 # |
| BNZ + QCN 100 | 5.8 ± 0.25 | 85 ± 3.0 # | 35.6 ± 7.2 # | 199 ± 33.7 # |
Asterisks are representing significant differences (p < 0.05) between the control and Benzene-exposed groups and #s are demonstrating significant differences between the Benzene-exposed group and those groups, which concurrently both were exposed to benzene and treated with various dose levels of quercetin. BNZ: Benzene; QCN: Quercetin; BWG: Body weight gain; ALT: alanine aminotransferase; AST: aspartate aminotransferase and ALP: alkaline phosphatase.
3.3. The Benzene-induced Micronucleus formation was declined in quercetin-received animals
The bone marrow cells from femur and also peripheral blood were smeared and stained by using May-Grunwald and Giemsa protocols. After the scoring at least 2000 (including polychromatic and normochromatic) erythrocytes per animal the percentage of micronucleated erythrocytes was calculated. Our findings showed that the percentage of MN formation in the BNZ-exposed group was significantly (p < 0.05) higher than the control group both in the bone marrow and peripheral blood samples. At the same time, administration of QCN alone for 28 days only reduced the MN formation in peripheral blood. Quercetin at all three given dose levels protected from the BNZ-induced MN formation in the bone marrow, while only at the medium and maximum dose levels was able to reduce significantly the MN formation in the peripheral blood (Table 3).
Table 3.
Effects of Quercetin on the Benzene-induced Micronucleus formation in the bone marrow and peripheral blood.
| Groups | MN (%) in bone marrow | MN (%) in peripheral blood |
|---|---|---|
| Control | 6.4 ± 1.1 | 1.15 ± 0.25 |
| BNZ | 10.7 ± 1.0 * | 1.8 ± 0.2 * |
| QCN | 4.4 ± 1.0 | 0.7 ± 0.07 * |
| BNZ + QCN 10 | 8.0 ± 0.5 # | 1.25 ± 0.3 |
| BNZ + QCN 50 | 6.7 ± 1.7 # | 1.14 ± 0.26 # |
| BNZ + QCN 100 | 7.1 ± 0.7 # | 1.2 ± 0.3 # |
Asterisks are representing significant differences (p < 0.05) between the control and Benzene-exposed groups and #s are demonstrating significant differences between the Benzene-exposed group and those groups, which concurrently both were exposed to benzene and treated with various dose levels of quercetin. BNZ: Benzene; QCN: Quercetin and MN: Micronucleus.
3.4. Quercetin lowered the Benzene-induced oxidative stress in the liver
To discover the impact of BNZ exposure and also the effect of QCN concurrent administration on oxidative stress status, total antioxidant capacity in serum and the concentration of total thiol molecules in the liver were assessed. Either biomarkers were declined remarkably in the BNZ-exposed animals when was compared with the corresponding control groups. Administration of QCN in non-BNZ-exposed mice resulted in a significant (p < 0.05) and insignificant (p > 0.05) elevation of TAC and TTM, respectively. Although QCN in BNZ-exposed mice enhanced the TAC and TTM in a dose-dependent manner, we however failed to show any statistically significant effect of QCN at the lowest given dose level on both TAC and TTM contents (Fig. 1A and B).
Fig. 1.
Effects of Quercetin on Benzene-induced oxidative stress; (A) total antioxidant capacity (TAC), (B) total thiol molecules (TTM), (C) MDA concentration and (D) Protein oxidation rate. Asterisks are representing significant differences (p < 0.05) between the control and Benzene-exposed groups and #s are demonstrating significant differences between the Benzene-exposed group and those groups, which concurrently both were exposed to benzene and treated with various dose levels of quercetin. BNZ: Benzene; QCN: Quercetin; MDA: Malondialdehyde.
The lipid peroxidation and protein oxidation in the liver were examined and results indicated that both oxidative biomarkers increased significantly in the BNZ-exposed animals. Twenty eight days administration of QCN alone very slightly elevated both factors in the liver tissue. Quercetin at 50 and 100 mg/kg dose levels significantly lowered both MDA and carbonylated protein levels in the liver. We found that QCN lowered the protein carbonylation and not lipid peroxidation in a dose-dependent fashion (Fig. 1C and D).
