Abstract
Background
Studies have shown that the Klotho gene has tremendous potential for future therapeutic purposes in both acute and chronic kidney diseases (CKD). This study aimed to investigate the possible protective mechanisms of the Klotho gene against acute kidney injury (AKI) induced by rhabdomyolysis (RM).
Methods
In this study, bone marrow mesenchymal stem cells (BMSCs) were transfected with recombinant adenoviruses expressing the Klotho gene (BMSCs-Klotho) and by those expressing empty vector (BMSCs-EV). After successful transfection, we tested the proliferation, secretion and migration abilities of the BMSCs-Klotho compared with those of the BMSCs-EV and BMSCs. Then, 30 male C57BL/6 mice were examined, with 6 mice randomly assigned to the control group (PBS injected into the tail vein, CON) or one of the four treatment groups treated with either BMSCs-Klotho (AKI+BMSCs-Klotho), BMSCs-EV (AKI+BMSCs-EV), BMSCs (AKI+BMSCs) or PBS (AKI+PBS) after induction of RM. Seventy-two h after treatment, serum creatinine (SCr) and blood urea nitrogen (BUN) levels were obtained to assess renal function, and renal tissue was obtained to measure kidney tissue damage. Additionally, kidney protective mechanism-related indexes, such as EPO, IGF-1, KIM-1 and HIF-1, were analysed using Western blot analysis and immunohistochemistry.
Results
The results obtained showed that the proliferation, secretory and migration abilities of the BMSCs were significantly increased after transfection with the Klotho gene. Treatment with BMSCs-Klotho, BMSCs-EV or BMSCs improved renal function compared to treatment with PBS. However, the improvement observed in renal function in the BMSCs-Klotho group was better than that of the other groups. Histological analysis demonstrated that tissue damage was significantly decreased in the mice in the AKI+BMSCs-Klotho, AKI+BMSCs-EV or AKI+BMSCs groups compared to that in the mice in the AKI+PBS group. However, the best recovery was observed in the mice treated with BMSCs-Klotho concomitantly. Furthermore, the expression of protective factors erythropoietin (EPO) and insulin-like growth factor 1 (IGF-1) increased obviously, and the injury biomarkers kidney injury molecule 1 (KIM-1) and hypoxia inducible factor 1 (HIF-1) decreased notably in the group of BMSCs-Klotho, BMSCs-EV and BMSCs. Additionally, the levels of the aforementioned protein indicators in the AKI+BMSCs-Klotho group were not different from those in the CON group.
Conclusion
Klotho overexpression exerted positive effects on BMSCs and markedly promoted recovery from RM-induced AKI. These findings suggest that the overexpression of the Klotho gene might be a good candidate for further therapy for AKI in clinical trials.
Keywords: Klotho, BMSCs, AKI, Renal function, Tissue damage, EPO, IGF-1, KIM-1, HIF-1
1. Background
Acute kidney injury (AKI) has been reported in up to 18% of patients admitted for acute medical care [1]. AKI is associated with significant short-term morbidity and mortality, with hospital mortality rates exceeding 50% when severe AKI is complicated with critical illness [2]. In 2001, The US National Center for Health Statistics estimated that roughly $10 billion of expenditures are attributed to hospital-acquired AKI annually [3]. A preponderance of evidence supports the presence of links between AKI and chronic kidney disease (CKD) [4]. Pre-AKI baseline CKD was associated with a two-fold increase in mortality and a four-to five-fold increase in risk of CKD outcomes compared with patients without AKI [5]. Therefore, strategies specifically for reducing renal damage and CKD risk for patients suffering from AKI are needed [6].
Rhabdomyolysis-induced by glycerol injection is a well-established model of experimental AKI [7]. It is characterized by intense acute tubular necrosis of the renal cortex and inflammatory cell infiltration [8,9].
Mesenchymal stem cells (MSCs), also known as multipotent mesenchymal stromal cells, originally isolated from bone marrow and have also been found in other organs and tissues [10]. In 2013, Chen K et al. evaluated the biodistributions of transplanted MSCs in the organs of mice with cisplatin-induced AKI and found that transplanted cells can enter the kidneys and survive [11]. MSCs have regenerative capability and exert paracrine actions on damaged tissues [10]. There is increasing interest in utilizing MSCs in a broad repertoire of cell-based therapies for the treatment of diseases, including osteogenesis imperfecta, diabetes, and acute graft-versus-host disease [10,[13], [14], [15], [16], [17], [18]]. It has also been demonstrated that MSCs can ameliorate AKI and improve renal function by reducing apoptosis and alleviating the inflammatory response and oxidative stress [19,20]. In recent years, MSCs have been used in clinical trials [21,22]. Scientists have established several stem cell-based therapy modalities for the treatment of AKI. Many different types of stem cells, including haematopoietic progenitor cells [23], adipose-derived stem cells [24] and umbilical cord-derived mesenchymal stem cells [25], have been investigated and found to have therapeutic effects against AKI [26]. Bone marrow mesenchymal stem cells (BMSCs) were used in the experiment in this study. The characteristics of MSCs described above set a premise for the success of this experiment.
Klotho was initially identified as a suppressor of ageing [27]. In addition, the 130 kD form is a single transmembrane protein encoded by the Klotho gene. The 70 kD Klotho isoform is a product of alternative splicing with the extracellular domain of the membrane, which can be released into the blood, functioning as a circulating soluble Klotho [28]. Circulating soluble Klotho in the bloodstream may act as an endocrine factor with multiple remote functions, including ion channel regulation, anti-insulin action, anti-Wnt signalling activity, suppression of cell senescence, and antioxidation [[29], [30], [31]]. Defects in Klotho gene expression in mice results in a syndrome that resembles human ageing, including a short lifespan, infertility, arteriosclerosis, skin atrophy, osteoporosis and emphysema. Klotho is predominantly expressed in distal renal tubules of the kidney, parathyroid glands, and choroid plexus of the brain [32,33] and may function as an endocrine or paracrine hormone [34]. Klotho is downregulated during kidney injury [35]. Klotho protein and gene therapy for kidney disease have been shown to be more effective in reducing apoptosis, alleviating renal fibrosis, and mitigating inflammatory cytokines and oxidative stress [[36], [37], [38], [39]]. However, the specific mechanism of the Klotho gene in renal injury mitigation remains largely unknown.
In this experiment, we investigated the mechanisms of the Klotho gene action on the treatment of AKI by measuring relevant indexes.
2. Materials and methods
2.1. Isolation and expansion of BMSCs
MSCs from the bone marrow of 6-week-old to 8-week-old male C57BL/6 mice (Animal Center of Xuzhou Medical University) were isolated and cultured. Briefly, the mice were euthanized by cervical dislocation, and their tibias and femurs were cleared of muscle and connective tissue. Bone marrow cells were aspirated using an 18-gauge needle with phosphate-buffered saline (PBS). The purge PBS mixture was collected, and a density gradient was used to isolate the blood mononuclear fraction from whole bone marrow. The isolated cells were cultured in DMEM-low glucose (KaiJi, Nanjing), 10% foetal bovine serum (FBS, Cyagen Biosciences, Guangzhou, China) and antibiotics at 37 °C and 5% carbon dioxide in air (Heal Force Development LTD, Hong Kong) [40]. The growth medium was changed every day, and the cells were sub cultured when 80–90% confluent.
2.2. Characterization of BMSCs
Flow cytometry analyses were performed to detect CD34, CD44, CD45, and CD29 cells (all from eBioscience, US) using a flow cytometer (Beckman Coulter, CA, USA). The cells were prepared at a concentration of 1.0 × 105 cells in 100 μl of PBS. Antibodies, including anti-CD34 conjugated to FITC, anti-CD29 conjugated to FITC, anti-CD44 conjugated to PE, and anti-CD45 conjugated to eFluor, were added to the cells and incubated at 4 °C for 30 min. The cells were acquired after two washes in PBS and analysed with FACSCalibur flow cytometer (BD Bioscience).
Differentiation potential was examined by culturing these cells under favourable conditions for adipogenic and osteogenic differentiation using the method of Fishbane Sand colleagues [41].