3.5. Quercetin lowered the BNZ-induced histopathological injuries in the liver
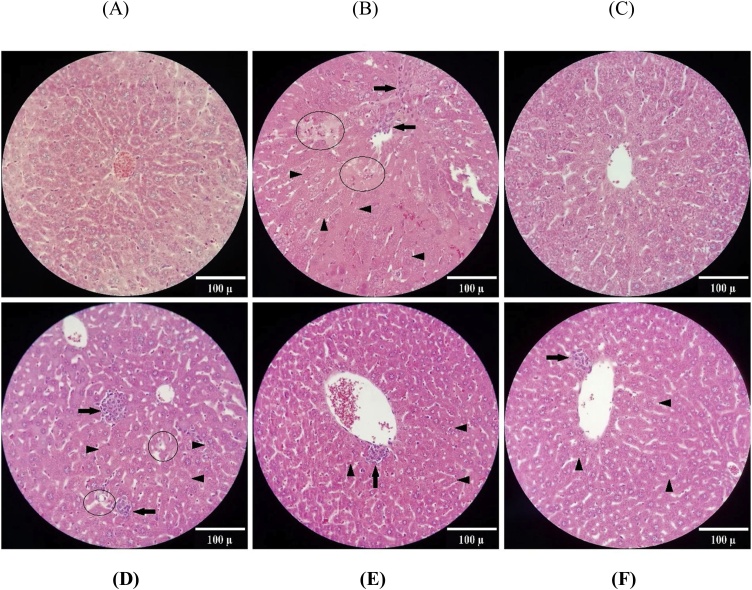
In the control group, the histological view of microscopic sections is normal and hepatocytes are representing normal membrane, cytoplasm and nucleus. No inflammatory cells infiltration is observed. In the BNZ-exposed group, small and abundant vacuoles inside the cytoplasm of hepatocytes have been formed. Moreover, necrosis of hepatocytes is also observed with hyaline cytoplasm, nuclei karyorrhexis, and disintegration of plasma membrane and extrusion of cytoplasm. Lobular inflammation of the liver in the BNZ-exposed group is characterized by the infiltration and aggregation of inflammatory cells, in particular close to the necrotic areas. In the group of animals that received only QCN, the histological view of the liver is normal like the control group. In the Q10 group, similar to the non-treated BNZ-exposed group, there are many histopathologic lesions such as necrotic damage, formation of small intracytoplasmic vacuoles and infiltration of inflammatory cells. Administration of 50 and 100 mg/kg QCN in the BNZ-exposed animals reduced the hepatic damages considerably. There is no remarkable difference between these two groups (Fig. 2A–F).
Fig. 2.
Photomicrographs of the liver sections from: (A) control, (B) BNZ-exposed, (C) QCN, (D) BNZ + QCN 10, (E) BNZ + QCN 50 and (F) BNZ + QCN 100. Arrow head: intracytoplasmic vacuoles, circle: necrotic areas, arrow: inflammatory cell infiltration (H&E staining 100 X). BNZ: Benzene; QCN: Quercetin.
4. Discussion
In this study the BNZ-induced genotoxicity in the bone marrow cells and also in peripheral erythrocytes were demonstrated. Moreover, hepatotoxicity of BNZ after 4 weeks exposure was also uncovered. Further the protective effects of QCN on the BNZ-induced hematoxicity and hepatotoxicity were characterized by its remarkable capacity in the reduction of BNZ-induced MN formation in the bone marrow cells and also in peripheral erythrocytes and the attenuation of the BNZ-induced hepatic functional and structural damages.