2.3. Generation of BMSCs overexpressing the Klotho gene
BMSCs overexpressing Klotho were generated by transducing BMSCs with lentiviral-based transfer of the Klotho gene (BMSC-Klotho-green fluorescent protein [GFP], BMSCs-Klotho) or empty vector gene (BMSC-empty vector-GFP, BMSCs-EV) (Shanghai GeneChem Co., Ltd.). The BMSCs were transfected according to a previous report [42]. Briefly, BMSCs were plated in 25-cm2 flasks and grown to 50% confluence (~106 cells). The Cells were incubated for 12 h with lentivirus at a multiplicity of infection (MOI) of 10 in the presence of 8 μg polybrene ml−1 (Sigma, USA). The next day, the medium was replaced with 5 ml of fresh medium. Three days later, the transfected cells were cultured in complete medium containing 2 μg/l puromycin. The medium was replaced every three days for four cycles. After screening by puromycin, the GFP-expressing cells were collected. In addition, PCR, ELISA and western blotting were used to assess the expression of Klotho i the n BMSCs transduced with an empty vector or Klotho.
2.4. CCK-8 cell viability assay
Cell viability was detected via the CCK-8 method (Dojindo, Japan). Cells were divided into three groups, BMSCs, BMSCs-Klotho and BMSCs-EV, and the cells were seeded into 96-well plates at a density of 3000 cells/100 μl medium. DMEM containing 10% FBS was used as the blank control. As soon as the cells were stable (approximately 2 h later), 10% CCK-8 was added to the medium, and the plates were incubated for 30 min. Cell proliferation was measured on days 1, 2, and 3, and absorption was measured at 450 nm.
2.5. Cell cycle analysis
BMSCs, BMSCs-Klotho and BMSCs-EV were harvested, washed with cold phosphate-buffered saline (PBS), and fixed in 1 ml of 70% ethanol. After incubation overnight at 4 °C in ethanol, the cells were washed in PBS and suspended in 500 ml of propidine iodide (PI) for 30 min before flow cytometry. The percentages of the cell populations in the G0–G1, S, and G2/M phases were determined by flow cytometry (BD Biosciences, USA), and the data were analysed using multicycle-DNA cell cycle analysis software. Each measurement was performed in triplicate.
2.6. Transwell cell invasion assay
BMSCs, BMSCs-Klotho and BMSCs-EV were collected, and the cells were suspended in serum-free medium at a density of 1 × 105 cells/ml. Subsequently, 200 μl of each cell suspension was added to the upper chamber of Transwell chambers (Corning Life Sciences, Tewksbury, MA, USA) pretreated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA), and 500 μl of DMEM supplemented with 30% FBS was added to each lower chamber. After incubation in the incubator for 24 h at 37 °C, the cells on the upper surface of the microporous membrane were removed with cotton swabs, whereas the cells on the lower surface of the membrane were fixed with a 4% paraformaldehyde solution (Sinopharm, Shanghai, China) for 30 min at room temperature and subsequently stained with 0.5% crystal violet solution (Amresco, Solon, OH, USA). Images of the stained cells from five selected visual fields were captured under a light microscope (AE31; Motic Incorporation, Ltd., Xiamen, China) at 200× magnification, and the number of cells that migrated through the microporous membranes was calculated. All assays were performed in duplicate.
2.7. Wound healing assay
The cells were seeded into 6-well plates (1 × 105 cells per well). When the cells reached 80–90% confluence, 200 -μl pipette tips were used to make scratches on the monolayer cell surface. The cells were subsequently washed with serum-free medium and then incubated with serum-free DMEM at 37 °C. At 0, 12, and 24 h, each sample image was captured, and the data were recorded to calculate the relative cell mobility based on the following formula: relative mobility = distance between the edges of the initial scratches - between the edges of migrated scratches.
2.8. Enzyme-linked immunosorbent assay
After being cultured for 2 days in each group, the medium was collected for secretory cell function analysis. Vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), and hepatocyte growth factor (HGF), which are secreted by BMSCs, were measured using ELISA kits (Abcam, United Kingdom) following the manufacturer's instructions.
2.9. Labelling of the BMSCs for histological cell tracking
In this experiment, the BMSCs were adjusted to 106/ml and labeled with 10 μM green fluorescent tracer 5-Chloromethylfluorescein diacetate (CMFDA, Invitrogen, San Diego, CA, USA) for 30 min at 37 °C. After further centrifugation, the cells were resuspended in PBS (BMSCs ≈ 106 in 200 μl PBS) and kept on ice until infusion. We infused CMFDA-labeled BMSCs via the tail vein, and examined the kidneys on days 3 by confocal microscopy. The tissue used for light microscopy was fixed in 10% neutral-buffered formalin for 12 h, then embedded in OCT and cryosectioned.
2.10. Experimental animals and procedures
Eight-to twelve-week-old C57BL/6 male mice were obtained from the Experimental Animal Center of Xuzhou Medical University. The mice were housed at a constant room temperature with a 12-h light/dark cycle. Standard rodent chow and water were provided ad libitum. The animals were acclimated for seven days prior to initiating the experiment. All animal protocols were approved by the Animal Ethics Committee of the Chinese PLA General Hospital and Military Medical College.
Animals were divided into five groups (six mice per group), namely, AKI+BMSCs-Klotho, AKI+BMSCs-EV, AKI+BMSCs and AKI+PBS. As described previously, AKI was induced by rhabdomyolysis (RM) [43]. C57BL/6 mice were deprived of water for 24 h and then administered one-half the dose of glycerol (50% v/v in sterile saline) in each hindlimb muscle under light sedation with pentobarbital. Dose-dependent studies defined an optimal glycerol dose of 8 ml/kg body weight. Six h later, the mice were given an intravenous injection of BMSCs (BMSCs ≈ 106 in 200 μl PBS), BMSCs-Klotho (BMSCs-Klotho ≈ 106 in 200 μl PBS) and BMSCs-EV (BMSCs-EV ≈ 106 in 200 μl PBS) or an equal volume of PBS (200 μl PBS) into the tail vein. Only the control (CON) group received an injection of 200 μl of PBS into the vein without RM-induced AKI. All blood and kidney samples were harvested for further processing at 72 h.
To determine the biochemical variables serum creatinine (SCr) and blood urea nitrogen (BUN) using a biochemistry Autoanalyser, blood and tissue samples were collected at 72 h and stored at −80 °C. For histological analysis, kidney tissue samples from each group were fixed with formalin, embedded in paraffin, and sectioned to a thickness of 5 μm.
2.11. Haematoxylin and eosin (H&E) staining
Kidney sections were sliced (5 microns), dewaxed using hydration and xylene, stained with haematoxylin (5 min), treated with hydrochloric acid ethanol (30 s), and soaked in water (15 min) before staining with eosin (2 min). After conventional dehydration, transparency, and sealing, the sections were analysed as described below.
2.12. Immunohistochemistry
Briefly, paraffin-embedded kidney sections were deparaffinized by xylene and rehydrated in an alcohol series and water. Samples were incubated overnight with anti-mouse primary antibodies including anti-VEGF (1:1000, Abcam, USA), anti–HIF–1 (1:1000, Abcam, USA), anti-EPO (1:200, PTG, China), anti-KIM-1 (1:200, Absin, China) and anti-IGF-1 (1:300, PTG, China) at 4 °C, rinsed with PBS three times and incubated with biotinylated secondary antibody. Nuclei were visualized by counterstaining with Harris haematoxylin. The colour intensity was measured using Image-Pro Plus 6.0.
2.13. Western blot analysis
Cells and tissues were washed twice in ice-cold PBS and then lysed with RIPA lysis buffer for 30 min on ice. The lysates were centrifuged at 12,000 rpm for 20 min at 4 °C, and the supernatants were collected and used for evaluation of the relevant protein levels. The protein concentrations in each sample were measured using a BCA protein assay kit (Kaiji Biotechnology, Nanjing, China). Western blotting was performed according to standard protocols. We used the following anti-mouse primary antibodies: anti-Klotho (1:1000, Proteintech, USA), anti-CXCR4 (1:500, Proteintech, USA), anti-SDF-1 (1:200, Proteintech, USA), anti-CyclinD1 (1:200, Proteintech, USA), anti-EGF (1:1000, Abcam, USA), anti–HIF–1 (1:1000, Abcam, USA), anti-EPO (1:200, PTG, China), anti-KIM-1 (1:200, Absin, China), anti-IGF-1 (1:300, PTG, China), and anti-β-actin (1:1000, Santa Cruz Biotechnology, USA)., and we used IgG secondary antibodies (1:200, Santa Cruz Biotechnology, USA).