Benzene is an aromatic hydrocarbon and a ubiquitous air pollutant with limited direct reactivity. There are two main benzene exposure sources of occupational and non-occupational for human beings. The occupational exposure mostly is reported in workers, which are working in crude oil and natural gas production, refinery industry, petrol and other petroleum products distribution and also in those are working in car repair shops and petrol stations. The mean exposure level between < 0.002 and 32.5 ppm in occupational exposure has been reported [20]. On the other hand cigarette smoke and ambient air are the well-known sources of BNZ non-occupational exposure. Inhalation is the most important route of BNZ absorption in occupational and non-occupational exposures with 30–52% absorption of inhaled BNZ in humans [21]. Although benzene penetrates via skin, however its dermal absorption in normal working conditions is not extensive.
The toxicity of BNZ largely depends on metabolism, which in mammalian takes place mainly in the liver, where it is primarily metabolized into benzene oxide by Cytochrome P450 2E1 (CYP 2E1), which is later bioconverted to muconaldehyde and phenol. The phenol metabolites undergo further CYP 2E1 metabolism processes and form catechol and hydroquinone, which the later one due to high level of myeloperoxidase in the bone marrow is converted into benzoquinones. Previous studies on animal models demonstrated that the hematotoxicity of BNZ is due to the bone marrow depression, which results in an aplastic anemia with reduced blood cells. One of the well-known reason for BNZ-induced hematoxicity is the BNZ-metabolism related metabolites that damage the bone marrow hematopoietic stem cells. It has been reported that the electrophilic quinones metabolite and muconaldehyde are directly reacting with proteins and cellular nucleophiles including DNA. At the same time, the bone marrow-related myeloperoxidase produce some quinones and semiquinones, which in addition of directly binding to macromolecular, are contributing in the production of oxygen radicals by redox reaction [22]. Hence, the observed hematoxicity including a remarkable leukopenia and also a significant elevation of micronucleated erythrocytes in the bone marrow and peripheral blood are explained by the BNZ-related directly and indirectly acting metabolites.
Liver on the other hand, is one of the most susceptible organs to the chemicals-induced injuries. The hepatic susceptibility to chemicals mainly is related to its crucial role in the biotransformation of chemicals, which mostly if not absolutely, results in markedly detrimental effects on biochemistry of hepatocytes such as excessive generation of reactive oxygen species (ROS) that ultimately causes oxidative damages not only in the liver but also in other organs including the kidneys and hematopoietic system [23]. As our findings showed the BNZ exposure resulted in a remarkable elevation of oxidative stress biomarkers including the rate of lipid peroxidation and simultaneously a significant reduction of total thiol molecules. Along with oxidative stress another mechanism of the BNZ-induced toxicity in general and hepatotoxicity in particular is associated to its metabolism-related reactive metabolites binding to cellular macromolecules including nucleic acids, proteins and lipids. When the produced ROS level in cell is more than the cell's antioxidant capacity, the oxidative stress-induced damages such as the genotoxic DNA damage, as well protein and lipids damages, result eventually in genomic instability and/or disturbances in the function of enzymes and other elements of cells [24]. Hence, the remarkable increase of micronucleated erythrocytes either in the bone marrow or in peripheral blood supported the BNZ-induced hematotoxicity. Previous reports showed that BNZ and its reactive metabolites via activation of PI3K-AKT, p38 MAPK, or JNK signaling pathways stimulate apoptosis of bone marrow cells or malignant increase of leukemia cells [25,26]. Recent study in rodent model showed that the deletion of pp2r1a gene in hepatocytes suppressed the cyp2e1 transcription and in turn reduced the BNZ-induced hematotoxicity, suggesting a close association between the BNZ-induced hepatotoxicity and hematotoxicity [27].