2.14. Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the cells using a total RNA extraction kit (Tiangen Biotech, Co., Ltd., Beijing, China) following the manufacturer's instructions. The extracted RNA from each group was reverse transcribed into cDNA using Super M-MLV reverse transcriptase (BioTeke Corporation, Beijing, China) and oligo (dT) 15. The mRNA expression levels of Klotho, CXCR4 and SDF-1 were detected by RT-qPCR on an Exicycler TM 96 Real-Time Quantitative PCR instrument (Bioneer Corporation, Daejeon, Korea) using cDNA as a template. The PCR conditions were as follows: 95 °C for 5 min; 95 °C for 20 s, 60 °C for 30 s, 72 °C for 20 s (40 cycles); 5 °C for 5 min. The relative mRNA levels in each sample were calculated using the 2-ΔΔCt method [44], with GAPDH as a reference. SYBR Green RT-PCR Master mix was purchased from Beijing Solarbio Science & Technology Co., Ltd. The following primers were used:
GAPDH forward AGGCCGGTGAGTATGTC and reverse 5′-TGCCTGCTTCACCACCTTCT-3′
Klotho forward ACTACGTTCAAGTGGACACTACT and reverse 5′-GATGGCAGAGAAATCAACACAGT-3′
CXCR4 forward GAAGTGGGGTCTGGAGACTAT and reverse 5′-TTGCCGACTATGCCAGTCAAG-3′
SDF-1 forward TGCATCAGTGACGGTAAACCA and reverse 5′-TTCTTCAGCCGTGCAACAATC-3′
3. Results
3.1. Bone mesenchymal stem cell phenotype
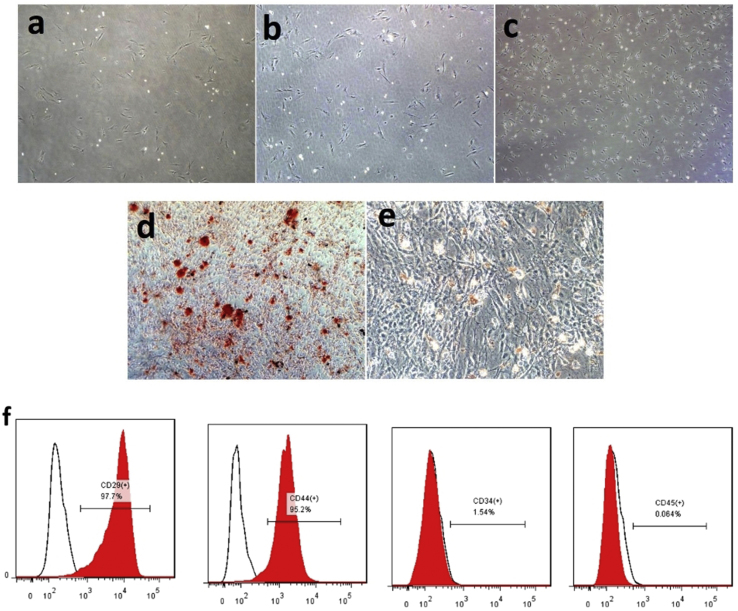
We verified the typical spindle-shaped morphology of the three generations of BMSCs under a light microscope (Fig. 1a–c). The BMSCs showed multiple differentiation appearances, and they were successfully differentiated into osteoblasts and adipocytes, as demonstrated by positive staining with Alizarin red and oil red O, respectively (Fig. 1d and e). The BMSCs were characterized by CD markers with FACS, which showed that the cells were positive for CD44 and CD29 and were negative for CD45 and CD34 (Fig. 1f). In this experiment, we used third-generation mesenchymal stem cells.
Fig. 1.
Characterization of BMSCs (a,b,c) Light microscopy revealed that the bone mesenchymal stem cells (BMSCs) were spindle-shaped. (a) (b) (c) represents the first generation, second generation and third generation of bone marrow mesenchymal stem cells respectively. (d) Alizarin Red S was used to show differentiation to osteocytes. (e) Oil Red O staining determined differentiation to adipocytes (Magnification, ×200). (f) Immunophenotype of isolated UC-MSCs. Isolated BMSCs were characterized by FACS. UC-MSCs were positive for CD29, CD44, and nearly negative for CD34 and CD45.
3.2. Efficient transfection of BMSCs by lentivirus overexpressing Klotho and an empty vector
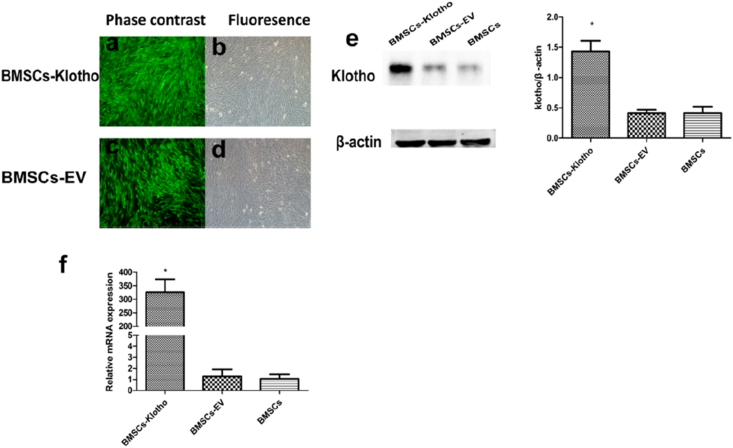
All cells, including BMSCs-Klotho and BMSCs-EV, significantly expressed GFP fluorescence, with a positivity rate of approximately 98%–100% (Fig. 2a–d). The protein expression of Klotho, measured using Western blot, and the mRNA expression of Klotho, quantified by qPCR, indicated that the expression levels of Klotho were significantly higher in the BMSCs-Klotho compared with those in the BMSCs and BMSCs-EV (P < 0.05; Fig. 2e and f). These results suggest that BMSCs-Klotho were successfully generated by retroviral infection.
Fig. 2.
Virus transduction ot BMSCs. BMSCs Transfected by lentivirus Klotho (a,b) and mediated-empty vector (c,d). In the BMSCs-Klotho and BMSCs-EV groups, cell were screened by Purinemycin with a concentration of 2 μg/ml. Western-blot (e) and qPCR (f) analyses were performed for high protein expression of Klotho in the Klotho-BMSCs group as compared with BMSCs-EV group and BMSCs group. Data presented as mean ± standard deviation (n = 3 each, ∗P ˂ 0.05 versus BMSCs-EV, BMSCs).
3.3. The overexpression of Klotho enhanced BMSC proliferation and secretory capacity
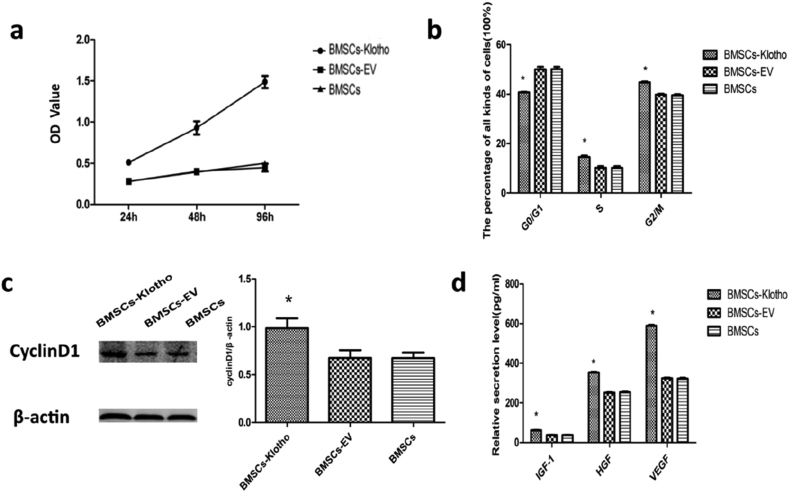
The proliferation ability of BMSCs overexpressing Klotho was evaluated using CCK-8 assay and cell cycle analysis. In addition, the expression of cyclin D1 protein, which promotes cellular proliferation [45], was also analysed. As shown by the CCK-8 results, viability of the BMSC-Klotho group cultured for 24 h was increased and the proliferation rate was significantly increased at 48 h and 96 h relative to the viability and proliferation of the BMSCs and BMSCs-EV (P < 0.05). The BMSC and BMSC-EV groups showed slower multiplication rates, and there was no difference between them (Fig. 3a).
Fig. 3.
Klotho enhance the proliferation ability and secretory levels of BMSCs. (a) The cell proliferation ability of BMSCs in three groups was determined by CCK-8 assay at 24 h, 48 h and 96 h (n = 9). (b) Cell cycle analysis of BMSCs, by comparing the percentage of cells, in each group, in S phase and G2/M phase and G0/G1 phase (n = 3). (c) Representative immunoblot for Cyclin D1 in each group. (d) The secretory levels of insulin-like growth factor-1 (IGF-1) Hepatocyte growth factor (HGF), vascular endothelial growth factor-A (VEGF-A) and by BMSCs in each group (n = 3). Data presented as mean ± standard deviation. ∗P < 0.05 versus BMSCs, BMSCs-EV.
Cell cycle analysis revealed that the transfected Klotho gene increased the percentage of cells in the S phase and G2/M phase compared with the percentages in the BMSC and BMSC-EV groups and a lower percentage of cells was in the G0/G1 phase (P < 0.05). There was no difference between the BMSC group and the BMSC-EV group (Fig. 3b). Consistent with these results, in the BMSC-Klotho group, Western blot analysis revealed a concomitant increase in the expression of cyclin D1 (P < 0.05; Fig. 3c). These results suggest that Klotho overexpression in BMSCs greatly increases cell proliferation in vitro. The proliferation capability of the BMSCs-Klotho showed a marked difference compared with that of the BMSCs and BMSCs-EV. In addition, there was no difference between BMSCs and BMSCs-EV.
The effects of Klotho gene overexpression on the secretory capacity of BMSCs in repairing AKI mechanisms (VEGF, IGF-1, and HGF) were also evaluated by ELISAs. As the results showed, VEGF, IGF-1, and HGF secreted by BMSCs-Klotho exhibited an increasing tendency (P < 0.05), and the secretory capacity of the BMSCs-EV and BMSCs did not show an obvious increase (Fig. 3d).
3.4. BMSC migration in vitro was enhanced by transfection with the Klotho gene
A wound-healing assay was performed to detect changes in the migration capacity of the cells, an essential early step in the paracrine process and differentiation of MSCs in injured organs. The results of the wound-healing assay demonstrated that the relative mobility of the BMSCs cells was significantly faster following the overexpression of the Klotho gene compared with that of the cells in the BMSC-EV and BMSC groups (P < 0.05; Fig. 4a and b). In addition, Transwell invasion assays were also performed to evaluate the effect of cells in the BMSC-Klotho group. Following transfection with Klotho, the results revealed that more cells migrated through the pores of the membrane from the bottom of the upper wells to the opposite side of the membrane at 24 h and 48 h (P < 0.05; Fig. 4c and d, respectively).
Fig. 4.
Klotho gene promotes the migration and invasion of BMSCs. (a,b) Following transfection, a wound-healing assay was performed to investigate changes in migration capacity (n = 3, magnification. ×200). (c,d) A Transwell invasion assay was performed to detect cell invasion capacity following transfection (n = 9). Images were captured under a light microscope (magnification, ×200). The experimental results are presented as the means ± standard deviation. ∗P < 0.05 versus BMSCs, BMSCs-EV.
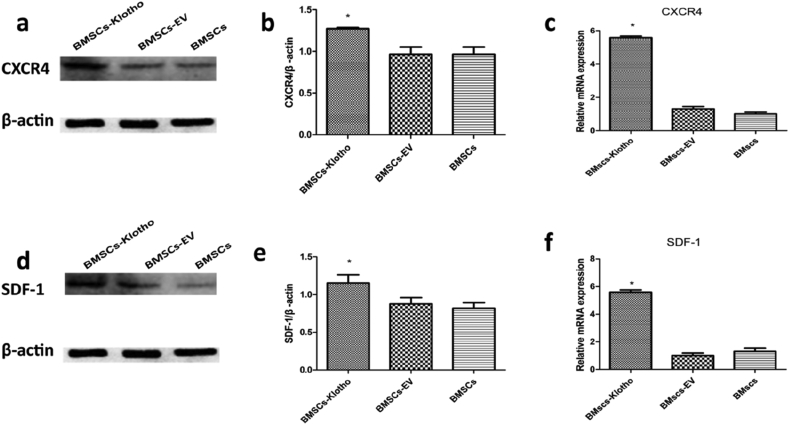
The expression of SDF-1 and its CXCR4, involved in the migration of cells to sites of injury, was tested by western blotting and qPCR [[46], [47], [48], [49]]. CXCR4 is normally expressed in bone marrow (BM) stem cells and was upregulated significantly in the BMSC-Klotho group compared to its expression in the BMSC-EV and BMSC groups (P < 0.05; Fig. 5a and b). The expression level of SDF-1 was highest in the BMSC-Klotho group relative to the BMSC-EV and BMSC groups (P < 0.05; Fig. 5c and d). In addition, the qPCR results were consistent with the Western blot results, and the mRNA expression of SDF-1 and CXCR4 was profoundly increased in the BMSC-Klotho group compared with the BMSC-EV and BMSC groups. There was no difference in the expression of mRNA between the BMSC-EV and BMSC groups (P < 0.05; Fig. 5e and f).
Fig. 5.
Klotho gene increased the relevant migration protein expression after rhabdomyolysis (RM)-induced acute kidney injury (AKI). (a,b) The expression of CXCR4 was tested by Western blot. (c.d) The expression of SDF-1 was tested by Western blot. (e,f) The mRNA expression of CXCR4 and SDF-1 was examined by qRCR respectively. Date presented as mean ± standard deviation. ∗P < 0.05 versus BMSCs-EV group and BMSCs group.
These results suggest that Klotho gene overexpression in BMSCs increases the expression of CXCR4 and SDF-1, which is beneficial for BMSC transplantation in the kidney. We can hypothesize that more cells can arrive at the kidney.
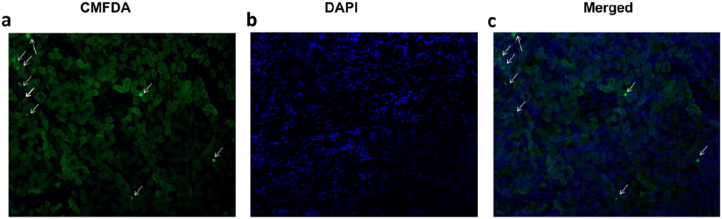
3.5. Chloromethylfluorescein diacetate-labeled BMSCs are recruited to the kidney
We observed that BMSCs were recruited to the kidneys, most of them localized to the cortical and medullary tubular tissue of kidneys. Enlarged views showed that every CMFDA-labeled BMSC presented a nucleus counterstained with 4′,6-diamidino- 2- phenylin -dole (DAPI, Fig. 6a–c) consistent with our previous study.
Fig. 6.
BMSCs are recruited to the kidney. (a) 5-Chloromethylfluorescein diacetate (CMFDA)-labeled BMSCs. (b) 4′,6-diamidino-2-phenylindole (DAPI), and (c) merged channels, (n = 3; ↓: CMFDA-labeled BMSCs; Magnification, ×200).