Our findings regarding the hepatotoxicity of BNZ in mice showed a marked reduction of total thiol molecules as a biomarker of glutathione level, which accompanied by significant elevation of protein carbonylation rate, supporting another mechanism of the BNZ-induced toxicity via glutathione depletion [28]. The reason for glutathione depletion could be related to the extra requirement of liver cells to glutathione for conjugation reactions in BNZ metabolism. It has been reported that benzene oxide and hydroquinone as BNZ metabolites, which strongly are conjugated with glutathione, in addition of having glutathione depletion effect, due to accumulation in the bone marrow cause hematoxicity in rodent model [29]. Another finding of the current study is that the BNZ-exposed animals showed significant elevation of hepatic enzymes including AST and ALT that accompanied by the increased microvacuoles inside the cytoplasm, infiltration and aggregation of inflammatory cells. A marked elevation of inflammatory cytokines including IL-1β and IL-6 along with degenerated hepatocytes and inflammatory cells infiltration have been reported in gasoline fumes exposed rats, supporting our findings and indicating a BNZ-induced inflammatory condition and damage to the intracellular membranes in the liver [30].
The second part of this study devoted to unveil the protective effects of QCN against the BNZ-induced hematotoxicity and hepatotoxicity. To reach these goals we administered QCN at three dose levels simultaneously with BNZ-exposure and the obtained results show that QCN reduced the BNZ-induced hematological damages including MN percentage and leukopenia. Moreover, QCN reduced hepatotoxicity as the BNZ-induced oxidative stress, hepatic enzymes level and the liver histology were improved. It has been frequently reported that the toxicity of BNZ mainly associates to its biotransformation and production of reactive metabolites in the bone marrow and also in other organs such as liver. Hence, any approach that able to reduce or inhibit the BNZ metabolism and/or attenuate the BNZ-metabolites produced damages, could be categorized medicinally beneficial agent in the management of BNZ toxicities. It has also been previously reported that QCN showed a strong inhibitory effect on CYP 2E1 activity on human hepatic microsomes [11]. It seems QCN not only by inhibition of CYP2E1 activity and consequently by reduction of BNZ-related metabolites resulted in attenuation of MN percentage in bone marrow and in peripheral erythrocytes, but also increased the RBC number. The possible mechanism for QCN effect on RBC number may be related to its stimulatory effect on erythropoietin secretion [31]. At the same time, one of the key factors in the pathogenesis of the BNZ-induced toxicity undoubtedly related to its capacity to produce oxidative stress and consequently hematological disorders along with hepatic damages that were characterized by reduction of WBC and RBC numbers and elevation of hepatic enzymes and structural injuries. Quercetin as a nutritional flavonoid does have capacity to act as a strong antioxidant and a great endogenous glutathione restoring agent. Its antioxidant effect has been documented in the prevention of Aβ-induced oxidative stress and Alzheimer's disease [10].
Another BNZ-induced toxicity was found to be hepatotoxicity, which was characterized by inflammatory cells infiltration and elevated hepatic enzymes concentrations in serum. Quercetin on the other hand was able to ameliorate both inflammatory biomarkers. Quercetin’s anti-inflammatory effects have been demonstrated in the carbon tetrachloride-induced inflammation and fibrosis by reduction of produced inflammatory cytokines including TNF-α, IL-1β, IL-6 [32]. A recent ex vivo study showed that QCN inhibited lipopolysaccharide-induced inflammation via suppression of TLR2 gene expression and STAT3 protein phosphorylation, suggesting QCN capacity for treating inflammation-associated diseases [33]. At the same time, protective effects of QCN on the Imidoclopride-induced hepato- and nephrotoxicity in adult rats through its antioxidant activity has been documented (Abdel moniem et al., 2019).
5. Conclusion
Taken all together our study showed the protective effects of QCN against the BNZ-induced hematotoxicyt and also hepatotoxicity. Quercetin’s protective effects were demonstrated in the amelioration of hematological disorders, reduction of micronucleoted erythrocytes, lowered hepatic enzymes level in the serum, improved antioxidant status and reduced histological damages in the liver.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgement
The authors are grateful to Dr. Vardast MR. and Miss. Shima Zeinali-Moghadam for their remarkable helps and emotional supports. This study was financially supported by Ardabil University of Medical Sciences.
Handling Editor: Dr. Aristidis Tsatsakis
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2021.08.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Duarte-Davidson R., Courage C., Rushton L., Levy L. Benzene in the environment: an assessment of the potential risks to the health of the population. Occup. Environ. Med. 2001;58:2–13. doi: 10.1136/oem.58.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacLeod M., Mackay D. An assessment of the environmental fate and exposure of benzene and the chlorobenzenes in Canada. Chemosphere. 1999;38:1777–1796. doi: 10.1016/s0045-6535(98)00394-4. [DOI] [PubMed] [Google Scholar]
- 3.Costantini A.S., Benvenuti A., Vineis P., Kriebel D., Tumino R., Ramazzotti V., Rodella S., Stagnaro E., Crosignani P., Amadori D. Risk of leukemia and multiple myeloma associated with exposure to benzene and other organic solvents: evidence from the Italian multicenter case–control study. Am. J. Ind. Med. 2008;51:803–811. doi: 10.1002/ajim.20592. [DOI] [PubMed] [Google Scholar]
- 4.Abd El-Shakour A., El-Ebiarie A.S., Ibrahim Y.H., Moneim A.E.A., El-Mekawy A.M. Effect of benzene on oxidative stress and the functions of liver and kidney in rats. J. Environ. Occupational Health. 2015;4:34–39. [Google Scholar]
- 5.Snyder R., Witz G., Goldstein B.D. The toxicology of benzene. Environ. Health Perspect. 1993;100:293–306. doi: 10.1289/ehp.93100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pal M., Sharma C.K., Pandey P., Singh R., Meena Anil.K. Benzene induced hematotoxicity and recent scenarios. Int. J. Scientific Innov. Res. 2016;4:51–55. [Google Scholar]
- 7.Abarikwu S.O., Simple G., Onuoha S.C., Mokwenye I., Ayogu J.F. Evaluation of the protective effects of quercetin and gallic acid against oxidative toxicity in rat’s kidney and HEK-293 cells. Toxicol. Rep. 2020;7:955–962. doi: 10.1016/j.toxrep.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleemann R., Verschuren L., Morrison M., Zadelaar S., van Erk M.J., Wielinga P.Y., Kooistra T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218:44–52. doi: 10.1016/j.atherosclerosis.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Heijnen C.G., Haenen G.R., Minou Oostveen R., Stalpers E.M., Bast A. Protection of flavonoids against lipid peroxidation: the structure activity relationship revisited. Free Radic. Res. 2002;36:575–581. doi: 10.1080/10715760290025951. [DOI] [PubMed] [Google Scholar]
- 10.Li Y.L., Guo H., Zhao Y.Q., Li A.F., Ren Y.Q., Zhang J.W. Quercetin protects neuronal cells from oxidative stress and cognitive degradation induced by amyloid β-peptide treatment. Mol. Med. Rep. 2017;16:1573–1577. doi: 10.3892/mmr.2017.6704. [DOI] [PubMed] [Google Scholar]
- 11.Östlund J., Zlabek V., Zamaratskaia G. In vitro inhibition of human CYP2E1 and CYP3A by quercetin and myricetin in hepatic microsomes is not gender dependent. Toxicology. 2017;381:10–18. doi: 10.1016/j.tox.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Jacquot L., Pourie G., Buron G., Monnin J., Brand G. Effects of toluene inhalation exposure on olfactory functioning: behavioral and histological assessment. Toxicol. Lett. 2006;165:57–65. doi: 10.1016/j.toxlet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Farris G.M., Wong V.A., Wong B.A., Janszen D.B., Shah R.S. Benzene-induced micronuclei in erythrocytes: an inhalation concentration-response study in B6C3F1 mice. Mutagenesis. 1996;11:455–462. doi: 10.1093/mutage/11.5.455. [DOI] [PubMed] [Google Scholar]
- 14.Hooftman R.N., De Raat W. Induction of nuclear anomalies (micronuclei) in the peripheral blood erythrocytes of the eastern mudminnow Umbra pygmaea by ethyl methanesulphonate. Mutat. Res. Lett. 1982;104:147–152. doi: 10.1016/0165-7992(82)90136-1. [DOI] [PubMed] [Google Scholar]