3.6. Bone mesenchymal stem cells transfected with the Klotho gene protected renal function against RM-induced AKI
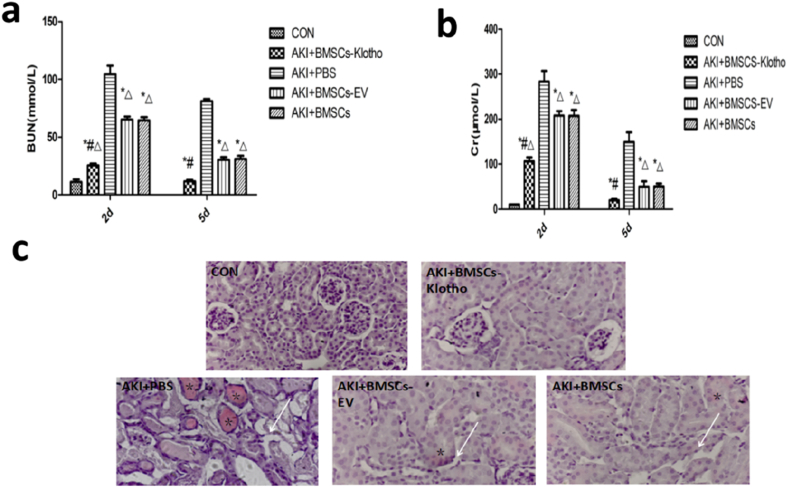
Renal function level was assessed in PBS-treated mice at 72 h (SCr, 149.50 ± 21.51 μmol/L; BUN, 81.12 ± 1.17 mmol/L), and it was significantly higher than that of the CON group. There were marked decreases in creatinine and BUN levels at 72 h in the BMSC-Klotho, BMSC-EV and BMSC groups compared with those of the PBS group (P < 0.05). However, renal function in the BMSC-Klotho group was not different from that of the CON group (P > 0.05). BMSCs-Klotho infusion markedly reduced the levels of SCr (Fig. 7a) and BUN (Fig. 7b) 72 h after glycerol injection compared with the levels in the mice administered BMCs, BMSCs-EV, or PBS(P < 0.05).
Fig. 7.
Klotho gene ameliorate rhabdomyolysis (RM)-induced acute kidney injury (AKI) and prevents tubular injury (a) Creatinine and (b) blood urea nitrogen (BUN) levels at 2 day and 5 day for Rhabdomyolysis-induced AKI model mice treated with PBS, BMSCs-Klotho, BMSCs-EV and BMSCs (n = 6/each group). Date presented as mean ± standard deviation. ∗P < 0.05 versus PBS group, #P < 0.05 versus BMSCs-EV group and BMSCs group, ΔP < 0.05 versus CON group at the corresponding times. (c) The overexpression of Klotho prevents tubular injury and attenuates kidney peritubular capillary loss. Representative light microscopy images of hematoxylin and eosin-stained kidney sections on day 3 in the CON group, BMSCs-Klotho group, BMSCs-EV group and BMSCs group (∗: vascular blockage; ↓: Vascular necrosis; Magnification, ×200).
3.7. Bone mesenchymal stem cells transfected with the Klotho gene alleviated renal tissue damage
Because maximum AKI was achieved at 72 h, according to Fu M a et al. [45], we selected a 72-h time point to evaluate kidney injury. To evaluate the therapeutic effect of BMSCs, BMSCs-EV and BMSCs-Klotho in the AKI model, the pathological changes in the kidney tubules, kidney glomeruli, and collecting tubules of each group were observed using H&E staining. Notable damage, including tubular necrosis, dilatation, and effusion in the kidney tubules, was observed in the AKI+PBS model group compared with the CON group. Various degrees of amelioration were observed in the AKI+BMSC, AKI+BMSC-EV and AKI+BMSC-Klotho groups compared with the AKI+PBS group. There was no difference between the BMSC group and the BMSC-EV group in terms of histopathology. The BMSC-Klotho group exhibited fewer necrotic and dilated tubules and less effusion in the tubules (Fig. 7c).
3.8. Bone mesenchymal stem cells transfected with the Klotho gene increased the protein expression of Klotho in renal tissues after RM-induced AKI
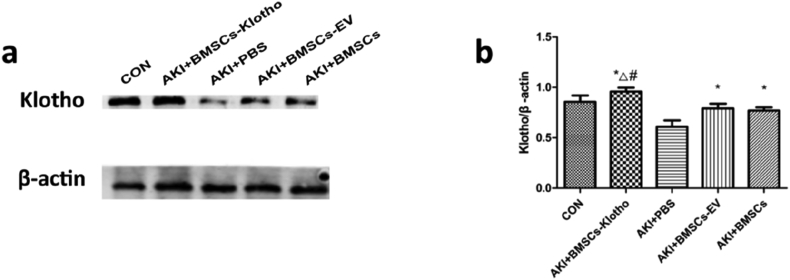
AKI reduces the expression of Klotho protein levels in renal tissue. However, protein expression in the BMSC-Klotho group was highest compared with the BMSC-EV, BMSC and PBS groups (P < 0.05) and was close to that of the control group. However, there was no difference between the BMSC group and the BMSC-vector group (P > 0.05) (Fig. 8a and b).
Fig. 8.
Analysis of Klotho expression in different groups by Western blot. (a) The expression of Klotho was tested by Western blot (n = 3). (b) Quantification of the result of Western blot. Date presented as mean ± standard deviation. ∗P < 0.05 versus PBS group, #P < 0.05 versus BMSCs-EV group and BMSCs group, ΔP < 0.05 versus CON group at the corresponding times.
3.9. Bone mesenchymal stem cells transfected with the Klotho gene increased EPO expression after RM-induced AKI
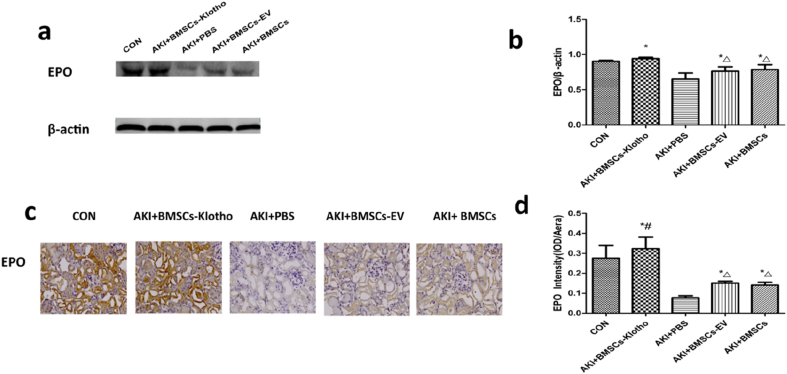
EPO was shown to be indispensable in the protection against AKI, as it helps enhance functional and morphologic tissue recovery [50]. Renal EPO protein levels decreased after the glycerol treatment. However, with BMSC-Klotho, BMC and BMSC-EV treatment, EPO increased at 72 h compared to EPO level after treatment with PBS (P < 0.05). The expression of EPO was significantly higher in the AKI+BMSC-Klotho group than in the AKI+BMSC-EV and AKI+BMSC groups, according to the Western blot analysis and immunohistochemical assay results (P < 0.05). However, there was no difference in EPO expression between the AKI+BMSC-EV group and AKI+BMSC group (P > 0.05) (Fig. 9a–d).
Fig. 9.
Klotho gene increased the EPO expression after rhabdomyolysis (RM)-induced acute kidney injury (AKI). (a,b) The expression of EPO was tested by Western blot. (c,d) The expression of EPO protein was examined by immunohistology (magnification, ×200). Date presented as mean ± standard deviation. ∗P < 0.05 versus PBS group. #P < 0.05 versus BMSCs-EV group and BMSCs group, ΔP < 0.05 versus CON group.
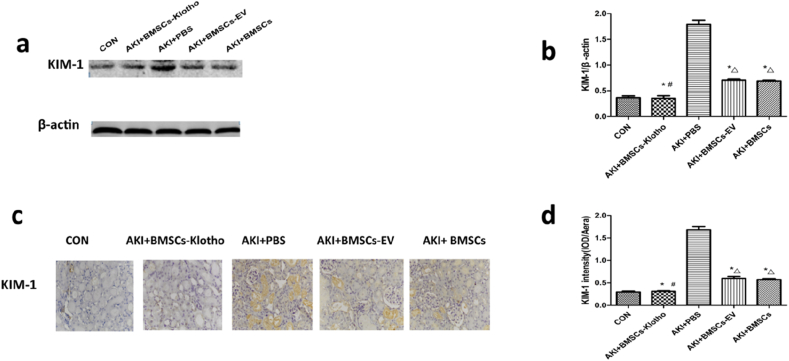
3.10. Bone mesenchymal stem cells transfected with the Klotho gene decreased KIM-1 expression after RM-induced AKI
The expression of KIM-1 protein in the kidney was elevated 72 h after treatment. With BMSC-Klotho, BMSC-EV and BMSC treatments, —renal KIM-1 level was lower than that after the PBS treatment following glycerol administration (P < 0.05; Fig. 10a and b). KIM-1 expression was negligibly detected by immunohistochemical staining in the untreated mouse kidneys. In the PBS-treated mouse kidneys, the KIM-1 staining was more intense and was localized mainly to the apical membranes of proximal tubular epithelial cells along in some damaged tubules. In contrast, the BMSC-EV- and BMSC-treated mice exhibited less extensive distribution of KIM-1 staining in the tubular epithelial cells, and there were fewer KIM-1-positive tubules in the mice treated with BMSCs-Klotho (P < 0.05; Fig. 10c and d).
Fig. 10.
Klotho gene decreased the KIM-1 expression after rhabdomyolysis (RM)-induced acute kidney injury (AKI). (a,b) The expression of KIM-1 was tested by Western blot. (c,d) The expression of KIM-1 protein was examined by immunohistology (magnification, ×200). Date presented as mean ± standard deviation. ∗P < 0.05 versus PBS group, #P < 0.05 versus BMSCs-EV group and BMSCs group, ΔP < 0.05 versus CON group.
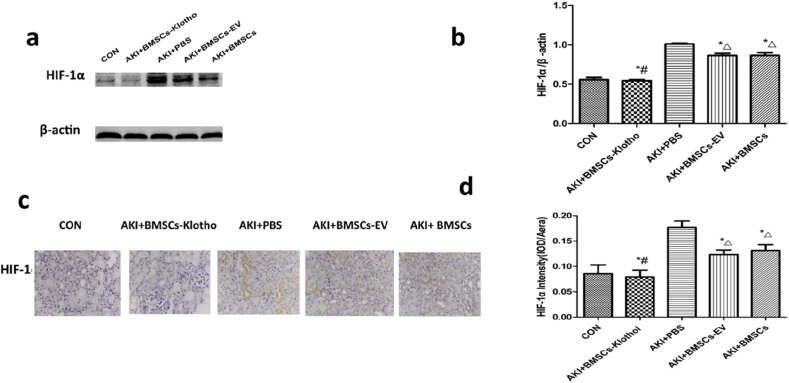
3.11. Bone mesenchymal stem cells transfected with the Klotho gene attenuated HIF-1 expression after rhabdomyolysis-induced AKI
Hypoxia-inducible factor (HIF) is a master regulator that mediates the adaptive response to hypoxia in cells and tissues. Under hypoxic conditions, HIF-1 protein accumulates [51]. In general, we did not find any differences in HIF-1 expression among the BMSC-EV and BMSC groups at 72 h (P > 0.05). BMSC-Klotho inhibited HIF-1 expression after glycerol treatment compared to the PBS, BMSC-EV and BMSC treatment groups (P < 0.05; Fig. 11a and b). HIF-1 localization at the basement membranes of proximal convoluted tubules was more clearly blocked by BMSC-Klotho treatment than it was after other treatments administered alone (P < 0.05). The expression of HIF-1 was similar to that of the CON group (P > 0.05) (Fig. 11c and d).
Fig. 11.
Klotho gene decreased the HIF-lα expression after rhabdomyolysis (RM)-induced acute kidney injury (AKI). (a,b) The expression of HIF-lα was tested by Western blot. (c,d) The expression of HIF-lα protein was examined by immunohistology (magnification, ×200). Date presented as mean ± standard deviation. ∗P < 0.05 versus PBS group. #P < 0.05 versus BMSCs-EV group and BMSCs group, ΔP < 0.05 versus CON group.
3.12. Bone mesenchymal stem cells transfected with the Klotho gene increased IGF-1 expression after rhabdomyolysis-induced AKI
Insulin-like growth factor-1 (IGF-1) is a multifunctional hormone that has pleiotropic effects on cellular proliferation, apoptosis, hypertrophy, and differentiation [52]. IGF-1 has been proven to have a remission effect on AKI [25]. We analysed the protein expression of IGF-1 after AKI. Decreased IGF-1 expression was observed after PBS, BMSC-EV and BMSC treatment and was negligibly decreased after the BMSC-Klotho intervention at the same time point (P < 0.05; Fig. 12a and b). Furthermore, in the BMSC-EV group and BMSC group, the protein expression of IGF-1 exceeded that of the PBS group. After BMSC-Klotho treatment, tissue IGF-1 staining was also more intense at 72 h compared with that of the mice receiving PBS, BMSC-EV and BMSC treatments (P < 0.05; Fig. 12c and d).
Fig. 12.
Klotho gene increased the IGF-1 expression after rhabdomyolysis (RM)-induced acute kidney injury (AKI). (a,b) The expression of IGF-l was tested by Western blot. (c,d) The expression of IGF-1 protein was examined by immunohistology (magnification, ×200). Date presented as mean ± standard deviation. ∗P < 0.05 versus PBS group. #P < 0.05 versus BMSCs-EV group and BMSCs group, ΔP < 0.05 versus CON group.
4. Discussion
Klotho not only serves as an early biomarker for AKI but also functions as a renal-protective factor with therapeutic potential. A study by Ming-Chang Hu et al., proved the protective effects of Klotho by supplementing recombinant Klotho protein in a Klotho gene deficiency model [53]. Although the therapeutic effect of human Klotho against AKI has been established, optimizations of Klotho gene overexpression therapy in renal repair remain limited.
Recently, several BMSC-based gene therapy studies in renal repair have been conducted, such as those looking at the therapeutic effects of HGF-modified MSCs in ischaemia/reperfusion-induced AKI rat models, overexpression of Nrf2 within MSC-protected rats against acute kidney injury, and enhanced renal protective effect of IGF-1-modified human umbilical cord-derived mesenchymal stem cells on gentamicin-induced acute kidney injury. In previous studies, we verified that most MSCs can be recruited to the kidneys and localize to the cortical and medullary tubular tissue of injured kidneys, especially in the outer medulla where the proximal tubules are located, and that the number of recruited MSCs decreased over time [25,[54], [55], [56]].
Considering the previous experiment, we modified the BMSCs with the Klotho gene. Enhancement of Klotho expression and secretion in BMSCs is a feasible strategy to improve the therapeutic effects against AKI. Additionally, after transfection with Klotho, the secretion of VEGF, IGF-1, and HGF and the proliferation ability of BMSCs, which are beneficial for the recovery of AKI, also significantly increased. On the other hand, in our study, BMSCs-Klotho also showed stronger migratory ability, compared with normal BMSCs and BMSCs-EV. As tested using Western blot and qPCR, SDF-1 and its ligand CXCR4 were upregulated by Klotho overexpression in the BMSCs, and this was considered the key mechanism of enhanced cell migration in our research. The increase in the migration capability of BMSCs-Klotho was confirmed by the Transwell and wound healing assays.
RM is a clinical syndrome characterized by injury to skeletal muscle fibres with disruption and release of its contents into the circulation. The severity of RM escalates from myoglobinuria, which can result in AKI. Glycerol-induced AKI is characterized by myoglobinuria, tubular necrosis and oxygen metabolites [57].
In this study, two essential indexes of renal function, SCr levels and BUN, were substantially lower in the BMSC-Klotho treatment group than in the other groups and were not different from the CON group. The levels of BUN and SCr in the AKI+BMSC-EV and AKI+BMSC groups were not different but were lower than that of the AKI+PBS group. Furthermore, histopathological renal injury sections also demonstrated that BMSCs modified by Klotho were superior to BMSCs and BMSCs-EV at improving impaired renal function and attenuating kidney damage.