- 15.Benzie I.F., Strain J. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth. Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 16.Miao-Lin H. Measurement of protein thiol groups and glutathione in plasma. Meth. Enzymol. 1994;233:380–383. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 17.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Niehaus Jr W., Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 19.Levine R. Carbonyl assay for determination of oxidatively modified proteins. Meth. Enzymol. 1994;233:246–257. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 20.Kirkeleit J., Riise T., Tore Gjertsen B., Moen B.E., Bråtveit M., Bruserud Ø. Effects of benzene on human hematopoiesis. Open Hematol. J. 2008;2:87–102. [Google Scholar]
- 21.Modjtahedi B.S., Maibach H.I. In vivo percutaneous absorption of benzene in man: forearm and palm. Food Chem. Toxicol. 2008;46:1171–1174. doi: 10.1016/j.fct.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Bolton J.L., Trush M.A., Penning T.M., Dryhurst G., Monks T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Checa J.C., Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol. Appl. Pharmacol. 2005;204:263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Vattanasit U., Navasumrit P., Khadka M.B., Kanitwithayanun J., Promvijit J., Autrup H., Ruchirawat M. Oxidative DNA damage and inflammatory responses in cultured human cells and in humans exposed to traffic-related particles. Int. J. Hyg. Environ. Health. 2014;217:23–33. doi: 10.1016/j.ijheh.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Jiang S., Chen Y., Ma R., Chen J., Qian S., Shi Y., Han Y., Zhang S., Yu K. Benzene metabolite hydroquinone induces apoptosis of bone marrow mononuclear cells through inhibition of β-catenin signaling. Toxicol. Vitr. 2018;46:361–369. doi: 10.1016/j.tiv.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Liu W.-H., Chou W.-M., Chang L.-S. p38 MAPK/PP2Acα/TTP pathway on the connection of TNF-α and caspases activation on hydroquinone-induced apoptosis. Carcinogenesis. 2013;34:818–827. doi: 10.1093/carcin/bgs409. [DOI] [PubMed] [Google Scholar]
- 27.Chen L., Guo P., Zhang H., Li W., Gao C., Huang Z., Fan J., Zhang Y., Li X., Liu X. Benzene-induced mouse hematotoxicity is regulated by a protein phosphatase 2A complex that stimulates transcription of cytochrome P4502E1. J. Biol. Chem. 2019;294:2486–2499. doi: 10.1074/jbc.RA118.006319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ola O.S., Sofolahan T.A. A monoterpene antioxidant, linalool, mitigates benzene-induced oxidative toxicities on hematology and liver of male rats. Egypt. J. Basic Appl. Sci. 2021;8:39–53. [Google Scholar]
- 29.Monks T.J., Butterworth M., Lau S.S. The fate of benzene-oxide. Chem. Biol. Interact. 2010;184:201–206. doi: 10.1016/j.cbi.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdrabouh A.E. Liver disorders related to exposure to gasoline fumes in male rats and role of fenugreek seed supplementation. Environ. Sci. Pollut. Res. - Int. 2019;26:8949–8957. doi: 10.1007/s11356-019-04307-x. [DOI] [PubMed] [Google Scholar]
- 31.Keskin E., Dönmez N., Kılıçarslan G., Kandır S. Beneficial effect of quercetin on some haematological parameters in streptozotocin-induced diabetic rats. Bull Environ. Pharmacol. Life Sci. 2016;5:65–68. [Google Scholar]
- 32.Li X., Jin Q., Yao Q., Xu B., Li L., Zhang S., Tu C. The flavonoid quercetin ameliorates liver inflammation and fibrosis by regulating hepatic macrophages activation and polarization in mice. Front. Pharmacol. 2018;9:72. doi: 10.3389/fphar.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao Y.-R., Lin J.-Y. Quercetin modulates cytokine expression and inhibits TLR2 expression and STAT3 activation in mouse activated inflammatory macrophages. J. Explor. Res. Pharmacol. 2020;5:31–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.