Some growth factors and chemokines such as EPO and IGF-1 that enhance epithelial proliferation, modulate inflammation, and promote angiogenesis, are therefore good candidates for AKI therapy. In this study, we found that the overexpression of the Klotho gene increased the expression of EPO and IGF-1 in kidney tissue compared with the other groups, as tested using western blotting and immunohistochemistry. It has also been reported that EPO has antiapoptotic and anti-inflammatory properties that protect tissues and enhance the tubular regeneration epithelium [58]. Therefore, it is reasonable to believe that the overexpression of Klotho can reduce inflammation and apoptosis by increasing EPO. Therefore, in further studies, we can test the relevant factors and indicators of inflammation and apoptosis, such as caspase 3, Bax, Fas, MCP-1 and IL-10.
KIM-1, which can estimate the degree of kidney injury, has been recognized as a sensitive biomarker of kidney injury after AKI [59]. We observed that the expression of KIM-1 increased in the group treated with PBS and decreased with BMSC-Klotho treatment. KIM-1 levels remained higher when treated with BMSCs-EV and BMSCs than with BMSCs-Klotho. Thus, we can conclude that Klotho played crucial roles in alleviating renal injury in this model.
Reviewing the relevant articles from the past 10 years, it appears that hypoxia occurs throughout the course of AKI. HIF-1 is activated under hypoxic conditions. Some scholars believe that HIF-1 is a core regulatory factor responding to oxygen deficiency and blood deficiency after AKI [60,61]. We found that, after PBS treatment, HIF-1 levels were higher than those after AKI+BMSC-EV, AKI+BMSC or AKI+BMSCs-Klotho treatment. This result indicates that the kidney tissues in the AKI+PBS group were in a period of repair after tissue injury. Tissue hypoxia was more serious than in the AKI+BMSC-EV, AKI+BMSC and AKI+BMSC-Klotho groups. Because the expression of HIF-1 was not different between the BMSC-Klotho group and the CON group, we can infer that the renal tissue environment of the AKI+BMSC-Klotho group was almost the same as that of the CON group. We can also conclude that the Klotho gene can accelerate the repair and improve the hypoxia of kidney tissues.
5. Conclusions
In summary, we conclude that Klotho overexpression exerts positive effects on BMSCs. The overexpression of Klotho enhanced the secretion, proliferation and migration of mesenchymal stem cells. Furthermore Klotho gene markedly promotes recovery from RM-induced AKI, by attenuating HIF-1 and KIM-1 expression, increasing EPO and IGF-1 expression at the same time.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
WenHui Ni, Email: 1512424536@qq.com.
Ying Zhang, Email: 2540122360@qq.com.
Zhongcheng Yin, Email: yzcxyfy@126.com.
References
- 1.Ftouh S., Lewington A. Prevention, detection and management of acute kidney injury: concise guideline. Clin Med. 2014;14(1):61. doi: 10.7861/clinmedicine.14-1-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellomo R., Kellum J.A., Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 3.Chertow G.M., Burdick E., Honour M., Bonventre J.V., Bates D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. [J] J Am Soc Nephrol: Jasn. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 4.Hsu C.Y. Yes, AKI truly leads to CKD[J] J Am Soc Nephrol. 2012;23(6):967–969. doi: 10.1681/ASN.2012030222. [DOI] [PubMed] [Google Scholar]
- 5.Rifkin D.E., Coca S.G., Kalantarzadeh K. Does AKI truly lead to CKD? [J] J Am Soc Nephrol: Jasn. 2012;23(6):979. doi: 10.1681/ASN.2011121185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belayev L.Y., Palevsky P.M. The link between AKI and CKD[J] Curr Opin Nephrol Hypertens. 2014;23(2):149. doi: 10.1097/01.mnh.0000441051.36783.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfert A.I., Oken D.E. Glomerular hemodynamics in established glycerol-induced acute renal failure in the rat. J Clin Invest. 1989;84(6):1967. doi: 10.1172/JCI114386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homsi E., Janino P., de Faria J.B. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure[J] Kidney Int. 2006;69(8):1385. doi: 10.1038/sj.ki.5000315. [DOI] [PubMed] [Google Scholar]
- 9.Liang X., Ding Y., Zhang Y., Tse H., Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 10.Ueno T., Nakashima A., Doi S., Kawamoto T., Honda K., Yokoyama Y. Mesenchymal stem cells ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-β1 signaling. Kidney Int. 2013;84(2):297–307. doi: 10.1038/ki.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xinaris C., Morigi M., Benedetti V., Imberti B., Fabricio A., Squarcina E. A novel strategy to enhance mesenchymal stem cell migration capacity and promote tissue repair in an injury specific fashion[J] Cell Transplant. 2013;22(3):423. doi: 10.3727/096368912X653246. [DOI] [PubMed] [Google Scholar]
- 13.Pereira R.F., O’Hara M.D., Laptev A.V., Halford K.W., Pollard M.D., Class R. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta[J] Proc Natl Acad Sci U S A. 1998;95(3):1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee R.H., Seo M.J., Reger R.L., Spees J.L., Pulin A.A., Olson S.D. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103(46):17438. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Ying, Xu Huitao, Xu Wenrong, Wang Bingying, Wu Huiyi, Tao Yang. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis[J] Stem Cell Res Ther. 2013;4(2):34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le B.K., Rasmusson I., Sundberg B., Götherström C., Hassan M., Uzunel M. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 17.Li H., Guo Z., Jiang X., Zhu H., Li X., Mao N. Mesenchymal stem cells alter migratory property of T and dendritic cells to delay the development of murine lethal acute graft-versus-host disease. Stem Cell. 2008;26(10):2531. doi: 10.1634/stemcells.2008-0146. [DOI] [PubMed] [Google Scholar]
- 18.Zappia E., Casazza S., Pedemonte E., Benvenuto F., Bonanni I., Gerdoni E. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy[J] Blood. 2005;106(5):1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 19.Djouad F., Fritz V., Apparailly F., Louis P.P., Bony C., Sany J. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52(5):1595–1603. doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- 20.Augello A., Tasso R., Negrini S.M., Cancedda R., Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56(4):1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 21.Li L., Black R., Ma Z., Wang A., Lin F. Use of mouse hematopoietic stem and progenitor cells to treat acute kidney injury[J] Am J Physiol Ren Physiol. 2012;302(1):F9. doi: 10.1152/ajprenal.00377.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y., Hogan W.J. Clinical application of mesenchymal stem cells in the treatment and prevention of graft-versus-host disease[J] Adv Hematol. 2011;2011:427863. doi: 10.1155/2011/427863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martell K., Trounson A., Baum E. Stem cell therapies in clinical trials: workshop on best practices and the need for harmonization. Cell Stem Cell. 2010;7(4):451. doi: 10.1016/j.stem.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Wang W.W., Li Z.Z., Wang W., Jiang Y., Cheng J., Lu S. Enhanced renoprotective effect of HIF-1α modified human adipose-derived stem cells on cisplatin-induced acute kidney injury in vivo[J] Sci Rep. 2015;5:10851. doi: 10.1038/srep10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P., Feng Y., Dong D., Liu X., Chen Y., Wang Y. Enhanced renoprotective effect of IGF-1 modified human umbilical cord-derived mesenchymal stem cells on gentamicin-induced acute kidney injury[J] Sci Rep. 2015;6:20287. doi: 10.1038/srep20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroo M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T. Mutation of the mouse klotho gene leads to a syndrome resembling ageing[J] Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 27.Hu M.C., Kuroo M., Moe O.W. Secreted Klotho and chronic kidney disease[J] Contrib Nephrol. 2013;180:47–63. doi: 10.1159/000346778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu M.C., Moe O.W. Klotho as a potential biomarker and therapy for acute kidney injury[J] Nat Rev Nephrol. 2012;8(7):423–429. doi: 10.1038/nrneph.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu M.C., Shi M., Han J.C., Zhang J.N., Pavlenco A., Liu S.Z. The erythropoietin receptor is a downstream effector of Klotho-induced cytoprotection[J] Kidney Int. 2013;84(3):468–481. doi: 10.1038/ki.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu M.C., Shiizaki K., Kuro M. Fibroblast growth factor 23 and klotho: physiology and pathophysiology of an endocrine network of mineral metabolism[J] Annu Rev Physiol. 2013;75(1):503. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurosu Hiroshi, Yamamoto Masaya, Clark Jeremy D., Pastor Johanne V., Nandi Animesh, Gurnani Prem. Suppression of aging in mice by the hormone klotho. Science (New York, N.Y.) 2005;309(5742):1829. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imura A., Tsuji Y., Murata M., Maeda R., Kubota K., Iwano A. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316(5831):1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 33.Kurosu H., Kuroo M. The Klotho gene family and the endocrine fibroblast growth factors. Curr Opin Nephrol Hypertens. 2008;17(4):368–372. doi: 10.1097/MNH.0b013e3282ffd994. [DOI] [PubMed] [Google Scholar]
- 34.Aizawa H., Saito Y., Nakamura T., Inoue M., Imanari T., Ohyama Y. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun. 1998;249(3):865–871. doi: 10.1006/bbrc.1998.9246. [DOI] [PubMed] [Google Scholar]
- 35.Sugiura H., Yoshida T., Mitobe M., Yoshida S., Shiohira S., Nitta K. Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70[J] Nephrol Dial Transplant. 2010;25(1):60–68. doi: 10.1093/ndt/gfp451. [DOI] [PubMed] [Google Scholar]
- 36.Doi S., Zou Y., Togao O., Pasto J.V., John G.B., Wang L. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286(10):8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno Juan A., Izquierdo Maria C., Sanchez-Niño Maria D., Suárez-Alvarez B., Lopez-Larrea C., Jakubowski A. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB.[J] J Am Soc Nephrol: JASN. 2011;22(7):1315. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito K., Ishizaka N., Mitani H., Ohno M., Nagai R. Iron chelation and a free radical scavenger suppress angiotensin II-induced downregulation of klotho, an anti-aging gene, in rat. FEBS Lett. 2003;551(1–3):58–62. doi: 10.1016/s0014-5793(03)00894-9. [DOI] [PubMed] [Google Scholar]
- 39.Mitobe M., Yoshida T., Sugiura H., Shirota S., Tsuchiya K., Nihei H. Oxidative stress decreases klotho expression in a mouse kidney cell line[J] Nephron Exp Nephrol. 2005;101(2):e67. doi: 10.1159/000086500. [DOI] [PubMed] [Google Scholar]
- 40.Altun B., Yilmaz R., Aki T., Akoglu H., Zeybek D., Piskinpasa S. Use of mesenchymal stem cells and darbepoetin improve ischemia-induced acute kidney injury outcomes. Am J Nephrol. 2012;35(6):531–539. doi: 10.1159/000339167. [DOI] [PubMed] [Google Scholar]
- 41.Fishbane S., Ragolia L., Palaia T., Johnson B., Elzein H., Maesaka J.K. Cytoprotection by darbepoetin/epoetin alfa in pig tubular and mouse mesangial cells[J] Kidney Int. 2004;65(2):452–458. doi: 10.1111/j.1523-1755.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang W.W., Wang W., Jiang Y., Li Z.Z., Cheng J., Liu N.M. Human adipose-derived stem cells modified by HIF-1α accelerate the recovery of cisplatin-induced acute renal injury in vitro[J] Biotechnol Lett. 2014;36(3):667. doi: 10.1007/s10529-013-1389-x. [DOI] [PubMed] [Google Scholar]
- 43.Geng Y., Zhang L., Fu B., Zhang J., Hong Q., Hu J. Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages[J] Stem Cell Res Ther. 2014;5(3):80. doi: 10.1186/scrt469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawyer M.G., Pfeiffer S., Sawyer A., Vierlinger K., Weinhäusel A. A survey of tools for the analysis of quantitative PCR (qPCR) data[J] Biomol Detect Quantif. 2014;1(1):23. doi: 10.1016/j.bdq.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu M., Rao M., Bouras T., Wang C., Wu K., Zhang X. Cyclin D1 inhibits peroxisome proliferator-activated receptor gamma-mediated adipogenesis through histone deacetylase recruitment[J] J Biol Chem. 2005;280(17):16934. doi: 10.1074/jbc.M500403200. [DOI] [PubMed] [Google Scholar]
- 46.Sabbahy M.E., Vaidya V.S. Ischemic kidney injury and mechanisms of tissue repair[J] Wiley Interdiscip Rev Syst Biol Med. 2011;3(5):606–618. doi: 10.1002/wsbm.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cell. 2010;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 48.Tögel F., Isaac J., Hu Z., Weiss K., Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67(5):1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 49.Lotan D., Sheinberg N., Kopolovic J., Dekel B. Expression of SDF-1/CXCR4 in injured human kidneys. [J] Pediatr Nephrol. 2008;23(1):71. doi: 10.1007/s00467-007-0648-2. [DOI] [PubMed] [Google Scholar]
- 50.Sharples E.J., Patel N., Brown P., Stewart K., Mota-Philipe H., Sheaff M. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion[J] J Am Soc Nephrol: Jasn. 2004;15(8):2115. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]
- 51.Hill P., Shukla D., Tran M.G.B., Aragones J., Cook H.T., Carmeliet P. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury[J] J Am Soc Nephrol: Jasn. 2008;19(1):39–46. doi: 10.1681/ASN.2006090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura T., Ebihara I., Nagaoka I., Tomino Y., Nagao S., Takahashi H. Growth factor gene expression in kidney of murine polycystic kidney disease. J Am Soc Nephrol: Jasn. 1993;3(7):1378–1386. doi: 10.1681/ASN.V371378. [DOI] [PubMed] [Google Scholar]
- 53.Hu M.C., Shi M., Zhang J., Quiñones H., Kuro-o M., Moe O.W. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78(12):1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xing L., Cui R., Peng L., Ma J., Chen X., Xie R.J. Mesenchymal stem cells, not conditioned medium, contribute to kidney repair after ischemia-reperfusion injury. Stem Cell Res Ther. 2014;5(4):1–12. doi: 10.1186/scrt489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y., Qian H., Zhu W., Zhang X., Yan Y., Ye S. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cell Dev. 2011;20(1):103. doi: 10.1089/scd.2009.0495. [DOI] [PubMed] [Google Scholar]
- 56.Mohammadzadehvardin M., Habibi R.M., Jahaniannajafabadi A. Adenovirus-mediated over-expression of Nrf2 within mesenchymal stem cells (MSCs) protected rats against acute kidney injury. Adv Pharmaceut Bull. 2015;5(2):201. doi: 10.15171/apb.2015.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh A.P., Junemann A., Muthuraman A., Jaggi A.S., Singh N., Grover K. Animal models of acute renal failure[J] Pharmacol Rep. 2012;64(1):31–44. doi: 10.1016/s1734-1140(12)70728-4. [DOI] [PubMed] [Google Scholar]
- 58.Spandou E., Tsouchnikas I., Karkavelas G., Dounousi E., Simeonidou C., Guiba-Tziampiri O. Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model[J] Nephrol Dial Transplant. 2006;21(2):330. doi: 10.1093/ndt/gfi177. official publication of the European Dialysis and Transplant Association - European Renal Association. [DOI] [PubMed] [Google Scholar]
- 59.Bonventre J.V. Kidney Injury Molecule-1 (KIM-1): a specific and sensitive biomarker of kidney injury[J] Scand J Clin Lab Investig Suppl. 2008;68(sup241):78–83. doi: 10.1080/00365510802145059. [DOI] [PubMed] [Google Scholar]
- 60.Guillemin K., Krasnow M.A. The hypoxic response: huffing and HIFing.[J] Cell. 1997;89(1):9. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- 61.Schödel J., Klanke B., Weidemann A., Buchholz B., Bernhardt W., Bertog M. HIF-prolyl hydroxylases in the rat kidney: physiologic expression patterns and regulation in acute kidney injury[J] Am J Pathol. 2009;174(5):1663. doi: 10.2353/ajpath.2009.080687. [DOI] [PMC free article] [PubMed] [Google Scholar]